Abstract
OBJECTIVE:
To review the effects, adverse consequences, and extent of energy drink consumption among children, adolescents, and young adults.
METHODS:
We searched PubMed and Google using “energy drink,” “sports drink,” “guarana,” “caffeine,” “taurine,” “ADHD,” “diabetes,” “children,” “adolescents,” “insulin,” “eating disorders,” and “poison control center” to identify articles related to energy drinks. Manufacturer Web sites were reviewed for product information.
RESULTS:
According to self-report surveys, energy drinks are consumed by 30% to 50% of adolescents and young adults. Frequently containing high and unregulated amounts of caffeine, these drinks have been reported in association with serious adverse effects, especially in children, adolescents, and young adults with seizures, diabetes, cardiac abnormalities, or mood and behavioral disorders or those who take certain medications. Of the 5448 US caffeine overdoses reported in 2007, 46% occurred in those younger than 19 years. Several countries and states have debated or restricted energy drink sales and advertising.
CONCLUSIONS:
Energy drinks have no therapeutic benefit, and many ingredients are understudied and not regulated. The known and unknown pharmacology of agents included in such drinks, combined with reports of toxicity, raises concern for potentially serious adverse effects in association with energy drink use. In the short-term, pediatricians need to be aware of the possible effects of energy drinks in vulnerable populations and screen for consumption to educate families. Long-term research should aim to understand the effects in at-risk populations. Toxicity surveillance should be improved, and regulations of energy drink sales and consumption should be based on appropriate research.
Keywords: energy drink, caffeine, taurine, children, adolescents, overdose
“Energy drinks” are beverages that contain caffeine, taurine, vitamins, herbal supplements, and sugar or sweeteners and are marketed to improve energy, weight loss, stamina, athletic performance, and concentration.1–3 Energy drinks are available in >140 countries and are the fastest growing US beverage market; in 2011, sales are expected to top $9 billion.4–10 Half of the energy drink market consists of children (<12 years old), adolescents (12–18 years old), and young adults (19–25 years old).7–10
Although healthy people can tolerate caffeine in moderation, heavy caffeine consumption, such as drinking energy drinks, has been associated with serious consequences such as seizures, mania, stroke, and sudden death.6–8,11–14 Numerous reports exist in the popular media, and there are a handful of case reports in the literature that associate such adverse events with energy drink consumption; it is prudent to investigate the validity of such claims (Appendix). Children, especially those with cardiovascular, renal, or liver disease, seizures, diabetes, mood and behavioral disorders, or hyperthyroidism or those who take certain medications, may be at higher risk for adverse events from energy drink consumption.6–8,14–24 Although the US Food and Drug Administration (FDA) limits caffeine content in soft drinks, which are categorized as food, there is no such regulation of energy drinks, which are classified as dietary supplements.1–3 Despite the large, unregulated market for energy drinks and reports in the literature and popular media of serious adverse events associated with their consumption, research into their use and effects has been sparse.25 However, schools, states, and countries increasingly are exploring content and sales regulations of these drinks.1,8,13,26–35
Given the rapidly growing market and popularity among youth, we reviewed the literature to (1) determine what energy drinks are, (2) compile consumption data of energy drinks by children, adolescents, and young adults, (3) compile caffeine and energy drink overdose data, (4) examine the physiologic effects of the ingredients in energy drinks, (5) identify potential problems of energy drinks among children and adolescents, (6) assess the marketing of energy drinks, (7) report current regulation of energy drinks, and (8) propose educational, research, and regulatory recommendations.
METHODS
We searched PubMed by using “energy drink,” “sports drink,” “guarana,” “caffeine,” “taurine,” “ADHD” (attention- deficit/hyperactivity disorder), “diabetes,” “children,” “adolescents,” “insulin,” “eating disorders,” and “poison control center” singly or in combination. We limited searches to English-language and foreign-language articles with English-language abstracts and selected articles by relevance to energy drink use in children and adolescents. We similarly searched Google for print and trade media. We reviewed articles and Internet sources by the above search through June 2010 and updated sections as new information became available through January 2011.
RESULTS
Two-thirds of the 121 references we found on energy drinks were in the scientific literature, although reports by government agencies and interest groups also contained much useful information (Table 1). Most information came from the United States, but European, Canadian, Australian, New Zealand, and Chinese sources are also represented.
TABLE 1.
Primary Literature and Media Sources Selected for Review, According to Relevance
| Source Description | No. of Results | Main Topics | Source Country |
|---|---|---|---|
| Primary literature | 81 | ||
| Systematic reviews | 0 | — | — |
| Review articles | 36 | Energy drinks are a growing problem; safety issues with energy drinks; stimulant adverse effects; caffeine dependence; caffeine and fluid-electrolyte balance; caffeine and exercise performance; caffeine and mental performance; caffeine and apnea of prematurity; caffeine and bronchopulmonary dysplasia; caffeine and coronary heart disease; herb adverse effects; adolescents with ADHD and misuse of medication; ADHD and substance use; cardiovascular effects of antidepressants in children and adolescents; caffeine consumption and eating disorders; caffeine and bone gain in children and adolescents; incidence of pediatric cardiomyopathy; frequency of myocardial injury in children; epidemiology of hypertrophic cardiomyopathy | US, Poland, United Kingdom, Germany, China |
| Randomized controlled trials | 0 | — | — |
| Experimental studies | 16 | Cognitive and physiological effects of energy drinks; caffeine and exercise performance, thermoregulation, and fluid-electrolyte balance; energy drink effects on hemodynamic and electrocardiographic parameters in young adults; caffeine effects in children; gender differences and caffeinated beverages; taurine and cardiac parameters; energy drinks and platelet and endothelial function; eye-tracking in adolescents viewing advertisements | United States, United Kingdom, Germany |
| Cohort studies | 2 | Caffeine and sleep patterns in children; caffeine exposure in children, adolescents, and young adults | United States |
| Surveys | 4 | Consumption by college students; consumption of caffeinated beverages by adolescents; energy drinks and risk-taking behavior; survey of obstetrician/gynecologist caffeine knowledge | United States, Germany |
| Case reports | 8 | Caffeine-induced psychosis; energy drinks and seizures; cardiac arrest after energy drink consumption; cardiac arrhythmias associated with energy drink consumption; caffeine fatalities; Red Bull and postural tachycardia; cardiomyopathy and pericarditis after methylphenidate; psychosis after energy drink consumption | United States |
| Perspectives | 10 | Caution reduces long-term health risks; pediatric cardiology; cardiovascular adverse effects of stimulants; prevention of hypertension; stimulants and sudden death; cardiovascular disease and stimulants in children; sugary beverages and childhood obesity; fluid replacement and exercise | United States |
| Basic science | 5 | ||
| Animal studies | 3 | Stimulants and insulin-stimulated glucose uptake in rats; adenosine receptors in rats; structural changes in rat heart tissue after oral methylphenidate | United States |
| Human studies | 2 | Caffeine modulates gene expression; guarana and platelet function | United States |
| Government agency reports | 10 | FDA overview of dietary supplements; energy drinks/high-caffeine beverages; caffeinated beverages and obesity; dietary supplements and military personnel; German assessment of energy drinks; Canadian assessment of energy drinks; health risks of energy shots; Poison Control Center data | Australia, Canada, European Germany, New Zealand, United States |
| Interest groups | 3 | Alcoholic energy drinks and youth; nutrition and energy drinks | United States |
| Popular media | 27 | ||
| Newspaper articles | 14 | Overuse of energy drinks and health consequences; school bans on energy drinks; racial differences in caffeine metabolism; energy drink dangers for youth; restriction of drugs in youth; preparticipation screening and congenital heart defects; regulation of energy drinks; Hong Kong's ban of Red Bull; questioning dietary supplements | United States |
| Web sites | 12 | Energy drink sales; French ban on Red Bull; Germany questions energy drink safety; a school's ban on energy drinks; marketing of energy drinks; high caffeine levels in energy drinks in Australia; Ireland's review of the safety of energy drinks; Dutch schools' ban on Red Bull; preparticipation sports physicals | Holland, United Kingdom, United States |
| Books | 1 | History of energy drinks and health consequences | United States |
| Total | 121 |
DISCUSSION
What Are Energy Drinks?
Energy drinks may contain caffeine, taurine, sugars and sweeteners, herbal supplements, and other ingredients (Table 2) and are distinct from sports drinks and vitamin waters (Table 3).6,8 In 2008, the National Federation of State High School Associations, while recommending water and sports drinks for rehydration, specifically did not recommend energy drinks and cited potential risks, the absence of benefit, and drug interactions (Table 4).36,37
TABLE 2.
Common Ingredients, Therapeutic Uses, and Adverse Effects of Energy Drink Ingredients8,14,25,30,49,53,82,83
| Ingredient | Description | Therapeutic Uses | Purported Effect From Energy Drinks | Adverse Effects (due to Idiosyncratic Reaction or Excessive Dosage) |
|---|---|---|---|---|
| Caffeine | An adenosine receptor antagonist: a central nervous system stimulant | As caffeine citrate, used to treat apnea and bronchopulmonary dysplasia in premature infants | Increases exercise endurance and improves cognition and mood when fatigued or sleep-deprived | Nervousness, irritability, anxiety, insomnia, tachycardia, palpitations, upset stomach, vomiting, abdominal pain, rigidity, hypokalemia, altered consciousness, paralysis, hallucinations, increased intracranial pressure, cerebral edema, seizures, rhabdomyolysis, supraventricular and ventricular tachyarrhythmias |
| Guarana | A South American plant that contains large amounts of caffeine, theobromine, and theophylline (a chronotrope and an inotrope) and tannins | None known | Stimulant, mainly through the effects of caffeine, and weight loss | Generally considered safe by the FDA Center for Food Safety and Applied Nutrition |
| Taurine | An abundant amino acid in the central nervous system; acts in neural growth and protection, cell metabolism, osmoregulation, antioxidation, and glycolysis; estimated daily intake is 400 mg/d | Infant formula has been supplemented with taurine since the 1980s because of evidence that it promotes healthy development; used to treat alcohol withdrawal, congestive heart failure, cystic fibrosis, palpitations/dysrhythmias, hypertension, diabetes, seizure disorders, hepatitis | Marketed to promote eye and biliary health and to prevent congestive heart failure by lowering blood pressure while improving cardiac contractility | Generally considered safe by the FDA |
| l-Carnitine | An amino acid involved in β-oxidation of fatty acids | Used as a therapeutic supplement in congenital and acquired-deficiency states, end-stage renal disease, valproate toxicity, and dementia; increases attention and decreases hyperactivity in certain populations of children; nonstimulant l-acetyl-carnitine is used to treat ADHD in boys with fragile X syndrome and, in 1 study, children with typical ADHD; it may also protect against heart disease | Added to promote fat metabolism and increase endurance | In high doses, can cause nausea, vomiting, abdominal pain, and diarrhea; has been reported to cause seizures in patients with no known disease and to increase seizure frequency in patients with seizure disorder |
| Ginseng | An East Asian herb | Believed to improve memory, increase stamina, and stimulate immune function | Improve physical performance | Reported symptoms of ginseng toxicity include diarrhea, vaginal bleeding, headache, vertigo, mania, hypertension, rashes, insomnia, irritability, Stevens-Johnson syndrome, and agranulocytosis; some of these symptoms may be related to contaminants, such as phenylbutazone and aminopyrine, used in its processing |
| Yohimbine | An alkaloid found in the plants Pausinystalia yohimbe and Rauwolfia serpentina | An herbal supplement believed to be an aphrodisiac and to relieve chest pain, diabetic complications, depression, and erectile dysfunction | Increase energy, metabolism, and stamina; promotes well-being | Can cause hypertension at usual doses and hypotension at high doses; tachycardia, death |
TABLE 3.
| Sports Drinks | Enhanced/Fortified Vitamin Waters | Energy Drinks | |
|---|---|---|---|
| Common brand names | Gatorade; Powerade | Glacéau Vitamin Water; SoBe Lifewater; Dasani Plus | Red Bull, Full Throttle, Monster Energy, Rockstar |
| Advertised purpose | To rehydrate the body after intense exercise for ≥1 h | Broad claims that range from improving endurance to strengthening immune defense to relaxation | To rapidly increase energy, endurance, and performance |
| Ingredients | Glucose, electrolytes | Water, vitamins, nutrients, sometimes sweeteners; no stimulants | Caffeine, sugar, herbal supplements, other substances (eg, taurine, glucuronolactone) |
| Effects | Replaces lost electrolytes and carbohydrates during sustained strenuous exercise; prevents dehydration | Prevents dehydration; may replace some nutrients; other than the caloric sweeteners, the contents are unlikely to present health risks, and there is no published evidence of benefit | Temporarily increases heart and respiratory rates and blood pressure; not designed to hydrate the body |
TABLE 4.
| Energy Drink Ingredient | Interactions |
|---|---|
| 5-Hydroxy tryptophan | Should not be combined with monoamine oxidase inhibitors |
| Vinpocetine | Increases the risk for excessive bleeding and should not be combined with aspirin, clopidogrel, warfarin, pentoxifylline, vitamin E, garlic, and gingko |
| Yohimbine | Affects cardiac function and should not be combined with tricyclic antidepressants, bupropion, phenothiazines, clonidine, stimulants, decongestants, or other blood pressure-lowering medications |
| Ginseng | Prolongs bleeding time and should not be combined with warfarin; interacts with phenelzine sulfate in patients being treated for mania; may interfere with estrogens or corticosteroids; may impede digoxin metabolism; reduces blood glucose levels |
Caffeine is the main active ingredient in energy drinks; many of them contain 70 to 80 mg per 8-oz serving (∼3 times the concentration in cola drinks) (Table 5).8,31 Caffeine content can be nearly 5 times greater than that in 8 oz of cola drinks when packaged as “energy shots” (0.8–3 oz) or as 16-oz drinks.6,29,38
TABLE 5.
Caffeine Content Reported by Manufacturers and Selected Additional Ingredients of Selected Products6,29,46
| Product | Selected Ingredients | Sugar Content per 8 oz, g | Average Caffeine Dose per 8 oz, mg | Amount per Serving, oz | Average Caffeine Dose per Container, mg |
|---|---|---|---|---|---|
| Comparison products | |||||
| NoDoz | Caffeine | 0 | NAa | NAa | 200 mg per tablet |
| Excedrin | Acetaminophen, aspirin, caffeine | 0 | NAa | NAa | 65 mg per tablet (serving size: 2 tablets) |
| Hershey's Kisses | Caffeine, sugar | 21 in 9 pieces | NAa | NAa | 9 mg in 9 pieces |
| Coca-Cola | Caffeine, sugar | 19–25 | 23 | 12 | 35 |
| Mountain Dew | Caffeine, sugar | 32 | 28 | 16 | 55 |
| Diet Pepsi Max | Ginseng | 0 | 47 | 12 | 69 |
| Tea, brewed | NAa | 0 | 54 | 8 | 54 |
| Coffee, generic brewed | NAa | 0 | 100 | 8 | 100 |
| Starbucks tall coffee | NAa | 0 | 174 | 12 | 260 |
| Energy drinks | |||||
| Low calorie | B vitamins, dextrose, fumarate, gingko, ginseng, glucuronolactone, guarana, glucose, inositol, l-arginine, l-carnitine, milk thistle, n-acetyl-l-tyrosine, sucrose, taurine, yohimbine HCL, vinpocetine, 5-hydroxy tryptophane | 0 | 77–19 200 | 0.17–16.00 | 80–400 |
| Regular | B vitamins, dextrose, fumarate, gingko, ginseng, glucuronolactone, guarana, high-fructose corn syrup, glucose, inositol, l-arginine, l-carnitine, milk thistle, n-acetyl-l-tyrosine, sucrose, taurine, yohimbine HCL, vinpocetine, 5-hydroxy tryptophane | 17–30 | 71–286 | 8.3–16.0 | 75–300 |
| Super-caffeinated | B vitamins, dextrose, fumarate, gingko, ginseng, glucuronolactone, guarana, glucose, inositol, l-arginine, l-carnitine, milk thistle, n-acetyl-l-tyrosine, sucrose, taurine, yohimbine HCL, vinpocetine, 5-hydroxy tryptophane | 0 | 315–19 200 | 0.17–2.50 | 80–400 |
NA indicates not applicable.
Product-specific information is from company Web sites; ingredients may vary according to country.
Energy drinks often contain additional amounts of caffeine through additives, including guarana, kola nut, yerba mate, and cocoa.6,7,14,25 Guarana (Paullinia cupana) is a plant that contains caffeine, theobromine (a chronotrope), and theophylline (an inotrope).7,8,14,39 Each gram of guarana can contain 40 to 80 mg of caffeine, and it has a potentially longer half-life because of interactions with other plant compounds.7,14 Manufacturers are not required to list the caffeine content from these ingredients.7,14 Thus, the actual caffeine dose in a single serving may exceed that listed.9,29
Consumption of Energy Drinks by Children, Adolescents, and Young Adults
In the United States, adolescent caffeine intake averages 60 to 70 mg/day and ranges up to 800 mg/day.24,40 Most caffeine intake among youth comes from soda; however, energy drinks are becoming increasingly popular.7,24,41,42 Several self-report studies have examined energy drink consumption by children, adolescents, and young adults.7,24,41,42
One study found that 28% of 12- to 14-year-olds, 31% of 12- to 17-year olds, and 34% of 18- to 24-year-olds reported regularly consuming energy drinks.5,43 Shortly after energy drinks were approved in Germany, a study of 1265 adolescents found that 94% were aware of energy drinks, 53% had tried them, 23% drank <1 can per week, and 3% drank 1 to 7 cans per week.44 Among 10- to 13-year-olds, 31% of girls and 50% of boys had tried energy drinks, and 5% of girls and 23% of boys reported drinking them regularly but at a rate of <1 can per week.44 Most children in the study consumed energy drinks in moderation, but a small group consumed extreme amounts.44
A survey of 496 college students found that 51% of those surveyed regularly consumed >1 energy drink per month; the majority of them habitually drank energy drinks several times per week.9 Insufficient sleep (67%) and the desire to increase energy (65%) were the most common reasons for use.9 In this study, 54% of the respondents reported mixing energy drinks with alcohol, and 49% drank ≥3 of them while partying.9 Another study of 795 college students found that 39% of the respondents had consumed an energy drink in the previous month and that, on average, men drank energy drinks 2.5 days/month, whereas women drank 1.2 days/month.45
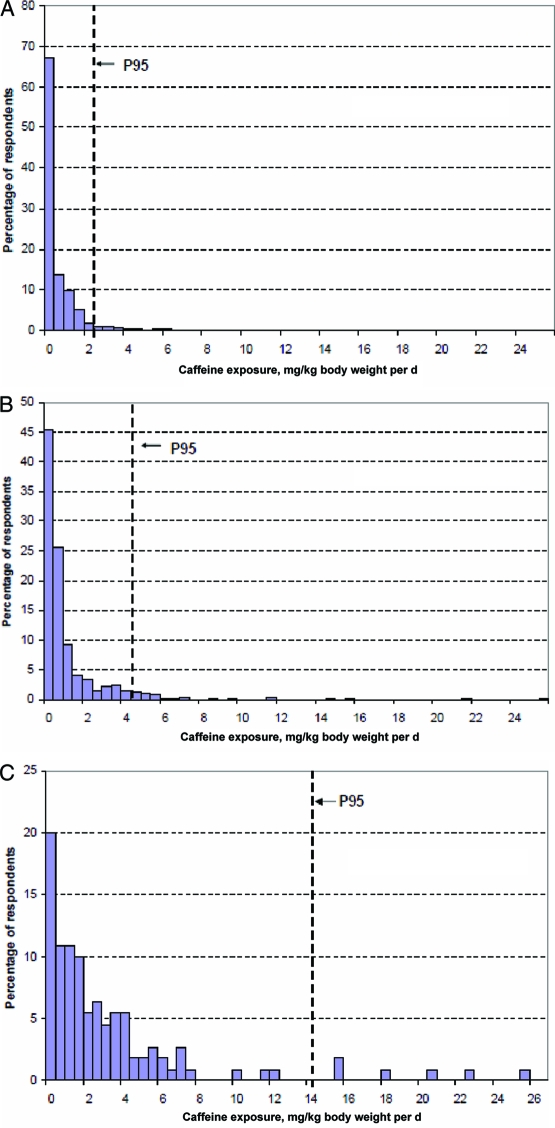
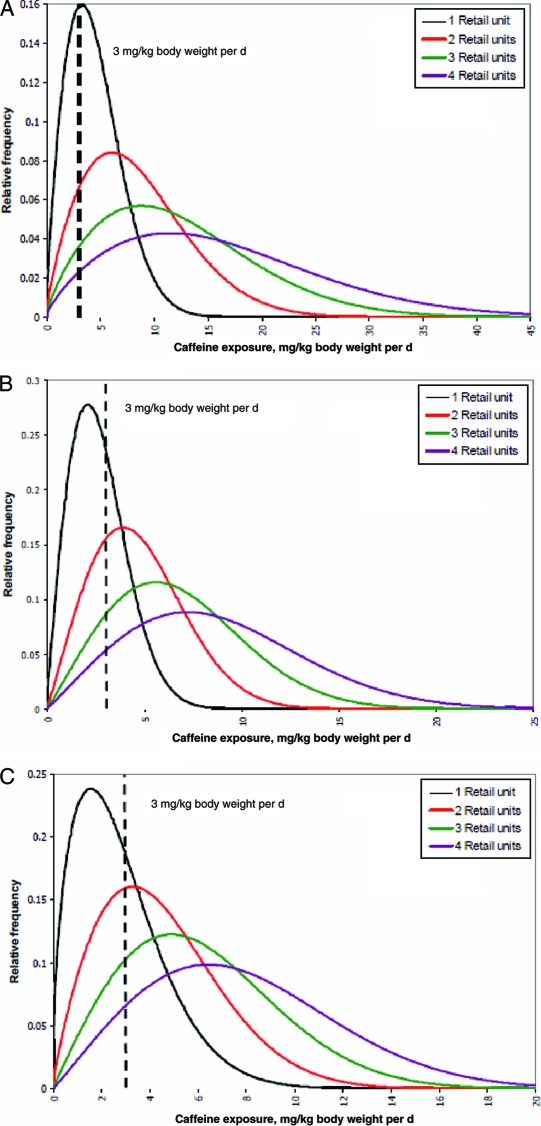
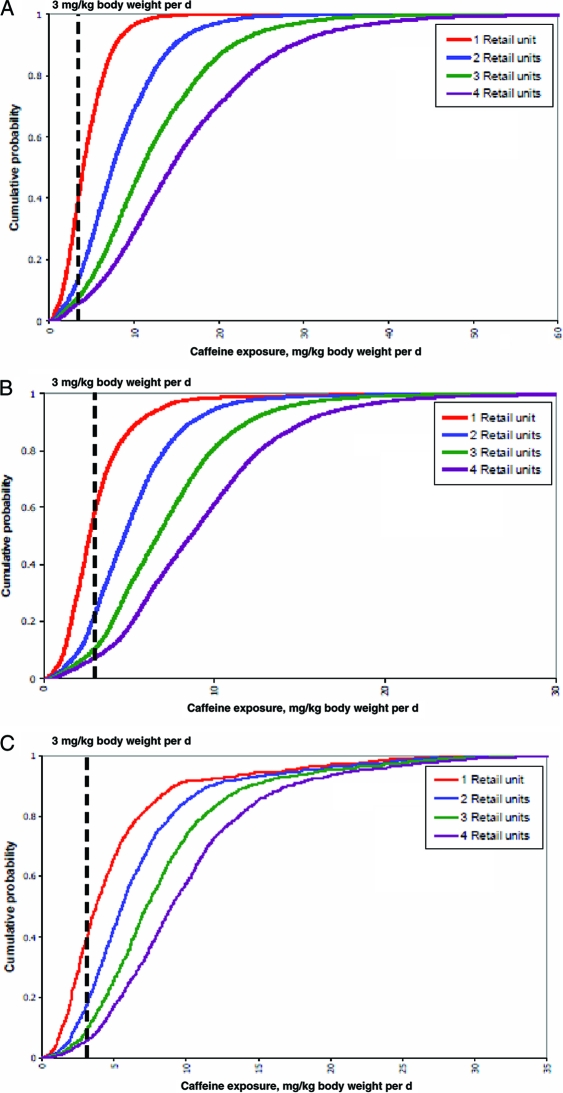
The estimated caffeine exposure of consuming energy drinks or energy shots was calculated for New Zealand children (5–12 years old), teenagers (13–19 years old), and young men (19–24 years old) (Figs 1–3).46 After consuming a single retail unit, 70% of the children and 40% of the teenagers who consumed caffeine were estimated to have exceeded the adverse-effect level of 3 mg/kg body weight per day beyond their baseline dietary exposure.46 An average child, teenager, or young man would all, on average, exceed the adverse-effect level after consuming a single retail unit of energy drink/energy shot above their baseline dietary caffeine exposure.46
FIGURE 1.
Mathematical model estimates for dietary consumption of caffeine and energy drinks in children aged 5 to 12 years (A), adolescents aged 13 to 19 years (B), and young males aged 19 to 24 years (C) using caffeine-concentration data from food and beverages combined with 24-hour diet-recall information from the 1997 New Zealand National Nutrition Survey and the 2002 New Zealand National Children's Nutrition Survey. A, Distribution of dietary baseline caffeine-exposure estimates for children (5–12 years old).46 P95 indicates the 95th percentile exposure and represents a high consumer. Caffeine-exposure units are mg/kg of body weight per day. B, Distribution of dietary baseline caffeine-exposure estimates for teenagers (13–19 years old).46 C, Distribution of dietary baseline caffeine-exposure for young males (19–24 years old).46 Reproduced with permission from David Crowe, manager of consumer communications for the New Zealand Food Safety Authority.
FIGURE 2.
A, Estimated distribution of caffeine exposure for children (5–12 years old) after the consumption of 1 to 4 retail units of energy drinks or energy shots.46 B, Estimated distribution of caffeine exposure for teenagers (13–19 years old) after the consumption of 1 to 4 retail units of energy drinks or energy shots.46 C, Estimated distribution of caffeine exposure for young males (19–24 years old) after the consumption of 1 to 4 retail units of energy drinks or energy shots.46 Caffeine-exposure units are mg/kg body weight per day. An adverse effect level of 3 mg/kg body weight per day is shown as a basis for risk evaluation. The area under the curves to the right of the adverse-effect lines represents the proportion of consumers potentially at risk from adverse effects of caffeine or the probability of a random consumer exceeding the adverse-effect level. Reproduced with permission from David Crowe, manager of consumer communications for the New Zealand Food Safety Authority.
FIGURE 3.
A, Cumulative probability curves of children (5–12 years old) consuming 1 to 4 retail units of energy drinks or energy shots in addition to baseline dietary exposure.46 B, Cumulative probability curve for teenagers (13–19 years old) consuming 1 to 4 retail units of energy drinks or energy shots in addition to baseline dietary exposure.46 C, Cumulative probability curve of young males (19–24 years old) consuming 1 to 4 retail units of energy drinks or energy shots in addition to baseline dietary exposure.46 Caffeine-exposure units are mg/kg body weight per day. An adverse-effect level of 3 mg/kg body weight per day is shown as a reference point. The portion of each curve to the right of the adverse-effect level represents the proportion of the population group potentially at risk from adverse effects of caffeine. The exposure of any percentile may be read off the x-axis by extrapolating from the intersection of the selected percentile on the y-axis with the curve of 1, 2, 3, or 4 retail units consumed; cumulative probability = 0.2 represents the 20th percentile, 0.4 = 40th percentile, etc. Reproduced with permission from David Crowe, manager of consumer communications for the New Zealand Food Safety Authority.
Caffeine and Energy Drink Overdoses
US poison control centers have not specifically tracked the prevalence of overdoses attributed to energy drinks, because exposures were coded as “caffeine” or “multisubstance exposures” and combined with other caffeine sources (Table 6) (American Association of Poison Control Centers Board of Directors, personal communication, 2010).47 Energy drinks were recently given unique reporting codes, so their toxicity can now be tracked (American Association of Poison Control Centers Board of Directors, personal communication, 2010).47
TABLE 6.
American Association of Poison Control Centers' Data on Caffeine Toxicity, 2006–200847
| Year | Total calls to PCCs to Report Caffeine Toxicity, n | Calls That Reported Caffeine Toxicity in Children <6 y old, n (% of Total Calls) | Calls That Reported Caffeine Toxicity in 6- to-19-Year-Olds, n (% of Total Calls) | Calls That Reported Caffeine Toxicity in Adults (>19 y old), n (% of Total Calls) | Patients Subsequently Treated, n (% of Total Calls) | Patients With Moderately Severe Symptoms, n (% of Total Calls) | Patients With Life-Threatening Effects, n (% of Total Calls) | Deaths, n (% of Total Calls) |
|---|---|---|---|---|---|---|---|---|
| 2008 | 4852 | 1208 (24.9) | 1170 (24.1) | 1090 (22.5) | 1281 (26.4) | 470 (9.7) | 11 (0.2) | 1 (0.02) |
| 2007 | 5448 | 1176 (21.6) | 1328 (24.4) | 1404 (25.8) | 1561 (28.7) | 544 (10.0) | 16 (0.3) | 1 (0.02) |
| 2006 | 5696 | 1247 (21.9) | 1427 (25.1) | 1427 (25.1) | 1799 (31.6) | 654 (11.5) | 18 (0.3) | 1 (0.02) |
PCC indicates poison control center.
Germany has tracked energy drink–related incidents since 2002.33 Reported outcomes include liver damage, kidney failure, respiratory disorders, agitation, seizures, psychotic conditions, rhabdomyolysis, tachycardia, cardiac dysrhythmias, hypertension, heart failure, and death.33 Ireland's poison center reported 17 energy drink adverse events including confusion, tachycardia, and seizures and 2 deaths between 1999 and 2005.25 New Zealand's poison center reported 20 energy drink/shot–related adverse events from 2005 to 2009; 12 cases were referred for treatment of vomiting, nausea, abdominal pain, jitteriness, racing heart, and agitation.46 The minimum and maximum symptomatic caffeine levels were 200 mg (4 mg/kg) in a 13-year-old with jitteriness and 1622 mg (35.5 mg/kg) in a 14-year-old. The maximum volume consumed was fifteen 250-mL cans (11.5 mg/kg caffeine) during 1 hour.46 One 23-year-old chronic energy drink consumer had a myocardial infarction.46
Physiologic Effects of the Ingredients in Energy Drinks
Caffeine Pharmacology and Physiology
Caffeine, the most commonly used psychoactive drug worldwide, may be the only psychoactive drug legally available over-the-counter to children and sold among food and beverage products.39,48 Caffeine is an adenosine and benzodiazepine receptor antagonist, phosphodiesterase inhibitor, and central nervous system stimulant.29,38,49 In healthy adults, a caffeine intake of ≤400 mg/day is considered safe; acute clinical toxicity begins at 1 g, and 5 to 10 g can be lethal.29
Physiologically, caffeine causes coronary and cerebral vasoconstriction, relaxes smooth muscle, stimulates skeletal muscle, has cardiac chronotropic and inotropic effects, reduces insulin sensitivity, and modulates gene expression in premature neonates.9,29,50–52 Large amounts of caffeine increase urine flow and sweat excretion and alter blood electrolyte levels.11,53 Although caffeine is a mild diuretic, consumption of ≤500 mg/day does not cause dehydration or chronic water imbalance.54,55
Caffeine is a ventilatory stimulant with anti-inflammatory and bronchoprotective effects.56 Caffeine has been linked to dyspnea on exertion from central and peripheral chemoreceptor stimulation.56 In addition, increased breathing work may divert blood flow away from locomotor muscles and negate any ergogenic advantage.56 Caffeine's cardiovascular effects include decreased heart rate from stimulation of medullary vagal nuclei and increased blood pressure.24,57–61
Adults who consume low-to-moderate amounts of caffeine (1–3 mg/kg or 12.5–100 mg/day) have improved exercise endurance, cognition, reaction time, and mood with sleep deprivation.9,24,56,62 However, these studies typically involve habitual caffeine consumers, and results reflect withdrawal-symptom reversal.58
Consuming 4 to 12 mg/kg of caffeine has been associated with undesirable symptoms, including anxiety and jitteriness.63 Headache and fatigue, common withdrawal symptoms, can occur after short-term, high-dose use.64 Caffeine intoxication is a clinical syndrome of nervousness, irritability, anxiety, insomnia, tremor, tachycardia, palpitations, and upset stomach.6,7,9,14,26,65 Additional adverse effects include vomiting and abdominal pain, hypokalemia, hallucinations, increased intracranial pressure, cerebral edema, stroke, paralysis, rhabdomyolysis, altered consciousness, rigidity, seizures, arrhythmias, and death.1,2,8,29,48
Caffeine intakes of >300 mg/day have been associated with miscarriage and low birth weight.38,66,67 Long-term caffeine consumption relates to a lower risk of Parkinson disease and a slower age-related cognitive decline.58
Effects of Caffeine in Children and Adolescents
Adolescent and child caffeine consumption should not exceed 100 mg/day and 2.5 mg/kg per day, respectively.7,38,63 For example, 8 oz of Red Bull (Fuschl am See, Austria) provides 77 mg of caffeine, or 1.1 mg/kg for a 70-kg male or 2.2 mg/kg for a 35-kg preteen.40 Whether the effects of caffeine in adults can be generalized to children remains unclear.63 In a study of 26 boys and 26 men, the same dose of caffeine affected blood pressure similarly, but heart rate was significantly lowered in boys, whereas there was no effect on heart rate in men.68 Boys also exhibited more increased motor activity and speech rates and decreased reaction time than did men.69
Caffeine can improve attention, but it also increases blood pressure and sleep disturbances in children.24,63,70,71 After cessation in children who habitually consume caffeine, attention decreases and reaction time increases transiently.24,39 Similarly, reaction time has been shown to decrease as the dose of caffeine in children increases.24
In a study of 9- to 11-year-olds with habitual (mean intake: 109 mg/day) and low (mean intake: 12 mg/day) caffeine consumption given 50 mg of caffeine after overnight abstention, habitual caffeine users reported withdrawal-symptom (headache and dulled cognition) reversal. The children who did not habitually consume caffeine reported no marked changes in cognitive performance, alertness, or headache.63
Caffeine may affect future food and beverage preferences by acting on the developing child's brain reward-and-addiction center; this effect may be gender specific.5 A study of 12- to 17-year-olds revealed that boys found caffeinated soda more reinforcing than did girls regardless of usual caffeine consumption.72
Physiologic Effects of Other Ingredients in Energy Drinks and Potential Synergistic Effects
Popular media and case reports have associated adverse events with energy drink consumption (Appendix). Yet, few studies have examined the physiologic effects of individual ingredients or potential synergistic effects; furthermore, results of experimental studies have been inconclusive and occasionally contradictory.24,25,59,73
Some studies of adults revealed improved mental alertness, reaction times, and concentration with energy drinks59,74; others revealed no improvement compared with caffeine or glucose alone.73 One study of 14 young adults compared a complete energy drink mixture to the glucose fraction, the caffeine fraction, and the herbal fraction.9,59 Although individual components did not enhance cognition, the combined ingredients did.9,59 Caffeine and taurine combined may synergistically decrease heart rate initially; one study found that 70 minutes after consumption, heart rate returned to normal and blood pressure increased.25,75 Taurine similarly produced a reflex bradycardia when injected into the rat cerebroventricular system.75 Another study of 15 healthy young adults in a 7-day trial in which they consumed 500 mL of an energy drink each day with 160 mg of caffeine and 2000 mg of taurine, reported an average increase in systolic blood pressure of 9 to 10 mm Hg and an average increased heart rate of 5 to 7 beats per minute 4 hours after consumption.25,38
Caffeine- and taurine-containing beverages increased left atrial contractility in 13 athletes, thereby increasing left ventricular end-diastolic volume and stroke volume.76 The caffeine-only group showed no changes in left ventricular function.76 Taurine may cause this increase in stroke volume by suppressing sympathetic nervous stimulation and influencing calcium stores in cardiac muscle.8 Results of human and animal studies have suggested that long-term taurine exposure may cause hypoglycemia25 but a decreased risk of coronary heart disease.77 In animal experiments, taurine also has shown anticonvulsive and epileptogenic properties.25
Among 50 young adults who drank one sugar-free energy drink, hematologic and vascular effects included increased platelet aggregation and mean arterial pressure and a decrease in endothelial function.78 Guarana has antiplatelet aggregation properties in vitro, but how it functions physiologically in energy drinks is unknown.79 A study of 20 healthy subjects revealed that caffeinated espresso had no effects on endothelial function.80 Caffeine alone did not affect platelet function.81
Ginseng, a common ingredient in many energy drinks, may lower blood glucose levels, but its actions in energy drinks are unclear.82
Potential Problems of Energy Drinks Among Children and Adolescents
Cardiovascular Effects of Energy Drinks on Children and Adolescents
High doses of caffeine may exacerbate cardiac conditions for which stimulants are contraindicated.17,18,83–86 Of particular concern are ion channelopathies and hypertrophic cardiomyopathy, the most prevalent genetic cardiomyopathy in children and young adults, because of the risk of hypertension, syncope, arrhythmias, and sudden death.11,86,87
Effects of Energy Drinks on Children and Adolescents With ADHD
ADHD occurs in 8% to 16% of US school-aged children and may be more prevalent in children with heart disease.88,89 Some 2.5 million US children take stimulants for ADHD, which may increase heart rate and blood pressure.89–91 Children with ADHD have higher rates of substance abuse, including the abuse of caffeine, which blocks the A2A adenosine receptors and thereby enhances the dopamine effect at the D2 dopamine receptor, similarly to the way guanfacine works for ADHD.92,93 For the subpopulation with methylphenidate cardiotoxicity, energy drink use may increase cardiac events.95,96 As with the ADHD stimulants, the combined effects of energy drinks and antidepressants are unknown.94
Energy Drink Use in Children and Adolescents With Eating Disorders
Children and adolescents with eating disorders, especially anorexia nervosa, may regularly consume high amounts of caffeine to counter caloric-restriction–associated fatigue, suppress appetite, and produce looser stools and some diuresis.97–100 Given that children and adolescents with eating disorders have a propensity for cardiac morbidity/mortality and electrolyte disorders, consumption of high-caffeine energy drinks may put them at further risk for cardiac dysrythmias and intracardiac conduction abnormalities.97–99
Effects on Caloric Intake and Diabetes
Because obesity is epidemic, caloric increases from energy drink consumption become important. Additional calories may increase blood pressure, blood glucose levels, BMI, calcium deficiency, dental problems, depression, and low self-esteem.4,101,102 Sugar and caffeine may also synergistically increase postprandial hyperglycemia, which is of concern for children with diabetes.38,51,52
Effects on Bone Mineralization
Early adolescence is the time of maximal calcium deposition in bone, and caffeine interferes with intestinal calcium absorption.103,104 It remains controversial whether caffeine itself has the most marked effect on bone acquisition during adolescence or whether replacement of milk intake by caffeinated beverages is the leading contributor.103,104
Marketing of Energy Drinks
Youth-targeted marketing strategies date to 1987 when Red Bull was introduced in Austria.100 When it took 5 years to get permission to export Red Bull to Germany, rumors about its legality and dangerous effects helped fuel its popularity, and it became known as “speed in a can,” “liquid cocaine,” and a “legal drug.”100
Energy drink marketing strategies include sporting event and athlete sponsorships, alcohol-alternative promotion, and product placement in media (including Facebook and video games) oriented to children, adolescents, and young adults.43,105 Newer alcoholic energy drinks, the cans of which resemble the nonalcoholic counterparts, target risk-taking youth.43
Contrasting with brand design is the voluntary fine-print warning label on some products, which state that they may not be safe for children, those who are sensitive to caffeine, or for pregnant or nursing women.105–107
Regulation of Energy Drinks
The FDA imposes a limit of 71 mg of caffeine per 12 fl oz of soda.1,2,6 Energy drink manufacturers may circumvent this limit by claiming that their drinks are “natural dietary supplements.”1,2 Thus, safety determinations of energy drinks are made solely by the manufacturers, and there are no requirements for testing, warning labels, or restriction against sales or consumption by minors.1–3 In contrast, over-the-counter dedicated caffeine stimulants (eg, No-Doz [Novartis Consumer Health, Parsippany, NJ]) must list the minimum age for purchase (12 years), adverse effects, cautionary notes, recommended dose, and the total daily recommended dose of caffeine. In November 2009, the FDA asked manufacturers of alcoholic energy drinks to prove their safety.108 The US Senate is considering a bill that would require supplement manufacturers to register annually with the FDA and allow FDA recalls of supplements suspected of being unsafe. Ingredients may also be restricted to those that have already been approved by the FDA.109
Regulatory controversies also extend internationally (Table 7). When France banned Red Bull, the manufacturers challenged the ban through the European Commission, which determined that the caffeine and taurine concentrations in energy drinks had not been proven to be health risks and ordered France to lift the ban; the European Food Safety Authority has encouraged international data-pooling to better assess risks in children, adolescents, and young adults.33,110 In 2008, authorities in Germany, Hong Kong, and Taiwan detected 0.13 μg per can of cocaine (average) in Red Bull Cola. Red Bull manufacturers insisted that active cocaine was removed from the coca leaf during processing and that the extract was used for flavoring. However, 11 of 16 German states banned the product.111
TABLE 7.
National and International Energy Drink Regulations
| Country | Bans on Energy Drinks | Restrictions | Proposed or Attempted Regulation |
|---|---|---|---|
| Argentina | — | — | Senate has proposed banning energy drinks in nightclubs5 |
| Australia | Recently banned 5 energy drinks on the basis of a caffeine content of >320 mg/L116 | — | Classifying energy drinks as pharmaceutical products, which are regulated, has been proposed116 |
| Canada | — | Requires warning labels, recommends a maximum daily consumption amount, and advises against mixing energy drinks with alcohol until further research has been conducted25 | — |
| Denmark | Prohibits energy drinks entirely5,25 | — | — |
| European Food Safety Authority | — | Beverages that contain >150 mg/L caffeine should be labeled “high caffeine content” and the exact amount present indicated on the label46 | — |
| France | — | — | Banned Red Bull but recently removed the ban after assessment by the European Food Safety Authority5 |
| Germany | 11 of 16 German states banned Red Bull Cola because of trace amounts of cocaine111 | — | Stricter regulations on warning labels have been requested by the government32; the German Federal Institute for Risk Assessment recommends that energy shots be banned because of the high risk of overdose38 |
| Ireland | — | — | Ireland is reviewing energy drink safety; Ireland's food-safety board has recommended that energy drinks be labeled as unsuitable for children <16 y old and that a ban be placed on the promotion of Red Bull in sporting events and in combination with alcohol118,120 |
| Netherlands | — | — | Reviewed energy drink safety and declared no risk118,120 |
| Norway | — | Energy drinks can only be sold in pharmacies5,25 | — |
| Sweden | — | Sales to children <15 y are banned; warning labels about consuming high caffeine after exercise and mixing energy drinks with alcohol are also present5 | — |
| Finland | — | — | Drinks that contain >150 mg/L of caffeine must be labeled “high caffeine content” and also must be labeled “not recommended for children, pregnant women, or people sensitive to caffeine”46; energy drinks must also state the maximum amount to be used daily”46 |
| Turkey | Ban on all high-caffeine energy drinks5,118 | — | — |
| United Kingdom | — | — | The UK's Committee on Toxicity investigated Red Bull and determined that it was safe for the general public but that children <16 y old or people sensitive to caffeine should avoid drinks with high caffeine content5,31 |
| Uruguay | Prohibits energy drinks entirely121 | — | — |
| United States | — | The FDA has listed caffeine as generally recognized as safe in concentrations of <200 mg/kg; in November 2009, the FDA announced plans to examine the safety of caffeinated alcoholic beverages46 | In 2008, Kentucky, Maine, and Michigan introduced legislation that would ban the sale of highly caffeinated drinks to children <18 y old, but the bills were defeated30; California is considering a bill to require special labels on alcoholic beverages to avoid confusion with nonalcoholic beverages43 |
CONCLUSIONS
On the basis of this review, we conclude that (1) energy drinks have no therapeutic benefit, and both the known and unknown pharmacology of various ingredients, combined with reports of toxicity, suggest that these drinks may put some children at risk for serious adverse health effects11,16,24,25,38; (2) typically, energy drinks contain high levels of caffeine, taurine, and guarana, which have stimulant properties and cardiac and hematologic activity,7,8,11 but manufacturers claim that energy drinks are nutritional supplements, which shields them from the caffeine limits imposed on sodas and the safety testing and labeling required of pharmaceuticals7,8,11; (3) other ingredients vary, are understudied, and are not regulated; (4) youth-aimed marketing and risk-taking adolescent developmental tendencies combine to increase overdose potential; (5) high consumption is suggested by self-report surveys but is underdocumented in children (deleterious associations with energy drink consumption have been reported globally in case reports and popular media7,8,11,25,31,38); and (6) interactions between compounds, additive and dose-dependent effects, long-term consequences, and dangers associated with risky behavior in children remain to be determined.5,14,25,38
EDUCATIONAL, RESEARCH, AND REGULATORY RECOMMENDATIONS
In the short-term, pediatric health care providers need to be aware of energy drink consumption by children, adolescents, and young adults and the potentially dangerous consequences of inappropriate use.112 Diet and substance-use histories should include screening for episodic/chronic energy drink consumption, both alone and with alcohol. Screening is especially important for athletes, children with high-risk behaviors, certain health conditions (eg, seizures, diabetes, hypertension, cardiac abnormalities), and children with behavioral changes, anxiety, poor nutrition, or sleep disturbances.11
For most children, adolescents, and young adults, safe levels of consumption have not been established. Yet, heavy use may be harmful or interact with medications and cause untoward adverse effects. Health care providers should educate families and children at risk for the potential adverse effects of energy drinks.
Routine high school athletic physicals do not identify everyone at risk for sudden cardiac death.11,113 Children with cardiac conditions should be counseled regarding the risks of caffeine-containing products, including irregular heart rhythms, syncope, dysrhythmias, and sudden death.17,18,85 Community partners, including schools, athletic groups, and regulatory bodies, also need to promote risk awareness.113 The fourth edition of the “Preparticipation Physical Evaluation” monograph will feature a revamped health questionnaire focused on cardiac health problems that may be exacerbated by physical activity; thus, adding questions about stimulant use, including energy drink consumption, becomes important.113
Long-term research objectives should aim to better define maximum safe doses, the effects of chronic use, and effects in at-risk populations (eg, those with preexisting medical conditions, those who consume energy drinks during and after exercise, or those who consume them in combination with alcohol), and better documentation and tracking of adverse health effects.47 Unless research establishes energy drink safety in children and adolescents, regulation, as with tobacco, alcohol, and prescription medications, is prudent.11
This approach is essential for reducing morbidity and mortality, encouraging research, and supporting families of children and young adults at risk for energy drink overdose, behavioral changes, and acute/chronic health consequences.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, CA068484, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, and HD80002; Health Resources and Services Administration grant HCOF-C76HF15614; the Children's Cardiomyopathy Foundation; and the Women's Cancer Association.
APPENDIX.
Representative Sample of Adverse Events Reported in Association With Nonalcoholic Energy Drink Consumption
| Source of Information | No. and of Age Patients | Previous Health Conditions | Symptoms | Reported Association With Energy Drink | Ref No. |
|---|---|---|---|---|---|
| Journal articles/case reports | One 25-y-old woman | Mitral valve prolapse | Unknown | Drank a bottle of Race 2005 Energy Blast with guarana and ginseng on the day that she had a cardiac arrest; the autopsy and toxicological screen results were negative except for a caffeine concentration of 19 mg/L in aortic blood | 29 |
| One 25-y-old man | None reported | Generalized seizures on 2 occasions over 4 mo; the seizures did not reoccur within 6 mo after abstaining from energy drinks | Reportedly drank energy drinks on an empty stomach; he reported consuming two 24-oz energy drinks 30–60 min before the seizure | 27 | |
| One 31-y-old man | None reported | Rhabdomyolysis and acute kidney failure with tubular necrosis | Active football referee drank 3 cans of Red Bull before a 3000-m competition; the authors stated that the taurine could have caused the rhabdomyolysis from hyperosmolarity because the distance was relatively short compared with his normal training | 25 and 38 | |
| One 43-y-old man | Paranoid-type schizophrenia and alcohol dependence in full, sustained remission | 6-wk history of worsening paranoia, delusions, and agitation resulting in hospitalization | Started drinking energy drinks 2 wk before becoming symptomatic; consumption increased to 8–10 cans per d; the cessation of caffeine reportedly improved symptoms | 114 | |
| One 47-y-old man | None reported | Delusions and paranoia; the psychosis resolved within 7 wk after reducing caffeine consumption | High caffeine intake | 13 | |
| 2 patients | Migraine headaches | Seizures | In 1 case, the energy drink was consumed on an empty stomach; in the other case, caffeine tablets were also consumed with the energy drink | 26 | |
| 2 depressed patients and 1 patient with no psychiatric illness | Started on ginseng for several months | Unknown | Mania, which resolved after stopping taking ginseng | 8 | |
| One young professional volleyball player | None reported | Developed orthostatic intolerance, postural tachycardia, and syncope, which resolved after energy drink consumption was stopped | 4–5 cans of Red Bull per d | 75 | |
| Newspaper articles | 4 middle school students | None reported | All transported to the hospital with tachycardia, hypertension, paresthesias, diaphoresis, jitters/anxiety; hypokalemia and hyperglycemia were diagnosed in the emergency department | All 4 shared 1 can of Redline energy drink | 16 |
| 7 high school students | None reported | Shortness of breath, heart palpitations, nausea; 2 students were treated in a hospital | SPIKE Shooter | 34 | |
| One teenaged boy | None reported | Severe stomach pain for ∼2 mo; endoscopic findings included severe inflammation, bleeding, and ulcerations in the duodenum | Several Redline energy drinks (250 mg of caffeine per 8-oz serving) per d | 32 | |
| Marin Institute | One 14-y-old girl | Diabetes | 2 d after drinking 1 can of SPIKE Shooter, she was hospitalized for a seizure | SPIKE Shooter | 43 |
| One 18-y-old girl | None reported | Died after sharing 4 cans of Red Bull with friends and then playing basketball | Red Bull | 43 | |
| Online news sources | One 14-y-old girl | Diabetes | Seizure | Reportedly drank 1 can of an energy drink before the seizure | 25 and 38 |
| One 17-y-old girl | None reported | Collapsed at the finish of a track race and was rushed to the emergency department after reporting chest pain and fatigue | Regularly skipped breakfast and drank 2 or 3 cans of Red Bull | 1 | |
| One 28-y-old man | None reported | The man reportedly drank energy drinks and then engaged in the strenuous physical activity of motocross; he subsequently died from a cardiac arrest, presumably from coronary vasospasm caused by the energy drinks | Unknown energy drink; unspecified but large numbers of energy drinks that contained high levels of caffeine and taurine were consumed | 28 | |
| One 47-y-old man | None reported | “I thought I was having a heart attack. I thought I was going to die.” The next day he reported feeling sore and exhausted from the experience | Two 8-oz cans of VPX Redline | 119 |
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- FDA
- Food and Drug Administration
- ADHD
- attention-deficit/hyperactivity disorder
REFERENCES
- 1. Lee J. Energy drinks vs. sports drinks: know thy difference. Available at: http://speedendurance.com/2009/07/09/energy-drinks-vs-sports-drinks-know-thy-difference Accessed January 17, 2011
- 2. McCarthy M. Overuse of energy drinks worries health pros. Available at: www.usatoday.com/sports/2009-07-01-Drinks_N.htm Accessed January 17, 2011
- 3. US Food and Drug Administration Overview of dietary supplements. Available at: www.fda.gov/Food/DietarySupplements/ConsumerInformation/ucm110417.htm Accessed January 17, 2011
- 4. Nitzke S, Tanumihardjo S, Salomon J, Coleman G. Energy drinks, sports drinks, and other functional/enhanced beverages are often a waste of money. Available at: www.uwex.edu/ces/wnep/specialist/nfl/mmpdfs/0810.pdf#page=1 Accessed January 17, 2011
- 5. Oddy WH, O'Sullivan TA. Energy drinks for children and adolescents, erring on the side of caution may reduce long term health risks. BMJ. 2009;339:b5268. [DOI] [PubMed] [Google Scholar]
- 6. Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks: a growing problem. Drug Alcohol Depend. 2009;99(1–3):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babu KM, Church RJ, Lewander W. Energy drinks: the new eye-opener for adolescents. Clin Pediatr Emerg Med. 2008;9(1):35–42 [Google Scholar]
- 8. Clauson KA, Shields KM, McQueen CE, Persad N. Safety issues associated with commercially available energy drinks. J Am Pharm Assoc (Wash DC). 2008;48(3):e55–e63; quiz e64–e67 [DOI] [PubMed] [Google Scholar]
- 9. Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutr J. 2007;6:35 Available at: www.nutritionj.com/content/6/1/35 Accessed January 17, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Press Office New report predicts energy drink sales in the U.S. to exceed $9 billion by 2011 [press release]. Available at: www.reportbuyer.com/press/new-report-predicts-energy-drink-sales-in-the-us-to-exceed-9-billion-by-2011 Accessed January 17, 2011
- 11. Lipshultz S. High risk: Ban energy drinks from schools. Miami Herald. April 20, 2008:4L, L4 [Google Scholar]
- 12. Broderick P, Benjamin AB. Caffeine and psychiatric symptoms: a review. J Okla State Med Assoc. 2004;97(12):538–542 [PubMed] [Google Scholar]
- 13. Hedges DW, Woon FL, Hoopes SP. Caffeine-induced psychosis. CNS Spectr. 2009;14(3):127–129 [DOI] [PubMed] [Google Scholar]
- 14. Heneman K, Zidenberg-Cherr S. Some facts about energy drinks. Available at: http://nutrition.ucdavis.edu/content/infosheets/EnergyDrinks.pdf Accessed January 17, 2011
- 15. Brecher EJ. Study: caffeine in sodas risky for black kids. Miami Herald. May 18, 2004:7E [Google Scholar]
- 16. Cohen H. Dangerous jolt: energy drink dangers for children. Miami Herald. April 1, 2008:E10, 10E [Google Scholar]
- 17. Frassica JJ, Orav EJ, Walsh EP, Lipshultz SE. Arrhythmias in children prenatally exposed to cocaine. Arch Pediatr Adolesc Med. 1994;148(11):1163–1169 [DOI] [PubMed] [Google Scholar]
- 18. Lipshultz SE, Frassica JJ, Orav EJ. Cardiovascular abnormalities in infants prenatally exposed to cocaine. J Pediatr. 1991;118(1):44–51 [DOI] [PubMed] [Google Scholar]
- 19. Lipshultz SE. Ventricular dysfunction clinical research in infants, children and adolescents. Prog Pediatr Cardiol. 2000;12(1):1–28 [DOI] [PubMed] [Google Scholar]
- 20. Lipshultz SE, Wilkinson JD, Messiah SE, Miller TL. Clinical research directions in pediatric cardiology. Curr Opin Pediatr. 2009;21(5):585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lipshultz SE. Realizing optimal care for children with cardiovascular disease: funding challenges and research approaches. Prog Pediatr Cardiol. 2005;20(1):71–90 [Google Scholar]
- 22. Mone SM, Gillman MW, Miller TL, Herman EH, Lipshultz SE. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004;113(4 suppl):1058–1069 [PubMed] [Google Scholar]
- 23. Chrissos J. Cold medicines taboo for kids under 4: further restricting the use of cold medicines for young children, drug companies now say they shouldn't be used in children younger than 4. Miami Herald. October 8, 2008: Living-Health [Google Scholar]
- 24. Temple JL. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci Biobehav Rev. 2009;33(6):793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Federal Institute for Risk Assessment New human data on the assessment of energy drinks. Available at: www.bfr.bund.de/cm/245/new_human_data_on_the_assessment_of_energy_drinks.pdf Accessed January 17, 2011
- 26. Health Canada Energy drinks safety and health effects. Available at: www.enotalone.com/article/10272.html Accessed January 17, 2011
- 27. Iyadurai SJ, Chung SS. New-onset seizures in adults: possible association with consumption of popular energy drinks. Epilepsy Behav. 2007;10(3):504–508 [DOI] [PubMed] [Google Scholar]
- 28. Berger AJ, Alford K. Cardiac arrest in a young man following excess consumption of caffeinated “energy drinks.” Med J Aust. 2009;190(1):41–43 [DOI] [PubMed] [Google Scholar]
- 29. Cannon ME, Cooke CT, McCarthy JS. Caffeine-induced cardiac arrhythmia: an unrecognised danger of healthfood products. Med J Aust. 2001;174(10):520–521 [DOI] [PubMed] [Google Scholar]
- 30. Bestervelt L. Raising the red flag on some energy drinks. Available at: www.nsf.org/media/enews/documents/energy_drinks.pdf Accessed January 17, 2011
- 31. Nordqvist C. French ban on Red Bull (drink) upheld by European Court. Available at: www.medicalnewstoday.com/articles/5753.php Accessed January 17, 2011
- 32. Parikh RK. Red alert on energy drinks. Los Angeles Times. September 1, 2008: Health [Google Scholar]
- 33. Starling S. Energy drinks safety questioned by German agency. Available at: www.beveragedaily.com/content/view/print/166290 Accessed January 17, 2011
- 34. High school students warned about energy drink: Smokey Hill student hospitalized after drinking SPIKE. Available at: www.thedenverchannel.com/news/11070908/detail.html Accessed January 17, 2011
- 35. Quinones D. Sidelined college football freshman plans medical path. Miami Herald. July 30, 2009: Sports [Google Scholar]
- 36. National Federation of State High School Associations, Sports Medicine Advisory Committee Position statement and recommendations for the use of energy drinks by young athletes. Available at: www.nfhs.org/search.aspx?searchtext=Energy%20Drinks Accessed January 17, 2011
- 37. National Federation of State High School Associations, Sports Medicine Advisory Committee Minimize the risk for dehydration and heat illness. Available at: www.nfhs.org/search.aspx?searchtext=Energy%20Drinks Accessed January 17, 2011
- 38. Federal Institute for Risk Assessment Health risks of excessive energy shot intake. December 2, 2009. Available at: www.bfr.bund.de/cm/245/health_risks_of_excessive_energy_shot_intake.pdf Accessed January 17, 2011
- 39. O'Connor E. A sip into dangerous territory. Monit Psychol. 2001;32(6). Available at: www.apa.org/monitor/jun01/dangersip.aspx Accessed January 17, 2011 [Google Scholar]
- 40. Pollak CP, Bright D. Caffeine consumption and weekly sleep patterns in US seventh-, eighth-, and ninth-graders. Pediatrics. 2003;111(1):42–46 [DOI] [PubMed] [Google Scholar]
- 41. Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME. Caffeine dependence in teenagers. Drug Alcohol Depend. 2002;66(1):1–6 [DOI] [PubMed] [Google Scholar]
- 42. Strain EC, Mumford GK, Silverman K, Griffiths RR. Caffeine dependence syndrome: evidence from case histories and experimental evaluations. JAMA. 1994;272(13):1043–1048 [PubMed] [Google Scholar]
- 43. Simon M, Mosher J. Alcohol, energy drinks, and youth: a dangerous mix. Available at: www.marininstitute.org/alcopops/resources/EnergyDrinkReport.pdf Accessed January 17, 2011
- 44. Viell B, Grabner L, Fruchel G, Boczek P. New caffeinated beverages: a pilot survey of familiarity and consumption by adolescents in north-Rhine Westphalia and Berlin and considerations of consumer protection [in German]. Z Ernahrungswiss. 1996;35(4):378–386 [DOI] [PubMed] [Google Scholar]
- 45. Miller KE. Wired: energy drinks, jock identity, masculine norms, and risk taking. J Am Coll Health. 2008;56(5):481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomson B, Schiess S. Risk profile: caffeine in energy drinks and energy shots. Available at: www.nzfsa.govt.nz/science/risk-profiles/fw10002-caffeine-in-beverages-risk-profile.pdf Accessed January 17, 2011
- 47. Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Heard SE; American Association of Poison Control Centers 2007 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila). 2008;46(10):927–1057 [DOI] [PubMed] [Google Scholar]
- 48. Holmgren P, Norden-Pettersson L, Ahlner J. Caffeine fatalities: four case reports. Forensic Sci Int. 2004;139(1):71–73 [DOI] [PubMed] [Google Scholar]
- 49. Greenwood MRC, Oria M. Use of dietary supplements by military personnel. Available at: www.nap.edu/catalog/12095.html Accessed January 17, 2011 [PubMed]
- 50. Connolly S, Kingsbury TJ. Caffeine modulates CREB-dependent gene expression in developing cortical neurons. Biochem Biophys Res Commun. 2010;397(2):152–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dworzanski W, Opielak G, Burdan F. Side effects of caffeine [in Polish]. Pol Merkur Lekarski. 2009;27(161):357–361 [PubMed] [Google Scholar]
- 52. Kolnes AJ, Ingvaldsen A, Bolling A, et al. Caffeine and theophylline block insulin-stimulated glucose uptake and PKB phosphorylation in rat skeletal muscles. Acta Physiol (Oxf). 2010;200(1):65–74 [DOI] [PubMed] [Google Scholar]
- 53. Del Coso J, Estevez E, Mora-Rodriguez R. Caffeine during exercise in the heat: thermoregulation and fluid-electrolyte balance. Med Sci Sports Exerc. 2009;41(1):164–173 [DOI] [PubMed] [Google Scholar]
- 54. Armstrong LE. Caffeine, body fluid-electrolyte balance, and exercise performance. Int J Sport Nutr Exerc Metab. 2002;12(2):189–206 [DOI] [PubMed] [Google Scholar]
- 55. Armstrong LE, Pumerantz AC, Roti MW, et al. Fluid, electrolyte, and renal indices of hydration during 11 days of controlled caffeine consumption. Int J Sport Nutr Exerc Metab. 2005;15(3):252–265 [DOI] [PubMed] [Google Scholar]
- 56. Chapman RF, Mickleborough TD. The effects of caffeine on ventilation and pulmonary function during exercise: an often-overlooked response. Phys Sportsmed. 2009;37(4):97–103 [DOI] [PubMed] [Google Scholar]
- 57. Rogers PJ, Dernoncourt C. Regular caffeine consumption: a balance of adverse and beneficial effects for mood and psychomotor performance. Pharmacol Biochem Behav. 1998;59(4):1039–1045 [DOI] [PubMed] [Google Scholar]
- 58. Rogers PJ. Caffeine, mood and mental performance in everyday life. Br Nutr Found Nutr Bull. 2007;32(suppl l):84–89 [Google Scholar]
- 59. Scholey AB, Kennedy DO. Cognitive and physiological effects of an “energy drink”: an evaluation of the whole drink and of glucose, caffeine and herbal flavouring fractions. Psychopharmacology (Berl). 2004;176(3–4):320–330 [DOI] [PubMed] [Google Scholar]
- 60. Lipshultz SE. Vote for children's health. Miami Herald. October 25, 2008; Opinion-Other Views-Healthcare [Google Scholar]
- 61. Whelton PK, He J, Appel LJ, et al. ; National High Blood Pressure Education Program Coordinating Committee. Primary prevention of hypertension: clinical and public health advisory from the national high blood pressure education program. JAMA. 2002;288(15):1882–1888 [DOI] [PubMed] [Google Scholar]
- 62. Burke LM. Caffeine and sports performance. Appl Physiol Nutr Metab. 2008;33(6):1319–1334 [DOI] [PubMed] [Google Scholar]
- 63. Heatherley SV, Hancock KM, Rogers PJ. Psychostimulant and other effects of caffeine in 9- to 11-year-old children. J Child Psychol Psychiatry. 2006;47(2):135–142 [DOI] [PubMed] [Google Scholar]
- 64. Griffiths RR, Woodson PP. Caffeine physical dependence: a review of human and laboratory animal studies. Psychopharmacology (Berl). 1988;94(4):437–451 [DOI] [PubMed] [Google Scholar]
- 65. Steinke L, Lanfear DE, Dhanapal V, Kalus JS. Effect of “energy drink” consumption on hemodynamic and electrocardiographic parameters in healthy young adults. Ann Pharmacother. 2009;43(4):596–602 [DOI] [PubMed] [Google Scholar]
- 66. Hinds TS, West WL, Knight EM, Harland BF. The effect of caffeine on pregnancy outcome variables. Nutr Rev. 1996;54(7):203–207 [DOI] [PubMed] [Google Scholar]
- 67. Dlugosz L, Belanger K, Hellenbrand K, Holford TR, Leaderer B, Bracken MB. Maternal caffeine consumption and spontaneous abortion: a prospective cohort study. Epidemiology. 1996;7(3):250–255 [DOI] [PubMed] [Google Scholar]
- 68. Turley KR, Desisso T, Gerst JW. Effects of caffeine on physiological responses to exercise: boys versus men. Pediatr Exerc Sci. 2007;19(4):481–492 [DOI] [PubMed] [Google Scholar]
- 69. Rapoport JL, Jensvold M, Elkins R, et al. Behavioral and cognitive effects of caffeine in boys and adult males. J Nerv Ment Dis. 1981;169(11):726–732 [DOI] [PubMed] [Google Scholar]
- 70. Bancalari E. Caffeine for apnea of prematurity. N Engl J Med. 2006;354(20):2179–2181 [DOI] [PubMed] [Google Scholar]
- 71. Bancalari E. Caffeine reduces the rate of bronchopulmonary dysplasia in very low birth weight infants. J Pediatr. 2006;149(5):727–728 [DOI] [PubMed] [Google Scholar]
- 72. Temple JL, Bulkley AM, Briatico L, Dewey AM. Sex differences in reinforcing value of caffeinated beverages in adolescents. Behav Pharmacol. 2009;20(8):731–741 [DOI] [PubMed] [Google Scholar]
- 73. Smit HJ, Cotton JR, Hughes SC, Rogers PJ. Mood and cognitive performance effects of “energy” drink constituents: caffeine, glucose and carbonation. Nutr Neurosci. 2004;7(3):127–139 [DOI] [PubMed] [Google Scholar]
- 74. Warburton DM, Bersellini E, Sweeney E. An evaluation of a caffeinated taurine drink on mood, memory and information processing in healthy volunteers without caffeine abstinence. Psychopharmacology (Berl). 2001;158(3):322–328 [DOI] [PubMed] [Google Scholar]
- 75. Terlizzi R, Rocchi C, Serra M, Solieri L, Cortelli P. Reversible postural tachycardia syndrome due to inadvertent overuse of red bull. Clin Auton Res. 2008;18(4):221–223 [DOI] [PubMed] [Google Scholar]
- 76. Baum M, Weiss M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids. 2001;20(1):75–82 [DOI] [PubMed] [Google Scholar]
- 77. Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis. 2010;208(1):19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Worthley MI, Prabhu A, De Sciscio P, Schultz C, Sanders P, Willoughby SR. Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med. 2010;123(2):184–187 [DOI] [PubMed] [Google Scholar]
- 79. Subbiah MT, Yunker R. Studies on the nature of anti-platelet aggregatory factors in the seeds of the Amazonian herb guarana (Paullinia cupana). Int J Vitam Nutr Res. 2008;78(2):96–101 [DOI] [PubMed] [Google Scholar]
- 80. Buscemi S, Verga S, Batsis JA, et al. Acute effects of coffee on endothelial function in healthy subjects. Eur J Clin Nutr. 2010;64(5):483–489 [DOI] [PubMed] [Google Scholar]
- 81. Natella F, Nardini M, Belelli F, et al. Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br J Nutr. 2008;100(6):1276–1282 [DOI] [PubMed] [Google Scholar]
- 82. Hui H, Tang G, Go VL. Hypoglycemic herbs and their action mechanisms. Chin Med. 2009;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dangerous supplements. Consum Rep. September 2010:16–20 [PubMed] [Google Scholar]
- 84. Lipshultz SE, Wong JC, Lipsitz SR, et al. Frequency of clinically unsuspected myocardial injury at a children's hospital. Am Heart J. 2006;151(4):916–922 [DOI] [PubMed] [Google Scholar]
- 85. Sokol KC, Armstrong FD, Rosenkranz ER, et al. Ethical issues in children with cardiomyopathy: making sense of ethical challenges in the clinical setting. Prog Pediatr Cardiol. 2007;23(1):81–87 [Google Scholar]
- 86. Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the united states. N Engl J Med. 2003;348(17):1647–1655 [DOI] [PubMed] [Google Scholar]
- 87. Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the pediatric cardiomyopathy registry. Circulation. 2007;115(6):773–781 [DOI] [PubMed] [Google Scholar]
- 88. Barbaresi WJ, Katusic SK, Colligan RC, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156(3):217–224 [DOI] [PubMed] [Google Scholar]
- 89. Vetter VL, Elia J, Erickson C, et al. ; American Heart Association, Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association, Council on Cardiovascular Nursing Cardiovascular monitoring of children and adolescents with heart disease receiving stimulant drugs: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young congenital cardiac defects committee and the council on cardiovascular nursing [published correction appears in Circulation. 2009;120(7):e55–e59]. Circulation. 2008;117(18):2407–2423 [DOI] [PubMed] [Google Scholar]
- 90. Wilens TE, Gignac M, Swezey A, Monuteaux MC, Biederman J. Characteristics of adolescents and young adults with ADHD who divert or misuse their prescribed medications. J Am Acad Child Adolesc Psychiatry. 2006;45(4):408–414 [DOI] [PubMed] [Google Scholar]
- 91. Wilens TE, Prince JB, Spencer TJ, Biederman J. Stimulants and sudden death: what is a physician to do? Pediatrics. 2006;118(3):1215–1219 [DOI] [PubMed] [Google Scholar]
- 92. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2). Available at: www.pediatrics.org/cgi/content/full/104/2/e20 Accessed January 17, 2011 [DOI] [PubMed] [Google Scholar]
- 93. Fredholm BB, Svenningsson P. Striatal adenosine A2A receptors: where are they? What do they do? Trends Pharmacol Sci. 1998;19(2):46–48 [DOI] [PubMed] [Google Scholar]
- 94. Wilens TE, Biederman J, Baldessarini RJ, et al. Cardiovascular effects of therapeutic doses of tricyclic antidepressants in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1996;35(11):1491–1501 [DOI] [PubMed] [Google Scholar]
- 95. Dadfarmay S, Dixon J. A case of acute cardiomyopathy and pericarditis associated with methylphenidate. Cardiovasc Toxicol. 2009;9(1):49–52 [DOI] [PubMed] [Google Scholar]
- 96. Take G, Bahcelioglu M, Oktem H, et al. Dose-dependent immunohistochemical and untrastructural changes after oral methylphenidate administration in rat heart tissue. Anat Histol Embryol. 2008;37(4):303–308 [DOI] [PubMed] [Google Scholar]
- 97. Krahn DD, Hasse S, Ray A, Gosnell B, Drewnowski A. Caffeine consumption in patients with eating disorders. Hosp Community Psychiatry. 1991;42(3):313–315 [DOI] [PubMed] [Google Scholar]
- 98. Stock SL, Goldberg E, Corbett S, Katzman DK. Substance use in female adolescents with eating disorders. J Adolesc Health. 2002;31(2):176–182 [DOI] [PubMed] [Google Scholar]
- 99. Striegel-Moore RH, Franko DL, Thompson D, Barton B, Schreiber GB, Daniels SR. Caffeine intake in eating disorders. Int J Eat Disord. 2006;39(2):162–165 [DOI] [PubMed] [Google Scholar]
- 100. Popkin B. We are what we drink. InThe World Is Fat: The Fads, Trends, Policies, and Products That Are Fattening the Human Race. New York, NY: Penguin Group; 2002:43–65 [Google Scholar]
- 101. Moreno MA, Furtner F, Frederick PR. Sugary drinks and childhood obesity. Arch Pediatr Adolesc Med. 2009;163(4):400. [DOI] [PubMed] [Google Scholar]
- 102. National Center for Chronic Disease Prevention and Health Promotion Obesity: halting the epidemic by making health easier. Available at: www.cdc.gov/nccdphp/publications/AAG/pdf/obesity.pdf Accessed January 17, 2011
- 103. Heaney RP. Effects of caffeine on bone and the calcium economy. Food Chem Toxicol. 2002;40(9):1263–1270 [DOI] [PubMed] [Google Scholar]
- 104. Lloyd T, Rollings NJ, Kieselhorst K, Eggli DF, Mauger E. Dietary caffeine intake is not correlated with adolescent bone gain. J Am Coll Nutr. 1998;17(5):454–457 [DOI] [PubMed] [Google Scholar]
- 105. Hein K. A bull's market: the marketing of Red Bull energy drink. Available at: http://findarticles.com/p/articles/mi_m0BDW/is_22_42/ai_75286777/?tag=content%3Bcol1 Accessed January 17, 2011
- 106. Thomsen SR, Fulton K. Adolescents' attention to responsibility messages in magazine alcohol advertisements: an eye-tracking approach. J Adolesc Health. 2007;41(1):27–34 [DOI] [PubMed] [Google Scholar]
- 107. Fischer PM, Richards JW, Jr, Berman EJ, Krugman DM. Recall and eye tracking study of adolescents viewing tobacco advertisements. JAMA. 1989;261(1):84–89 [PubMed] [Google Scholar]
- 108. Weise E. Petition calls for FDA to regulate energy drinks. USA Today. October 22, 2008: News, Health & Behavior [Google Scholar]
- 109.Our view on pills and potions: do you really know what's in that dietary supplement? [Accessed January 17, 2011]. Available at: www.usatoday.com/news/opinion/editorials/2010-06-07-editorial07_ST_N.htm?loc=interstitialskip.
- 110. European Food Safety Authority EFSA adopts opinion on two ingredients commonly used in some energy drinks [press release]. Available at: www.efsa.europa.eu/en/press/news/ans090212.htm Accessed January 17, 2011
- 111. Red Bull pulled from shelves in Hong Kong. Available at: http://content.usatoday.com/communities/ondeadline/post/2009/06/67549503/1 Accessed October 27, 2009
- 112. Anderson BL, Juliano LM, Schulkin J. Caffeine's implications for women's health and survey of obstetrician-gynecologists' caffeine knowledge and assessment practices. J Womens Health (Larchmt). 2009;18(9):1457–1466 [DOI] [PubMed] [Google Scholar]
- 113. Pepine CJ. Panel endorses preparticipation sports physicals for every child. Cardiol Today. 2010:13(6). Available at: www.cardiologytoday.com/print.aspx?rid=65042 Accessed January 17, 2011 [Google Scholar]
- 114. Cerimele JM, Stern AP, Jutras-Aswad D. Psychosis following excessive ingestion of energy drinks in a patient with schizophrenia. Am J Psychiatry. 2010;167(3):353. [DOI] [PubMed] [Google Scholar]
- 115. Convertino VA, Armstrong LE, Coyle EF, et al. American college of sports medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 1996;28(1):i–vii [DOI] [PubMed] [Google Scholar]
- 116. Bruce B. Energy drinks banned over caffeine levels in Australia. Available at: www.foodbev.com/news/energy-drinks-banned-over-caffeine-levels-in-australia Accessed January 17, 2011
- 117. Neuman W. “Energy shots” stimulate power drink sales. Available at: www.nytimes.com/2009/07/11/business/11energy.html Accessed January 17, 2011
- 118. Derbyshire D. Energy drinks “should have caffeine health warning on cans.” MailOnline. Available at: www.dailymail.co.uk/news/article-1060705/Energy-drinks-caffeine-health-warning-cans.html Accessed January 17, 2011
- 119. Energy drinks' caffeine buzz can land the unwary in the ER. REDORBIT News. 2005. Available at: www.redorbit.com/news/display/?id=833954 Accessed January 17, 2011
- 120. Nutra Ingredients.com Ireland to review safety of energy drinks. Available at: www.nutraingredients.com/Regulation/Ireland-to-review-safety-of-energy-drinks Accessed January 17, 2011
- 121. Red Bull banned in Dutch schools Dutch Daily News. Available at: www.dutchdailynews.com/red-bull-banned-in-dutch-schools Accessed January 17, 2011