Abstract
OBJECTIVE
To protect granulosa cells from chemotherapy-induced toxicity by retrovirus-mediated multidrug resistance gene (MDR1) transfection.
DESIGN
Laboratory study.
SETTING
Academic research laboratory in a university hospital.
INTERVENTION(S)
KK15 immortalized murine granulosa cell line was transiently transduced with sf91m3 retrovirus vector carrying MDR1 cDNA that encodes P-glycoprtoein (P-gp). Transduced cells were selected with colchicine and treated with doxorubicin or paclitaxel for 24–72 hours. The expression and function of MDR1 and the mRNA expression of selected steroidogenesis enzymes were evaluated by flow cytometry, cell viability assays, Western blot, and RT-PCR.
MAIN OUTCOME MEASURE(S)
Viability of sf91m3-transduced KK15 cells after treatment with doxorubicin and paclitaxel.
RESULT(S)
sf91m3-transduced KK15 demonstrated high expression of biologically active MDR1 as shown by flow cytometry analysis and immunoblotting using P-gp monoclonal antibody and Rhodamine 123 efflux assays. sf91m3-transduced KK15 exhibited significant resistance to toxicity of 10uM paclitaxel(p≤0.001). MDR1-transduced KK15 cells were also protected from doxorubicin toxicity (10nM to 2.5uM) as shown by cell viability assay (p≤0.02). Both flow cytometry and cell viability assay showed that the protection of KK15 from doxorubicin toxicity was lost at 5 uM of doxorubicin; equivalent to 500 times LD50 (p≥0.05). sf91m3-transduced KK15 showed normal mRNA expression of a panel of selected steroidogenesis enzymes.
CONCLUSION(S)
Retroviral gene delivery of human MDR1 inhibited chemotherapy- induced granulosa cell toxicity and offered chemoprotection in an in vitro model.
INTRODUCTION
Recent improvements in cancer diagnosis and therapeutics have made it possible for many women to survive their initial cancer diagnosis. Many female cancer survivors are however faced with loss of fertility due to chemotherapy-induced premature ovarian failure.(1) Alternative therapy to supplement current tools of fertility and reproductive health preservation in female cancer survivors is urgently needed.(2) (3) (4)
Upregulation of phase one and phase two drug metabolizing enzymes (such as cytochrome P450 and glutathione-S-transferace) and phase three drug transporters (such as MDR1) lead to development of tumor resistance to chemotherapy.(5) (6) Phase three drug transporters, in particular, play a critical role in the metabolism, elimination, and detoxification of chemotherapy. Cancer resistance to chemotherapy is often coupled with MDR1-enhanced drug efflux. (7) MDR1 is an adenosine triphosphate-binding cassette (ABC) transporter of the ABC transporter superfamily. Members of this superfamily are involved in transport processes with substrates ranging from small ions to large polypeptides and polysaccharides.(8) (9) MDR1 encodes the 170 kDa P-glycoprotien (P-gp), an adenosine triphosphate (ATP)-dependent transmembrane efflux pump for a diverse group of lipophilic compounds. (10) As a xenobiotic transporter, P-gp is the predominant molecule responsible for chemoresistance by promoting the transport and efflux of various anticancer agents.(11) (12) (13) (14) P-gp is widely expressed in normal cells and tissues. (15) (13) (16) P-gp expression was shown in ovarian surface epithelium and Fallopian tubes, porcine follicles, porcine granulosa cells, rat preovulatory follicles, (17) and in vitro matured oocytes. (18) Progesterone decreases P-gp MDR1 expression; (14, 18) (19) and estrogen mediates post-transcriptional down-regulation of P-gp. (20) Upregulation of P-gp in normal tissues is thought to serve as body’s defense against xeneobiotic toxicity. The distribution of P-gp on the luminal surfaces of reproductive tissues is consistent with its proposed role in excretory transport and protection of these tissues from cytotoxic agents and xenobiotics and may play a role in the blood-placenta (13) and blood-follicle barriers.
Upregulation of P-gp by MDR1-retroviral transduction techniques has been investigated in ex-vivo and in-vivo bone marrow stem cell protection from chemotoxicity. (15, 16, 21, 22) Here, we examine the potential role of MDR1 as a chemoprotective agent in normal granulosa cells. We show that upregulation of MDR1 in granulosa cells prior to chemotherapy protects from chemotherapy toxicity without adversely affecting the expression of steroidogenic enzymes. Our findings have implication on the potential role of phase 3 drug metabolizing enzymes, such as MDR1, in mitigating chemotherapy toxicity in granulosa cells in the ovary.
MATERIAL AND METHODS
Vectors and cell lines
The proviral vector SF91m3 carrying the human MDR1 cDNA was a kind gift from Christopher Baum. (23) Cell lines used in this study included the GPenv+ AM12 packaging cell line (Genetix Pharmaceuticals, Cambridge, MA), the PG13 mouse embryo fibroblast producer cell line (ATCC, Manassas, VA), and the KK15 immortalized murine granulosa cell line. (24) Cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEMF12) supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin and streptomycin (Invitrogen, Carlasbad, CA).
Retroviral transductions and creation of PG13 and KK15 transduced with psf91m3 and expressing P-gp
Bacterial transformation to propagate psf91m3 plasmid, transduction of AM-12 packaging cells with pSF91m3 plasmid using lipofectamine, cell sorting and creation of stably transfected PG13 clones was performed as previously described (see supplemental data). (23) (25) KK15 cells were co-cultivated on the top of PG13 - pSF91m3 viral vector-producing PG13 cells in transwell plates to produce transiently transfected KK15 expressing functional P-gp (see supplemental material and methods). (13)
Flow cytometry analysis
MDR1-transduced KK15 cells expressing P-gp and control cells were grown in 12 well plates and treated with vehicle control, 5uM doxorubicin or 10uM paclitaxel for 72 hours. For cell viability assay, cells were harvested for staining with annexin V and Propidium iodide as per protocol using annexin V-FITC apoptosis detection kit (BD Pharmingen, San Diego, CA). In MDR1 expression experiments, MDR1 was detected by incubating with a phycoerythrin-conjugated monoclonal MDR1 antibody (UA12-PE) at 1:10 dilution for 30 minutes. (26) Goat anti-mouse immunoglobulin G2A (Jackson Immunoresearch Lab, West Grove, PA) was used as an isotype control. In the Rhodamine-123 (Rh-123; Sigma, St. Louis, MO) efflux assay, MDR1 gene expression was determined in MDR1-transduced cell lines by their ability to efflux the fluorescent dye Rh-123 resulting in an Rh-123 dull phenotype.
Cell viability assay using ATP production
KK15 cells expressing P-gp or control cells were treated with vehicle control or various doses of chemotherapy drugs for 24 to 72 hours. ATP production was measured directly using luciferase reagent to quantify the number of live cells in culture using CellTiter-Glo® Luminescent Cell Viability Assay per protocol (Promega, Madison, WI). Luminescence was read in a 96 well plate using a Synergy plate reader (PerkinElmer Life & Analytical Sciences, Shelton, CT).
Western blot, reverse transcription and polymerase chain reaction (RT-PCR)
Western blot and RT-PCR were performed as described previously. (27)
Statistical analysis
Experiments were performed a minimum of three independent times and when appropriate, the data was analyzed using unpaired t-test or one-way ANOVA followed by Tukey test as a post-ANOVA multiple comparison tests. P < 0.05 was considered statistically significant.
RESULTS
Generation of psf91m3-PG13 stable clones and transient transduction of KK15 granulosa cells
AM-12 packaging cells were transduced with sf91m3 retrovirus vector carrying a human MDR1 cDNA to produce replication-incompetent human MRD1-retroviruses particles. Flow cytometry using the MDR1-spcific antibody UIC2-PE showed that 99.69% of control untransduced AM-12 cells were negative for P-gp, while 97.6% of AM-12 cells transduced with psf91m3 express MDR1 (supplemental figure 1). Cell-free culture supernatant from AM-12 cells transduced with pSF91m3 was used to retrovirally transduce PG13 cells to produce stable clones (supplemental figure 2). Transduced PG13 cells were harvested and either stained with UIC2-PE monoclonal antibody or assayed for Rhodamine123 efflux, followed by flow cytometry. 1.64% of untransduced control PG13 cells were positive for P-gp expression while 15.4% of the transduced PG13 cells stained positive for MDR1 as shown by UIC2-PE antibody staining (supplemental figure 2). In Rhodamine efflux assay for P-gp function, cells expressing zero to low levels of MDR1 retained Rhodamine dye and showed stronger bright fluorescence, whereas cells with high expression of P-gp efflux pump efficiently extruded the dye during staining and appeared dull (supplemental figure 3). Only 1.57% of the control untransduced PG13 were dull following incubation with Rhodamine while 19.8 % of the transduced PG13 were Rhodamine dull indicating that these cells had acquired functional MDR1 (supplemental figure 3).
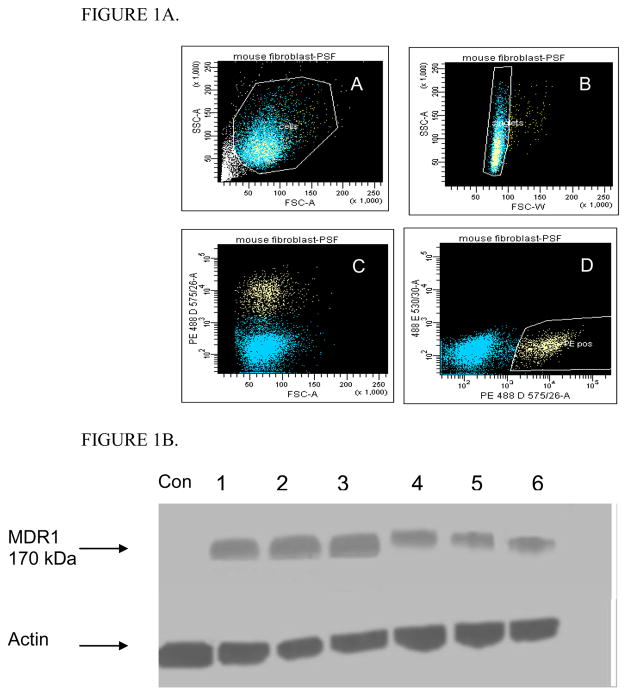
FACSCalibur flow cytometer was used to sort single P-gp positive PG13 cells. There was clear separation of the two cell population following transduction of PG13 cells with SF91m3 (Figure 1A). Sorted PG13 cells were clonally expanded and assessed for P-gp expression by Western blot; Western blot confirmed P-gp expression in the selected clones (Figure 1B).
Figure 1. SF91m3 transfection of PG13 and KK15 cells.
Figure 1A. Categorization and expansion of clonal PG13 packaging cells that produce SF91m3 retrovirus. SF91m3-producing PG13 cells were sorted for single cells expressing MDR1 as defined by staining with phycoerythrin-tagged monoclonal MDR1 antibody (UIC2-PE). A: forward and side scatter revealed that 90 % of the cells were alive, B: live cells gated and sorted for single cells (blue), C: live cells stained with U1C-PE showed two cell population; blue color represent unstained cells while yellow color represent MDR1 carrier cells, D: MDR1 positive cells (yellow) gated and sorted for further cell expansion.
Figure 1B. Relative band intensity for Western blot of MDR1 expressing clonal PG13 packaging cells that was established from sorted cells expressing SF91m3. Six clones (1–6) were screened and confirmed to release high titers of 170-kDa MDR1, while untransduced control PG13 did not express MDR1 protein. B-actin is the internal control. Control (Con) indicates untransduced PG13 cells.
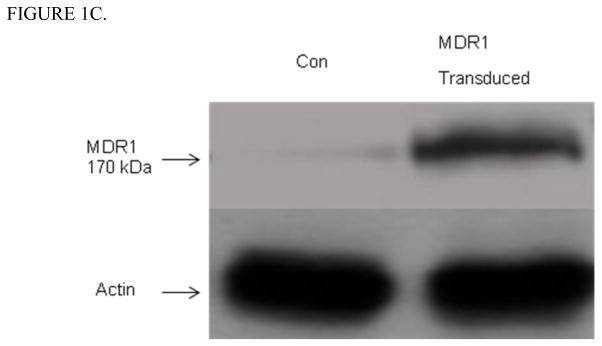
Figure 1C. MDR1 protein expression in in vitro transduced KK15 murine granulosa cells. Western blot of KK15 cells transduced with SF91m3 retrovirus at 100 PFU and co-cultured with colchicine to select for MDR1 positive clones. Tranduced KK15 showed an intense 170-kDa MDR1 band while there was a very faint shadow band in control KK15 cells (Con). B-actin was used as an internal loading control. Control (Con) indicates untransduced KK15 cells.
KK15 cells (28) were transiently transduced with psf91m3 plasmid carrying MDR1 cDNA by co-culture with either PG13 or cell free supernatants containing psf91m3 plasmid and selected for MDR1 expression with colchicine. Western blots showed that P-gp expression was absent in control KK15 cells, while there was a 170 kDa band consistent with the expression of P-gp in transduced KK15 (Figure 1C).
Transduced KK15 cells exhibited protection from paclitaxel and doxorubicin chemotherapy toxicity
Control or KK15 cells expressing human MDR1 were treated with vehicle control or various concentrations of chemotherapy drugs. Cell viability was assessed by flow cytometry following annexin V and Propidium iodide staining or by measuring ATP production. The LD50 (lethal dose 50) of KK15 for doxorubicin and paclitaxel was 50 nM and 1uM respectively (supplemental table 1A) and was higher than that of primary murine granulosa cells (pmGC) likely due to slower rate of growth and cell doubling time of pmGC. Chemotherapy doses equivalent to or higher than the LD50 were used for further experiments. For flow cytometry experiments, control or KK15 cells expressing human MDR1 were treated with vehicle control, 5uM doxorubicin, or 10uM paclitaxel for 72 hours. Cell survival was assessed with FACSCalibur flow cytometer analysis for live/dead cells ratio. MDR1-transiently transduced granulosa cell showed increased cell survival following chemotherapy treatment with 10uM paclitaxel (93±3.2% vs. 42±2.9%, p≤0.001). 5uM Doxorubicin was lethal to KK15 cells and only 12±4.2% of untransduced cells survived (61%±5.3 survival vs. 12%±4.2, p = 0.27) when compared to untransduced cells, (supplemental table 1B and figures 2A).
Figure 2.
Chemotherapy treatment of control or MDR1-transduced KK15 cells with vehicle (control), 5uM doxorubicin, or 10uM paclitaxel. MDR1 transduction completely protected KK15 cell from paclitaxel chemotherapy toxicity, while the protection of KK15 was only partial in the doxorubicin group. Cells were stained with Propodium Iodide for live/dead cell count and live cells were FACS-gated and results presented as histograms. Left peaks on the histograms correspond to live cells and right peaks correspond to dead cells. A and C are MDR1-negative untransduced KK15, B and D are MDR1-positive transduced KK15 cells. Control (Con) indicates vehicle treatment, doxorubicin (Dox), or paclitaxel (Pac).
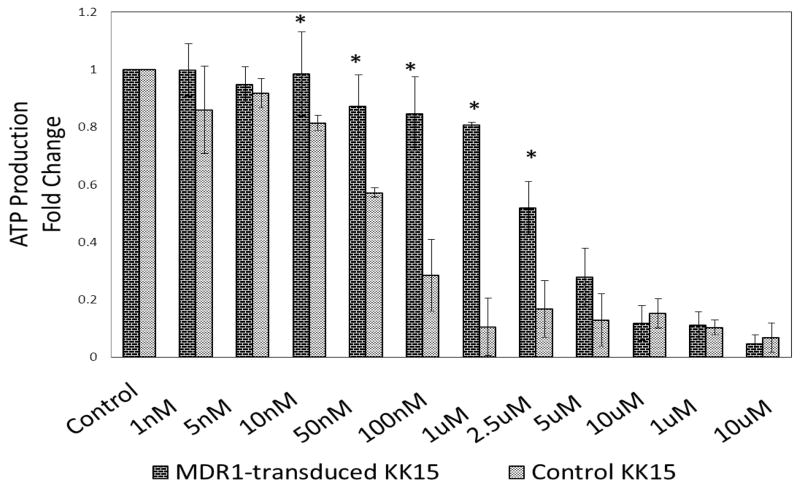
To further examine the potential effects of MDR1 overexpression in KK15 on doxorubicin toxicity, we titrated the effect of doxorubicin treatment in a cell viability assay. Control or MDR1- overexpressing KK15 cells were treated with vehicle control or various concentrations of doxorubicin and assessed for cell viability via ATP production. There was no difference between MDR-transduced and control cells at lower concentrations of doxorubicin chemotherapy (1nM to 5nM) since KK15 cell viability was unchanged for both (P≥0.05, figure 3). Protection from doxorubicin toxicity was seen at 10 nM and at higher concentrations up to 2.5uM, a dose that is 50 times the LD50 (p≤0.02, figure 3). Protection diminished with doxorubicin doses 5uM or higher (p≥0.05, figure 3).
Figure 3.
Cell viability assay of MDR1-transduced (dark-hatched bars) or control (light-hatched bars) KK15 cells treated with different concentrations of doxorubicin (1 nM to 1mM). Cell viability was measure by ATP production using luciferase reagent assay. MDR1 transduction protected KK15 cells from the toxicity of doxorubicin at concentrations as high as 2.5uM (~50 times LD50). Control (Con) indicates vehicle treatment. * = P≤0.05. Data was expressed as mean±sem.
MDR1-transduced KK15 and primary murine granulosa cells showed normal expression of a panel of steroidogenesis enzymes
Control and human MDR1-transduced KK15 cells expressed equivalent amounts of SF-1, CYP1A1, CYP1A2, CYP1B1, HSD17B1, HSD17B2, aromatase, NQO1, and COMT mRNA as shown by semi-quantitative RT-PCR assay. Actin was used as an internal control (Figure 4).
Figure 4.
RT-PCR of mRNA expression levels of selected panel of steroidogenesis enzymes in control or SF91m3- transduced KK15. Control and MDR1-transduced KK15 cells had equivalent expression levels of the tested panel of genes as indicated by equal relative band intensities for RT-PCR results. B-actin was used as the internal control. (C) Indicates control untransduced KK15 cells; (T) indicates MDR1-transduced KK15 cells.
Discussion
One untapped area of research in the field of fertility preservation in cancer patients is the concept of molecular shielding of the ovary to rescue ovarian follicular and steroidogeneic function from chemotoxicity. Very few models of protection of the ovary from chemotherapy toxicity have been reported in the literature. 2-mercaptoethane sulfonate, sphingosine-1-phosphate, Bcl-2 overexpression and down regulation of Bax, and administration of Imatinib have all been investigated as protective agents. (29) (30) (31) (32) (33) (34) (35) Protection of granulosa cells from chemotoxicity by manipulating phase three drug metabolizing enzymes such as MDR1 constitute another potential venture for chemoprotection of the ovary. Lipid soluble drugs, such as vincristine, doxorubicin, etoposide, and paclitaxel, enter the cell by diffusion across the plasma membrane. Contrary to water soluble drugs that are execrated ready from the cells, chemoprotection from lipid soluble drugs can only be achieved by activation of energy-dependent transport systems such as MDR1 gene. (36) (37)
Here, we investigated the effects of MDR1 upregulation on granulosa cell survival following chemotherapy treatment as a surrogate marker for the potential role of drug transporters in shielding and rescuing ovarian granulosa cells from chemotherapy toxicity. We genetically modified granulosa cells in vitro by transient transduction with retrovirus containing human MDR1 to overexpress P-gp. We used Sf91m3 retroviral vector containing human MDR1 to transfect murine granulosa cells because of the high homology of the DNA binding domains between human and murine MDR1 genes. Hybrid Sf91m3 retroviral vectors used in this study circumvent the species-specific block and were active in both human progenitor hemobiotic cells and murine spermatogenic cells. (38, 39) Here, we report for the first time that Sf91m3 retrovirus vector is able to transduce KK15 granulosa cells in vitro and to produce high levels of functional P-gp expression. We showed that P-gp is naturally very minimally expressed in untransduced control KK15 murine granulosa cells as shown by a faint MDR-1 band on Western blot. Transduction with MDR1-retroviral vector rendered KK15 resistant to P-gp-transported chemotherapy agents and protected granulosa cells from paclitaxel toxicity as well as doxorubicin toxicity at doses as high as 50 times LD50 as measured by ATP production assay. LD50 dose calculations indicate that KK15 granulosa cells are very sensitive to doxorubicin chemotherapy when compared to other cell lines. (40) Our study showed protection of granulosa cells by MDR1 upregulation was dose dependent as evident by the protection from a wide range of doxorubicin toxicity and confirmed loss of protection at higher doses of doxorubicin, 500 times higher than the LD50. (41) Stereoidogenic gene expression in MDR1- retrovirally-transduced granulosa cells was maintained as shown by the ready detection of a selected panel of stereoidogenesis enzymes by RT-PCR.
For ovarian protection in cancer patients, MDR1 upregulation and P-gp expression need to be restricted to the ovaries to avoid resistance of the primary tumor to chemotherapy. Ovarian-specific targeted delivery of chemoprotective agents prior to chemotherapy can potentially rescue the ovaries from chemotherapy-induced toxicity without jeopardizing the response of the primary tumor to treatment. For example, while enhancing liver metabolism of chemotherapy could protect the ovary indirectly by decreasing the systemic level of the chemotherapy and hence the amount of drug that reaches the ovary, low systemic levels of the chemotherapy may increase the resistance of the original cancer to chemotherapy making this a net undesirable outcome. Molecular shielding of the ovary provides a unique opportunity to rescue ovarian tissues from chemotherapy-induced cytotoxic damage. Ovarian structures lend a spectacular opportunity to this concept since gametes develop in special niche and are surrounded by specialized cells that nurture and protect them (granulosa/theca in females and Leydig/Sertoli cells in males). Oocytes do not have a direct blood supply; they depend on diffusion of nutrients through gap junctions of adjacent protective cells. This yields a unique opportunity for shielding by molecularly modifying adjacent cells. Although oocytes are the primary goal for chemoprotection and fertility preservation, gene transduction could potentially modify the oocytes genetically and adversely affect future progeny. In addition, In vitro studies on oocytes are complicated by the limited number of oocytes and the need for very sensitive assays to detect transfected proteins. Hence granulosa cells are a better alternative for gene therapy to build a defense barrier and eliminate the toxic effects in cells surrounding the oocytes. The utility of granulosa cell rescue on preserving fertility will depend on the mechanism of chemotherapy toxicity in the ovary. Whether chemotherapy-induced destruction of granulosa cells is the primary cause of the subsequent premature ovarian failure or whether the germ cells are the primary target is still unknown. Although granulosa cells make an inviting target in view of their juxta proximity to the oocytes, vascular, theca, and stromal ovarian cells could also potentially serve as targets. The best cell type to target, the best technique to utilize, and the ideal signaling pathway to interrogate for molecular shielding of the ovary still awaits discovery. The ultimate impact of MDR1 over expression by granulosa cells on protecting the oocytes will depend on the direction of efflux of MDR-1 by the granulosa cells and the extracellular concentrations of anti-cancer drugs in the vicinity of the oocyte and will need further validation.
Although the results from this study are promising, many safety issues need to be addressed and clarified prior to the translation of this concept to human therapy. Despite the vast improvement in retrovirus vectors and gene therapy, viral gene transfer to future children is of primary concern. The effects of retroviral transfer techniques on follicular growth, steroid production, germ-line transmission, tumor resistance, viral activation, cell toxicity, and oncogenic potential need to be scrutinized. Despite the limitations of this study, we demonstrated for the first time the utility of drug transporters such as MDR1 in protection of normal granulosa cells from chemotoxicity. This could serve as a surrogate model for the potential role of ovarian metabolism in fertility preservation in cancer patients. Further studies are however needed to answer the many unresolved questions in the field of molecular shielding of the ovary from chemotherapy toxicity prior to clinical applications of such therapy. Key questions include the choice and effectiveness of protecting agents as well as selective delivery to the ovary to prevent tumor resistance to chemotherapy.
Supplementary Material
Supplemental Figure 1. PG13 packaging cells producing SF91m3 retrovirus were established by infection from AM-12 amphotropic producer cells. Figure 1 show Flow cytometry of AM12 cells transduced with psf91m3 and stained for MDR1 expression using P-gp monoclonal antibody (UIC2) tagged with phycoerthrosin (UIC2-PE). A and B are scatter blot and histogram of untransduced control AM12 cells, C and D are scatter blot and histogram of psf91m3-transduced AM12 cells. MDR1 expression increased from 0.31 to 97.6% following transduction with psf91m3.
Supplemental Figure 2. U1C2-PE staining of PG13 cells. Expression of MDR1 by PG13 cells was confirmed by staining with phycoerythrin-tagged monoclonal MDR1 antibody (UIC2-PE) three days following transduction with SF91m3. A and B are scatter blot and histogram of untransduced PG13 showing that only 1.64% of control PG13 cells were positive for P-gp expression. C and D are scatter blot and histogram of MDR1- transduced PG13. Two cell populations seen in C corresponding to two histogram peaks in D indicating that 15.4% of transduced PG13 cells were positive for P-gp expression.
Supplemental Figure 3. Rhodamine 123 efflux assay of PG13 cells: Expression of MDR1 by PG13 cells was confirmed by staining with Rhodamine 123 efflux assay three days following transduction with SF91m3. A and B are scatter blot and histogram of untransduced PG13 cells respectively showing that only 1.57% of control PG13 cells were positive for Rhodamine efflux and have functional P-gp expression. C and D are scatter blot and histogram of transduced PG13. Two cell populations seen in C correspond to two peaks in D indicating that 19.8% of transduced PG 13 cells have functional P-gp expression MDR1.
Supplemental Table 1A. Cell viability measured by ATP production was used to find the LD50 for the indicated chemotherapy agents in KK15 and primary murine granulosa cells (pmGC). KK15 cells were treated with various concentrations of the chemotherapy for 24–72 hours. The LD50 of KK15 for doxorubicin and paclitaxel were calculated and compared to LD50 of pmGC. KK15 were more sensitive to chemotherapy toxicity when compared to pmGC.
Supplemental Table 1B. Percentage of live cells after chemotherapy treatment in control and transduced kk15 cells. Control KK15 or MDR1-transduced KK15 cells were treated with vehicle control, doxorubicin, or paclitaxel chemotherapy at the indicated doses. Cells were stained with annexin V and Propidium iodide and cell viability was assessed by flow cytometry. In vitro transduction of KK15 murine granulosa cells with MDR1 protected the cells from the toxicity of paclitaxel and failed to protect the cells from the toxicity of 5uM of doxorubicin. Control (Con) indicates vehicle treatment, doxorubicin (Dox), paclitaxel (Pac). *Indicates P value ≤ 0.05.
Acknowledgments
This work will not have been possible without the University of Wisconsin Comprehensive Cancer Center and the University of Wisconsin 3P laboratory shared core facilities. This work was supported by the University of Wisconsin startup funds and NIH K12 HD0558941 to SMS, and UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources.
Footnotes
Conflict of interest:
The author has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lo Presti A, Ruvolo G, Gancitano RA, Cittadini E. Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol. 2004;113 (Suppl 1):S33–40. doi: 10.1016/j.ejogrb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt M, Breen N, Devesa S. Cancer prevalence and survivorship issues: analyses of the 1992 National Health Interview Survey. J Natl Cancer Inst. 1999;91(17):1480–6. doi: 10.1093/jnci/91.17.1480. [DOI] [PubMed] [Google Scholar]
- 3.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. The New England journal of medicine. 2009;360(9):902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maman E, Prokopis K, Levron J, Carmely A, Dor J, Meirow D. Does controlled ovarian stimulation prior to chemotherapy increase primordial follicle loss and diminish ovarian reserve? An animal study Hum Reprod. 2009;24(1):206–10. doi: 10.1093/humrep/den337. [DOI] [PubMed] [Google Scholar]
- 5.Leonard GD, Polgar O, Bates SE. ABC transporters and inhibitors: new targets, new agents. Curr Opin Investig Drugs. 2002;3(11):1652–9. [PubMed] [Google Scholar]
- 6.Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6(2):115–38. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 7.Fojo AT, Ueda K, Slamon DJ, Poplack DG, Gottesman MM, Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987;84(1):265–9. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velamakanni S, Wei SL, Janvilisri T, van Veen HW. ABCG transporters: structure, substrate specificities and physiological roles: a brief overview. J Bioenerg Biomembr. 2007;39(5–6):465–71. doi: 10.1007/s10863-007-9122-x. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 10.Schiedlmeier B, Schilz AJ, Kuhlcke K, Laufs S, Baum C, Zeller WJ, et al. Multidrug resistance 1 gene transfer can confer chemoprotection to human peripheral blood progenitor cells engrafted in immunodeficient mice. Hum Gene Ther. 2002;13(2):233–42. doi: 10.1089/10430340252769761. [DOI] [PubMed] [Google Scholar]
- 11.Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94(8):4028–33. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai K, Kamatani N, Georges E, Ling V. Identification of a membrane glycoprotein overexpressed in murine lymphoma sublines resistant to cis-diamminedichloroplatinum(II) The Journal of biological chemistry. 1990;265(22):13137–42. [PubMed] [Google Scholar]
- 13.Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol. 2003;285(3):C584–91. doi: 10.1152/ajpcell.00418.2002. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda H, He PJ, Yokota K, Soh T, Yamauchi N, Hattori MA. Progesterone-dependent and -independent expression of the multidrug resistance type I gene in porcine granulosa cells. Mol Cell Biochem. 2007;298(1–2):179–86. doi: 10.1007/s11010-006-9364-7. [DOI] [PubMed] [Google Scholar]
- 15.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–8. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86(2):695–8. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finstad CL, Saigo PE, Rubin SC, Federici MG, Provencher DM, Hoskins WJ, et al. Immunohistochemical localization of P-glycoprotein in adult human ovary and female genital tract of patients with benign gynecological conditions. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1990;38(11):1677–81. doi: 10.1177/38.11.1976674. [DOI] [PubMed] [Google Scholar]
- 18.Arai M, Yamauchi N, Fukuda H, Soh T, Hattori MA. Development of multidrug resistance type I P-glycoprotein function during in vitro maturation of porcine oocyte. Reprod Toxicol. 2006;21(1):34–41. doi: 10.1016/j.reprotox.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Keating E, Goncalves P, Lemos C, Costa F, Campos I, Smith SB, et al. Progesterone inhibits folic acid transport in human trophoblasts. J Membr Biol. 2007;216(2–3):143–52. doi: 10.1007/s00232-007-9057-5. [DOI] [PubMed] [Google Scholar]
- 20.Kizaki K, Ito R, Okada M, Yoshioka K, Uchide T, Temma K, et al. Enhanced gene expression of myocardial matrix metalloproteinases 2 and 9 after acute treatment with doxorubicin in mice. Pharmacological research: the official journal of the Italian Pharmacological Society. 2006;53(4):341–6. doi: 10.1016/j.phrs.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Miller DG, Adam MA, Miller AD. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10(8):4239–42. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehgal A, Weeratunge N, Casimir C. Retroviral transduction of quiescent haematopoietic cells using a packaging cell line expressing the membrane-bound form of stem cell factor. Gene Ther. 1999;6(6):1084–91. doi: 10.1038/sj.gt.3300932. [DOI] [PubMed] [Google Scholar]
- 23.Birner P, Ritzi M, Musahl C, Knippers R, Gerdes J, Voigtlander T, et al. Immunohistochemical detection of cell growth fraction in formalin-fixed and paraffin-embedded murine tissue. Am J Pathol. 2001;158(6):1991–6. doi: 10.1016/S0002-9440(10)64670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kananen K, Markkula M, Rainio E, Su JG, Hsueh AJ, Huhtaniemi IT. Gonadal tumorigenesis in transgenic mice bearing the mouse inhibin alpha-subunit promoter/simian virus T-antigen fusion gene: characterization of ovarian tumors and establishment of gonadotropin-responsive granulosa cell lines. Mol Endocrinol. 1995;9(5):616–27. doi: 10.1210/mend.9.5.7565808. [DOI] [PubMed] [Google Scholar]
- 25.Turner SD, Rafferty JA, Fairbairn LJ, Ashby J, Tinwell H, Eckert HG, et al. The effects of dose, route of administration, drug scheduling and MDR-1 gene transfer on the genotoxicity of etoposide in bone marrow. Leukemia. 2000;14(10):1796–802. doi: 10.1038/sj.leu.2401810. [DOI] [PubMed] [Google Scholar]
- 26.Mechetner EB, Roninson IB. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci U S A. 1992;89(13):5824–8. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salama SA, Kamel MW, Diaz-Arrastia CR, Xu X, Veenstra TD, Salih S, et al. Effect of tumor necrosis factor-alpha on estrogen metabolism and endometrial cells: potential physiological and pathological relevance. J Clin Endocrinol Metab. 2009;94(1):285–93. doi: 10.1210/jc.2008-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Molecular and cellular endocrinology. 2004;228(1–2):67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 29.McDaid HM, Lopez-Barcons L, Grossman A, Lia M, Keller S, Perez-Soler R, et al. Enhancement of the therapeutic efficacy of taxol by the mitogen-activated protein kinase kinase inhibitor CI-1040 in nude mice bearing human heterotransplants. Cancer research. 2005;65(7):2854–60. doi: 10.1158/0008-5472.CAN-04-4391. [DOI] [PubMed] [Google Scholar]
- 30.Gonfloni S, Di Tella L, Caldarola S, Cannata SM, Klinger FG, Di Bartolomeo C, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nature medicine. 2009;15(10):1179–85. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]
- 31.Dai C-l, Tiwari AK, Wu C-P, Su X-D, Wang S-R, Liu D-g, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer research. 2008;68(19):7905–14. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. American journal of obstetrics and gynecology. 2008;198(4):463.e1–6. doi: 10.1016/j.ajog.2007.12.027. discussion 463.e6–7. [DOI] [PubMed] [Google Scholar]
- 33.Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nature medicine. 2000;6(10):1109–14. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 34.Teneriello MG, Tseng PC, Crozier M, Encarnacion C, Hancock K, Messing MJ, et al. Phase II evaluation of nanoparticle albumin-bound paclitaxel in platinum-sensitive patients with recurrent ovarian, peritoneal, or fallopian tube cancer. J Clin Oncol. 2009;27(9):1426–31. doi: 10.1200/JCO.2008.18.9548. [DOI] [PubMed] [Google Scholar]
- 35.Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, et al. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci U S A. 2007;104(12):5229–34. doi: 10.1073/pnas.0608557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Annereau JP, Szakacs G, Tucker CJ, Arciello A, Cardarelli C, Collins J, et al. Analysis of ATP-binding cassette transporter expression in drug-selected cell lines by a microarray dedicated to multidrug resistance. Mol Pharmacol. 2004;66(6):1397–405. doi: 10.1124/mol.104.005009. [DOI] [PubMed] [Google Scholar]
- 37.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 38.Eckert HG, Stockschlader M, Just U, Hegewisch-Becker S, Grez M, Uhde A, et al. High-dose multidrug resistance in primary human hematopoietic progenitor cells transduced with optimized retroviral vectors. Blood. 1996;88(9):3407–15. [PubMed] [Google Scholar]
- 39.Danno S, Itoh K, Baum C, Ostertag W, Ohnishi N, Kido T, et al. Efficient gene transfer by hybrid retroviral vectors to murine spermatogenic cells. Hum Gene Ther. 1999;10(11):1819–31. doi: 10.1089/10430349950017509. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA, Ngo H, Martin MC, Wolf JK. An evaluation of cytotoxicity of the taxane and platinum agents combination treatment in a panel of human ovarian carcinoma cell lines. Gynecologic oncology. 2005;98(1):141–5. doi: 10.1016/j.ygyno.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Knipper R, Kuehlcke K, Schiedlmeier B, Hildinger M, Lindemann C, Schilz AJ, et al. Improved post-transcriptional processing of an MDR1 retrovirus elevates expression of multidrug resistance in primary human hematopoietic cells. Gene therapy. 2001;8(3):239–46. doi: 10.1038/sj.gt.3301384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PG13 packaging cells producing SF91m3 retrovirus were established by infection from AM-12 amphotropic producer cells. Figure 1 show Flow cytometry of AM12 cells transduced with psf91m3 and stained for MDR1 expression using P-gp monoclonal antibody (UIC2) tagged with phycoerthrosin (UIC2-PE). A and B are scatter blot and histogram of untransduced control AM12 cells, C and D are scatter blot and histogram of psf91m3-transduced AM12 cells. MDR1 expression increased from 0.31 to 97.6% following transduction with psf91m3.
Supplemental Figure 2. U1C2-PE staining of PG13 cells. Expression of MDR1 by PG13 cells was confirmed by staining with phycoerythrin-tagged monoclonal MDR1 antibody (UIC2-PE) three days following transduction with SF91m3. A and B are scatter blot and histogram of untransduced PG13 showing that only 1.64% of control PG13 cells were positive for P-gp expression. C and D are scatter blot and histogram of MDR1- transduced PG13. Two cell populations seen in C corresponding to two histogram peaks in D indicating that 15.4% of transduced PG13 cells were positive for P-gp expression.
Supplemental Figure 3. Rhodamine 123 efflux assay of PG13 cells: Expression of MDR1 by PG13 cells was confirmed by staining with Rhodamine 123 efflux assay three days following transduction with SF91m3. A and B are scatter blot and histogram of untransduced PG13 cells respectively showing that only 1.57% of control PG13 cells were positive for Rhodamine efflux and have functional P-gp expression. C and D are scatter blot and histogram of transduced PG13. Two cell populations seen in C correspond to two peaks in D indicating that 19.8% of transduced PG 13 cells have functional P-gp expression MDR1.
Supplemental Table 1A. Cell viability measured by ATP production was used to find the LD50 for the indicated chemotherapy agents in KK15 and primary murine granulosa cells (pmGC). KK15 cells were treated with various concentrations of the chemotherapy for 24–72 hours. The LD50 of KK15 for doxorubicin and paclitaxel were calculated and compared to LD50 of pmGC. KK15 were more sensitive to chemotherapy toxicity when compared to pmGC.
Supplemental Table 1B. Percentage of live cells after chemotherapy treatment in control and transduced kk15 cells. Control KK15 or MDR1-transduced KK15 cells were treated with vehicle control, doxorubicin, or paclitaxel chemotherapy at the indicated doses. Cells were stained with annexin V and Propidium iodide and cell viability was assessed by flow cytometry. In vitro transduction of KK15 murine granulosa cells with MDR1 protected the cells from the toxicity of paclitaxel and failed to protect the cells from the toxicity of 5uM of doxorubicin. Control (Con) indicates vehicle treatment, doxorubicin (Dox), paclitaxel (Pac). *Indicates P value ≤ 0.05.