Abstract
Context
Relatively little is known about genetic determinants of cognitive dysfunction in schizophrenia. Recent studies suggest that a BDNF prodomain SNP, resulting in valine to methionine substitution, is associated with impaired declarative memory in healthy volunteers and in schizophrenia patients. These studies indicate that BDNFMet variant may mediate hippocampal cognitive functions by modulating intracellular trafficking and activity-dependent BDNF release. How this functional SNP affects other neurocognitive measures have not been examined. Its role in determining cognitive deficits in schizophrenia has not been systematically studied either.
Objective
To characterize the neurocognitive and brain morphometric phenotypic correlates of BDNF val66met polymorphism, and test the specificity of BDNFMet variant on cognitive dysfunction in schizophrenia.
Design, Setting and Participants
A comprehensive battery of standardized neuropsychological tests was administered to 144 healthy volunteers and 293 schizophrenia-spectrum disorder patients at a tertiary-care university hospital. Approximately two-thirds of the sample also underwent high-resolution MRI brain scans.
Main Outcome Measures
Genotype effects (Met-allele-carriers versus Val homozygotes) on five cognitive domain z-scores and MRI gray matter (GM) brain volume measures (Talairach-atlas based cerebral lobes and optimized voxel-based morphometry (VBM)) were examined using general linear models.
Results
On verbal memory, there was a significant genotype effect but no genotype-by-diagnosis effects. In both patients and healthy volunteers, Met-allele-carriers had poorer verbal memory performance than their Val/Val counterparts. On visuospatial abilities, there were significant genotype and genotype-by-diagnosis effects. Met allele-associated visuospatial impairment was specific to patients, but not healthy volunteers. There were significant genotype effects on GM volumes within brain regions known to subserve these two cognitive domains, with Met-allele-carriers having smaller temporal and occipital lobar GM. Optimized VBM further suggests that parietal heteromodal cortical GM deficits may underlie visuospatial impairment in Met-allele-carrier patients.
Conclusions
We replicated the association between BDNFMet variant and poor medial temporal lobe-related memory performance. The consonance of our cognitive and brain morphology findings further suggests that BDNFMet variant may have a specific role in conferring visuospatial dysfunction in schizophrenia.
Schizophrenia is a complex genetic disorder characterized clinically by a heterogeneous syndrome comprising delusions, hallucinations and cognitive impairment. These myriad signs and symptoms arise from dysfunction in distributed neural circuits, and from complex genes-environment interactions. One approach toward tackling the complex genetics and phenotypic heterogeneity1, 2 has been to better understand the genetic basis of cognitive impairment, a core endophenotypic feature of schizophrenia. Using a large cohort of schizophrenia patients and healthy volunteers, the present report pursues this research by investigating the phenotypic correlates (cognition and MRI brain gray matter (GM) volumes) of a SNP within the brain-derived neurotrophic factor (BDNF) gene.
BDNF, a vital trophic protein for neuronal survival and differentiation in the developing nervous system, is also important in modulating activity-dependent synaptic plasticity among mature neurons 3, 4. Stimulation of hippocampal and visual cortical neurons increases BDNF gene transcription, protein secretion and synaptic signaling. Activity-dependent BDNF secretion is required for long-term potentiation and long-term depression, which are cellular mechanisms underlying learning and memory 5, 6. BDNF’s role in cognition is further supported by studies of BDNF-mutant mice, which show hippocampal-dependent learning deficits and impaired pattern discrimination 7, 8.
Recent studies indicate that a SNP (rs6265), producing a valine to methionine substitution in the proBDNF protein at codon 66 (Val66Met), is related to hippocampus-mediated memory performance in humans 9–12. Hippocampal neurons transfected with BDNFMet variant show less depolarization-induced BDNF secretion 9, 13. It is postulated that methionine substitution leads to inefficient trafficking of BDNF to secretory granules, reduced activity-dependent BDNF release, and in turn, poorer hippocampus-mediated memory. Thus, met allele carriers, regardless of healthy human volunteers, schizophrenia probands or their unaffected siblings, have poorer episodic memory than their respective Val homozygous counterparts 9, 11, 12. Heterozygotes also have significantly lower left hippocampal N-acetyl aspartate levels 9. Healthy volunteers who are met allele carriers have lower hippocampal fMRI BOLD response while performing a declarative memory task 10, and have smaller hippocampal and prefrontal GM volumes 14, 15.
Thus, BDNF val66met polymorphism appears to be important in hippocampus-dependent memory in humans, possibly regardless of schizophrenia affectation status. However, its effects on other cognitive domains in healthy individuals have not been systematically studied. Whether this functional SNP has a specific role in conferring cognitive dysfunction in schizophrenia is also unknown. Therefore, we characterized the neurocognitive phenotype of BDNF val66met polymorphism by assessing the genotype effects on 1) a comprehensive battery of standardized neuropsychological tests, and 2) MRI GM brain volumes. Since larger GM volumes in specific brain regions have been associated with better performance in different cognitive domains 16, using these two complementary approaches will provide convergent evidence regarding genotype-phenotype relationships.
Methods
Subjects
Two hundred and ninety-three patients with schizophrenia-spectrum disorders and 144 healthy volunteers were obtained through the University of Iowa Mental Health Clinical Research Center (MHCRC). These subjects have participated in various MHCRC research studies approved by the University of Iowa institution review board. All subjects gave written informed consent to undergo research assessments. Patients were evaluated using a semi structured interview instrument, Comprehensive Assessment of Symptoms and History (CASH) 17, from which schizophrenia (N=264), schizoaffective disorder (N=23) or schizotypal personality disorder (N=6) diagnoses meeting DSM-III-R or DSM-IV criteria were based. Healthy volunteers were recruited from the community through newspaper advertisements. They were initially screened by telephone, and further evaluated using an abbreviated version of the CASH to exclude subjects with current or past medical, neurological or psychiatric illnesses.
Genetic Analyses
DNA was prepared by high-salt extraction from whole blood 18. BDNF val66met genotyping was performed using the fluorogenic 5′ nuclease method (TaqMan®, Applied Biosystems, Foster City, CA) using reagents obtained from Applied Biosystems (ABI), including VIC and FAM labeled probes and TaqMan® Universal PCR Master Mix. PCR and allele calling were performed on a StrataGene Mx3000P qPCR thermocycler. Replicate samples were included on all genotyping plates to ensure accurate allele calling.
Neurocognitive Assessment
All study subjects were administered a comprehensive cognitive battery by psychometrists who have been trained in standardized assessment and scoring procedures. Testing generally took approximately 4 hours to complete and, when necessary, occurred over several sessions.
In order to provide comprehensive yet efficient assessment of the relationships between cognitive performance and BDNF polymorphism, the neuropsychological tests were grouped into cognitive domains on the basis of a priori theoretical considerations19–23. These five domains were: verbal memory, processing speed/attention, problem solving, language skills, and visuospatial skills. These theoretical groupings of cognitive domains have good internal consistency, and their internal reliability have been tested using Cronbach’s alpha 19, 24. The median Cronbach’s alpha for the five cognitive domains was 0.80 (range=0.75 to 0.85).
The verbal memory domain is comprised of the following neuropsychological tests: Rey Auditory Verbal Learning Test (RAVLT) – trials 1–5, RAVLT – trial 7, RAVLT – delayed recall, Wechsler Memory Scale – Revised (WMS-R) Logical Memory – immediate recall, WMS-R Logical Memory – delayed recall. The component neuropsychological tests for visuospatial skills were Rey-Osterrieth Complex Figure Test – copy, WAIS-R Block Design subtest, WAIS-R Object Assembly subtest, Benton Judgment of Line Orientation. The remaining 3 cognitive domains, computed using 18 other neuropsychological test variables, have been previously described 19.
Prior to deriving cognitive domain scores for subjects in this study, the raw test score from each of the neuropsychological test variable was converted to a z score (Mean=0, SD=1) based on norms established using our database on 576 healthy volunteers. Since these almost 600 healthy volunteers were recruited from the same geographical area and were tested by the same psychometrists, our normative dataset provides a consistent and uniform basis for deriving the z-scores. Scores were reversed where necessary so that a larger negative score indicates poorer performance below the mean. Using these z scores, each domain score is the summed average of its component neuropsychological test variables.
MRI acquisition and image processing
Magnetic resonance images of the whole brain were obtained on a 1.5-Tesla GE (General Electric Medical Systems, Milwaukee, Wisconsin) Signa MR scanner. Three different MR sequences were acquired for each subject (i.e. T1-weighted spoiled grass, proton density (PD) and T2-weighted images). The images were processed using the locally developed BRAINS (Brain Research: Analysis of Images, Networks, and Systems) software package. The imaging parameters as well as detailed descriptions of image analysis methods for measuring lobar gray matter (GM) volumes have been provided elsewhere 25–28.
In brief, the T1-weighted images were spatially normalized and re-sampled so that the anterior-posterior axis of the brain was realigned parallel to the anterior-posterior commissure line, and the interhemispheric fissure was aligned on the other two axes. The T2 and PD weighted images were aligned to the spatially normalized T1 weighted image using an automated image registration program 29. These images were then subjected to a linear transformation into standardized stereotaxic Talairach atlas space 30 to generate automated measurements of frontal, temporal, parietal, and occipital lobes, cerebellum, and subcortical regions 27. To further classify tissue volumes into GM, white matter (WM) and cerebrospinal fluid (CSF), we employed a discriminant analysis method of tissue segmentation based on automated training class selection that utilized data from the T1, T2 and PD sequences 28. In this study, we examined GM volumes in the frontal, temporal, parietal and occipital lobes.
A second set of image analysis used a voxel-based morphometry (VBM) approach to estimate GM brain volumes. VBM is a whole-brain method that involves voxel-wise statistical inference about regional GM concentration or volume in relationship to group membership or other variables of interest. VBM analysis was implemented on a Linux workstation that runs Matlab 6.5, R13 (The MathWorks Inc., Natick, MA, USA) to host the SPM package (SPM2, Wellcome Department of Cognitive Neurology, Institute of Neurology, London). VBM was performed as described by Good et al. 31. This “optimized” VBM method is based on the previously described VBM protocol 32 with some modifications that include the creation of study-specific templates and a modulation step. T1 MR images were first registered and spatially normalized to the default SPM T1 template to create study-specific templates for the control and patient groups. After tissue segmentation, the study-specific templates consisted of an average T1 image and a priori probability gray, white and CSF images. T1 images of patients and control subjects were then registered and spatially normalized to the study-specific templates created in the previous step, and these warped images were segmented into GM, WM and CSF. Only the GM images were used for the statistical analyses. The spatial normalization step involved both linear and non-linear transformations. As a result of the non-linear transformations, the volumes of certain brain regions may grow while others may shrink. The modulation step multiplies the image intensity at each voxel by the corresponding Jacobean determinant derived from the non-linear normalization step. This modulation step effectively converts the concentration (relative amount of GM in each voxel) to absolute amounts (volumes) of GM in a voxel. Finally, the normalized modulated GM images were smoothed with a 12 mm FWHM isotropic Gaussian kernel. The modulated smoothed GM image data were used for statistical analysis with SPM2 employing the framework of the general linear model 33.
Statistical analyses
BDNF val66met allele and genotype frequencies were compared between patients and healthy volunteers using Chi-square tests. Both heterozygotes and Met homozygotes have been previously associated with impaired BDNF secretion 9, 13. Furthermore, in our sample of patients and healthy volunteers, heterozygotes and Met homozygotes did not differ significantly with regard to cognitive domain scores (T109’s≤1.15, p’s≥0.25 among patients; T47’s≤1.00, p’s≥0.32 among healthy volunteers) or lobar GM volumes (T73’s≤0.70, p’s≥0.49 among patients; T21’s≤1.14, p’s≥0.27 among healthy volunteers). Thus, heterozygotes and Met homozygotes were combined and categorized as the ‘Met’ BDNF group. Val homozygotes were categorized as ‘Val’ BDNF group.
Analyses of the relationships between BDNF grouping and the 5 cognitive domain scores were performed using ANCOVAs. By summarizing our neuropsychological battery into cognitive domains and by performing the statistical analyses in two steps, we limit Type I errors and reduce capitalizing on chance associations. The first step involved an ANCOVA test for each cognitive domain as the dependent variable. Each model included 6 factors: age, gender, full scale IQ, diagnostic grouping, genotype grouping and a diagnostic group-by-genotype interaction term. Main effects, i.e. remaining variance after accounting for the other 5 factors in the model, for diagnostic grouping, genotype grouping and diagnostic group-by-genotype interaction are presented. The diagnostic group-by-genotype interaction term in the model detects the differential effects alleles might have on cognitive scores between diagnostic groups. For cognitive domains where the ANCOVA had significant genotype main effects, a second step of follow-up analyses was then performed using the component neuropsychological test scores.
The main effects of BDNF genotype on lobar GM volumes were also analyzed using ANCOVAs. In each general linear model, the respective lobar GM measure was the dependent measure. Intracranial volume, age, gender and diagnostic grouping, genotype grouping and diagnostic group-by-genotype interaction were entered as factors into each model. VBM statistical analyses, which also employed the framework of general linear models, was performed using SPM2 33.
Results
Sociodemographic characteristics of the sample are summarized in Table 1. Healthy volunteers and patients were of comparable age. A significantly greater proportion of patients were male. Patients also had fewer years of education and lower full scale IQ. However, parental educational attainment was comparable between the diagnostic groups.
Table 1.
Sociodemographic characteristics and BDNF allele and genotype distributions in healthy volunteers and schizophrenia paitents.
| Healthy volunteers | Patients | Statistica (p) | |
|---|---|---|---|
| N | 144 | 293 | |
| % Male | 45.1 | 72.7 | 31.7 (<.0001) |
| Mean age (SD) (years) | 27.9 (8.19) | 27.4 (9.21) | 0.56 (0.58) |
| Education (years) | 15.0 (1.79) | 12.9 (2.19) | 10.1 (<0.0001) |
| Parental education (years) | 13.4 (2.14) | 13.4 (2.57) | 0.14 (0.89) |
| Full scale IQ | 110 (11.7) | 91.5 (13.5) | 14.4 (<0.0001) |
| Allele frequency (%) | |||
| Met | 19.1 | 20.8 | 0.35 (0.55) |
| Val | 80.9 | 79.2 | |
| Genotype frequency (N (%)) | |||
| Met/Met | 6 (4.2) | 11 (3.8) | Healthyb: 0.54 (0.77) |
| Met/Val | 43 (29.9) | 100 (34.1) | Patientsb: 0.26 (0.88) |
| Val/Val | 95 (65.9) | 182 (62.1) | |
Chi-square tests for categorical measures; T-tests for continuous measures
Hardy-Weinberg expectation
BDNF allele and genotype frequencies are summarized in Table 1. Met allele frequency was not statistically significant across diagnostic groups. Genotype distributions in healthy volunteers and schizophrenia patients did not deviate from Hardy-Weinberg expectations (χ2=0.35 and 0.76 respectively, p’s≥0.35).
The mean age of illness onset among patients was 21.5 years (SD=6.74), and mean duration of illness 6.70 years (SD=6.75). Met and Val patient groups did not differ significantly with respect to age of illness onset, premorbid social adjustment, or severity of positive symptoms (T291’s≤0.89, p’s≥0.37). Met-allele-carrier patients had significantly less severe negative symptoms (Mean=10.9 (SD=3.99) versus 11.8 (SD=3.63) in Val homozygous patients; T291=2.00, p=0.02).
Genotype effects on cognitive domain scores
The effects of BDNF val66met polymorphism on full scale IQ and on the five neurocognitive domains are summarized in Table 2. There were significant genotype effects on verbal memory performance (F1,436=4.51, p=0.03) and on visuospatial abilities (F1,436=4.12, p=0.04). No statistically significant genotype effects or genotype-by-diagnosis effects were observed with regard to full scale IQ, processing speed/attention, problem solving, or language cognitive domain scores.
Table 2.
Comparison of full-scale IQ and cognitive domain scores (z-scores) by diagnostic and genotype groupings
| Neurocognitive Domains | Healthy volunteers | Patients | Genotype F * (p) | Genotype by Diagnosis F * (p) | ||
|---|---|---|---|---|---|---|
| Met (N=49) | Val (N=95) | Met (N=111) | Val (N=182) | |||
| WAIS-R FSIQ | 110.9 (12.9) | 110.2 (11.0) | 91.9 (14.5) | 91.3 (12.9) | 0.42 (0.52) | 0.07 (0.78) |
| Verbal memory | 0.03 (0.80) | 0.19 (0.75) | −1.38 (1.04) | −1.31 (0.95) | 4.51 (0.03) | 0.00 (0.95) |
| Processing speed/Attention | 0.07 (0.75) | 0.13 (0.60) | −1.31 (1.12) | −1.20 (0.99) | 2.77 (0.10) | 0.64 (0.42) |
| Problem solving | 0.06 (0.52) | −0.05 (0.56) | −1.20 (1.24) | −1.23 (1.03) | 0.23 (0.63) | 0.55 (0.46) |
| Language | 0.01 (0.89) | −0.02 (0.77) | −1.10 (1.36) | −1.24 (1.10) | 0.10 (0.75) | 0.97 (0.33) |
| Visuospatial abilities | 0.07 (0.70) | 0.04 (0.64) | −1.14 (1.55) | −0.77 (1.14) | 4.12 (0.04) | 5.58 (0.02) |
Met: Met/Met or Met/Val genotypes, Val: Val homozygotes;
Main effects on cognitive domain scores (df=1, 436)
Healthy volunteers with Val/Val genotype had higher verbal memory cognitive domain scores than Met-allele-carrier healthy volunteers. Similarly, the mean verbal memory score in Val/Val patients was also higher than the Met patient group. In addition to a significant genotype effect, there was also a statistically significant genotype-by-diagnosis effect on visuospatial abilities (Table 2; F1,436=5.58, p=0.02). Patients with Met allele were significantly more impaired than Val/Val patients (F1,292=10.46, p=0.001). There were, however, no group differences in visuospatial performance among healthy volunteers (F1,143=0.27, p=0.61).
Approximately one-third of patients were not receiving antipsychotic treatment at the time of neurocognitive testing (33 patients were antipsychotic-naïve and 59 patients had been noncompliant or had undergone a medication washout prior to functional neuroimaging studies). Almost half of the patients were treated with the newer atypical antipsychotics (132 patients on monotherapy and 9 patients received a newer atypical plus a typical antipsychotic); 23 patients were on typical antipsychotic monotherapy and 37 patients required clozapine treatment. BDNF genotype frequency did not differ significantly across antipsychotic treatment groups (χ2=0.70, df=2, p=0.70). Antipsychotic treatment (treatment versus no treatment or atypicals versus typicals) had no significant main effects on verbal memory or visuospatial cognitive domain scores (p’s≥0.09). When antipsychotic treatment was entered into the general linear models, the main effects of genotype remained statistically significant (p’s<0.05). Details of analyses are available upon request.
Genotype effects on component neuropsychological tests within verbal memory and visuospatial cognitive domains
For the two cognitive domains in which there were significant genotype effects, the mean z-scores for individual component neuropsychological tests, broken down by diagnostic groupings and by genotype, are summarized in Table 3 and Figure 1a.
Table 3.
Comparison of verbal memory and visuospatial neuropsychological tests (z-scores) by diagnostic and genotype groupings
| Component neuropsychological tests | Healthy volunteers | Patients | Genotype F (p) * | ||
|---|---|---|---|---|---|
| Met | Val | Met | Val | ||
| Verbal memory domain | |||||
| RAVLT – trials 1–5 | −0.01 (1.03) | 0.14 (0.87) | −1.64 (1.31) | −1.50 (1.15) | 5.80 (0.02) |
| RAVLT – trial 7 | 0.01 (1.07) | 0.03 (0.92) | −1.41 (1.36) | −1.35 (1.15) | 1.40 (0.24) |
| RAVLT – delayed recall | 0.02 (1.05) | 0.03 (0.94) | −1.48 (1.20) | −1.30 (1.13) | 3.85 (0.05) |
| WMS-R Logical Memory – immediate recall | −0.03 (0.78) | 0.25 (0.92) | −1.26 (1.11) | −1.23 (1.14) | 4.57 (0.03) |
| WMS-R Logical Memory – delayed recall | 0.03 (0.79) | 0.28 (0.82) | −1.07 (1.04) | −1.13 (1.03) | 2.72 (0.09) |
| Visuospatial abilities domain | |||||
| ROCFT – copy | 0.05 (0.79) | 0.16 (0.77) | −1.33 (2.55) | −0.99 (1.95) | 3.60 (0.05) |
| WAIS-R Block Design subtest | 0.00 (1.04) | 0.02 (0.95) | −1.12 (1.46) | −0.84 (1.32) | 6.33 (0.01) |
| WAIS-R Object Assembly subtest | 0.04 (1.00) | 0.02 (0.88) | −0.91 (1.33) | −0.75 (1.19) | 2.44 (0.12) |
| Judgment of Line Orientation | 0.17 (0.88) | −0.04 (1.01) | −1.01 (2.11) | −0.48 (1.48) | 4.80 (0.03) |
Met: Met/Met or Met/Val; Val: Val/Val only
effects covarying for gender, FSIQ, age and diagnostic grouping (df=1, 436)
RAVLT = Rey Auditory Verbal Learning Test; WMS-R = Wechsler Memory Scale – Revised; ROCFT = Rey-Osterrieth Complex Figure Test; WAIS-R = Wechsler Adult Intelligence Scale – Revised; Judgment of Line Orientation
Figure 1.
Figure 1a. Comparison of neuropsychological test scores (z-scores) within visuospatial cognitive domain between genotype groupings in healthy volunteers and schizophrenia patients (p values for genotype effects covarying for gender, FSIQ and age; ROCFT = Rey-Osterrieth Complex Figure Test; WAIS = Wechsler Adult Intelligence Scale – Revised).
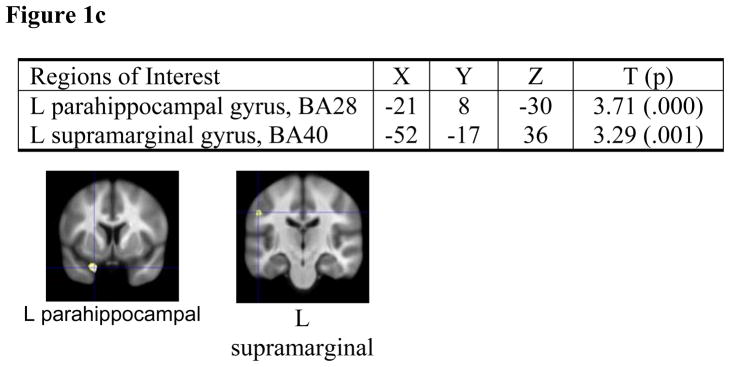
Optimized voxel-based morphometry analyses of healthy volunteers (Figure 1b) and schizophrenia patients (Figure 1c): Brain regions in which Val homozygotes had greater gray matter volumes than Met allele carriers.
Within the verbal memory cognitive domain, there were significant genotype effects on two of the three RAVLT test scores (Mean of trials 1 to 5 and delayed recall), and on WMS-R Logical Memory (immediate recall) (F1,436’s≥3.85, p’s≤0.05). The effects of genotype on WMS-R Logical Memory (delayed recall) approached, but did not achieve statistical significance (F1,436=2.72, p=0.09).
For the component neuropsychological tests in the visuospatial abilities domain, BDNF genotype had significant effects on the ROCFT Copy, WAIS-R Block Design and Judgment of Line Orientation tests (F1,436’s≥3.60, p’s≤0.05). More interestingly, compared to Val homozygous patients, the Met patient group consistently had greater impairment in all 4 tests that assessed visuospatial abilities (Figure 1a; significant main effects of genotype for WAIS-R Block Design subtest and Judgment of Line Orientation: F1,292’s≥7.49, p’s≤0.007). Among healthy volunteers, on the other hand, there were no statistically significant genotype effects on any of the 4 component neuropsychological tests used in computing the visuospatial abilities domain (Figure 1a; F1,143’s≤2.50, p’s≥0.16).
MRI brain GM volume correlates
Eighty healthy volunteers and 183 schizophrenia patients received multi-spectral MRI brain scans. The remaining 64 healthy volunteers and 110 patients did not have available MRI scans because they were claustrophobic in the scanner, had poor quality scans, or had scans obtained using non-comparable imaging parameters (either on an older or newer scanning protocol). There were no statistically significant differences between healthy volunteers with and without MR data or between patients with and without MR data with regard to gender composition, BDNF genotype distribution, age, clinical characteristics or cognitive domain scores (details of statistical analyses available upon request).
We first compared Talairach-based lobar GM volumes between genotype groupings. We further explored genotype effects on GM volumes from smaller brain regions using optimized voxel-based morphometry (VBM). There were significant genotype effects on GM volumes within brain regions known to subserve verbal memory and visuospatial abilities (Table 4; Temporal lobar GM: F1,262=5.44, p=0.02; Occipital lobar GM: F1,262=5.73, p=0.02). Patient and healthy volunteer met-allele-carriers had smaller temporal and occipital lobar GM volumes than their respective Val/Val counterparts. There were no significant genotype-by-group interactions on any of the lobar GM volume measures.
Table 4.
BDNF: Comparison of MRI lobar gray matter (GM) brain volumes (cc) by diagnostic and genotype groupings
| ROIs | Healthy volunteers | Patients | Genotype F (p) * | Genotype by Diagnosis F (p) * | ||
|---|---|---|---|---|---|---|
| Met (N=23) | Val (N=57) | Met (N=72) | Val (N=111) | |||
| Frontal GM | 266.5 (34.9) | 270.0 (35.5) | 263.8 (37.3) | 266.3 (34.1) | 0.06 (0.80) | 0.18 (0.67) |
| Temporal GM | 154.7 (17.8) | 161.0 (17.3) | 155.8 (18.9) | 158.2 (17.8) | 5.44 (0.02) | 0.80 (0.37) |
| Parietal GM | 143.1 (19.2) | 143.5 (16.0) | 140.7 (17.9) | 141.0 (16.1) | 0.31 (0.58) | 0.23 (0.63) |
| Occipital GM | 70.4 (11.0) | 73.6 (9.1) | 69.3 (8.9) | 72.0 (10.0) | 5.73 (0.02) | 0.00 (0.99) |
Met: Met/Met or Met/Val; Val: Val/Val only
Main effects on lobar gray matter volumes (df=1, 262)
Since our automated Talairach-based method provides only lobar GM volumes, we further explored BDNF genotype effects using VBM. An SPM2-based implementation of VBM compared 57 Val/Val healthy volunteers against 23 healthy volunteer met-allele-carriers. The Val group had significantly larger GM volumes in regions that mediate verbal memory encoding (left inferior temporal gyrus, Brodmann area (BA) 37; left prefrontal cortex, BA9) and object perception (left lateral occipital cortex/extrastriate cortex, BA18) (Figure 1b). A separate VBM comparing 111 Val/Val patients versus 72 met-allele-carrier patients indicated that Val patients had significantly larger left parahippocampal (known to mediate verbal memory encoding) and left supramarginal gyral (visuomotor control) GM volumes (Figure 1c).
Discussion
In this study, we investigated the roles of BDNF in human cognition and in cognitive dysfunction among schizophrenia patients. Using a large sample of healthy volunteers and schizophrenia patients, we systematically examined the effects of BDNF Val66Met gene polymorphism on a comprehensive battery of standardized neuropsychological tests, and on high-resolution MRI gray matter (GM) brain volume measures. Besides replicating the association between BDNFMet variant and poor declarative memory 9–12, 14, 15, we also found BDNFMet variant to correlate with reduced GM volumes within brain regions known to participate in verbal memory and visuospatial abilities 34–36. The consonance of these cognitive and brain morphology findings suggests that BDNF val66met polymorphism influences specific aspects of human cognition. Genotype status did not appear to impact general intellectual abilities, or affect attention, problem solving or language skills. Of greater potential significance, we believe, is that BDNFMet variant may have a specific role for conferring visuospatial dysfunction in schizophrenia. Unlike healthy volunteers, Met-allele-carrier patients consistently performed worse than their Val homozygous counterparts on all four tests of visuospatial abilities. Met-allele-carrier patients also had GM brain volumetric deficits in both the ventral as well as the dorsal visual pathways.
Neurocognitive impairment, a hallmark of schizophrenia 37–40, is not restricted to a small subset of patients. Measurable deficits are present in 40%–60% of schizophrenia patients 41. Impairment tends to be generalized and involves dysfunction in multiple cognitive domains including visuospatial abilities 42. A recent meta-analysis found that compared to healthy volunteers, the mean effect sizes for impairment in Judgment of Line Orientation and WAIS Block Design tests were 0.60 and 0.46 respectively 43. Such deficits in visuospatial performance have often been attributed to failures of attention or short-term visual memory 44, 45. However, impairment in the perception of visual stimuli 46 and in other early-stage processing of visual information 47–52 may also contribute to poor visuospatial abilities. Visuospatial performance is subserved by large-scale, distributed neuronal networks. Object perception involves the ventral occipito-temporal pathway whereas the dorsal occipito-parietal stream is associated with spatial information 34, 36, 53, 54. Therefore, brain regions that mediate visuospatial abilities include the secondary visual cortices (BA 18), inferior temporal regions (BA 37) and the parietal heteromodal association cortices.
In our study, we found that Met allele carriers, regardless of healthy volunteers or schizophrenia patients, had smaller occipital and temporal lobar GM volumes than their respective Val homozygous counterparts. Additionally, from our VBM analyses, Met-allele-carrier patients had smaller left supramarginal gyral GM volume than Val homozygous patients. Healthy volunteers, on the other hand, did not show any parietal GM volume differences across genotype groupings. Because visuospatial abilities involve widely distributed neural circuits, healthy volunteers with BDNFMet variant may have sufficient cognitive reserve to compensate for deficits within the occipital and temporal lobes. Thus, without parietal GM volume deficits, Met-allele-carrier healthy volunteers were able to perform comparably as their Val/Val counterparts in neuropsychological tests assessing visuospatial abilities. On the other hand, having deficits in both the ventral occipito-temporal as well as dorsal occipito-parietal visual pathways, met-allele-carrier schizophrenia patients may be less able to compensate for global reductions in occipital and temporal lobar GM volumes. This may, in turn, translate into poorer performance on neuropsychological tests assessing visuospatial abilities.
Among its diverse functions of neuronal survival and in mediating synaptic plasticity, BDNF is also known to play important roles during the development of the visual cortex 55 as well as in modulating visual functions 56–59. Visual experience-regulated secretion of BDNF is an essential molecular signal for normal visual cortical maturation. High levels of BDNF and TrkB mRNA expression have been found in the higher-order visual areas of adult macaque monkeys, which further suggest that BDNF may continue to regulate visual functions beyond neurodevelopment. While the importance of BDNF in mediating visual cortical neuronal survival and synaptic plasticity within visual pathways is well established, how would a single nucleotide substitution in the gene encoding the proBDNF protein affect cognitive abilities and GM brain volumes?
As with other neurotrophins, the BDNF gene encodes a precursor peptide (proBDNF). During intracellular trafficking through the Golgi apparatus and trans-Golgi network, the proBDNF domain is subsequently cleaved off so that the remaining mature BDNF protein is packaged into secretory vesicles. Compared to the wild-type BDNF (BDNFVal), there is relatively less efficient localization of mature BDNF to secretory vesicles when hippocampal neurons 9 or cerebral cortical neurons 13 were transfected with BDNFMet variant. Since pro-neurotrophins are important for proper folding, dimerization and targeting of the mature neurotrophins 60, Egan et al and Chen et al 9, 13 have postulated that substitution of valine with methionine in the proBDNF may result in defective intracellular protein trafficking, and perturb BDNF synthesis. This would, in turn, lead to decreased activity-dependent BDNF secretion and impairment in hippocampus-mediated memory functions. Like others 9–11, 14, 15, we also found BDNFMet variant to be associated with poorer declarative memory and reduced temporal lobar GM volumes. Although similar expression studies 9, 13 have not been performed on neurons from the visual brain regions, it is not inconceivable that BDNFMet variant could cause analogous intracellular protein trafficking defects in neurons within distributed neural circuits subserving visuospatial abilities. With less BDNF available for activity-dependent secretion in neurons from all three nodes of the visuospatial neural circuits (i.e. occipital, temporal and parietal), Met-allele-carrier patients may therefore show greater impairment in visuospatial test performance.
The mechanisms by which BDNFMet variant affects GM brain volumes are not well understood. This may be mediated neurodevelopmentally through the neurotrophic effects of BDNF 61. If BDNFMet variant results in reduced BDNF synthesis, neuronal proliferation and neuronal survival may be decreased in a BDNF-deficient milieu. Besides having fewer neurons, the surviving neurons may also have small soma size and diminished dendritic growth 62, which would further contribute to smaller gross GM brain volumes. Alternatively, BDNFMet variant may influence GM brain volumes beyond neurodevelopment through modulating synaptic activity in mature neurons 63.
To our knowledge, this is the first study showing that the effects of a common SNP on cognitive function in schizophrenia patients may be different from those in healthy volunteers. Among SNPs known to contribute to variance in human cognition, polymorphisms in the cathecol-O-methyltransferase (COMT), BDNF, metobotropic glutamate receptor (GRM3) and disrupted-in-schizophrenia 1 (DISC1) genes appear to have similar effects in healthy volunteers and in schizophrenia patients 9, 64–67. None of these previous studies have found significant genotype-by-diagnostic grouping effects with regard to working memory or verbal memory. Clearly, our finding that BDNFMet variant may have a specific role for conferring visuospatial dysfunction in schizophrenia needs replication.
Our study is also limited by the inherent low specificity of standardized neuropsychological tests to isolate individual cognitive processes. Future studies may want to explore BDNF genotype effects on experimental cognitive tasks (e.g. visual backward masking), where sub-processes within the visual information processing stream could be better isolated. Additionally, our Talairach-atlas based MR image analyses tested hypotheses at the level of cerebral lobes, and may miss smaller brain regions. We addressed the low regional specificity of Talairach-based lobar GM measurements by performing a second set of MR image analyses using optimized VBM. With our VBM analyses, we were able to replicate two previous studies which examined MRI brain morphometric correlates of BDNF val66met polymorphism 14, 15. Pezawas et al study found reduced GM volumes in the prefrontal regions and smaller hippocampus volumes among Met-allele-carrier healthy volunteers 14. Szeszko and colleagues reported of smaller hippocampus volumes among Met-allele-carriers and that the genotype effect on hippocampus volume was greater among schizophrenia patients than in healthy volunteers 15. Our VBM analyses also indicate that brain regions important in verbal memory that are impacted by BDNFMet variant may be different across diagnostic groups. Met-allele-carrier healthy volunteers had relatively smaller left inferior temporal and left superior frontal gyral GM volumes. Met-allele-carrier patients, on the other hand, only had smaller left parahippocampal gyrus. The absence of frontal lobe GM volume differences between patient genotype groupings may be related to the complex genetics and heterogeneity of schizophrenia. Other schizophrenia susceptibility genes 68 may have greater effects on reducing frontal lobe volume than BDNF val66met polymorphism, thereby obscuring the influence of BDNFMet variant on reducing prefrontal GM volume among schizophrenia patients. Additionally, the limitations of VBM analytic methods 69, 70 and our choice of VBM analysis may have also contributed to these observed differences in brain regions associated with Met allele across patients and healthy volunteers.
Our findings of BDNFMet variant associated verbal memory and visuospatial impairment may have potential treatment implications in schizophrenia. Since the greater part of persistent psychosocial impairment in schizophrenia patients is attributable to cognitive deficits 71, there has been increased interest in developing novel treatments that specifically target neurocognitive dysfunction 72. It is hoped that cognition enhancing treatments may then translate into improved social and vocational outcome for schizophrenia patients. Thus, BDNF and its receptors (i.e. tyrosine kinase receptor TrkB and p75) are potential molecular targets for developing treatments specific against cognitive dysfunction in schizophrenia. Strategies for exploiting this potential include pharmacological agents that elevate endogenous BDNF 73, BDNF-mimetic peptides 74, implantation of genetically engineered cells that produce and release BDNF, or intrathecal infusion of recombinant BDNF. Such novel treatments targeting BDNF transmission may have a small effect on enhancing cognition, and could be especially beneficial for Met-allele-carrier patients. Although many hurdles will need to be overcome before such strategies will benefit patients clinically, it is hoped that as we gradually chip away at the genetic complexity and phenotypic heterogeneity of schizophrenia, individually-tailored and biologically-informed therapies of greater precision will replace our current approaches to the pharmacological treatment of schizophrenia.
Acknowledgments
This research was supported in part by NIMH Grants MH68380, MH31593, MH40856 and MH43271
Footnotes
Parts of this research were presented at the 43rd American College of Neuropsychopharmacology Annual Meeting, San Juan, Puerto Rico, 12/14/04, and Xth International Congress on Schizophrenia Research, Savannah, GA, April 2–6, 2005
References
- 1.Moldin SO. Indicators of liability to schizophrenia: perspectives from genetic epidemiology. Schizophr Bull. 1994;20:169–84. doi: 10.1093/schbul/20.1.169. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 3.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–74. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 5.Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 2004;101:15788–92. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 7.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci U S A. 1996;93:12547–52. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–54. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 9.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 10.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134:73–5. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- 12.Tan YL, Zhou DF, Cao LY, Zou YZ, Wu GY, Zhang XY. Effect of the BDNF Val66Met genotype on episodic memory in schizophrenia. Schizophrenia Research. 2005;77:355–6. doi: 10.1016/j.schres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–11. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Kane JM, Goldman D, Malhotra AK. Brain-derived neurotrophic factor Val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–6. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 16.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19:4065–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 20.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res. 2004;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 21.Kareken DA, Gur RC, Mozley D, Mozley LH, Saykin AJ, Shtasel DL, Gur RE. Cognitive functioning and neuroanatomic volume measures in schizophrenia. Neuropsychology. 1995;9:211–219. [Google Scholar]
- 22.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 23.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Ho BC, Alicata D, Ward J, Moser DJ, O’Leary DS, Arndt S, Andreasen NC. Untreated initial psychosis: relation to cognitive deficits and brain morphology in first-episode schizophrenia. Am J Psychiatry. 2003;160:142–8. doi: 10.1176/appi.ajp.160.1.142. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen NC, Cizadlo T, Harris G, Swayze V, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993;5:121–30. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- 26.Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, 2nd, O’Leary DS, Ehrhardt JC, Yuh WT. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA. 1994;272:1763–9. [PubMed] [Google Scholar]
- 27.Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VW, 2nd, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WT. Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23:144–54. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 31.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 32.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 33.Friston KJ, Holmes AP, Worsley K, Poline J-B, Frith CD, Frackowiak RSJ. Statistic parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 34.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 35.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 36.Ng VW, Bullmore ET, de Zubicaray GI, Cooper A, Suckling J, Williams SC. Identifying rate-limiting nodes in large-scale cortical networks for visuospatial processing: an illustration using fMRI. J Cogn Neurosci. 2001;13:537–45. doi: 10.1162/08989290152001943. [DOI] [PubMed] [Google Scholar]
- 37.Kraepelin E. In: Dementia Praecox and Paraphrenia. Barclay RM, Robertson GM, translators. Edinburgh, Scotland: E&S Livingstone; 1919. [Google Scholar]
- 38.Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin J, translator. New York, New York: International Universities Press; 1950. [Google Scholar]
- 39.Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: The relevance of working memory. In: Carroll BJ, Barrett JE, translators. Psychopathology and the Brain. New York, NY: Raven Press; 1990. pp. 1–23. [Google Scholar]
- 40.Gur RC, Ragland JD, Gur RE. Cognitive changes in schizophrenia - A critical look. Int Rev Psychiatry. 1997;9:449–457. [Google Scholar]
- 41.Goldberg TE, Kelsoe JR, Weinberger DR, Pliskin NH, Kirwin PD, Berman KF. Performance of schizophrenic patients on putative neuropsychological tests of frontal lobe function. Int J Neurosci. 1988;42:51–8. doi: 10.3109/00207458808985758. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed S, Paulsen J, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: A study of first-episode patients. Arch Gen Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 44.Nestor PG, Faux SF, McCarley RW, Shenton ME, Sands SF. Measurement of visual sustained attention in schizophrenia using signal detection analysis and a newly developed computerized CPT task. Schiz Res. 1990;3:329–332. doi: 10.1016/0920-9964(90)90018-3. [DOI] [PubMed] [Google Scholar]
- 45.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–82. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–92. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 47.Miller S, Saccuzzo D, Braff D. Information processing deficits in remitted schizophrenics. J Abnorm Psychol. 1979;88:446–9. [PubMed] [Google Scholar]
- 48.Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia. New findings using schizophrenic subgroups and manic control subjects. Arch Gen Psychiatry. 1981;38:175–9. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- 49.Knight RA, Elliott DS, Freedman EG. Short-term visual memory in schizophrenics. J Abnorm Psychol. 1985;94:427–42. doi: 10.1037//0021-843x.94.4.427. [DOI] [PubMed] [Google Scholar]
- 50.Green M, Walker E. Symptom correlates of vulnerability to backward masking in schizophrenia. Am J Psychiatry. 1986;143:181–6. doi: 10.1176/ajp.143.2.181. [DOI] [PubMed] [Google Scholar]
- 51.Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43:132–8. doi: 10.1016/S0006-3223(97)00316-8. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz BD, Winstead DK. Visual processing deficits in acute and chronic schizophrenics. Biol Psychiatry. 1982;17:1377–87. [PubMed] [Google Scholar]
- 53.Kanwisher N, Downing P, Epstein R, Kourtzi Z. Functional neuroimaging of human visual recognition. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 54.Potts GF, Gugino LD, Leventon ME, Grimson WE, Kikinis R, Cote W, Alexander E, Anderson JE, Ettinger GJ, Aglio LS, Shenton ME. Visual hemifield mapping using transcranial magnetic stimulation coregistered with cortical surfaces derived from magnetic resonance images. J Clin Neurophysiol. 1998;15:344–50. doi: 10.1097/00004691-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang B, Kitamura A, Yasuda H, Sohya K, Maruyama A, Yanagawa Y, Obata K, Tsumoto T. Brain-derived neurotrophic factor acutely depresses excitatory synaptic transmission to GABAergic neurons in visual cortical slices. Eur J Neurosci. 2004;20:709–18. doi: 10.1111/j.1460-9568.2004.03523.x. [DOI] [PubMed] [Google Scholar]
- 57.Desai NS, Rutherford LC, Turrigiano GG. BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem. 1999;6:284–91. [PMC free article] [PubMed] [Google Scholar]
- 58.Berardi N, Maffei L. From visual experience to visual function: roles of neurotrophins. J Neurobiol. 1999;41:119–26. [PubMed] [Google Scholar]
- 59.Okuno H, Tokuyama W, Li YX, Hashimoto T, Miyashita Y. Quantitative evaluation of neurotrophin and trk mRNA expression in visual and limbic areas along the occipito-temporo-hippocampal pathway in adult macaque monkeys. J Comp Neurol. 1999;408:378–98. [PubMed] [Google Scholar]
- 60.Rattenholl A, Lilie H, Grossmann A, Stern A, Schwarz E, Rudolph R. The pro-sequence facilitates folding of human nerve growth factor from Escherichia coli inclusion bodies. Eur J Biochem. 2001;268:3296–303. doi: 10.1046/j.1432-1327.2001.02232.x. [DOI] [PubMed] [Google Scholar]
- 61.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–65. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. Cortical Degeneration in the Absence of Neurotrophin Signaling: Dendritic Retraction and Neuronal Loss after Removal of the Receptor TrkB. Neuron. 2000;26:233–45. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
- 64.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive Subprocesses in Working Memory: Relationship to Catechol-O-methyltransferase Val158Met Genotype and Schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- 66.Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, Balkissoon R, Vakkalanka RK, Weinberger DR, Rapoport JL, Straub RE. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD(67)), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–8. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- 69.Bookstein FL. “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–62. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 70.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 72.Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schiz Res. 2004;72:5. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 73.Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol. 2001;21:484–7. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 74.O’Leary PD, Hughes RA. Design of potent peptide mimetics of brain-derived neurotrophic factor. J Biol Chem. 2003;278:25738–44. doi: 10.1074/jbc.M303209200. [DOI] [PubMed] [Google Scholar]