Abstract
Since the isolation and purification of erythropoietin (EPO) in 1977, the essential role of EPO for mature red blood cell production has been well established. The cloning and production of recombinant human EPO led to its widespread use in treating patients with anaemia. However, the biological activity of EPO is not restricted to regulation of erythropoiesis. EPO receptor (EPOR) expression is also found in endothelial, brain, cardiovascular and other tissues, although at levels considerably lower than that of erythroid progenitor cells. This review discusses the survival and proliferative activity of EPO that extends beyond erythroid progenitor cells. Loss of EpoR expression in mouse models provides evidence for the role of endogenous EPO signalling in nonhaematopoietic tissue during development or for tissue maintenance and/or repair. Determining the extent and distribution of receptor expression provides insights into the potential protective activity of erythropoietin in brain, heart and other nonhaematopoietic tissues.
Erythropoietin (EPO) is the hormone necessary for production of red blood cells that function primarily to transport oxygen from the lungs to tissues. EPO is synthesised in the adult kidney and can be induced by anaemia or hypoxic stress to increase the number of red blood cells and oxygen transport. EPO secreted into the circulation targets the haematopoietic stem cell in the bone marrow to stimulate differentiation along the erythroid lineage and acts as a survival factor for erythroid progenitor cells. EPO is essential for regulation of haematocrit since the red blood cell lifespan of 120 days requires the production of about two million red blood cells per second. Human EPO was first purified in 1977 from the urine of aplastic anaemia patients (Ref. 2). Cloning of the EPO gene led to the production of recombinant EPO (Ref. 3, 4) and its clinical use for the past two decades for the treatment of anaemia such as that associated with chronic kidney disease. Increased availability of the recombinant protein has facilitated structure and function studies of EPO and its receptor, and the discovery of its pleiotropic activity.
Erythropoietin and red blood cell production
EPO protein
Structural studies of human EPO revealed that it is a single polypeptide of 166 amino acids folded into four α-helices that are linked by two disulphide bridges between cysteines 6 and 161 and between cysteines 29 and 33 (Ref. 3, 4, 6). EPO shares structural homology with growth hormone and other members of the haematopoietic class 1 cytokine superfamily (Ref. 8). The molecular mass varies from 30 to 34 kDa, depending on the carbohydrate content. The composition of EPO is about 60% protein and 40% carbohydrate, with three N-glycosylation sites at asparagines 24, 38 and 83, and one O-glycosylation site at serine 126 (Ref. 9). Each N- and O-glycosylation site can accommodate up to four and two sialic acid residues, respectively, although O-linked glycosylation of EPO does not appear to be required for in vivo biological activity (Ref. 10, 11). Nonsialated EPO is rapidly cleared from the circulation via the galactose receptor in the liver (Ref. 12, 13).
Site of EPO production
During development, definitive erythropoiesis takes place in the fetal liver. EPO is required for survival of erythroid progenitor cells at the CFU-E (eythroid colony-forming unit) stage and is produced primarily in the fetal liver by hepatocytes (Ref. 14, 15). In addition to EPO production in fetal liver, EPO gene expression in kidney begins from about 18 weeks in humans, and, at birth, when the site of haematopoiesis switches to the bone marrow, the kidney becomes the predominant EPO-producing organ (Ref. 16, 17, 18).
EPO expression in fetal liver, adult liver and adult kidney is inducible by hypoxia (Ref. 16, 19, 20). In mouse during hypoxic induction of Epo, the adult liver can contribute as much as 20–50% of the total Epo produced (Ref. 21, 22). In situ hybridisation and studies in transgenic mice, including mice carrying a reporter gene inserted into a large Epo gene fragment, were useful in localising specific Epo-producing cells; in the kidney these have been identified as the interstitial peritubular cells with neural characteristics (Ref. 16, 23, 24). Hypoxic induction of EPO production up to 150-fold or more in the kidney results from an increase in the number of EPO-producing cells rather than from an increase in EPO production per cell (Ref. 16, 21, 25). Regulation of EPO expression in the liver and kidney appears to require different flanking gene elements, which are located immediately 3′ for liver expression and 6–14 kb 5′ for kidney expression (Ref. 19, 26).
Regulation of EPO
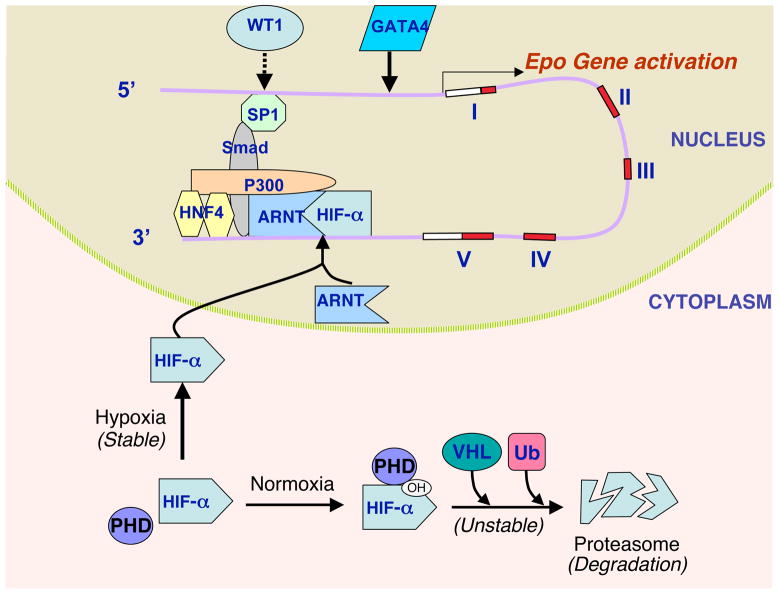
EPO induction by hypoxia is a critical response for regulation of red blood cell production by anaemic stress or chronic hypoxia. At the cellular level, EPO production is mainly determined by its gene transcriptional activity. The EPO gene has a 3′ enhancer active in hepatocytes that contains a hypoxia-responsive element (HRE) and provides hypoxic induction via interaction with multiple transcription factors including the hypoxia-inducible factor HIF (Ref. 27, 28, 29, 30). HIF is an important regulatory factor for activation of genes in response to physiological stress or hypoxia, such as vascular endothelial growth factor (VEGF), EPO and the glucose transporter GLUT-1 (SLC3A1). HIF consists of a constitutive β-subunit (also called the arylhydrocarbon receptor nuclear translocator; ARNT) that localises in the nucleus, and an α-subunit [HIF-1α (HIF1A), HIF-2α (EPAS1) or HIF-3α (HIF3A)] found in the cytoplasm (Ref. 30, 31). At normoxia, the α-subunit is proline hydroxylated, which leads to ubiquitination by interaction with the von Hippel–Lindau protein and rapid degradation by the proteasome. The α-subunit is stabilised by hypoxia and is transported to the nucleus where it forms a dimer with ARNT to affect specific gene activation, depending in part on the α-subunit (Ref. 4, 32) (Fig. 1). Induction of transcription through the 3′ HRE in the EPO gene in Hep3G hepatocytes has provided much insight on the regulation of HIF-1 activity by hypoxia (Ref. 29). Human genetic mutations that disrupt the normal function of proline hydroxylase or von Hippel–Lindau protein have been identified as causes for abnormally high levels of EPO production that result in polycythaemia (Ref. 33, 34). Manipulating levels of HIF-1α and HIF-2α using RNA interference and genetically modified mouse models indicate that HIF-2α plays a critical role in erythropoiesis and Epo hypoxic induction (Ref. 35, 36, 37, 38), while HIF-1α preferentially activates VEGF and GLUT-1 (Ref. 39). In patients with excessive erythrocytosis, a gain of function mutation in the HIF-2α gene has been determined to be a genetic lesion in one family, demonstrating that HIF-2α is an important contributor to EPO regulation (Ref. 40).
Figure 1. Hypoxia induction of erythropoietin gene expression.
(a) At normoxia, the hypoxia-inducible factor HIF-α is hydroxylated at its prolines by prolyl hydroxylase (PHD), which enables interaction with the von Hippel–Lindau (VHL) protein and ubiquitin (Ub); HIF-α is then polyubiquitinated and degraded by the proteasome. (b) Under hypoxic conditions, HIF-α is stabilised and translocated to the nucleus, where it binds its partner ARNT/HIF-1β to activate erythropoietin (EPO) gene transcription via binding to a 3′ enhancer (Ref. 27). Other factors also contribute to EPO transcription through stabilisation of a macromolecular complex at this 3′ site and reinforcement of enhancer–promoter contact: these include HNF4, p300 (EP300), SMAD3 and SP1 ((Ref. 29, 47, 210) (Ref. 55) Please fix reference format. The open arrow indicates that GATA factors can bind to the 5′ promoter of EPO, and can act as an inducer (GATA4) or repressor (GATA2) in EPO-expressing cells and as a repressor (GATA2/GATA3) in nonexpressing cells (Ref. 16, 55). The dashed arrow indicates that WT1 contributes to tissue-specific EPO expression in hepatocytes (Ref. 46). The five exons of the EPO gene (shaded) and the 3′ transcribed untranslated region (open) are shown schematically on the representation of DNA. Abbreviations: ARNT, arylhydrocarbon receptor nuclear translocator; p300, E1A binding protein p300 (EP300; also known as CBP/p300); HNF4, hepatocyte nuclear factor 4; SMAD3, SMAD family member 3; SP1, Sp1 transcription factor.
Hypoxic regulation of EPO also requires hepatocyte nuclear factor 4 [HNF-4 (HNF4A)], which is constitutively expressed in the major sites of EPO production: the fetal liver and the adult kidney. HNF-4 binds to the EPO 3′ enhancer adjacent to the HIF-binding site (Ref. 41). HIF and HNF-4 interact to form a macromolecular complex with transcription coactivator EP300 (also known as p300) for activation of stimulus-specific and tissue-specific gene transcription (Ref. 29, 42). In the fetal liver during early definitive erythropoiesis, the transcription factor GATA4 (see next paragraph) and the retinoic acid receptor RXRA contribute to high EPO gene activation (Ref. 43). RXRA competes with HNF-4 for occupancy in the 3′ EPO enhancer, and is no longer necessary later in development as HNF-4 activity in the fetal liver increases (Ref. 44). The Wilms tumour suppressor, WT1, directly affects EPO expression in hepatocytes and its targeted deletion in mice decreases erythropoiesis in fetal liver (Ref. 45) (Ref. 46) Reformat references. Transcription factors SMAD3, which binds to the 3′ EPO enhancer, and SP1, which binds to the promoter region, also cooperate with HIF, HNF-4 and EP300 in EPO gene transcription and induction by hypoxia (Ref. 47). Together these factors act to stabilise the multifactorial complex interacting with the 3′ enhancer and reinforce the promoter–enhancer contact.
The GATA transcription factors bind the DNA motif GATA, and belong to the zinc finger protein family. GATA1, GATA2 and GATA3 are primarily involved in haematopoiesis, and their expression is also seen in some nonhaematopoietic tissues such as the kidney, urinary tract and nervous system (Ref. 48, 49, 50, 51). GATA4, GATA5 and GATA6 are found in the heart, gut and extraembryonic endoderm (Ref. 52, 53, 54). In fetal liver, GATA4 activates EPO gene expression, recruits chromatin-modifying factors to the EPO promoter to confer an open chromatin configuration and may participate in the switch of EPO gene expression from fetal liver to adult kidney (Ref. 55). GATA1, GATA2 and GATA3 can inhibit EPO expression through binding in the 5′ promoter region (Ref. 56). GATA2 recruits histone deacetylases to downregulate EPO expression and mediates suppression of expression by proinflammatory cytokines (Ref. 57). Transgenic mouse studies showed that a single nucleotide mutation in the GATA box of the EPO gene promoter abolishes the repression by GATA2 and GATA3, and causes ectopic expression (Ref. 16). Finally, it was shown that EPO itself regulates expression of GATA1 and GATA2 during erythroid differentiation (Ref. 58) and GATA3 in neural cells and skeletal muscle myoblasts (Ref. 59, 60).
EPO receptor
During erythroid differentiation of haematopoietic stem cells, EPO acts through binding of its receptor (EPOR) on the surface of early erythroid progenitor cells to promote cell survival, proliferation and differentiation down the erythroid lineage (Ref. 61, 62). EPOR is expressed at low levels on early erythroid progenitor cells, the BFU-E (erythroid burst-forming unit) (Ref. 63). It is then upregulated during erythroid differentiation and increases by tenfold or more on the erythroid progenitor cells by the CFU-E stage, which requires EPO signalling for protection against apoptosis. EPO binding to erythroid progenitor cells upregulates expression of its own receptor, which increases EPO response as well as expression of erythroid-specific transcription factors such as GATA1, SCL/TAL1 and EKLF (KLF1), and other erythroid-specific genes (Ref. 64). During late erythropoiesis, EPOR expression is downregulated, and EPO is no longer required for cell survival (Ref. 58). The erythroid precursors enucleate to form reticulocytes that continue to synthesise haemoglobin, and then enter into the circulation to mature into red blood cells. EPO activity in haematopoietic tissue indicates that productive EPO signalling depends on both the level of EPOR cell-surface expression and on the local EPO concentration.
The expression of EPOR extends beyond haematopoietic cells and includes endothelial, neural, muscle, cardiovascular and renal tissues (Ref. 60, 65, 66, 67, 68). EPO acting as a survival factor and promoting proliferation and differentiation of the CFU-E provides insight on its role in nonhaematopoietic cells. EPO is associated with cytoprotective and/or proliferative activity in these nonhaematopoietic tissues (Ref. 69), and thus, may contribute to EPO activity to improve oxygen delivery via a direct effect on nonhaematopoietic tissue in addition to increased red blood cell production, increase cell survival and/or progenitor cell proliferation relating to ischaemic response or tissue repair.
EPOR in erythroid progenitor cells
EPOR on the surface of erythroid progenitor cells binds to EPO as a homodimer (Fig. 2). The EPOR polypeptide has a single transmembrane domain and a cytoplasmic domain that lacks tyrosine kinase activity (Ref. 70). The human EPOR gene product is encoded as 508 amino acids from eight exons spanning over 6.5 kb (Ref. 71). The exons vary in length from 29 to 203 amino acids, with the membrane-spanning region encoded in exon 6 and much of the cytoplasmic region encoded in exon 8. The extracellular domain consists of a short α-helix followed by two seven-stranded β-sheet domains (Ref. 72). Four of the five cysteine residues in the extracellular domain and a Trp-Ser-X-Trp-Ser motif located proximal to the membrane are conserved across the haematopoietic cytokine superfamily receptors that include the receptors for interleukin (IL)-2, -3 and -4, prolactin and thrombopoietin (Ref. 73, 74). The Trp-Ser-X-Trp-Ser motif contributes to the EPOR expression on the cell surface, EPO binding and EPOR activation (Ref. 75). With high levels of EPOR expression, only a small fraction is processed into mature EPOR on the cell surface, with a half-life of 1 h or less (Ref. 74). Moved from above
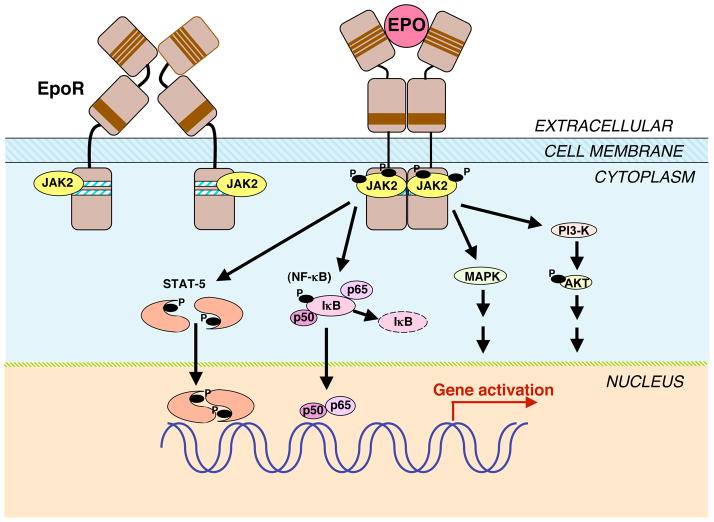
Figure 2. Erythropoietin signalling pathways.
The binding of erythropoietin (EPO) to its homodimeric receptor (EPOR) results in a conformation change and transphosphorylation of associated tyrosine kinase JAK2. JAK2 activation results in phosphorylation of EPOR and STATs. Activated STATs dimerise and translocate to the nucleus to affect specific downstream gene transcription. JAK2 also activates other signal transduction pathways, including the phosphoinositide 3-kinase (PI3K/AKT), mitogen-activated protein kinase (MAPK), and nuclear factor (NF) κB (p50 and p65) pathways in nonhaematopoietic cells. In EPOR, the four conserved cysteines (thin lines) and the proximal conserved Trp-Ser-X-Trp-Ser motif (thick line) in the extracellular domain, as well as the conserved Box1/Box2 regions (heavy lines) in the membrane-proximal cytoplasmic domain are indicated. Abbreviations: IκB, inhibitor of κB; JAK, Janus kinase; p50 and p65, NF-κB subunits STAT, signal transducer and activator of transcription’.
As EPOR lacks an intrinsic tyrosine kinase domain, the nonreceptor tyrosine kinase JAK2 is essential for signalling of EPO (Fig. 2) – as is the case for signalling by other ligands of the haematopoietic cytokine receptor superfamily members, such as thrombopoietin and IL-3 (Ref. 76). Studies of JAK2 mutations indicate that JAK2 binding to EPOR at its Box1/Box2 regions (Fig. 2) allows for the progression of JAK2 from an inactive state when not in contact with EPOR to an active state with the potential to be activated following EPO stimulation (Ref. 1). EPO binding to the EPOR dimer induces a conformational change that brings the cytoplasmic domains and hence the associated JAK2 proteins in closer proximity to each other resulting in the transphosphorylation and activation of JAK2 and EPOR (Fig. 2) (Ref. 5). Targeted deletion of JAK2 resembles deletion of EPO or EPOR (Ref. 7). Mutations in exon 12 of JAK2 that increase JAK2 activity are associated with polycythaemia vera and cytokine-independent growth of bone marrow cells in culture (Ref. 77, 78, 79). These include the substitution of phenylalanine for valine at position 617 detected in approximately 95% of polycythaemia vera patients and other somatic gain-of-function mutations (Ref. 80).
Epo activation of JAK2 via EpoR gives rise to tyrosine phosphorylation of STAT5, its translocation to the nucleus and induction of STAT5 target genes. Complete knockout of STAT5 results in loss of the anti-apoptotic proteins and marked decrease in the trans-acting iron regulatory protein, IRP2 in the fetal liver accompanied with reduction in the transferin receptor and iron uptake (Ref. 81). Mice that lack STAT5 develop a severe embryonic microcytic anemia and die prior to or shortly after birth. Conversely, activation of STAT5 rescues fetal liver cells from the JAK2 or EpoR knockout mice and allows erythropoiesis and erythroid progenitor cell proliferation to proceed (Ref. 82).
The EPOR cytoplasmic domain contains tyrosines that provide phosphorylation sites with EPO signalling that act as docking sites for signalling molecules such as the mitogen-activated protein kinase (MAPK), but deletion of the distal cytoplasmic region and associated tyrosines indicate that this region is not required for erythroid mitogenic activity of EPOR (Ref. 83). The EPOR C-terminal region also contains a negative-growth-regulatory domain that affects signal transduction or receptor processing (Ref. 84), and can hamper EPOR exit from the endoplasmic reticulum and contribute to its degradation (Ref. 85, 86). EPO binding to its receptor mediates endocytosis and EPOR is subsequently either resecreted intact or degraded in lysosomes at a rate determined by its dissociation from its receptor (Ref. 87, 88). Following EPO binding, EPOR can also be ubiquitinated and the C-terminal portion degraded by proteasomes while still on the cell surface via β-transducin repeat-containing protein (β-TRCP/BTRC) (Ref. 89). Deletion of the C-terminal region that includes the β-TRCP-interacting region and the proximal docking site for the negative regulator SHP1 (a protein tyrosine phosphatase) has been identified in congenital polycythaemia patients (Ref. 90, 91, 92).
EPOR transcriptional regulation
EPOR is expressed in a cell-restricted fashion and expression depends on the stage of differentiation. The EPOR proximal promoter is characterised by an inverted SP1-binding site (CCGCCC) and an inverted GATA1-binding site (TTATCT) located at positions −151 and −179 from the first codon of the human EPOR gene, respectively (Ref. 71). The SP1 motif that binds the SP1 transcription factor is required for high-level EPOR promoter activity (Ref. 93), and the induction of EPOR by stem cell factor (SCF) has been suggested to be mediated by SP1 (Ref. 94). GATA1 binds to the GATA motif and transactivates the EPOR promoter (Ref. 93, 95). In the absence of GATA1, GATA2 can also activate EPOR expression (Ref. 96). However, during erythroid differentiation, GATA1 downregulates GATA2 and EPOR expression follows GATA1 induction by EPO (Ref. 58), indicating that EPO regulates the expression of its own receptor. Additional upregulation of EPOR mediated via antisense EPOR transcripts has recently been suggested (Ref. 97). During terminal differentiation, EPOR is downregulated with decreasing levels of GATA1. CACCC and GC-box motifs are located in the upstream 5′ region, which negatively regulates EPOR promoter activity (Ref. 71, 98). A human EPOR genomic fragment extending 2 kb 5′ and 7 kb 3′ of the coding region was sufficient to provide EPOR expression in haematopoietic tissue in transgenic mice (Ref. 99).
EPOR and development
During embryogenesis, erythropoiesis is initiated in the extra-embryonic yolk sac giving rise to primitive erythropoiesis. In mice, EpoR is expressed in the yolk sac blood islands, but Epo expression is not detected until the shift to definitive erythropoiesis in the fetal liver (Ref. 100). In targeted deletion of Epo or EpoR in mice, primitive erythropoiesis proceeds with BFU-E and CFU-E accumulating in the fetal liver (Ref. 61, 62). However, CFU-E require EPO for survival, and progression beyond the CFU-E stage is markedly inhibited, which results in severe anaemia and death around embryonic day E13.5. Close examination of embryos lacking Epo or EpoR also reveals ventricular hypoplasia at embryonic day E12, increased apoptosis in the myocardium and endocardium, and a reduction in the number of cardiac myocytes and endothelial cells (Ref. 59, 67, 101). In EpoR-null mice, increased apoptosis is seen in the fetal brain as early as embryonic day E10.5, prior to the onset of anaemia (Ref. 59), indicating that EPO may contribute to selective cell survival during organ development. As with erythroid differentiation, EPO may act as an antiapoptotic and mitogenic factor and contribute to progenitor cell survival and proliferation during development. Studies of nonhaematopoietic tissues in animal models and cell culture provide increasing evidence for EPOR expression and EPO activity beyond erythroid cell differentiation (Ref. 69, 102, 103) (Fig. 3).
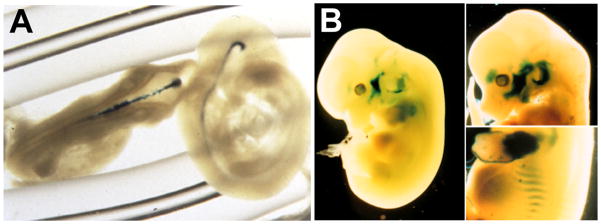
Figure 3. Embryonic erythropoietin receptor expression beyond erythroid tissues.
(a) To visualise erythropoietin receptor (EPOR) expression in nonhaematopoietic tissue, a lacZ reporter gene driven by the EPOR promoter was used to produce transgenic mice. In brain, these mice exhibit expression in the neural tube at embryonic day E10.5 (from (Ref. 131) with permission). (b) At embryonic day 12.5 (left) and 13.5 (right), reporter gene expression resembles developing skeletal muscle (from. (Ref. 60) with permission).
Erythropoietin and vascular endothelium
EPO activity in endothelial cells
The availability of recombinant EPO as a laboratory reagent provided the opportunity to examine its activity in nonhaematopoietic tissue (Fig. 4). EPO stimulated dose-dependent proliferation and enhanced migration of endothelial cells that expressed the mature erythroid form of EPOR (Ref. 65, 104). EPO treatment of endothelial cell cultures stimulated JAK2, the production of matrix metalloproteinase 2 (MMP2), and the formation of vascular structures (Ref. 105). EPO protected endothelial cell cultures from anoxia-induced injury, an effect dependent on AKT1 activation and on the maintenance of mitochondrial membrane potential (Ref. 106). In vivo, EPO induced a strong proangiogenic response in the chick embryo chorioallantoic membrane (Ref. 105).
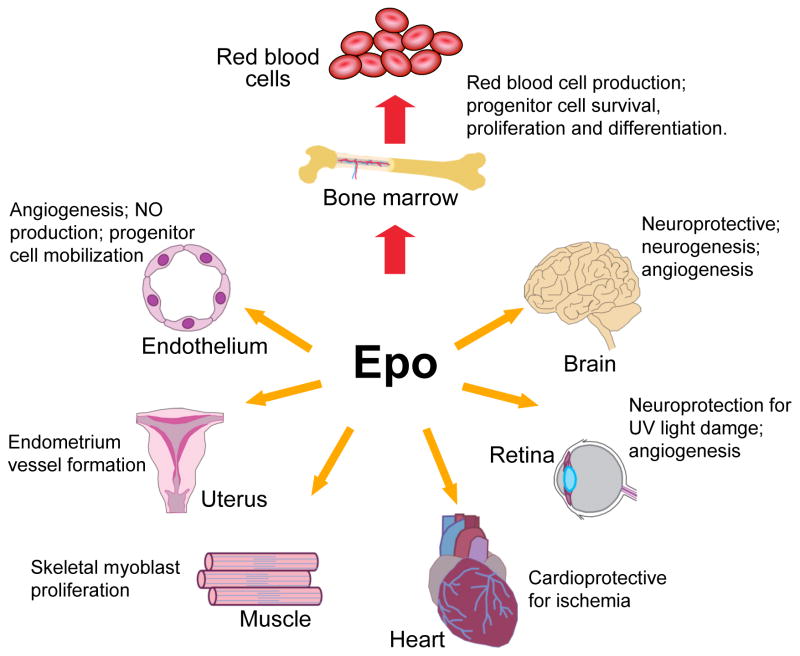
Figure 4. Erythropoietin activity in multiple tissues.
Erythropoietin (EPO) acts primarily to regulate erythropoiesis in the bone marrow by stimulating erythroid progenitor cell survival, proliferation and differentiation to produce mature red blood cells. EPO receptor expression on endothelial cells, the endometrium (lining) of the uterus, skeletal muscle myoblasts, the heart, and endothelial cells and neural cells in the retina and brain allows EPO to also act as a survival or mitogenic factor on these nonhaematopoietic cells, providing the potential for a response to EPO in multiple tissues.
Nitric oxide production in EPO-stimulated endothelial cells
Endothelial cells express endothelial nitric oxide synthase (eNOS/NOS3), which produces nitric oxide (NO) to regulate vascular tone, blood pressure homeostasis and blood flow. In endothelial cell cultures, EPO upregulated EPOR and increased endothelial cell sensitivity and response to EPO (Ref. 107). The combination of EPO and hypoxia together induced EPOR, modulated eNOS expression and stimulated NO release in human endothelial cells from umbilical vein and artery (Ref. 107). Animal studies provide evidence for EPO stimulation of NO production in vivo. Rats treated for 14 days with EPO exhibited elevated haematocrit and vascular expression of eNOS and basal vasodilation (Ref. 108). Transgenic mice expressing high levels of human EPO resulting in elevated erythrocytosis had increased eNOS and NO production, and NO relaxation (Ref. 109). Treatment with the NO synthase inhibitor L-NAME (N-nitro-L-arginine methyl ester) resulted in vasoconstriction, hypertension and death. By contrast, increased erythrocytosis alone without elevated EPO was not associated with increased NO production. Polycythaemia patients with low EPO levels showed no endothelium-dependent vasodilation response to acetylcholine but became responsive with haemodilution (Ref. 110). These patients remain responsive to NO and show induced pulmonary vasodilation with inhaled NO. In the transgenic mice with high EPO levels, the increased bioavailability of NO counteracted some of the adverse effects of the very high haematocrit (around 80%) to maintain normotension and to prevent cardiovascular dysfunction (Ref. 109).
Oestrogen-stimulated EPO production
The angiogenic activity of EPO has been suggested to play a major role in the female oestrus cycle (Ref. 111). In rodents, oestrogen stimulated Epo production in the uterus and Epo interacted with EpoR in the microvascular endothelial cells in the endometrium to promote angiogenesis (Ref. 112). Exogenous EPO injected into the uterine cavity of oestrogen-stimulated ovariectomised female mice stimulated blood vessel formation in the endometrium. The endometrial transition from diestrus to proestrus was blocked by inhibition of endometrial growth and angiogenesis via injection of soluble EPOR that can bind EPO and block its activity. Oestrogen activation of EPO production and the resultant angiogenic response suggest that EPO is one of the oestrogen-regulated angiogenic factors that along with VEGF maintain the cyclic uterine angiogenesis in the oestrus cycle (Ref. 111).
Embryonic angiogenesis and mobilisation of endothelial progenitor cells
EPO also appears to contribute to embryonic angiogenesis. During mouse development, the vasculature and heart exhibit high levels of Epo and EpoR expression (Ref. 67, 101, 113). In mice, targeted deletion of Epo or EpoR severely affected angiogenesis and decreased the complexity of the vessel networks, resulting in narrower vessel diameter and fewer branches (Ref. 113). These changes were observed two days prior to the severe anaemia associated with loss of definitive erythropoiesis in the Epo- or EpoR-null mice, and are consistent with the pattern of Epo and EpoR expression observed in wild-type embryos (Ref. 67, 101, 113).
This angiogenic activity associated with endogenous EPO during development may relate to the EPO stimulation of endothelial progenitor cell mobilisation or proliferation (Ref. 114). In adult mice, EPO treatment increased the number of stem and progenitor cells including endothelial progenitor cells in the bone marrow, spleen and peripheral blood (Ref. 114). Patients with coronary heart disease showed a significant association of EPO and VEGF with the number of circulating endothelial progenitor cells (Ref. 114), and EPO treatment in patients with renal anaemia or normal volunteers significantly increased mobilisation of circulating endothelial progenitor cells in the peripheral blood (Ref. 115). In a mouse model of vascular injury, EPO treatment for three days following wire injury of the femoral artery induced the AKT–eNOS pathway and NO synthesis, increased mobilisation of circulating endothelial progenitor cells, promoted re-endothelialisation, and inhibited neointimal hyperplasia (Ref. 116). Mobilisation of endothelial progenitor cells and their recruitment to the pulmonary vasculature and hypoxia activation of NOS in lung were greatly impaired in mice that lack EpoR in the cardiovascular system (Ref. 117). The development of pulmonary hypertension and pulmonary vascular remodelling were accelerated upon exposure to reduced oxygen for 3 weeks compared with wild-type mice. These mice also exhibited poorer response in a model for hind-limb ischaemia using femoral artery ligation. Blood flow recovery, activation of VEGF or its receptor VEGFR-2 (KDR), and mobilisation of endothelial progenitor cells were also impaired (Ref. 118). These differences persisted even after transplantation of wild-type bone marrow. Mice expressing EpoR only in haematopoietic tissue also exhibited reduction in VEGF-induced growth of blood vessels. These vascular responses relating to EPO activation of endothelial progenitor cells or loss of EPOR in nonhaematopoietic tissue indicate that activity of endogenous and exogenous EPO extends beyond erythropoiesis and that EPO can mediate significant vascular effects.
EPO and retinopathy
Anaemia in preterm infants is associated with reduced EPO levels (Ref. 119, 120), and EPO with supplemental iron reduced transfusion needs in infants with birthweights <1000 g without development of severe retinopathy of prematurity (Ref. 121, 122). However, the progression of retinopathy of prematurity has been linked to extremely low birth weight and exposure to exogenous EPO (Ref. 123). Retinopathy of prematurity begins with reduced vascular growth followed by hypoxia-induced neovascularisation that correlates with elevated VEGF levels (Ref. 124). In a mouse model of retinopathy of prematurity, knockdown of HIF-2α decreased the hypoxic induction of Epo and protected the retina from neovascularisation, suggesting that EPO is the HIF-2α target gene that is responsible for retinopathy of prematurity (Ref. 125). Therefore, although early EPO stimulation of endothelial cells as a survival factor has the potential to be protective, as a mitogenic factor EPO may contribute to later development of retinopathy (Ref. 126).
In diabetic retinopathy, EPO administration was observed to improve macular oedema, stable vision and fluorescein angiography (Ref. 127). However, elevated EPO and VEGF were found in the vitreous fluid of patients with proliferative diabetic retinopathy and branch retinal vein occlusion (Ref. 127). A recently discovered polymorphism in the EPO gene promoter is linked to elevated EPO levels in the vitreous body and to diabetic retinopathy, suggesting a possible causal relationship (Ref. 128). In a murine model, blocking Epo signalling inhibited ischaemia-induced retinal neovascularisation (Ref. 129). The vitreous and plasma EPO levels do not correlate, suggesting a local source for elevated EPO in the vitreous fluid. The association of increased circulating endothelial progenitor cells with diabetic retinopathy suggests systemic vasculogenesis contributes to retina neovascularisation rather than local angiogenesis (Ref. 130).
The mouse model of oxygen-induced retiopathy illustrates that early EPO administration when endogenous Epo level in the retina is low protected the retina from hyperoxia-induced vaso-obliteration and reduced hypoxia-induced retinal neuron apoptosis (Ref. 126). This contrasts adverse effects observed with later EPO administration when endogenous Epo level is elevated and increased neovascularization is observed (Ref. 126). Therefore, the protective and adverse effects associated with EPO in retinopathy depends on the timing of EPO elevation and indicates that variation in EPO response depends on the local bioavailability of endogenous and exogenous EPO.
Erythropoietin and skeletal muscle
EPOR expression in myocytes
Transgenic mice carrying a reporter gene driven by the EPOR promoter showed reporter gene expression in the developing embryo (Ref. 60, 131). In particular, transgene expression appeared to follow expression of the basic-helix–loop–helix muscle transcription factor MYF5 (Ref. 60, 132) (Fig. 3b). Cultures of primary mouse myoblasts from skeletal muscle demonstrated EpoR expression and a proliferative response to EPO (Ref. 60). EPO stimulated expression of EpoR, and the transcription factors, MYF5, MYOD1 and GATA3. As observed during erythroid differentiation, EpoR was downregulated during myoblast differentiation and was not detected on mature skeletal muscle. This contrasts with EPOR in myocardium where EpoR expression has been found to persist in adult myocardiocytes (Ref. 133).
Erythropoietin and heart
Endogenous EPO and heart development
In heart, EPOR is first detected during midgestation (Ref. 67) and persists through adulthood (Ref. 133, 134). Mice with targeted deletion of Epo or EpoR exhibited apoptosis in endocardium and myocardium, ventricular hypoplasia and a reduction in the number of proliferating cardiomyocytes at embryonic day 12–13 (Ref. 59, 67, 101). Although haematopoietic rescue of the EpoR-null genotype resulted in adult mice with no apparent heart morphological defect (Ref. 135), after myocardial ischaemia–reperfusion these mice showed an increase in infarct size and cardiomyocyte apoptosis compared with wild-type mice (Ref. 136). Therefore, endogenous EPO may contribute to heart tissue maintenance or repair in rodents, suggesting the potential for cardioprotection with exogenous EPO administration.
EPO and cardioprotection
Animal models of heart ischaemia or ischaemia–reperfusion injury provide further evidence for the cardioprotective activity of EPO. In rabbits, injection of EPO at the onset of permanent occlusion of the coronary artery reduced infarct size, reduced left ventricle size and improved left ventricle ejection fraction (Ref. 137). After myocardial ischaemia–reperfusion injury in rats, EPO injected intraperitoneally for 7 days reduced loss of cardiomyocytes by 50% (Ref. 133). In a mouse model for myocardial infarction, a single injection of EPO intraperitoneally following permanent ligation of the left descending coronary artery reduced apoptosis in the area at risk 24 h later and even reduced infarct size at 8 weeks after insult (Ref. 138). EPO administration protected against the myocardial ischaemia–reperfusion injury and inflammatory response by phosphoinositide 3-kinase (PI3K) activation of the transcription factor AP-1 (c-fos/c-jun) and increasing eNOS and NO production (Ref. 139). In contrast, EPO protection was lost in mice with targeted deletion of cardiac AP-1. The requirement for eNOS in the cardioprotective activity of EPO was demonstrated by the significant reduction in EPO protection in myocardial ischemia–reperfusion injury in eNOS-null mice (Ref. 140).
The myocardio protective effects of EPO may result from a direct antiapoptotic effect on cardiomyocytes and/or from an indirect EPO response by endothelial cells in the heart (Ref. 141). In mice, EPO treatment initiated 3 weeks after coronary artery ligation as a model for chronic heart failure induced mobilisation of endothelial progenitor cells from the bone marrow, increased their homing to cardiac microvasculature, and increased myocardial VEGF expression and neovascularisation (Ref. 142). In a rat model of postmyocardial infarction heart failure, initiation of EPO treatment 3 weeks after insult promoted neovascularisation, and improved cardiac function and capillary density at 9 weeks, although no difference in infarct size was observed (Ref. 143). Therefore, EPO cardioprotective activity in rodents may be effective even after the initial 24 h following insult and may improve cardiac function beyond a reduction in infarct size.
Erythropoietin and brain
EPO production and EPOR expression in brain
EPO is produced by astrocytes and neurons and regulated by HIF-2α in a hypoxia-dependent manner (Ref. 37, 144), and thus provides a source for EPO on the brain side of the blood–brain barrier. EPO is expressed in human fetal and adult brain and in brains of nonhuman primates and other mammals, and is found in cerebrospinal fluid (Ref. 68, 145, 146). Examination of EpoR in the developing mouse revealed EpoR expression in regions associated with neurogenesis (Ref. 59); expression localises to the neural tube at mid gestation (Fig. 3a) at levels comparable to adult bone marrow (Ref. 131). Epo expression follows EpoR expression by ~24 h in neural crest cells and mesenchyme-derived cells (Ref. 147). EpoR was downregulated as neural progenitor cells differentiated to mature neurons (Ref. 148) and by birth was about 1% or less than the level at day E10.5 (Ref. 131). EPOR mRNA in human fetal brain is the mature erythroid form but is processed less efficiently than in erythroid cells (Ref. 149). In adult brain from humans, nonhuman primates and rodents, the hippocampus, cortex and midbrain exhibit EPO binding (Ref. 15, 146).
EPO and neuroprotection
EPO stimulated neural progenitor cell proliferation, and promoted neural differentiation and mature neuron survival (Ref. 59, 66, 148, 150, 151). EPO was neuroprotective against glutamate toxicity and hypoxia (Ref. 59, 66). In NT2 neural cells, EPO induced GATA3, a GATA factor required for normal brain development, and GATA3 transactivated EPOR expression via the GATA1-binding motif (Ref. 50, 59). Hypoxia increased EPOR expression in neural cell cultures, and therefore EPO response (Ref. 59, 149), although EPOR does not appear to be regulated by HIF. During development, EpoR-null mice had smaller brain size with a thinner layer of neuroepithelium prior to death in utero from severe anaemia (Ref. 59). Neural cells from these mice proliferated more slowly and were less tolerant to hypoxia. Mice with EpoR expression restricted to haematopoietic tissue survived through adulthood with normal haematocrit but exhibited increased apoptosis in the developing brain, neural cells with increased sensitivity to hypoxia, and decreased neural cell proliferation and increased sensitivity to glutamate toxicity in adult brain (Ref. 135). Adult mice with loss of EpoR brain expression showed defective neural progenitor cell migration to the peri-infarct cortex in response to ischaemic stroke (Ref. 147). The perturbation in neural cell response with loss of EPOR expression suggests that endogenous Epo is neuroprotective in embryonic and adult mice.
In gerbils, infusion of soluble EPOR in brain during mild brain ischaemia resulted in neural degeneration and impaired learning (Ref. 152). Rodents show increased EpoR in the periphery of ischaemic brain injury and EPO administration in brain reduced injury and improved performance in the Morris water maze test (Ref. 153, 154). In mice, the increased EpoR expression in ischaemic stroke was followed by Epo production in brain, and exogenous EPO reduced infarct size (Ref. 155). These studies suggest the induction of EPOR followed by endogenous EPO is neuroprotective in response to ischaemic injury and that increasing EPO bioavailability can significantly reduce injury and promote repair. In ischaemic preconditioning, a period of mild brain ischaemia provides resistance to severe ischaemic insult. HIF activation in preconditioning increased VEGF and EPO, which can contribute to tolerance to brain ischemia (Ref. 154). Soluble EPOR directly administered to the brain blocked the protective effect of preconditioning (Ref. 156, 157, 158). EPO administration in the retina mimicked hypoxia preconditioning and protected photoreceptors from ultraviolet light damage (Ref. 159). Protection was also observed in mice expressing high levels of human EPO (Ref. 160).
EPO levels in the central nervous system did not correlate with serum levels without blood–brain barrier dysfunction in patients with traumatic brain injury (Ref. 145). In patients administered large doses of EPO intravenously, increase of EPO in the cerebrospinal fluid depended on disruption of the blood–brain barrier (Ref. 161). Animals treated with systemic EPO showed brain uptake of less than 0.1% in the absence of injury to the blood–brain barrier (Ref. 162). Nevertheless, systemic or intraperitoneal EPO administration in rodent models of brain ischaemia or trauma was neuroprotective (Ref. 163, 164, 165), suggesting that the blood–brain barrier was compromised for EPO permeability, or that EPO neuroprotective activity is indirect.
EPO signalling in neural cells
EPO stimulates JAK2 activation in both erythroid progenitor cells and in neural cells. Brain ischaemia in transgenic mice with increased EPO in brain resulted in increased activation of JAK2, ERK1/2 (MAPK1/3) and AKT, and induction of Bcl-xL (Ref. 166). Hippocampal neuron cultures showed EPO treatment maintained mitochondrial membrane potential and inhibited cytochrome c release and caspase activity (Ref. 167). In mice with targeted deletion of STAT5, hippocampal neurons showed loss of EPO neurotrophic activity but EPO neuroprotection remained (Ref. 168). The PI3K signalling pathway was implicated in EPO protection of hippocampal CA1 neurons in a rat model for ischaemic stroke (Ref. 169). While EPO signalling in neural cells is similar to that in erythroid progenitor cells, the participation of NF-κB is only associated with EPO neuroprotection (Ref. 170) (Fig. 2). EPO antisense RNA decreased JAK2/STAT and NF-κB in hypoxia preconditioning and blocked the protective activity of hypoxia preconditioning (Ref. 171).
EPO neuroprotection and angiogenesis
In brain, EPOR is expressed on both endothelial and neural cells (Ref. 102). The mild brain phenotype in mice that lack EpoR in neural cells may result from EPO response by endothelial cells in the brain (Ref. 172). In a rodent model of ischaemic stroke, EPO treatment increased VEGF, brain-derived neurotrophic factor (BDNF), neurogenesis and angiogenesis. EPO treatment of endothelial cells cocultured with neural progenitor cells increased matrix metalloproteinase production by endothelial cells and neural cell migration (Ref. 173). EPO stimulation of neural progenitor cells without hypoxic stress increased VEGF production modestly and induced endothelial expression of VEGFR2, resulting in enhanced angiogenesis by VEGF (Ref. 174). These studies suggest the possibility that EPO neuroprotection may result from a direct effect of EPO on neural progenitor cells and neurons or may be mediated through other cell types such as endothelial cells. Endothelial cells can also increase eNOS activity in response to EPO, and therefore can also affect oxygen delivery to the brain beyond red blood cell production.
Therapeutic potential in brain diseases
High-dose EPO administration provided long-term neuroprotection and neurogenesis in a neonatal model of ischaemic stroke in rodents (Ref. 175). Juvenile rats subjected to acute brain injury and treated with EPO for 2 weeks showed prevention of cognitive dysfunction and brain atrophy 3 and 9 months after injury (Ref. 176), providing support for use of EPO for progressive neurodegenerative disorders. A proof-of-concept study for EPO treatment in ischaemic stroke was initiated and indicated the potential for EPO benefit (Ref. 177). Similarly, EPO showed promise for improving clinical outcome in a pilot study for EPO treatment in multiple sclerosis (Ref. 178). A Phase II study of 39 schizophrenic patients demonstrated an association between EPO treatment and improved cognitive function (Ref. 179). Elucidation of the mechanisms for EPO neuroprotective effects in brain disorders to reduce injury and possibly improve psychopathology will give new insight for potential treatment.
Other forms of EPO and its receptor
Modifications of EPO protein
Recombinant human EPO (rhEPO), which is about 40% carbohydrate and 60% protein, is produced as a combination of several glycoforms that are distinct from human serum EPO (Ref. 180). Although not required for in vitro binding, carbohydrate moieties provide in vivo stability to EPO protein and resistance to thermal inactivation (Ref. 12, 13). The addition of N-linked carbohydrates decreased receptor-binding activity but increased rhEPO serum half-life and in vivo activity (Ref. 181). Prolonged in vivo activity reduced the frequency required for therapeutic EPO administration in adult patients with chronic kidney disease and a single dose in anaemic preterm neonates accelerated effective erythropoiesis (Ref. 182, 183). Other approaches to extend the in vivo half-life of EPO include homodimerisation by linking EPO via a 17 amino acid flexible peptide, or multimerisation to dimers and trimers via chemical crosslinking; these increased the biological activity of EPO in vitro and in vivo (Ref. 184, 185). A fully synthetic EPO of 166 amino acids similar to the native protein with two branched polymer moieties covalently attached significantly increased EPO activity in vitro and extended the half-life in vivo (Ref. 186, 187). A third generation of EPO includes modification by polyethelene glycone to also extend in vivo half-life and, like the first generation of pharmacological EPO, was shown to reduce anaemia in patients with renal disease (Ref. 187, 188).
Non-EPO-based erythroid-stimulating agents
Small peptides, or EPO mimetics with sequences unrelated to EPO, can activate the EPOR homodimer (Ref. 5, 189). An antibody that targets the extracellular domain of human EPOR was also used as the basis to develop an EPO mimic that was active in vivo in a mouse model expressing human EPOR and appeared to bind in a symmetric manner to EPOR distinct from the two asymmetric binding sites for EPO (Ref. 190). An alternate therapeutic strategy for anaemia is to activate the hypoxic regulatory mechanisms associated with activation of EPO production, a treatment approach that was shown to induce endogenous EPO production in rhesus macaques (Ref. 191, 192).
Nonerythroid forms of EPO and its receptor
Efforts to segregate EPO activity in erythropoiesis from its nonhaematopoietic protective effects have led to investigation of alternative forms of EPO. Excessive EPO can result in high haematocrit and high blood viscosity, which in an animal model can lead to degenerative processes and increased vascular permeability in liver and kidney, degeneration of nerve and muscle fibres and decreased neuromuscular junctions, and a general reduction in life expectancy (Ref. 193). The short half-life of EPO stripped of sialic acid (asialoerythropoietin) is not sufficient to increase erythropoiesis in vivo but has been reported to be neuroprotective in animal models (Ref. 194). Other EPO modifications such as carbamylation have been proposed to result in EPO that acts act specifically in nonhaematopoietic cells without stimulation of erythropoiesis (Ref. 195). Carbamylated EPO did not bind the classical EPOR or stimulate haematopoietic activity in select animal models (Ref. 196). Neuroprotective activity observed with carbamylated EPO appears to be associated with upregulation of the extracellular signalling molecule, SHH (sonic hedgehog), and this activity does not appear to require EPOR (Ref. 197). The structure of the receptor for carbamylated EPO and the role of EPOR in the receptor complex have not yet been determined.
A specific nonhaematopoietic form for the receptor to native EPO has been proposed consisting of a heteromeric complex between EPOR and the common cytokine β receptor (Ref. 198). Beyond this initial report, the precise structure for this alternate EPO receptor heterocomplex has not yet been elucidated. Interestingly, this alternative form of EPO receptor may not be necessary for EPO neuroprotective activity since neural cells that lack the common cytokine β receptor exhibit EPO protection and, unlike EPOR, the common cytokine β receptor is barely detected in astrocytes and neurons in the hippocampus that exhibits EPO protection in a neurodegenerative rodent model (Ref. 199, 200). Nevertheless, efforts continue in searching for a nonhaematopoietic specific EPO receptor and in developing agents like asialoerythropoietin and peptide mimetics capable of stimulating an EPO response in nonhaematopoietic cells/tissue without increasing erythropoiesis.
Adverse effects associated with EPO therapy
EPO, anaemia and chronic renal disease
The potential for adverse effects of EPO action was suggested in patient studies in which exogenous EPO was used to elevate haematocrit to bring it within normal range. For example, EPO therapy in two trials of chronic renal disease was able to correct anaemia, but normalising haematocrit did not reduce the risk of cardiovascular events (Ref. 201, 202). Treatment of patients with EPO to reach a target haemoglobin level of 11.3 g/dl or 13.5 g/dl showed an increase incidence of composite events (death, myocardial infarction, hospitalisation for congestive heart failure and stroke) in the high haemoglobin group, and more patients in this group had one or more serious events (Ref. 202). In a separate study involving patients with a milder anaemia, correction of anaemia to the 13 to 15 g/dl range compared with a subnormal range of 10.5 to 11.5 g/dl, was associated with improved quality of life but did not change the incidence of the first cardiovascular event or combined adverse events, and was related to increased hypertensive episodes, headaches and the number of patients requiring dialysis (Ref. 201). Studies such as these illustrate the difficulty in determining that appropriate target haemoglobin level in EPO therapy and the potential for increased adverse effects using target haemoglobin levels in or close to the normal range.
Anaemia in cancer patients and EPO therapy
Several clinical studies have examined EPO therapy to treat anaemia in cancer patients and these are reviewed in greater detail elsewhere (Ref. 203). For example, EPO treatment in anaemic cancer patients receiving nonplatinum chemotherapy was associated with reduced transfusion requirements, increased haemoglobin and improvement of quality of life (Ref. 204). In a specific cancer type, patients receiving chemoradiotherapy for squamous cell carcinoma of the oral cavity and oropharynx followed by resection of the primary tumour bed and neck dissection, showed increased cancer control and survival rates with EPO treatment without supplemental iron (Ref. 205). The associated benefit of EPO treatment in cancer patients with anaemia led to efforts to maintain haemoglobin in the normal range.
By contrast to earlier studies, EPO treatment to increase or maintain haemoglobin levels close to or in the normal range drew attention to potential adverse effects. In patients with metastatic breast cancer irrespective of chemotherapy, a trial to maintain normal haemoglobin concentration was terminated early due to significant increase in disease progression and thrombotic and vascular events (Ref. 206). In anaemic patients with head and neck cancer undergoing radiotherapy, a double-blind placebo-control trial using EPO therapy to normalise haemoglobin resulted in bringing haemoglobin into the normal range (>14 g/dl in women and >15 g/dl in men), but also showed decreased benefit in cancer control and survival in the EPO treatment group compared with placebo (Ref. 207). Despite imbalance of risk factors and heterogeneity of the study population, these studies illustrate the adverse effects associated with high haemoglobin targets in cancer patients, particularly in metastatic breast cancer and head and neck cancer.
An evaluation of clinical trials comparing EPO treatment with placebo or standard of care for anaemia in cancer patients underscored the associated risk of venous thromboembolism with EPO administration and increased mortality in select solid cancers (Ref. 208). The recommendations for EPO treatment for low haemoglobin level (approaching or <10g/dl) for cancer patients with chemotherapy-related anaemia are reviewed elsewhere (Ref. 209). While EPO has been shown to be useful in increasing haemoglobin level in cancer patients with chemotherapy-associated anaemia, awareness of potential adverse events raises several questions and concerns including the appropriate target haemoglobin level, the role of supplemental iron, specific tumour-type response to EPO, variation in individual response, and the direct effect of EPO in stimulating nonhaematopoietic tissue response or cancer cell growth (Ref. 203).
Conclusions
CFU-Es are highly sensitive to EPO due to the markedly elevated level of EPOR expression. EPO itself induces expression of its receptor in CFU-E via induction of the erythroid transcription factor GATA1. By contrast, EPOR expression in nonhaematopoietic cells lacks the transactivation by GATA1 characteristic of erythroid gene expression. As a consequence, EPOR levels and EPO response are more modest in endothelial cells, cardiomyocytes and neurons. Nevertheless, the survival and proliferative activities of EPO that are required for red blood cell formation appear to extend to other EPOR-expressing cells, resulting in EPO protective activity associated with stress or ischaemia in nonhaematopoietic tissue such as heart and brain. Tissue-specific induction of EPOR may promote nonhaematopoietic EPO responses such as cardioprotection or neuroprotection. Identification of the spatial and temporal expression of EPOR will provide insight into the full range of tissues that may exhibit a protective response to EPO.
Erythroid, endothelial and other cellular responses determine the overall effects of exogenous EPO administration. To what extent can the nonerythroid EPO response such as cardioprotection or neuroprotection be utilised without increasing haematocrit? Conversely, the nonhaematopoietic responses to EPO raise further concern about adverse events in treatment of anaemia with high-dose EPO. Although initially considered primarily an erythroid cytokine, EPO function as a survival and proliferative factor for progenitor cells beyond the erythroid lineage is apparent. The extent to which exogenous EPO can be used to promote repair in ischaemic stroke or cardiovascular events as indicated by culture studies and animal models warrants further validation in clinical studies.
Acknowledgments
We thank Alan N. Schechter and Blanche Alter for insightful discussions, and the peer reviewers for their helpful comments and suggestions. Current funding is provided by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Funakoshi-Tago M, et al. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol. 2008;28:1792–1801. doi: 10.1128/MCB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 3.Lin FK, et al. Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci U S A. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs K, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 5.Livnah O, et al. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 6.Lai PH, et al. Structural characterization of human erythropoietin. J Biol Chem. 1986;261:3116–3121. [PubMed] [Google Scholar]
- 7.Neubauer H, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 8.Wen D, et al. Erythropoietin structure-function relationships. Identification of functionally important domains. J Biol Chem. 1994;269:22839–22846. [PubMed] [Google Scholar]
- 9.Sasaki H, et al. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem. 1987;262:12059–12076. [PubMed] [Google Scholar]
- 10.Wasley LC, et al. The importance of N- and O-linked oligosaccharides for the biosynthesis and in vitro and in vivo biologic activities of erythropoietin. Blood. 1991;77:2624–2632. [PubMed] [Google Scholar]
- 11.Higuchi M, et al. Role of sugar chains in the expression of the biological activity of human erythropoietin. J Biol Chem. 1992;267:7703–7709. [PubMed] [Google Scholar]
- 12.Fukuda MN, et al. Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood. 1989;73:84–89. [PubMed] [Google Scholar]
- 13.Tsuda E, et al. The role of carbohydrate in recombinant human erythropoietin. Eur J Biochem. 1990;188:405–411. doi: 10.1111/j.1432-1033.1990.tb15417.x. [DOI] [PubMed] [Google Scholar]
- 14.Zanjani ED, et al. Studies on the liver to kidney switch of erythropoietin production. J Clin Invest. 1981;67:1183–1188. doi: 10.1172/JCI110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dame C, et al. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood. 1998;92:3218–3225. [PubMed] [Google Scholar]
- 16.Obara N, et al. Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood. 2008;111:5223–5232. doi: 10.1182/blood-2007-10-115857. [DOI] [PubMed] [Google Scholar]
- 17.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 18.Koury ST, et al. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood. 1991;77:2497–2503. [PubMed] [Google Scholar]
- 19.Kochling J, Curtin PT, Madan A. Regulation of human erythropoietin gene induction by upstream flanking sequences in transgenic mice. Br J Haematol. 1998;103:960–968. doi: 10.1046/j.1365-2141.1998.01081.x. [DOI] [PubMed] [Google Scholar]
- 20.Bondurant MC, Koury MJ. Anemia induces accumulation of erythropoietin mRNA in the kidney and liver. Mol Cell Biol. 1986;6:2731–2733. doi: 10.1128/mcb.6.7.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan CC, Eckardt KU, Ratcliffe PJ. Organ distribution of erythropoietin messenger RNA in normal and uremic rats. Kidney Int. 1991;40:69–76. doi: 10.1038/ki.1991.181. [DOI] [PubMed] [Google Scholar]
- 22.Fandrey J, Bunn HF. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 1993;81:617–623. [PubMed] [Google Scholar]
- 23.Maxwell PH, et al. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int. 1993;44:1149–1162. doi: 10.1038/ki.1993.362. [DOI] [PubMed] [Google Scholar]
- 24.Koury ST, Bondurant MC, Koury MJ. Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood. 1988;71:524–527. [PubMed] [Google Scholar]
- 25.Eckardt KU, et al. Distribution of erythropoietin producing cells in rat kidneys during hypoxic hypoxia. Kidney Int. 1993;43:815–823. doi: 10.1038/ki.1993.115. [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL, et al. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc Natl Acad Sci U S A. 1991;88:8725–8729. doi: 10.1073/pnas.88.19.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galson DL, et al. The orphan receptor hepatic nuclear factor 4 functions as a transcriptional activator for tissue-specific and hypoxia-specific erythropoietin gene expression and is antagonized by EAR3/COUP-TF1. Mol Cell Biol. 1995;15:2135–2144. doi: 10.1128/mcb.15.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood. 1999;94:1864–1877. [PubMed] [Google Scholar]
- 30.Metzen E, Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biol Chem. 2004;385:223–230. doi: 10.1515/BC.2004.016. [DOI] [PubMed] [Google Scholar]
- 31.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008;141:325–334. doi: 10.1111/j.1365-2141.2008.07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu CJ, et al. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Percy MJ, et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ang SO, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 35.Warnecke C, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. Faseb J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 36.Rankin EB, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavez JC, et al. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruber M, et al. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zelzer E, et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. Embo J. 1998;17:5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Percy MJ, et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanchard KL, et al. Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol Cell Biol. 1992;12:5373–5385. doi: 10.1128/mcb.12.12.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebert BL, Bunn HF. Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein. Mol Cell Biol. 1998;18:4089–4096. doi: 10.1128/mcb.18.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makita T, Duncan SA, Sucov HM. Retinoic acid, hypoxia, and GATA factors cooperatively control the onset of fetal liver erythropoietin expression and erythropoietic differentiation. Dev Biol. 2005;280:59–72. doi: 10.1016/j.ydbio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Makita T, et al. A developmental transition in definitive erythropoiesis: erythropoietin expression is sequentially regulated by retinoic acid receptors and HNF4. Genes Dev. 2001;15:889–901. doi: 10.1101/gad.871601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alberta JA, et al. Role of the WT1 tumor suppressor in murine hematopoiesis. Blood. 2003;101:2570–2574. doi: 10.1182/blood-2002-06-1656. [DOI] [PubMed] [Google Scholar]
- 46.Dame C, et al. Wilms tumor suppressor, Wt1, is a transcriptional activator of the erythropoietin gene. Blood. 2006;107:4282–4290. doi: 10.1182/blood-2005-07-2889. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Elsner T, et al. A cross-talk between hypoxia and TGF-beta orchestrates erythropoietin gene regulation through SP1 and Smads. J Mol Biol. 2004;336:9–24. doi: 10.1016/j.jmb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Ma GT, et al. GATA-2 and GATA-3 regulate trophoblast-specific gene expression in vivo. Development. 1997;124:907–914. doi: 10.1242/dev.124.4.907. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y, Yamamoto M, Engel JD. GATA2 is required for the generation of V2 interneurons. Development. 2000;127:3829–3838. doi: 10.1242/dev.127.17.3829. [DOI] [PubMed] [Google Scholar]
- 50.Pandolfi PP, et al. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 51.Hoshino T, et al. Reduced BMP4 abundance in Gata2 hypomorphic mutant mice result in uropathies resembling human CAKUT. Genes Cells. 2008;13:159–170. doi: 10.1111/j.1365-2443.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 52.Gao X, et al. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18:2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterkin T, Gibson A, Patient R. Redundancy and evolution of GATA factor requirements in development of the myocardium. Dev Biol. 2007;311:623–635. doi: 10.1016/j.ydbio.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capo-Chichi CD, et al. Perception of differentiation cues by GATA factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev Biol. 2005;286:574–586. doi: 10.1016/j.ydbio.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Dame C, et al. Hepatic erythropoietin gene regulation by GATA-4. J Biol Chem. 2004;279:2955–2961. doi: 10.1074/jbc.M310404200. [DOI] [PubMed] [Google Scholar]
- 56.Imagawa S, Yamamoto M, Miura Y. Negative regulation of the erythropoietin gene expression by the GATA transcription factors. Blood. 1997;89:1430–1439. [PubMed] [Google Scholar]
- 57.La Ferla K, et al. Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-kappaB. Faseb J. 2002;16:1811–1813. doi: 10.1096/fj.02-0168fje. [DOI] [PubMed] [Google Scholar]
- 58.Rogers HM, et al. Hypoxia alters progression of the erythroid program. Exp Hematol. 2008;36:17–27. doi: 10.1016/j.exphem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X, et al. Erythropoietin receptor signalling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 60.Ogilvie M, et al. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754–39761. doi: 10.1074/jbc.M004999200. [DOI] [PubMed] [Google Scholar]
- 61.Wu H, et al. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 62.Lin CS, et al. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 63.Broudy VC, et al. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991;77:2583–2590. [PubMed] [Google Scholar]
- 64.Chiba T, Ikawa Y, Todokoro K. GATA-1 transactivates erythropoietin receptor gene, and erythropoietin receptor-mediated signals enhance GATA-1 gene expression. Nucleic Acids Res. 1991;19:3843–3848. doi: 10.1093/nar/19.14.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anagnostou A, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morishita E, et al. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 67.Wu H, et al. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597–3605. doi: 10.1242/dev.126.16.3597. [DOI] [PubMed] [Google Scholar]
- 68.Juul SE, et al. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 70.Youssoufian H, et al. Structure, function, and activation of the erythropoietin receptor. Blood. 1993;81:2223–2236. [PubMed] [Google Scholar]
- 71.Noguchi CT, et al. Cloning of the human erythropoietin receptor gene. Blood. 1991;78:2548–2556. [PubMed] [Google Scholar]
- 72.Syed RS, et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 73.D’Andrea AD, Zhu Y. Cloning and functional analysis of erythropoietin-, interleukin-3- and thrombopoietin-inducible genes. Stem Cells. 1996;14(Suppl 1):82–87. doi: 10.1002/stem.5530140710. [DOI] [PubMed] [Google Scholar]
- 74.Hilton DJ, et al. Increased cell surface expression and enhanced folding in the endoplasmic reticulum of a mutant erythropoietin receptor. Proc Natl Acad Sci U S A. 1995;92:190–194. doi: 10.1073/pnas.92.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshimura A, et al. Mutations in the Trp-Ser-X-Trp-Ser motif of the erythropoietin receptor abolish processing, ligand binding, and activation of the receptor. J Biol Chem. 1992;267:11619–11625. [PubMed] [Google Scholar]
- 76.Parganas E, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 77.Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 78.Levine RL, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 79.Horn T, et al. Detection of the activating JAK2 V617F mutation in paraffin-embedded trephine bone marrow biopsies of patients with chronic myeloproliferative diseases. J Mol Diagn. 2006;8:299–304. doi: 10.2353/jmoldx.2006.050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott LM, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerenyi MA, et al. Stat 5 regulates cellular iron uptake of erythroid cells via IRP-2 and TfR-1. Blood. 2008 doi: 10.1182/blood-2008-02-138339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grebien F, et al. Stat5 activation enables erythropoiesis in the absence of EpoR and Jak2. Blood. 2008;111:4511–4522. doi: 10.1182/blood-2007-07-102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miura Y, et al. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- 84.D’Andrea AD, et al. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991;11:1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Supino-Rosin L, et al. A cytosolic domain of the erythropoietin receptor contributes to endoplasmic reticulum-associated degradation. Eur J Biochem. 1999;263:410–419. doi: 10.1046/j.1432-1327.1999.00516.x. [DOI] [PubMed] [Google Scholar]
- 86.Nahari T, et al. A transplanted NPVY sequence in the cytosolic domain of the erythropoietin receptor enhances maturation. Biochem J. 2008;410:409–416. doi: 10.1042/BJ20071297. [DOI] [PubMed] [Google Scholar]
- 87.Gross AW, Lodish HF. Cellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP) J Biol Chem. 2006;281:2024–2032. doi: 10.1074/jbc.M510493200. [DOI] [PubMed] [Google Scholar]
- 88.Sawyer ST, Krantz SB, Goldwasser E. Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem. 1987;262:5554–5562. [PubMed] [Google Scholar]
- 89.Meyer L, et al. beta-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood. 2007;109:5215–5222. doi: 10.1182/blood-2006-10-055350. [DOI] [PubMed] [Google Scholar]
- 90.Arcasoy MO, Harris KW, Forget BG. A human erythropoietin receptor gene mutant causing familial erythrocytosis is associated with deregulation of the rates of Jak2 and Stat5 inactivation. Exp Hematol. 1999;27:63–74. doi: 10.1016/s0301-472x(98)00003-4. [DOI] [PubMed] [Google Scholar]
- 91.Kralovics R, Prchal JT. Genetic heterogeneity of primary familial and congenital polycythemia. Am J Hematol. 2001;68:115–121. doi: 10.1002/ajh.1162. [DOI] [PubMed] [Google Scholar]
- 92.Kralovics R, et al. Two new EPO receptor mutations: truncated EPO receptors are most frequently associated with primary familial and congenital polycythemias. Blood. 1997;90:2057–2061. [PubMed] [Google Scholar]
- 93.Chin K, et al. Regulation of transcription of the human erythropoietin receptor gene by proteins binding to GATA-1 and Sp1 motifs. Nucleic Acids Res. 1995;23:3041–3049. doi: 10.1093/nar/23.15.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato T, et al. Induction of the erythropoietin receptor gene and acquisition of responsiveness to erythropoietin by stem cell factor in HML/SE, a human leukemic cell line. J Biol Chem. 1998;273:16921–16926. doi: 10.1074/jbc.273.27.16921. [DOI] [PubMed] [Google Scholar]
- 95.Zon LI, et al. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991;88:10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiss MJ, Keller G, Orkin SH. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, et al. Synergistic upregulation of erythropoietin receptor (EPO-R) expression by sense and antisense EPO-R transcripts in the canine lung. Proc Natl Acad Sci U S A. 2008;105:7612–7617. doi: 10.1073/pnas.0802467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maouche L, et al. A CCACC motif mediates negative transcriptional regulation of the human erythropoietin receptor. Eur J Biochem. 1995;233:793–799. doi: 10.1111/j.1432-1033.1995.793_3.x. [DOI] [PubMed] [Google Scholar]
- 99.Liu ZY, Chin K, Noguchi CT. Tissue specific expression of human erythropoietin receptor in transgenic mice. Dev Biol. 1994;166:159–169. doi: 10.1006/dbio.1994.1304. [DOI] [PubMed] [Google Scholar]
- 100.Lee R, et al. Erythropoietin (Epo) and EpoR expression and 2 waves of erythropoiesis. Blood. 2001;98:1408–1415. doi: 10.1182/blood.v98.5.1408. [DOI] [PubMed] [Google Scholar]
- 101.Yu X, et al. The human erythropoietin receptor gene rescues erythropoiesis and developmental defects in the erythropoietin receptor null mouse. Blood. 2001;98:475–477. doi: 10.1182/blood.v98.2.475. [DOI] [PubMed] [Google Scholar]
- 102.Chen ZY, Warin R, Noguchi CT. Erythropoietin and normal brain development: receptor expression determines multi-tissue response. Neurodegener Dis. 2006;3:68–75. doi: 10.1159/000092096. [DOI] [PubMed] [Google Scholar]
- 103.Noguchi CT, et al. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anagnostou A, et al. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ribatti D, et al. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 106.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 107.Beleslin-Cokic BB, et al. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood. 2004;104:2073–2080. doi: 10.1182/blood-2004-02-0744. [DOI] [PubMed] [Google Scholar]
- 108.Kanagy NL, et al. Erythropoietin administration in vivo increases vascular nitric oxide synthase expression. J Cardiovasc Pharmacol. 2003;42:527–533. doi: 10.1097/00005344-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 109.Quaschning T, et al. Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. Faseb J. 2003;17:259–261. doi: 10.1096/fj.02-0296fje. [DOI] [PubMed] [Google Scholar]
- 110.Defouilloy C, et al. Polycythemia impairs vasodilator response to acetylcholine in patients with chronic hypoxemic lung disease. Am J Respir Crit Care Med. 1998;157:1452–1460. doi: 10.1164/ajrccm.157.5.9702061. [DOI] [PubMed] [Google Scholar]
- 111.Sasaki R. Pleiotropic functions of erythropoietin. Intern Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. [DOI] [PubMed] [Google Scholar]
- 112.Yasuda Y, et al. Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J Biol Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- 113.Kertesz N, et al. The role of erythropoietin in regulating angiogenesis. Dev Biol. 2004;276:101–110. doi: 10.1016/j.ydbio.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 114.Heeschen C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 115.Bahlmann FH, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 116.Urao N, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 117.Satoh K, et al. Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation. 2006;113:1442–1450. doi: 10.1161/CIRCULATIONAHA.105.583732. [DOI] [PubMed] [Google Scholar]
- 118.Nakano M, et al. Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ Res. 2007;100:662–669. doi: 10.1161/01.RES.0000260179.43672.fe. [DOI] [PubMed] [Google Scholar]
- 119.Saito T, et al. Serum erythropoietin titres in the anaemia of premature infants. Br J Haematol. 1983;54:53–58. doi: 10.1111/j.1365-2141.1983.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 120.Stockman JA, 3rd, et al. Anemia of prematurity: determinants of the erythropoietin response. J Pediatr. 1984;105:786–792. doi: 10.1016/s0022-3476(84)80308-x. [DOI] [PubMed] [Google Scholar]
- 121.Ohls RK, et al. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double-blind, placebo-controlled study. J Pediatr. 1997;131:661–665. doi: 10.1016/s0022-3476(97)70089-1. [DOI] [PubMed] [Google Scholar]
- 122.Turker G, et al. The effect of early recombinant erythropoietin and enteral iron supplementation on blood transfusion in preterm infants. Am J Perinatol. 2005;22:449–455. doi: 10.1055/s-2005-918888. [DOI] [PubMed] [Google Scholar]
- 123.Fortes Filho JB, et al. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye. 2007 doi: 10.1038/sj.eye.6702924. [DOI] [PubMed] [Google Scholar]
- 124.Aiello LP, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morita M, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. Embo J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen J, et al. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedman EA, Brown CD, Berman DH. Erythropoietin in diabetic macular edema and renal insufficiency. Am J Kidney Dis. 1995;26:202–208. doi: 10.1016/0272-6386(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 128.Tong Z, et al. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci U S A. 2008;105:6998–7003. doi: 10.1073/pnas.0800454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watanabe D, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 130.Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye. 2006;20:546–552. doi: 10.1038/sj.eye.6701920. [DOI] [PubMed] [Google Scholar]
- 131.Liu C, et al. Regulated human erythropoietin receptor expression in mouse brain. J Biol Chem. 1997;272:32395–32400. doi: 10.1074/jbc.272.51.32395. [DOI] [PubMed] [Google Scholar]
- 132.Patapoutian A, et al. Isolated sequences from the linked Myf-5 and MRF4 genes drive distinct patterns of muscle-specific expression in transgenic mice. Development. 1993;118:61–69. doi: 10.1242/dev.118.1.61. [DOI] [PubMed] [Google Scholar]
- 133.Calvillo L, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wright GL, et al. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. Faseb J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 135.Suzuki N, et al. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- 136.Tada H, et al. Endogenous erythropoietin system in non-hematopoietic lineage cells plays a protective role in myocardial ischemia/reperfusion. Cardiovasc Res. 2006;71:466–477. doi: 10.1016/j.cardiores.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 137.Parsa CJ, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moon C, et al. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci U S A. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rui T, et al. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res. 2005;65:719–727. doi: 10.1016/j.cardiores.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 140.Burger D, et al. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006;72:51–59. doi: 10.1016/j.cardiores.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 141.Narmoneva DA, et al. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Westenbrink BD, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 143.van der Meer P, et al. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol. 2005;46:125–133. doi: 10.1016/j.jacc.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 144.Masuda S, et al. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem. 1994;269:19488–19493. [PubMed] [Google Scholar]
- 145.Marti HH, et al. Detection of erythropoietin in human liquor: intrinsic erythropoietin production in the brain. Kidney Int. 1997;51:416–418. doi: 10.1038/ki.1997.55. [DOI] [PubMed] [Google Scholar]
- 146.Marti HH, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 147.Tsai PT, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen ZY, et al. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- 149.Chin K, et al. Production and processing of erythropoietin receptor transcripts in brain. Brain Res Mol Brain Res. 2000;81:29–42. doi: 10.1016/s0169-328x(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 150.Shingo T, et al. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Studer L, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sakanaka M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sadamoto Y, et al. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 154.Bernaudin M, et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 155.Bernaudin M, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 156.Prass K, et al. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 157.Malhotra S, et al. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006;83:19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- 158.Ruscher K, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Grimm C, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 160.Grimm C, et al. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xenocostas A, et al. The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. Eur J Clin Pharmacol. 2005;61:189–195. doi: 10.1007/s00228-005-0896-7. [DOI] [PubMed] [Google Scholar]
- 162.Banks WA, et al. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol. 2004;505:93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 163.Brines ML, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun Y, et al. Mechanisms of erythropoietin-induced brain protection in neonatal hypoxia-ischemia rat model. J Cereb Blood Flow Metab. 2004;24:259–270. doi: 10.1097/01.WCB.0000110049.43905.AC. [DOI] [PubMed] [Google Scholar]
- 165.Yatsiv I, et al. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. Faseb J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- 166.Kilic E, et al. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. Faseb J. 2005;19:2026–2028. doi: 10.1096/fj.05-3941fje. [DOI] [PubMed] [Google Scholar]
- 167.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Byts N, et al. Essential role for Stat5 in the neurotrophic but not in the neuroprotective effect of erythropoietin. Cell Death Differ. 2008;15:783–792. doi: 10.1038/cdd.2008.1. [DOI] [PubMed] [Google Scholar]
- 169.Zhang F, et al. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- 170.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 171.Liu J, et al. Neuroprotection by hypoxic preconditioning involves oxidative stress-mediated expression of hypoxia-inducible factor and erythropoietin. Stroke. 2005;36:1264–1269. doi: 10.1161/01.STR.0000166180.91042.02. [DOI] [PubMed] [Google Scholar]
- 172.Wang L, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 173.Wang L, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang L, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–1368. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gonzalez FF, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 176.Siren AL, et al. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 2006;129:480–489. doi: 10.1093/brain/awh703. [DOI] [PubMed] [Google Scholar]
- 177.Ehrenreich H, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 178.Ehrenreich H, et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain. 2007;130:2577–2588. doi: 10.1093/brain/awm203. [DOI] [PubMed] [Google Scholar]
- 179.Ehrenreich H, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- 180.Skibeli V, Nissen-Lie G, Torjesen P. Sugar profiling proves that human serum erythropoietin differs from recombinant human erythropoietin. Blood. 2001;98:3626–3634. doi: 10.1182/blood.v98.13.3626. [DOI] [PubMed] [Google Scholar]
- 181.Elliott S, et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol. 2003;21:414–421. doi: 10.1038/nbt799. [DOI] [PubMed] [Google Scholar]
- 182.Ling B, et al. Darbepoetin alfa administered once monthly maintains hemoglobin concentrations in patients with chronic kidney disease. Clin Nephrol. 2005;63:327–334. doi: 10.5414/cnp63327. [DOI] [PubMed] [Google Scholar]
- 183.Warwood TL, et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25:725–730. doi: 10.1038/sj.jp.7211387. [DOI] [PubMed] [Google Scholar]
- 184.Sytkowski AJ, et al. An erythropoietin fusion protein comprised of identical repeating domains exhibits enhanced biological properties. J Biol Chem. 1999;274:24773–24778. doi: 10.1074/jbc.274.35.24773. [DOI] [PubMed] [Google Scholar]
- 185.Sytkowski AJ, et al. Human erythropoietin dimers with markedly enhanced in vivo activity. Proc Natl Acad Sci U S A. 1998;95:1184–1188. doi: 10.1073/pnas.95.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kochendoerfer GG, et al. Design and chemical synthesis of a homogeneous polymer-modified erythropoiesis protein. Science. 2003;299:884–887. doi: 10.1126/science.1079085. [DOI] [PubMed] [Google Scholar]
- 187.Topf JM. CERA: third-generation erythropoiesis-stimulating agent. Expert Opin Pharmacother. 2008;9:839–849. doi: 10.1517/14656566.9.5.839. [DOI] [PubMed] [Google Scholar]
- 188.Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. [PubMed] [Google Scholar]
- 189.Wrighton NC, et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 190.Liu Z, et al. A potent erythropoietin-mimicking human antibody interacts through a novel binding site. Blood. 2007;110:2408–2413. doi: 10.1182/blood-2007-04-083998. [DOI] [PubMed] [Google Scholar]
- 191.Bernhardt WM, et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 192.Hsieh MM, et al. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Heinicke K, et al. Excessive erythrocytosis in adult mice overexpressing erythropoietin leads to hepatic, renal, neuronal, and muscular degeneration. Am J Physiol Regul Integr Comp Physiol. 2006;291:R947–956. doi: 10.1152/ajpregu.00152.2006. [DOI] [PubMed] [Google Scholar]
- 194.Erbayraktar S, et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci U S A. 2003;100:6741–6746. doi: 10.1073/pnas.1031753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fiordaliso F, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Leist M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 197.Wang L, et al. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–32470. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- 198.Brines M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 200.Nadam J, et al. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol Dis. 2007;25:412–426. doi: 10.1016/j.nbd.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 201.Drueke TB, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 202.Singh AK, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 203.Jelkmann W, et al. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol. 2008;67:39–61. doi: 10.1016/j.critrevonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 204.Littlewood TJ, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 205.Glaser CM, et al. Impact of hemoglobin level and use of recombinant erythropoietin on efficacy of preoperative chemoradiation therapy for squamous cell carcinoma of the oral cavity and oropharynx. Int J Radiat Oncol Biol Phys. 2001;50:705–715. doi: 10.1016/s0360-3016(01)01488-2. [DOI] [PubMed] [Google Scholar]
- 206.Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 207.Henke M, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 208.Bennett CL, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. Jama. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 209.Rizzo JD, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26:132–149. doi: 10.1200/JCO.2007.14.3396. [DOI] [PubMed] [Google Scholar]
- 210.Bunn HF, et al. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998;201:1197–1201. doi: 10.1242/jeb.201.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading, resources and contacts
Publications
- Hodges VM, et al. Pathophysiology of anemia and erythorcytosis. Crit Rev Oncol Hematol. 2007;64:139–158. doi: 10.1016/j.critrevonc.2007.06.006. This review describes anaemia and erythrocytosis in relation to erythropoietin (EPO) levels in diagnosis and treatment. [DOI] [PubMed] [Google Scholar]
- Sasaki R. Pleiotropic functions of erythropoietin. Intern Med. 2003;42:142–149. doi: 10.2169/internalmedicine.42.142. This review highlights the early observations of EPO production in the central nervous system and reproductive organs, including the author’s pioneering work on EPO expression and function in these tissues. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, et al. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol/Hematol. 2008;67:39–61. doi: 10.1016/j.critrevonc.2008.03.006. This paper reviews EPO receptor expression and function in tumour tissues and the implications for administration of EPO to treat anaemia in cancer patients. [DOI] [PubMed] [Google Scholar]
- Sytkowski AJ. Erythropoietin: Blood, Brain and Beyond. Wiley-VCH; Weinheim, Germany: 2004. This book provides an overview of the history, developmental biology, transcription regulation and biochemistry of EPO in the context of erythropoiesis and EPO therapy in anaemia in relation to a variety of clinical conditions. Other topics include EPO in nonhaematopoietic tissues and in elite athletics. [Google Scholar]
- Websites:This page from the Information Center for Sickle Cell and Thalassemic Disorders website includes an overview of the relationship between iron and erythropoietin activity in red blood cell production: http://sickle.bwh.harvard.edu/iron_epo.html