Abstract
OBJECTIVE:
Recent pesticide-monitoring results suggest that a shift in residential pesticide exposure from organophosphorus insecticides to pyrethroid insecticides has occurred. Pyrethroid insecticides are potential neurodevelopmental toxicants and have not been evaluated for developmental toxicity. Our objective was to explore the association between prenatal exposure to permethrin (a common pyrethroid) and piperonyl butoxide (a pyrethroid synergist) and 36-month neurodevelopment.
METHODS:
Participants is this study were part of a prospective cohort of black and Dominican mothers and newborns living in low-income neighborhoods in New York City. We examined 36-month cognitive and motor development (using the Bayley Scales of Infant Development, second edition) as a function of permethrin levels measured in maternal and umbilical cord plasma collected on delivery and permethrin and piperonyl butoxide levels measured in personal air collected during pregnancy. All models were controlled for gender, gestational age, ethnicity, maternal education, maternal intelligence, quality of the home environment, and prenatal exposure to environmental tobacco smoke and chlorpyrifos.
RESULTS:
Prenatal exposure to permethrin in personal air and/or plasma was not associated with performance scores for the Bayley Mental Developmental Index or the Psychomotor Developmental Index. After data adjustment, children more highly exposed to piperonyl butoxide in personal air samples (>4.34 ng/m3) scored 3.9 points lower on the Mental Developmental Index than those with lower exposures (95% confidence interval: −0.25 to −7.49).
CONCLUSIONS:
Prenatal exposure to piperonyl butoxide was negatively associated with 36-month neurodevelopment.
Keywords: prenatal pesticide exposure, neurodevelopment
WHAT'S KNOWN ON THIS SUBJECT:
Pyrethroid insecticides and the pyrethroid synergist piperonyl butoxide are potential neurodevelopmental toxicants and have not been evaluated for developmental toxicity. In this study the effects of prenatal exposure to piperonyl butoxide and permethrin on neurodevelopment in children aged 36 months were examined.
WHAT THIS STUDY ADDS:
A significant inverse association was observed between prenatal exposure to piperonyl butoxide, a pyrethroid synergist, and 36-month neurodevelopment. No significant association was observed between prenatal exposure to permethrin and adverse neurodevelopment.
Widespread residential pesticide use in urban communities in the United States has resulted in ubiquitous, low-level exposure of pregnant women and children.1–4 Data from a substantial body of experimental and epidemiological research suggest that low-level prenatal and early postnatal pesticide exposures adversely affect neurodevelopment.5
In previous epidemiological studies associations between prenatal organochlorine and/or organophosphorus (OP) insecticide exposures and neurodevelopment were evaluated.3,6–8 For example, results of our research at the Columbia Center for Children's Environmental Health (CCCEH) demonstrated adverse neurodevelopmental sequelae in an urban cohort after prenatal exposure to chlorpyrifos, an OP insecticide.9 With increasing pesticide regulations, residential exposures to organochlorine and OP insecticides have decreased.10–12 Current data from sales and environmental monitoring suggest that pyrethroid insecticides replaced OP insecticides for residential pest control as uses of chlorpyrifos and other OP insecticides were phased out.12,13 Pyrethroid insecticides have not been evaluated for long-term neurotoxic effects after low-level exposure in humans, but experimental data raise concerns about the safety of prenatal and early childhood exposures.14
We examined associations between prenatal exposure to pyrethroid insecticides and 36-month neurodevelopment among children in the CCCEH cohort, using the Bayley Scales of Infant Development, second edition (BSID-II).15 Permethrin was selected as the target pyrethroid because it was recently one of the most common insecticides detected in a nationally representative sample of residential homes in the United States,16 as well as the most commonly sold pesticide in the catchment area of the CCCEH according to results of a survey completed in 2007.17 Permethrin was the only pyrethroid detected in maternal and cord plasma samples using a novel method developed to measure levels of 12 pyrethroid insecticides in human plasma.18 Environmental and biological monitoring of pyrethroid insecticides present challenges. Most pyrethroid insecticides are difficult to measure in air because they are not volatile19 and are difficult to measure in bodily fluids because they are rapidly metabolized, often to nonselective metabolites.20,21 Consequently, we measured piperonyl butoxide (PBO), the most common pyrethroid insecticide synergist, in personal air samples collected during pregnancy as an indicator of maternal pyrethroid insecticide use. PBO is commonly formulated with pyrethrin and a number of pyrethroid insecticides, including permethrin and tetramethrin.17,22 Notably, PBO is not used as a synergist in OP or carbamate insecticide formulations.17
We hypothesized that prenatal exposure to permethrin, as indicated by analysis of personal air and/or plasma samples collected at delivery, was associated with lower neurocognitive scores in children aged 36 months. In addition, we hypothesized that prenatal exposure to PBO, an indicator of pyrethroid insecticide use in the maternal environment, was also associated with delayed neurocognitive development.
METHODS
Study Subjects
Subjects in this report were selected from the CCCEH Mothers and Newborns cohort, a prospective longitudinal study of black and Dominican women living in upper Manhattan and the South Bronx. The CCCEH study has been extensively described previously.23 Briefly, mothers were enrolled from prenatal clinics at Harlem and New York Presbyterian hospitals. Enrollment was restricted to self-reported nonsmoking women aged 18 to 35 years, who self-identified as African American or Dominican and registered for care by the 20th week of pregnancy. Eligible women were free of diabetes mellitus, hypertension, known HIV infection, and documented/reported drug use and had resided in the area for at least 1 year. Between 1998 and 2006, 725 subjects were enrolled into the CCCEH cohort and complete prenatal questionnaire data and environmental and/or biological monitoring data on insecticide exposure were obtained. From these participants, we selected all children enrolled in the CCCEH study who had reached the age of 36 months and for whom these data were available: (1) prenatal maternal interview data; (2) environmental markers of PBO and/or environmental and/or biological markers of permethrin exposure levels; (3) neurobehavioral outcome assessment at 3 to 4 years; and (4) assessment of covariates (see statistical analysis section). Evidence of exposure to PBO was available only in personal air samples, not in maternal or cord plasma samples. To eliminate subjects who smoked during pregnancy, we selected subjects with plasma cotinine levels of < 15 ng/mL at delivery. Developmental outcome measures and 1 or more of the pesticide dosimeters were available for 348 subjects. The numbers of subjects included in multivariate analyses were: (1) 342 subjects for permethrin in personal air; (2) 272 subjects for permethrin in plasma; and (3) 230 subjects for PBO in personal air. The sample size for each statistical analysis varied because of variation in the availability of samples for pesticide analysis. The institutional review board of Columbia University approved this study, and informed consent was obtained from all participants.
Maternal Prenatal Questionnaire
A trained research worker administered a 45-minute questionnaire to each woman in her home during the third trimester of pregnancy. Data collected included demographic information, home characteristics, residential history, active and passive smoking, occupational history, alcohol and drug use, residential insecticide use, and frequency of pest sightings in the home during pregnancy.11,24–28 Prenatal questionnaire data were obtained for all subjects.
Environmental and Biological Pesticide Exposure Assessment
Umbilical cord plasma was collected at delivery and maternal plasma collected within 48 hours of delivery, according to the CCCEH protocol.29 Plasma samples were analyzed for concentrations of cis- and trans-permethrin and chlorpyrifos by gas chromatography–high-resolution mass spectrometry at the Centers for Disease Control and Prevention (Atlanta, Georgia). Methods for the laboratory measurements, including quality control, reproducibility, and limits of detection (LOD) were published previously.30
Maternal personal air samples were collected during the third trimester of pregnancy. Subjects were asked to wear a small backpack holding a personal ambient air monitor during daytime hours, and to place the monitor near the bed at night, for a period of 48 consecutive hours. Concentrations of cis- and trans- permethrin, PBO, and chlorpyrifos were measured in personal air samples by gas chromatography–mass spectrometry at Southwest Research Institute (San Antonio, Texas). A description of the gas chromatography–mass spectrometry assay, including quality control, reproducibility, and LOD, was published previously.26
Child Behavior and Neurodevelopmental Outcome Assessment
The BSID-II was used to assess cognitive and psychomotor development among children aged 36 months.15 Use of this test to identify developmental delay in the CCCEH cohort has been previously described.9 The BSID-II was selected because it is widely used and norm referenced, can be used to diagnose developmental delay, and is known to be sensitive to effects of neurotoxicants.31 Each test generates a continuous Mental Developmental Index (MDI) and Psychomotor Developmental Index (PDI). Children are classified as normal or at risk for delay on the basis of standardized cut-points (normal: ≥85; at risk for delay: <85). Trained bilingual research assistants tested each child under controlled conditions in the study office and were monitored for interrater reliability.
Description of Potential Covariates
Covariates included known predictors of development and factors that potentially influenced the association between permethrin and/or PBO exposure and neurodevelopmental outcomes. Covariates were assessed through maternal self-report in the prenatal questionnaire, including ethnicity, education, age, and prenatal exposure to environmental tobacco smoke (ETS).32 Gestational age (determined by date of the woman's last menstrual period and/or ultrasound), birth outcome data (birth weight, length, and head circumference), and gender were obtained from the medical record. Education was categorized as high school graduate or nongraduate at the time of the interview. When the child was aged 2 to 3 years, the mother's nonverbal intelligence was measured by using the Test of Nonverbal Intelligence (TONI), second edition, a 15-minute language-free measure of general intelligence that is relatively stable and free of cultural bias.33 The quality of the care-taking environment, measured by use of the Home Observation for Measurement of the Environment (HOME), was also assessed when the child was aged 2 to 3 years.34 The HOME score was used to assesses the physical and interactive aspects of the home environment35 and has been widely used in studies of neurotoxicity.36 TONI and/or HOME scores were missing for 37 and 26 women, respectively. The sample mean was substituted for those missing values to maximize sample size. Analyses conducted with and without the imputed values showed no differences in the magnitude of the covariate or the exposure effects.
Our own recent findings linked adverse neurodevelopmental sequelae with prenatal chlorpyrifos exposure.8,9 However, in 2000–2001 the US Environmental Protection Agency withdrew the residential registrations for chlorpyrifos. After these restrictions were imposed, levels of chlorpyrifos in personal air and maternal and cord plasma samples dropped significantly with little variability, and the lower levels are no longer associated with adverse outcomes. In the current study, participants were enrolled after enactment of the US Environmental Protection Agency regulations. Among participants selected for the current analyses, chlorpyrifos is not associated with our exposure dosimeters (ie, PBO and permethrin), and inclusion of chlorpyrifos in the regression models did not improve the model fit. Therefore, chlorpyrifos was not included as a covariate in our final models.
Statistical Analysis
We estimated the effects of prenatal permethrin exposure on neurocognitive development using multivariate linear regression for continuous BSID-II scores and multivariate logistic regression for categorical BSID-II scores. In maternal personal air samples and maternal/cord plasma samples, detection of cis- and trans-permethrin was low (range: 5%–18%). Consequently, we created dichotomous variables (0, permethrin levels less than the LOD; 1, permethrin levels greater than the LOD).
We developed 3 models to examine the association between PBO and neurodevelopment in children aged 36 months. PBO was detected in the majority of personal air samples (75%). Levels below the LOD (0.1 ng/m3) were assigned a value of one-half the detection limit concentration. Levels were logarithmically transformed to normalize a positively skewed distribution. We used model 1 to examine associations between log-transformed PBO exposure and continuous BSID-II scores. With model 2 we used 5 categories of PBO exposure to assess the potential dose-response relationships between exposure and developmental outcomes. The lowest exposure group included subjects with PBO levels below the LOD; subjects with other values for PBO levels were categorized into 4 equally sized exposure groups. The 5 resulting categories were: reference, levels < LOD; first quartile, lowest PBO exposure; second quartile, low-to-mid PBO exposure; third quartile, midhigh PBO exposure; fourth quartile, highest quartile of PBO exposure. Indicator variables were used in the multiple regression analyses to compare mean developmental scores among subjects within each exposure group (second to fifth categories) to those in the reference group. For MDI, the only group for which mean 36-month BSID-II scores were significantly lower was the group exposed to the highest levels of PBO (fifth category). Therefore, we computed a dichotomous exposure variable to classify subjects from the fifth category into those exposed to high levels of PBO (>4.34 ng/m3) versus those exposed to all lower levels (4.34≤ ng/m3) (model 3).
On the basis of standardized MDI and PDI cut-points, we used multiple logistic regression to examine the odds of 36-month developmental delay. The exposure measures for multiple logistic regression were the same as those used for multiple linear regression. For permethrin in personal air and maternal/cord plasma, we used the dichotomous exposure variables. For PBO in personal air, we use the 3 regression models described previously.
The final approach included exposure variables for both permethrin and PBO. On the basis of determinants of neurodevelopment described in the literature, we explored the significance of 2-way interactions of (1) air levels of permethrin isomers and PBO, (2) air levels of PBO and education status (self-reported high school education), and (3) air levels of PBO and ETS exposure.
RESULTS
Table 1 presents the demographic characteristics of 348 subjects and their children. In general, these subjects do not differ from those in the parent cohort. However, subjects reported ETS exposure less frequently than those not selected (27.0% in the selected cohort versus 41.3% in the parent cohort, chi-squared = 15.3, P < .01). Among the 348 subjects, there were differences in demographic characteristics according to level of pesticide exposure. Women with PBO exposure levels in the highest quartile were less likely to have a high school diploma (64.0% vs 34.0%, χ2 = 13.1, P < .05) and scored lower on the home inventory (37.2 vs 39.6, t test, P < .05) than women with PBO exposure levels in the lower quartiles.
TABLE 1.
Demographic Characteristics of Mothers (N = 348) and Birth Outcome Data of Children
| Characteristic | Proportion, % | |
|---|---|---|
| Race/ethnicity | ||
| African-American | 32.0 | |
| Dominican | 68.0 | |
| Maternal age, mean (range), y | 25.4 (18–38) | |
| Maternal education <high school degree at delivery | 39.1 | |
| Maternal nonverbal intelligence, mean (SE) | — | 84.2 (0.75) |
| HOME score, mean (SE) | — | 39.5 (0.3) |
| Birth weight, mean (SE), g | — | 3377.8 (24.1) |
| Birth length, mean (SE), cm | — | 50.3 (0.2) |
| Birth head circumference, mean (SE), cm | — | 34.1 (0.1) |
| Gestational age, mean (SE), wk | — | 39.3 (0.1) |
| Gender (male) | 46.8 | |
| Prenatal exposure to ETS | 26.7 |
Missing values: birth weight, 4; birth length, 26; birth head circumference, 46.
Table 2 presents data for the distribution of pesticide concentrations in personal air samples collected during pregnancy and maternal/cord plasma samples collected on delivery. PBO was weakly associated with cis- and trans-permethrin in personal air (Spearman's r = 0.29; P < .001 and r = 0.23; P < .001, respectively). Air levels of cis-and trans-permethrin isomers were strongly intercorrelated (Spearman's r = 0.86; P < .001). Both isomers of permethrin were correlated across maternal and cord plasma samples (cis-permethrin Spearman's r = 0.30, P < .001 and trans-permethrin r = 0.23; P = .002). In plasma, cis- and trans-permethrin isomers were not significantly intercorrelated.
TABLE 2.
Prenatal Exposure to Pesticides in Personal Air Samples Collected During the Third Trimester of Pregnancy and Maternal/Cord Samples Collected at Delivery
| n | LODa | % >LOD | 25% | 50% | 75% | 95% | |
|---|---|---|---|---|---|---|---|
| Personal air, ng/m3 | |||||||
| Piperonyl butoxide | 230 | 0.10 | 75.2 | 0.18 | 0.42 | 1.12 | 7.94 |
| Cis-permethrin | 342 | 0.18 | 18.7 | <LOD | <LOD | 0.21 | 1.39 |
| Trans-permethrin | 340 | 0.36 | 16.5 | <LOD | <LOD | 0.39 | 2.10 |
| Plasma, pg/g | |||||||
| Maternal cis-permethrin | 272 | 1.0 | 12.9 | <LOD | <LOD | <LOD | 1.66 |
| Cord cis-permethrin | 240 | 1.0 | 7.1 | <LOD | <LOD | <LOD | 1.48 |
| Maternal trans-permethrin | 272 | 1.0 | 18.0 | <LOD | <LOD | <LOD | 4.06 |
| Cord trans-permethrin | 240 | 1.0 | 5.4 | <LOD | <LOD | <LOD | 1.07 |
LOD for personal air was determined as nanograms per extract. On calculation of nanograms per cubic meter, LODs varied slightly depending on the concentration of the extract and the amount of air sample collected during a 48-hour period. The mean volume of air samples was 11 ± 0.6 m3.
Table 3 shows the covariate models used in multiple regression analyses to examine associations between PBO or permethrin exposure and 36-month BSID-II. The covariates explained 25% and 13%, respectively, of the total variance in MDI and PDI scores.
TABLE 3.
Multiple Linear Models of Covariates Included in Analyses of Associations Between Prenatal Pesticide Exposure and 36-Month BSID-II Scores (N = 348)
| 36-Month MDI Score |
36-Month PDI Scorea |
|||
|---|---|---|---|---|
| β, Mean ± SE | P | β, Mean ± SE | P | |
| Genderb | −3.86 ± 1.18 | .001 | −5.28 ± 1.43 | <.001 |
| Ethnicityc | 6.61 ± 1.42 | <.001 | 4.66 ± 1.71 | .002 |
| Gestational age | 0.11 ± 0.47 | .82 | 0.91 ± 0.57 | .12 |
| Maternal TONI score | ||||
| Indicator 1 | 0.52 ± 1.50 | .72 | 1.52 ± 1.81 | .40 |
| Indicator 2 | −0.38 ± 1.68 | .83 | −1.23 ± 2.04 | .54 |
| Indicator 3 | −1.09 ± 2.22 | .62 | −0.93 ± 2.68 | .73 |
| Maternal educationd | −2.19 ± 1.27 | .09 | 1.04 ± 0.03 | .91 |
| HOME score | 0.46 ± 0.11 | <.001 | 0.40 ± 0.13 | .002 |
| ETSe | 0.65 ± 1.38 | .64 | 2.59 ± 1.66 | .12 |
Two subjects had 36-month PDI scores below the minimal obtainable score of 50 and were therefore considered untestable.
0 indicates male; 1, female.
0 indicates Dominican; 1, African American.
0 indicates less than high school education; 1, equivalent to or greater than high school education.
0 indicates no ETS; 1, ETS.
Prenatal Exposure to Permethrin and 36-month BSID-II
In multivariate linear and logistic regression analyses in which we controlled for gender, ethnicity, gestational age, maternal intelligence (assessed with the TONI), education, quality of the home environment (HOME), and prenatal exposure to ETS there were no significant associations between cis- or trans-permethrin measured in personal air samples and mental or motor development at 36 months (data not shown). Similarly, there were no significant associations between cis- or trans-permethrin measured in maternal and/or umbilical cord plasma samples and mental or motor development at 36 months (data not shown).
Prenatal Piperonyl Butoxide and 36-month BSID-II
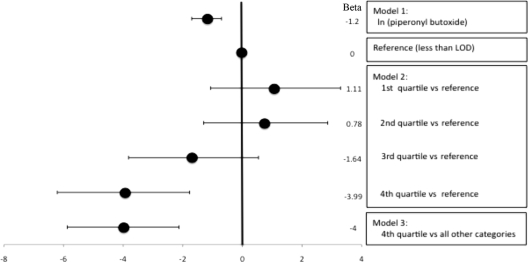
Figure 1 displays results of multiple linear regression models used to estimate the effects of prenatal PBO exposure on 36-month MDI and PDI with adjustment for covariates. Model 1 demonstrates a significant inverse association between prenatal PBO exposure and 36-month MDI. MDI scores declined 1.2 points per log-unit increase in PBO exposure (95% confidence interval [CI]: −0.33 to −2.25; P = .008). Model 2, in which we used PBO exposure as a 5-category variable, demonstrates an adverse effect of PBO on 36-month MDI, with the strongest effect in the highest quartile versus the referent group (odds ratio: −4.57; 95% CI: −0.30 to −8.84; P = .04). In model 3, the highest category of PBO exposure versus all other groups is associated with a 3.9-point mean decrement in MDI score (95% CI: −0.25 to −7.49; P = .04).
FIGURE 1.
Linear regression models to test the effects of prenatal exposure to PBO measured in personal air on the risk of delayed mental development in children aged 36 months. Data were adjusted for gender, ethnicity, prenatal ETS exposure, gestational age at birth, maternal nonverbal intelligence (TONI), maternal education, and quality of the home environment (HOME) (n = 230). Coding of covariates: gender (0 indicates male; 1, female), ethnicity (0 indicates Dominican; 1, African American), prenatal ETS (0 indicates no ETS; 1, yes ETS), maternal education (0 indicates high school or equivalent; 1, less than high school).
Table 4 displays the results of multiple logistic regression analyses performed to estimate the odds of delayed mental development as a function of prenatal PBO exposure, with adjustment for covariates. Each log unit increase in PBO exposure was associated with a 1.3-fold increased odds of delayed mental development (95% CI: 1.06–1.66; P = .01). The most highly exposed children experienced 3.11-fold (95% CI: 1.38–6.98; P = .006) increased odds of mental delay compared with the referent group (all other categories of exposure).
TABLE 4.
Multiple Logistic Regression Models Testing Effects of Piperonyl Butoxide in Personal Air on the Odds of Delayed Mental Development (MDI ≤85) (n = 230)
| Median Piperonyl Butoxide, ng/m3a | Subjects With MDI Score >85, n | Subjects With MDI Score ≤85, n | Odds Ratio (95% CI) | P | |
|---|---|---|---|---|---|
| Model 1a | |||||
| (ln)PBO | 0.51 | 170 | 60 | 1.32 (1.06–1.66) | 0.01 |
| Model 2b | |||||
| Reference | — | 42 | 15 | — | — |
| 1st Quartile vs reference | 0.27 | 32 | 8 | 0.61 (0.21–1.74) | 0.36 |
| 2nd Quartile vs reference | 0.45 | 41 | 8 | 0.58 (0.21–1.64) | 0.31 |
| 3rd Quartile vs reference | 0.87 | 32 | 10 | 0.97 (0.95–2.60) | 0.95 |
| 4th Quartile vs reference | 4.33 | 23 | 19 | 2.49 (0.95–6.54) | 0.06 |
| Model 3c | |||||
| 4th Quartile vs all other categories | 4.33 | 23 | 19 | 3.11 (1.38–6.98) | 0.006 |
All models adjusted for gender, ethnicity, gestational age, maternal nonverbal intelligence (TONI), education, quality of the home environment (HOME), and prenatal exposure to ETS and plasma chlorpyrifos.
Exposure assessed as (ln)PBO; geometric mean is presented.
5 categories of PBO exposure: 0 indicates reference (less than LOD); quartiles (1–4) of values in the detectable range.
2 categories of PBO exposure: 0 indicates reference (less than LOD) and lowest 3 quartiles; 1, highest quartile of values.
PBO was not significantly associated with 36-month PDI either as a continuous or a categorical variable (Mean β ± SE = −0.79 ± 0.64; OR [95% CI]: 0.98 [0.73–1.30]). In this cohort, only 26 children demonstrated any evidence of motor delay (PDI ≤ 85). Because of this small number, no additional analyses of the association between PBO and PDI were undertaken.
Prenatal Exposure to PBO and Permethrin and BSID-II at 36 Months
We examined the effects of combined exposure to PBO and permethrin on 36-month mental development of children. Addition of cis- or trans-permethrin in personal air to the PBO model did not change the magnitude of the effect of PBO on 36-month MDI or PDI. There were no significant interactions between PBO and permethrin (data not shown), nor were any interactions between pesticide exposure and potential effect modifiers including education and ETS significant (data not shown).
DISCUSSION
Our results demonstrate a significant association between PBO measured in personal air collected during the third trimester of pregnancy and delayed mental development at 36 months among children in this cohort. Previous data showed a significant increase in PBO in maternal personal air samples after the residential restriction on OP insecticides and also a significant association between personal air PBO levels and use of spray insecticides within the home during pregnancy.12 To our knowledge, no previous data suggest an adverse effect of PBO exposure on neurocognitive development. PBO works as a synergist by potentiating the insecticidal action of pyrethroid insecticides. Most pyrethroid insecticides are detoxified by cytochrome P450 enzymes. PBO inhibits cytochrome P450 enzyme activity, thus delaying the detoxification of active pyrethroid parent compounds.22 Few studies have investigated the developmental toxicity of PBO. In a 2-generation study of dietary PBO exposure, F1-generation mice displayed restrained olfactory orientation. In addition, dietary PBO exposure resulted in increased movement (average speed and total distance) in a dose-response manner among male mice.37 No human studies have examined potential reproductive and neurobehavioral effects. Because the systemic toxicity of PBO in humans is relatively low, concern for health effects could arise from its potential to interfere with P450 activity and alter the metabolism of other xenobiotic compounds, including pharmaceutical medications38 and other pesticides. In addition, exposure to PBO has been shown to generate reactive oxygen species via metabolic pathways and to induce oxidative DNA damage.39 Oxidative stress could be a mechanism of altered neurologic development. Alternatively, PBO may interfere with detoxification of endogenous or exogenous neurotoxic substances and these substances have an impact on nervous system development at higher PBO concentrations.
We did not observe significant associations between cis- and/or trans-permethrin measured in either prenatal personal air or maternal/cord plasma and 36-month BSID-II scores. However, measuring permethrin in environmental and biological samples is challenging. Most pyrethroid insecticides, including permethrin, are not volatile19; thus our ability to detect them in personal air samples is fairly low. In addition, pyrethroid insecticides, including permethrin, are rapidly metabolized.32 Residues of parent compounds stay in the body <24 hours. Difficulties measuring permethrin in maternal and cord plasma may prevent us from seeing significant associations with neurodevelopmental outcomes. Such difficulties also explain the lack of association between PBO and cis- and trans-permethrin in air. Because PBO is volatile and permethrin is not volatile, we would not expect to find a strong association between the 2 compounds.
With the exception of the increased odds of motor delay in the lowest PBO exposure group, prenatal exposure to PBO seems to have an impact on cognitive rather than motor development. This finding is worrisome because MDI scores are more predictive of school readiness.40,41 Although effects of PBO exposure on MDI in this study were modest, they were comparable in magnitude to reports from studies of other prenatal neurotoxicants that affect development in young children.42–45
The authors acknowledge several limitations of the study design. Although we evaluated multiple covariates as potential confounders in the current study, it is possible that unmeasured factors confound the relationship between PBO and 36-month developmental outcomes. For example, we observed an unexpected increase in the odds of motor delay among children in the lowest PBO exposure group and did not observe an effect on motor delay in any of the higher exposure groups. Although this could be a chance finding attributable to small sample size, it is possible that uncontrolled exposures to other pesticides or neurodevelopmental toxicants may have confounded these results. In addition, this study would have benefitted from an internal measure of PBO exposure; however, at the time of publication no validated laboratory methods existed to measure PBO or its metabolites in blood or urine samples.
CONCLUSIONS
We observed a significant inverse association between prenatal exposures to PBO, a pyrethroid synergist, and 36-month neurodevelopment. We did not observe a significant association between prenatal exposure to permethrin and adverse neurodevelopment. These findings should be considered preliminary and may be useful for generating future hypotheses.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Environmental Health Sciences (grants 5P01ES09600, P50ES015905, 5R01ES08977), US Environmental Protection Agency (grants R827027, 8260901, and RR00645), Educational Foundation of America, John and Wendy Neu Family Foundation, New York Community Trust, and Trustees of the Blanchette Hooker Rockefeller Fund.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- OP
- organophosphorus
- CCCEH
- Columbia Center for Children's Environmental Health
- BSID-II
- Bayley Scales of Infant Development, second edition
- PBO
- piperonyl butoxide
- LOD
- limits of detection
- MDI
- Mental Developmental Index
- PDI
- Psychomotor Developmental Index
- ETS
- environmental tobacco smoke
- TONI
- Test of Nonverbal Intelligence
- HOME
- Home Observation for Measurement of the Environment
- CI
- confidence interval
REFERENCES
- 1. Adgate JL, Kukowski A, Stroebel C, et al. Pesticide storage and use patterns in Minnesota households with children. J Expo Anal Environ Epidemiol. 2000;10(2):159–167 [DOI] [PubMed] [Google Scholar]
- 2. Berkowitz GS, Obel J, Deych E, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111(1):79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107(suppl 3):409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naeher LP, Tulve NS, Egeghy PP, et al. Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United States city. Sci Total Environ. 2010;408(5):1145–1153 [DOI] [PubMed] [Google Scholar]
- 5. Bjørling-Poulsen M, Andersen HR, Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health. 2008;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berkowitz GS, Wetmur JG, Birman-Deych E, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112(3):388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engel SM, Berkowitz GS, Barr DB, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165(12):1397–1404 [DOI] [PubMed] [Google Scholar]
- 8. Young JG, Eskenazi B, Gladstone EA, et al. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209 [DOI] [PubMed] [Google Scholar]
- 9. Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6). Available at: www.pediatrics.org/cgi/content/full/118/6/e1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone DL, Sudakin DL, Jenkins JJ. Longitudinal trends in organophosphate incidents reported to the National Pesticide Information Center, 1995–2007. Environ Health. 2009;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whyatt RM, Camann D, Perera FP, et al. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol Appl Pharmacol. 2005;206(2):246–254 [DOI] [PubMed] [Google Scholar]
- 12. Williams MK, Rundle A, Holmes D, et al. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ Health Perspect. 2008;116(12):1681–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Power LE, Sudakin DL. Pyrethrin and pyrethroid exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J Med Toxicol. 2007;3(3):94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shafer TJ, Meyer DA, Crofton KM. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect. 2005;113(2):123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bayley N. Balyey Scales of Infant Development. 2nd ed San Antonio, TX: Psychological Corporation; 1993 [Google Scholar]
- 16. Stout DM, 2nd, Bradham KD, Egeghy PP, et al. American Healthy Homes Survey: a national study of residential pesticides measured from floor wipes. Environ Sci Technol. 2009;43(12):4294–4300 [DOI] [PubMed] [Google Scholar]
- 17. Horton MK, Jacobson JB, McKelvey W, et al. Characterization of residential pest control products used in inner city communities in New York City. J Expo Sci Environ Epidemiol. 2010; June 16 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pérez JJ, Williams MK, Weerasekera G, et al. Measurement of synthetic pyrethroid, organophosphorous, and carbamate insecticides in human plasma using isotope dilution gas chromatography-high resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(27):2554–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laskowski DA. Physical and chemical properties of pyrethroids. Rev Environ Contam Toxicol. 2002;174:49–170 [DOI] [PubMed] [Google Scholar]
- 20. Leng G, Gries W. Determination of pyrethroids in blood plasma and pyrethroid/pyrethrin metabolites in urine by gas chromatography-mass spectrometry and high resolution GC-MS . In: Vidal JL, Frenich AG. eds. Methods in Biotechnology. Totowa, NJ: Humana Press; 2005:17–33 [DOI] [PubMed] [Google Scholar]
- 21. Leng G, Kuhn KH, Idel H. Biological monitoring of pyrethroids in blood and pyrethroid metabolites in urine: applications and limitations. Sci Total Environ. 1997;199(1–2):173–181 [DOI] [PubMed] [Google Scholar]
- 22. Farnham AW. The mode of action of piperonyl butoxide with reference to studying pesticide resistance In: Jones DG. ed. Piperonyl Butoxide: The Insecticide Synergist. San Diego, CA: Academic Press; 1998:199–213 [Google Scholar]
- 23. Perera FP, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107(suppl 3):451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perera FP, Rauh V, Tsai WY, et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111(2):201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whyatt RM, Barr DB, Camann DE, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111(5);749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whyatt RM, Camann DE, Kinney PL, et al. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;110(5):507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whyatt RM, Garfinkel R, Hoepner LA, et al. Within- and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City. Environ Health Perspect. 2007;115(3):383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whyatt RM, Camann DE, Kinney PK, Reyes A, Ramirez J, Dietrick J, Diaz D, Holmes D, Perera FP. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ Health Perspect. 2002;5:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perera FP. Molecular epidemiology of environmental carcinogenesis. Recent Results Cancer Res. 1998;154:39–46 [DOI] [PubMed] [Google Scholar]
- 30. Barr DB, Barr JR, Maggio VL, et al. A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high-resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778(1–2):99–111 [DOI] [PubMed] [Google Scholar]
- 31. Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C. Low-level lead exposure and children's cognitive function in the preschool years. Pediatrics. 1991;87(2):219–227 [PubMed] [Google Scholar]
- 32. Soderlund DM, Clark JM, Sheets LP, et al. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171(1):3–59 [DOI] [PubMed] [Google Scholar]
- 33. Brown L, Sherbenou RJ, Johnson SK. Test of Non-verbal Intelligence: A Language-Free Measure of Cognitive Ability. 2nd ed Austin, TX: PRO-ED; 1990 [Google Scholar]
- 34. Caldwell DM, Bradley RH. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas Press; 1979 [Google Scholar]
- 35. Bradely R, Caldwell B, Rock S, et al. Home-environment and cognitive-development in the 1st 3 years of life: a collaborative study involving 6 sites and 3 ethnic-groups in North America. Dev Psychol. 1989;25(2):217–235 [Google Scholar]
- 36. Bellinger D, Leviton A, Waternaux C, Needleman H, Rabinowitz M. Low-level lead exposure, social class, and infant development. Neurotoxicol Teratol. 1988;10(6):497–503 [DOI] [PubMed] [Google Scholar]
- 37. Tanaka T. Reproductive and neurobehavioural effects of piperonyl butoxide administered to mice in the diet. Food Addit Contam. 2003;20(3);207–214 [DOI] [PubMed] [Google Scholar]
- 38. Franklin MR. Methylenedioxyphenyl insecticide synergists as potential human health hazards. Environ Health Perspect. 1976;14:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muguruma M, Unami A, Kanki M, et al. Possible involvement of oxidative stress in piperonyl butoxide induced hepatocarcinogenesis in rats. Toxicology. 2007;236(1–2):61–75 [DOI] [PubMed] [Google Scholar]
- 40. Aylward G, Verhurlst S, Bell S, Gyurke J. Cognitive and motor score differences in biologically at-risk infants. Dev Med Child Neurol. 1995;18(1):43–52 [Google Scholar]
- 41. Cole KN, Harris SR. Instability of the intelligence quotient-motor quotient relationship. Dev Med Child Neurol. 1992;34(7):633–641 [DOI] [PubMed] [Google Scholar]
- 42. Delaney-Black V, Covington C, Templin T, et al. Expressive language development of children exposed to cocaine prenatally: literature review and report of a prospective cohort study. J Commun Disord. 2000;33(6):463–480; quiz 480–461 [DOI] [PubMed] [Google Scholar]
- 43. Hurt H, Brodsky NL, Betancourt L, Braitman LE, Malmud E, Giannetta J. Cocaine-exposed children: follow-up through 30 months. J Dev Behav Pediatr. 1995;16(1):29–35 [PubMed] [Google Scholar]
- 44. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26(3):373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. Relationship of prenatal cocaine exposure and maternal postpartum psychological distress to child developmental outcome. Dev Psychopathol. 1997;9(3):473–489 [DOI] [PMC free article] [PubMed] [Google Scholar]