Abstract
OBJECTIVE:
To determine if beneficial effects of a weight-management program could be sustained for up to 24 months in a randomized trial in an ethnically diverse obese population.
PATIENTS AND METHODS:
There were 209 obese children (BMI > 95th percentile), ages 8 to 16 of mixed ethnic backgrounds randomly assigned to the intensive lifestyle intervention or clinic control group. The control group received counseling every 6 months, and the intervention group received a family-based program, which included exercise, nutrition, and behavior modification. Lifestyle intervention sessions occurred twice weekly for the first 6 months, then twice monthly for the second 6 months; for the last 12 months there was no active intervention. There were 174 children who completed the 12 months of the randomized trial. Follow-up data were available for 76 of these children at 24 months. There were no statistical differences in dropout rates among ethnic groups or in any other aspects.
RESULTS:
Treatment effect was sustained at 24 months in the intervention versus control group for BMI z score (−0.16 [95% confidence interval: −0.23 to −0.09]), BMI (−2.8 kg/m2 [95% confidence interval: −4.0–1.6 kg/m2]), percent body fat (−4.2% [95% confidence interval: −6.4% to −2.0%]), total body fat mass (−5.8 kg [95% confidence interval: −9.1 kg to −2.6 kg]), total cholesterol (−13.0 mg/dL [95% confidence interval: −21.7 mg/dL to −4.2 mg/dL]), low-density lipoprotein cholesterol (−10.4 mg/dL [95% confidence interval: −18.3 mg/dL to −2.4 mg/dL]), and homeostasis model assessment of insulin resistance (−2.05 [95% confidence interval: −2.48 to −1.75]).
CONCLUSIONS:
This study, unprecedented because of the high degree of obesity and ethnically diverse backgrounds of children, reveals that benefits of an intensive lifestyle program can be sustained 12 months after completing the active intervention phase.
Keywords: adolescents, children, BMI, obesity, behavior modification, exercise, insulin resistance
WHAT'S KNOWN ON THIS SUBJECT:
Long-term pediatric weight management studies have been predominantly carried out with affluent, white populations. Thus, evidence on successful management of childhood obesity is limited, especially in inner-city, ethnically diverse populations.
WHAT THIS STUDY ADDS:
This randomized controlled trial bridges the gap in the pediatric obesity treatment literature by revealing a sustained treatment effect on anthropometric and metabolic markers after a family-based lifestyle intervention versus traditional clinical care in inner-city, ethnically diverse populations.
Considering the high global prevalence of childhood obesity, it is surprising how little published evidence exists regarding successful treatment programs, especially in ethnic minorities and inner-city impoverished populations, which have the highest rates of childhood obesity. The first, a 2003 Cochrane review1 on treatment of childhood obesity, referenced only 18 randomized controlled trials. Most of these studies had few subjects and targeted populations of white, educated, middle-class families. The Yale Bright Bodies Weight Management Program was developed to fill the unmet needs of obese, inner-city children who were primarily black and Hispanic. This family-based, intensive lifestyle program has been an ongoing treatment option available to overweight and obese children in the Greater New Haven area since 1998.
To evaluate the effectiveness of the Bright Bodies program, a 2-year, nonrandomized pilot study2 was conducted that had lasting positive effects on body composition. Armed with this success, the next step was to design and implement an ambitious 12-month randomized controlled trial that compared the effects of Bright Bodies with outcomes of conventional clinical management in 209 children and adolescents. At the end of 12 months, virtually all anthropometric and metabolic parameters,3 including increased insulin sensitivity and improved glucose tolerance,4 favored the Bright Bodies groups over the clinic control group. The results were cited in the most recent Cochrane review and an evidence report prepared for the US Department of Health and Human Services as 1 of the best outcomes of intensive lifestyle interventions in children and adolescents.5,6 However, it also was noted that longer-term follow-up of 2 years or more is critical to confirm maintenance of treatment effects and that such data are limited in the research literature on childhood obesity interventions.5,6 Studies by Epstein et al7–9 have included the longest observational study to date (10-year follow-up) of a behavioral intervention but were excluded by the Cochrane6 and US Department of Health and Human Services5 reports because they lacked comparison control groups. Consequently, in this article we will present the analysis and comparison of 24-month clinical and metabolic outcomes of the participants in the 2 groups in our randomized clinical trial that completed the 12 month extension phase of that study.
METHODS
Participants
Eligibility criteria, recruitment, and enrollment of participants in the randomized clinical trial were reported in detail elsewhere.3 Subjects were recruited from the Yale Pediatric Obesity Clinic by clinicians who were coinvestigators. Major inclusion criteria included English-speaking, 8- to 16-year-old children with a BMI ≥ 95th percentile.10 Exclusion criteria included serious medical conditions that would preclude participation in the program, use of medications that may cause significant weight gain/loss, or involvement in a coexisting weight management program. Participants randomly assigned to the control group were offered participation in the weight management program after 12 months, but only 1 family elected to do so. The rest were encouraged to continue with standard clinic appointments every 6 months and return for a final 24-month outcome measurement at clinic. The study was approved by the Yale Human Investigation Committee, and written informed assent and consent were obtained from participants and parents.
Study Design
The study was designed to be a 12-month parallel-group, randomized controlled trial with a 12-month extension phase. Recruitment occurred between May 2002 and September. 2004; 12-month follow-up ended in September 2005, and 24-month follow-up ended in September 2006. Participants were randomly assigned 2:1 by using a permuted block design to the weight management or clinic control group by the same coinvestigators who recruited the subjects. Random assignments were generated by computer and concealed by the study statistician (Dr Dziura). Initially, a second randomization (1:1) occurred within the weight management group to investigate types of diet intervention children received (ie, structured meal plan versus better food choices). However, randomization to the structured meal plan group was discontinued because of an 83% dropout rate at 6 months. After completion of the initial 12 months of the study, the weight management group participants were referred back to the Yale Pediatric Obesity Clinic for 6-month clinic appointments and received no additional intensive lifestyle intervention during the extension phase. The control group continued to attend 6-month clinic appointments for the last year.
Study Groups
Bright Bodies Weight Management Group
During the intervention phase of the program, participants randomly assigned to the Bright Bodies group attended the program at a nearby school twice a week for 6 months and then every other week for an additional 6 months. This setting was chosen with respect to the limited transportation options of the socioeconomically diverse families. The program consisted of exercise twice (50 minutes each) and nutrition/behavior modification once (40 minutes each) per week, described in detail previously.3 Parents did not participate in the exercise component. Parents attended classes of nutrition-related topics, but did not attend behavior-modification-related topics with their child (they alternately attended their own parent class). Nutrition and behavior modification topics were based on the Smart Moves Workbook, a curriculum designed for overweight and obese children and written by 1 of the authors (Ms Savoye). Sample topics in the behavior modification component included “Ready, Set, Goal!,” “Risky Business: Identifying High-risk Situations,” “Mirror, Mirror on the Wall,” “Bullies, Teasers, and Other Annoying People,” and “Oops I Slipped: Understanding a Relapse.” Techniques included self-awareness, goal-setting, stimulus control, coping-skills training, and cognitive behavior strategies. Behavior modification classes were facilitated by the registered dietitian or social worker. Parent classes included topics that reflected the challenges parents verbalized. These classes emphasized the importance of the parents' role in modeling healthy behavior change.
The nutrition education component of the program used a nondiet approach that emphasized low-fat, nutrient-dense foods of moderate portions. Topics included “Determining Portion Sizes,” “Better Food Choices: A NonDiet Approach,” “Making Sense of a Food Label,” and “Bag It! Pros to Bringing Lunch to School.” A favorite nutrition topic for parents and children alike was “Recipes Dear to the Heart,” which includes a recipe for collard greens that has been trimmed of calories and fat. The topic also involves sharing traditional family recipes and working together as a team to modify it to be healthier. This topic is an example of how the program was tailored to inner-city, ethnically diverse populations. In addition, the dietitian was bilingual (Spanish) for parents who needed additional explanation of nutrition concepts.
The exercise component of the program was facilitated by exercise physiologists. Each class consisted of a warm-up, high-intensity aerobic exercise, and cool down. The high-intensity exercise consisted of a variety of children games, obstacle courses, basketball, flag football, sprinting games, and sport drills. The objective of the high intensity exercise was to sustain 65% to 80% of the age-adjusted maximal heart rate for the duration of the exercise. Participants were also encouraged to exercise 3 additional days at home per week and to decrease sedentary behaviors. The minimum activity that each participant completed was 100 minutes per week (2 50-minute sessions) for the first 6 months and 100 minutes twice per month for the last 6 months.
After completing 1 year of the program, participants were encouraged to stay active and apply the knowledge gained during the program when making food choices (nondiet approach) throughout the next year. They were referred back to the Yale Pediatric Obesity Clinic for follow-up where end of study assessments were made at 24 months.
Control Group
The control group participants were followed in the Yale Pediatric Obesity Clinic every 6 months and received general diet and exercise counseling (∼30 minutes) by dietitians and physicians along with brief psychosocial counseling by a social worker (∼30 minutes). At 24 months, the participants were asked to return for end-of-study assessments.
Outcomes
Outcome measures for 24 months were obtained at the Yale Pediatric Obesity Clinic with the same scale and instruments that were used in all subjects in both groups for the 24 months of the study. Outcome measures included weight (kg), height (cm), BMI (kg/m2), percent body fat (%), total body fat (kg), blood pressure (mm Hg), lipid profile (total, high-density lipoprotein and low-density lipoprotein cholesterol, and triglycerides in mg/dL), fasting plasma glucose (mg/dL), fasting insulin (μIU/mL), and homeostasis model assessment of insulin resistance (HOMA-IR).
Weight was measured (participants in socks, no shoes, wearing light gown) to the nearest 0.1 kg using a medical weight scale (CN20, Detecto; Webb City, MO). Height was measured with a stadiometer (Harpenden; Cambridge, MD), calibrated in 0.1-cm intervals. BMI was calculated as weight in kg divided by height in meters squared. Percent body fat was obtained by a body fat analyzer (TBF 300, Tanita Corporation, Arlington Heights, IL). Total body fat was calculated by multiplying percent body fat by actual weight in kg. Blood pressure was measured automatically with a sphygmomanometer (01-752, American Diagnostics; Hauppauge, NY) 3 times after participants sat still for 5 minutes; second and third measurements were averaged for outcome.
Blood samples were obtained after a 10-hour overnight fast. Plasma glucose levels were measured with a chemistry analyzer (YSI 2700 STAT Analyzer, Yellow Springs Instruments; Yellow Springs, OH), and plasma insulin levels were measured by radiommunoassay (Linco Laboratories, St Charles, MO). Plasma lipid levels were measured with an autoanalyzer (747-200, Roche-Hitachi; Indianapolis, IN). HOMA-IR was calculated from fasting plasma glucose and insulin as follows: fasting plasma insulin (in μIU/mL) + fasting plasma glucose (in mmol/L)/22.5.11
Sample Size and Statistical Analysis
Group comparisons were made on the basis of the intent-to-treat principle, whereby all subjects were analyzed in the group to which they were randomly assigned. Baseline characteristics were compared by using t tests for continuous variables and χ2 tests for nominal variables. Mixed model repeated measures analysis incorporating all available data was used to examine treatment differences in outcomes over time. In these models we assume missing data are missing at random, ie, missing values may depend on observed but not unobserved data.12 The models included adjustment for baseline outcome, treatment assignment, time (6, 12, and 24 months), and a treatment by time interaction as fixed effects. The correlation of repeated measures was modeled using an unstructured covariance pattern whereby the correlation between alternative time-lags was allowed to vary. Log transformations were used for positively skewed variables. Mean changes from baseline and 95% confidence intervals derived from the mixed models are presented.
Before analysis, missing data patterns were evaluated using simple graphical and tabular methods. The pattern of missing data was the result of dropout where those missing an outcome at a time point had missing outcomes at all subsequent times. However, the missing levels of the outcomes were not related to observed previous levels of the outcomes or any baseline covariates for both treatment groups. For instance, those who dropped out had similar BMI profiles compared with those who did not drop out (data not shown). In addition, Pattern Mixture models, including dropout as a covariate, were used to examine the potential influence of dropout on outcome measures.13 No effect by dropout was found on estimates of treatment, time, and treatment by time interaction, which means that the estimates have little dependency on a subject's completion of the study. Therefore, results of the pattern mixture models are not shown. All analysis was performed using SAS 9.1 (SAS Institute, Inc, Cary, NC), with statistical significance set at P < .05 using 2-sided tests.
RESULTS
Participants
As seen in Table 1, treatment groups were similar with regard to baseline characteristics. Dropouts were similar to completers with the exception of baseline diastolic blood pressure (Table 2). Of the 75 weight management group participants reported on at 12 months, 45 were available for outcome measures at 24 months (60% retention rate). Of the 44 control group participants reported on at 12 months, 31 were available for outcome measures at 24 months (70% retention rate).
TABLE 1.
Baseline Characteristics of Children Randomly Assigned to Weight Management and Control Group
| All Randomly Assigned Participants |
Completers at 24 Mo |
|||||
|---|---|---|---|---|---|---|
| Weight Management Group (n = 105) | Control Group (n = 69) | P | Weight Management Group (n = 45) | Control Group (n = 31) | P | |
| Race/ethnic group, n (%) | ||||||
| Non-Hispanic White | 40 (38.1) | 24 (34.8) | .89 | 16 (35.6) | 14 (45.2) | .42 |
| Non-Hispanic Black | 40 (38.1) | 27 (39.1) | 16 (35.6) | 12 (38.7) | ||
| Hispanic | 25 (23.8) | 18 (26.1) | 13 (28.9) | 5 (16.1) | ||
| Gender, n (%) | ||||||
| Female | 58 (55.2) | 47 (68.1) | .09 | 23 (51.1) | 22 (71.0) | .08 |
| Male | 47 (44.8) | 22 (31.9) | 22 (48.9) | 9 (29.0) | ||
| Age, y | 12.0 (2.5) | 12.5 (2.3) | .19 | 12.0 (2.2) | 12.2 (2.3) | .71 |
| Weight, kg | 87.0 (25.1) | 91.2 (23.3) | .27 | 87.6 (20.8) | 90.4 (23.1) | .58 |
| Height, cm | 155.0 (11.6) | 157.7 (11.6) | .15 | 156.1 (10.0) | 157.2 (11.5) | .67 |
| BMI | 35.7 (7.5) | 36.2 (6.2) | .68 | 35.6 (6.4) | 36.1 (5.3) | .75 |
| BMI z score | 2.47 (0.34) | 2.48 (0.27) | .80 | 2.47 (0.34) | 2.49 (0.26) | .72 |
| Body fat, % | 47.0 (8.7) | 45.8 (7.2) | .37 | 46.9 (7.8) | 46.2 (7.1) | .72 |
| Body fat mass, kg | 42.1 (18.1) | 42.4 (14.9) | .91 | 41.5 (13.4) | 42.6 (16.3) | .76 |
| Blood pressure, mm Hg | ||||||
| Systolic | 123 (13.6) | 122 (14.0) | .74 | 123 (15.4) | 122 (15.4) | .70 |
| Diastolic | 66 (9.5) | 67 (11.1) | .66 | 64 (9.3) | 62 (9.7) | .47 |
| Cholesterol, mg/dL | ||||||
| Total | 167 (34.5) | 158 (33.5) | .07 | 167 (34.6) | 165 (34.9) | .80 |
| HDL | 44 (10.8) | 43 (16.5) | .78 | 42 (10.0) | 43 (21.8) | .81 |
| LDL | 98 (33.4) | 92 (28.0) | .16 | 98 (30.8) | 96 (29.2) | .72 |
| Triglyceridesa | 104 (1.8) | 101 (1.6) | .79 | 112 (1.8) | 112 (1.7) | .99 |
| Fasting glucose | 92 (8.3) | 89 (8.5) | .09 | 92 (6.9) | 90 (7.4) | .17 |
| Fasting insulina | 23 (1.8) | 24 (1.7) | .48 | 23.5 (1.62) | 24.3 (1.55) | .76 |
| HOMA-IRa | 5.07 (1.87) | 5.23 (1.7) | .74 | 5.34 (1.64) | 5.30 (1.61) | .95 |
Data are presented as mean (SD) unless otherwise indicated. HDL indicates high-density lipoprotein; LDL, low-density lipoprotein.
Data are presented as geometric means with geometric SDs.
TABLE 2.
Baseline Characteristics of Children Who Dropped Out at Any Time Point and Nondropouts at All Time Points (Completed 24-Mo. Assessment)
| Nondropouts (n = 76) | Dropouts (n = 98) | P | |
|---|---|---|---|
| Race/ethnic group, n (%) | |||
| Non-Hispanic White | 30 (39.5) | 34 (34.7) | .81 |
| Non-Hispanic Black | 28 (36.8) | 39 (39.8) | |
| Hispanic | 18 (23.7) | 25 (25.5) | |
| Gender, n (%) | |||
| Female | 45 (59.2) | 60 (61.2) | .79 |
| Male | 31 (40.8) | 38 (38.8) | |
| Age, y | 12.1 (2.3) | 12.2 (2.6) | .73 |
| Weight, kg | 88.7 (21.7) | 88.6 (26.5) | .97 |
| Height, cm | 156.6 (10.6) | 155.7 (12.4) | .63 |
| BMI | 35.8 (6.0) | 36.0 (7.8) | .84 |
| BMI z score | 2.48 (0.31) | 2.47 (0.32) | .91 |
| Body fat, % | 46.6 (7.5) | 46.5 (8.6) | .91 |
| Body fat mass, kg | 42.0 (14.5) | 42.4 (18.6) | .87 |
| Blood pressure, mm Hg | |||
| Systolic | 123 (15.3) | 122 (12.3) | .92 |
| Diastolic | 63 (9.4) | 69 (10.1) | .0004 |
| Cholesterol, mg/dL | |||
| Total | 166 (34.5) | 162 (34.3) | .44 |
| HDL | 43 (15.8) | 44 (11.1) | .58 |
| LDL | 97 (30.0) | 95 (32.7) | .60 |
| Triglyceridesa | 112 (1.7) | 96 (1.8) | .08 |
| Fasting glucose | 91 (7.2) | 90 (9.3) | .47 |
| Fasting insulina | 24 (1.6) | 22 (1.9) | .47 |
| HOMA-IRa | 5.33 (1.62) | 4.99 (1.94) | .45 |
Data are presented as mean (SD) unless otherwise indicated. HDL indicates high-density lipoprotein; LDL, low-density lipoprotein.
Data are presented as geometric means with geometric SDs.
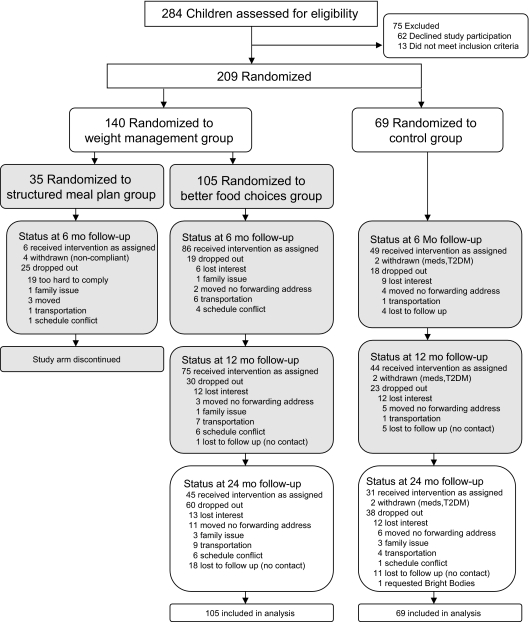
As shown in the consort diagram in Fig 1, the most common reasons for dropping out were similar in both groups (moved with no forwarding address and not interested). The 1 control group participant who decided to receive the intervention at 12 months was considered a “drop out” from the control group. The category “lost to follow-up” includes those who could not be contacted because their telephone was out of service or the caregivers did not respond to telephone calls/letters. The percentage of participants lost to follow-up was similar in both groups.
FIGURE 1.
Flowchart for enrollment, randomization, and follow-up of study participants.
Effects of Treatment
Anthropometric
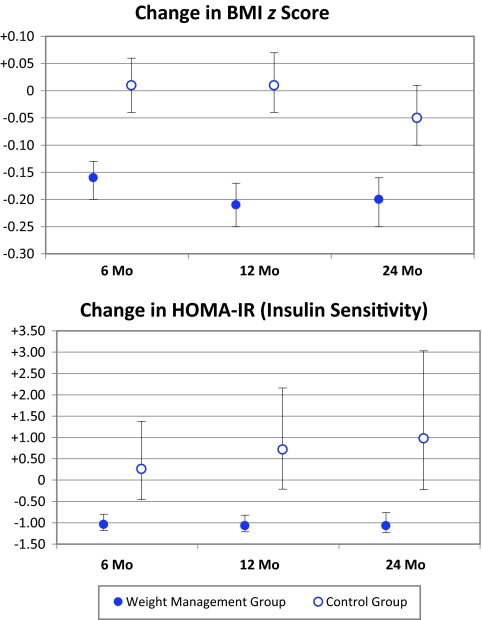
Changes from baseline in BMI, BMI z score, and body fat are shown in Table 3. The treatment effect between the weight management group and control group for BMI z score (see Fig2) and BMI was sustained at 24 months (P < .0001). Likewise, both body fat percent (P < .0001) and total body fat (P < .0001) also resulted in a sustained treatment effect favoring the weight management group.
TABLE 3.
Changes From Baseline in Body Composition, Cardiovascular, and Insulin Sensitivity Parameters for Weight Management and Control Groups at 6, 12, and 24 Mo
| Outcome | Month | Weight Management Group, Mean (95% CI) | Control Group, Mean (95% CI) | Treatment Effect (Intervention-Control), Mean (95% CI) | Between-Group P |
|---|---|---|---|---|---|
| Weight, kg | 6 | −2.4 (−4.0 to −0.8) | 4.6 (2.5 to 6.8) | −7.0 (−9.8 to −4.3) | <.0001a |
| 12 | 0.3 (−1.4 to 2.0) | 8.3 (6.1 to 10.6) | −8.0 (−10.9 to −5.2) | <.0001a | |
| 24 | 5.9 (4.0 to 7.9) | 12.0 (9.5 to 14.6) | −6.1 (−9.3 to −2.9) | .0002a | |
| Height, cm | 6 | 2.5 (1.8 to 3.2) | 2.0 (1.1 to 3.0) | 0.4 (−0.7 to 1.6) | .45 |
| 12 | 4.5 (3.8 to 5.2) | 3.5 (2.5 to 4.4) | 1.0 (−0.2 to 2.2) | .10 | |
| 24 | 7.7 (6.8 to 8.6) | 6.4 (5.2 to 7.5) | 1.3 (−0.1 to 2.8) | .07 | |
| BMI | 6 | −2.1 (−2.7 to −1.5) | 0.9 (0.1 to 1.7) | −3.0 (−4.0 to −2.0) | <.0001a |
| 12 | −1.8 (−2.4 to −1.1) | 1.9 (1.1 to 2.8) | −3.7 (−4.8 to −2.6) | <.0001a | |
| 24 | −0.9 (−1.7 to −0.1) | 1.9 (0.9 to 2.9) | −2.8 (−4.0 to −1.6) | <.0001a | |
| BMI z score | 6 | −0.16 (−0.20 to −0.13) | 0.01 (−0.04 to 0.06) | −0.18 (−0.24 to −0.12) | <.0001a |
| 12 | −0.21 (−0.25 to −0.17) | 0.01 (−0.04 to 0.07) | −0.23 (−0.29 to −0.16) | <.0001a | |
| 24 | −0.20 (−0.25 to −0.16) | −0.05 (−0.10 to 0.01) | −0.16 (−0.23 to −0.09) | <.0001a | |
| Body fat, % | 6 | −2.7 (−3.9 to −1.6) | 2.1 (0.6 to 3.6) | −4.9 (−6.7 to −3.0) | <.0001a |
| 12 | −3.9 (−5.1 to −2.8) | 2.1 (0.6 to 3.6) | −6.0 (−7.9 to −4.1) | <.0001a | |
| 24 | −3.6 (−4.9 to −2.3) | 0.6 (−1.2 to 2.3) | −4.2 (−6.4 to −2.0) | .0002a | |
| Body fat mass, kg | 6 | −3.6 (−5.3 to −1.9) | 4.3 (2.1 to 6.6) | −7.9 (−10.8 to −5.1) | <.0001a |
| 12 | −3.7 (−5.4 to −1.9) | 5.7 (3.4 to 8.1) | −9.4 (−12.3 to −6.5) | <.0001a | |
| 24 | −0.6 (−2.6 to 1.4) | 5.2 (2.6 to 7.8) | −5.8 (−9.1 to −2.6) | .0005a | |
| Blood pressure, mm Hg systolic | 6 | −1.5 (−3.7 to 0.7) | 0.4 (−2.6 to 3.3) | −1.9 (−5.6 to 1.8) | .31 |
| 12 | −1.3 (−3.7 to 1.1) | −0.3 (−3.4 to 2.8) | −1.0 (−4.9 to 2.9) | .62 | |
| 24 | −2.5 (−5.5 to 0.5) | −1.9 (−5.8 to 1.9) | −0.6 (−5.5 to 4.3) | .81 | |
| Blood pressure, mm Hg diastolic | 6 | −2.2 (−4.3 to −0.2) | 1.4 (−1.3 to 4.1) | −3.6 (−7.0 to −0.3) | .04a |
| 12 | 1.3 (−0.9 to 3.5) | 2.3 (−0.5 to 5.2) | −1.1 (−4.6 to 2.5) | .56 | |
| 24 | 0.8 (−1.9 to 3.5) | 2.8 (−0.7 to 6.3) | −2.0 (−6.4 to 2.4) | .38 | |
| Cholesterol, mg/dL total | 6 | −4.2 (−8.5 to 0.1) | 4.6 (−1.2 to 10.3) | −8.8 (−15.9 to −1.6) | .02a |
| 12 | −4.7 (−9.2 to −0.1) | 6.5 (0.5 to 12.4) | −11.2 (−18.7 to −3.6) | .004a | |
| 24 | −8.0 (−13.5 to −2.4) | 5.0 (−1.8 to 11.8) | −13.0 (−21.7 to −4.2) | .004a | |
| Cholesterol, mg/dL HDL | 6 | 1.5 (−0.4 to 3.4) | −0.7 (−3.2 to 1.9) | 2.2 (−1.0 to 5.4) | .18 |
| 12 | 2.0 (0.1 to 4.0) | 0.3 (−2.3 to 2.9) | 1.8 (−1.5 to 5.0) | .29 | |
| 24 | 3.7 (1.5 to 5.9) | 1.3 (−1.6 to 4.1) | 2.5 (−1.1 to 6.1) | .18 | |
| Cholesterol, mg/dL LDL | 6 | −0.7 (−4.5 to 3.2) | 5.3 (0.2 to 10.4) | −6.0 (−12.3 to 0.4) | .07 |
| 12 | 0.3 (−3.8 to 4.3) | 5.8 (0.5 to 11.1) | −5.5 (−12.2 to 1.2) | .11 | |
| 24 | −4.4 (−9.5 to 0.6) | 5.9 (−0.2 to 12.1) | −10.4 (−18.3 to −2.4) | .01a | |
| Triglycerides, mg/dLb | 6 | −12.5 (−17.2 to −6.3) | −0.7 (−10.3 to 12.9) | −11.8 (−20.8 to −0.5) | .04a |
| 12 | −14.5 (−18.9 to −8.6) | −4.2 (−13.1 to 8.7) | −10.4 (−19.5 to 0.6) | .06 | |
| 24 | −17.0 (−21.2 to −10.6) | −6.6 (−15.7 to 7.4) | −10.4 (−20.2 to 1.3) | .07 | |
| Fasting glucose, mg/dL | 6 | −1.9 (−3.4 to −0.3) | −0.9 (−2.9 to 1.1) | −0.9 (−3.5 to 1.6) | .46 |
| 12 | −2.4 (−4.1 to −0.8) | −1.1 (−3.2 to 1.1) | −1.4 (−4.1 to 1.3) | .32 | |
| 24 | −1.7 (−3.9 to 0.4) | −1.5 (−4.1 to 1.2) | −0.3 (−3.7 to 3.1) | .87 | |
| Fasting insulin, μIU/mLb | 6 | −4.5 (−5.2 to −3.5) | 1.3 (−1.9 to 6.1) | −5.8 (−7.9 to −4.5) | .0001a |
| 12 | −4.6 (−5.3 to −3.4) | 3.4 (−0.7 to 9.6) | −8.0 (−10.0 to −6.9) | <.0001a | |
| 24 | −4.7 (−5.5 to −3.2) | 4.5 (−0.8 to 13.2) | −9.2 (−11.0 to −7.5) | .0001a | |
| HOMA-IRb | 6 | −1.04 (−1.18 to −0.80) | 0.26 (−0.46 to 1.38) | −1.29 (−1.78 to −1.02) | .0001a |
| 12 | −1.07 (−1.21 to −0.82) | 0.72 (−0.21 to 2.16) | −1.78 (−2.24 to −1.60) | <.0001a | |
| 24 | −1.07 (−1.23 to −0.76) | 0.98 (−0.22 to 3.03) | −2.05 (−2.48 to −1.75) | .0001a |
Data are presented as mean (SD) unless otherwise indicated. HDL indicates high-density lipoprotein; LDL, low-density lipoprotein.
Statistically significant.
Data are presented as geometric means with geometric SDs.
FIGURE 2.
Changes from 6, 12, and 24 months are from baseline. At 6 months (weight management group, n = 86; control group, n = 49) P < .0001 for BMI z score, and P = .0001 for HOMA-IR; at 12 months (weight management, n = 75; control group, n = 44) P < .0001 for BMI z score and HOMA-IR; and at 24 months (weight management group, n = 45; control group, n = 31) P < .0001 for BMI z score, and P = .0001 for HOMA-IR.
Metabolic
As with the 6- and 12-month data, there was no statistical significance in treatment effect for systolic and diastolic blood pressures at 24 months (P = .81 and P = .38, respectively). Treatment effect for total cholesterol (P = .004) and low-density-lipoprotein cholesterol (P = .01) were both significant, whereas high-density lipoprotein was not (P = .18). Although fasting triglycerides and glucose also were not significantly different, fasting insulin was lowered, and HOMA-IR (see Fig 2) markedly improved with the Bright Bodies intervention (P = .0001).
DISCUSSION
The results of this study reveal that the beneficial effects of the Bright Bodies weight management program on body weight, body composition, plasma lipids and insulin, and insulin sensitivity were sustained in comparison to the clinic control group for 24 months, although the lifestyle intervention phase of the study ended after 12 months. Although the differences in outcomes between the 2 treatment groups were greater at 12 than at 24 months, the 24-month results indicate that education, behavior modification, and exercise training had a long-lasting effect on the food choices and physical activity in these ethnically diverse, primarily low-income, obese children. These results are particularly noteworthy because no randomized control trials in pediatrics have demonstrated long-term maintenance of positive outcomes beyond the first 6 to 12 months in disadvantaged, inner-city populations.
Although the attrition rate was high at 24 months, the dropout rates were similar in both treatment groups, according to baseline characteristics. Likewise, follow-up outcomes were similar among those who dropped out compared with those who completed the 24-month follow-up. In addition, in analyzing the 24-month data, statistical measures were employed to evaluate the effect of attrition, including comparing results in completers-only with results from methods that are more robust to missing data (ie, mixed models and pattern-mixture models). Conclusions were unaltered by these various approaches.
Of the 54 lifestyle modification trials included in the most recent Cochrane review,6 only 16 studies reported 24-month data, only 4 studies14–17 had baseline BMIs as high as our subjects, only 1 study targeted non-white populations,17 and all had high dropout rates. Moreover, in the 1 study that targeted black youth,17 the treatment effect was lost by 24 months.18 One aim of future studies is to determine whether the program would be equally effective if the subjects met only once versus twice per week. This reduced program “dose” might ease the burden on the families and perhaps lower the overall drop-out rate for these interventions. Although annual program satisfaction questionnaires indicated that twice-weekly sessions were not a burden, we should not ignore that 6% of the overall intervention group dropouts were because of schedule conflicts.
In addition to reduction of adiposity, other major clinical benefits of this program were the continuous improvements of HOMA-IR and fasting insulin because severe insulin resistance is so common in prediabetic obese youth.19 Indeed, during the initial 12-month randomized phase of this study, oral glucose tolerance tests were performed in a subgroup of 13 Bright Bodies and 10 control subjects. After 12 months of the Bright Bodies program, the 3 subjects with prediabetes reverted to normal glucose tolerance, whereas 4 subjects in the control groups progressed from normal to impaired glucose tolerance.4 In response to these promising results, a larger study in which oral glucose tolerance test outcomes in prediabetic, obese children randomly assigned to Bright Bodies or clinic control group interventions are compared is currently under way. Additional longitudinal investigation of the clinical significance of reductions in adiposity and HOMA-IR reveals that there is a relationship to these 2 biological markers. In fact, Morrison et al20 found HOMA-IR and insulin levels at 9 to 10 years old to predict increased BMI and impaired fasting glucose and type 2 diabetes mellitus, respectively, 10 years later. In addition, the Bogalusa Heart Study has recently confirmed that adiposity and HOMA-IR are interrelated.21
Programs that include increased activity and behavior modification have traditionally resulted in more improvements in childhood obesity.6 During the development of the program, the major goal was to provide the child and parent with tools to empower them to make better decisions that would promote a healthier lifestyle. Leaving the families “to their own devices” between 12 and 24 months with the result of a sustained treatment effect highly suggests that the child and parent adopted healthier behaviors. However, in an attempt to avoid characteristics of dieting, we deliberately avoided requesting food diaries, which resulted in a lack of measurement in diet change. Similarly, we lack measurement of activity change because we did not collect activity records when requesting an additional 3 days of activity.
The lack of psychosocial measures should also be included as a limitation of this study. Lastly, a study of this nature would not be complete without mention of the cost-effectiveness and cost/benefit of the weight management program verses clinical treatment approach. Such an analysis has been completed on the 12-month data (Nowicka P, Price G, Vipin S, Shaw M, Mercado J, Tamborlane WV, Savoye M, unpublished data, 2010).
CONCLUSION
The Bright Bodies intensive lifestyle modification program has demonstrated sustained treatment effects up to 24 months when compared with a standard clinical care. Although the attrition rate was high, this study is unique and, more importantly, unprecedented in that we targeted ethnically diverse children with very high BMIs. This gives us hope that behavior change is possible, even in the most challenging populations. Although global focus is shifting to the prevention of childhood obesity, it is equally urgent to pursue treatment models that are effective for those who need immediate treatment.
ACKNOWLEDGMENTS
This work was supported by a Clinical Translation Science Award to Dr Sherwin (UL1-RR024139) from the National Center for Research Resources, a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research; a NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant to Dr Sherwin (P30-DK-45735); NIH grants to Dr Caprio (R01-HD28016 and R01-HD40787), Yale University School of Medicine; an unrestricted gift from the McPhee Foundation (Bristol, CT) awarded to Ms Savoye; the Tegger Foundation for funds granted to Dr Nowicka; and the Fulbright Commission for funds granted to Ms O'Malley.
We thank the families who participated in this study (all clinic and Bright Bodies participants received no compensation for their involvement) and the clinical staff of the Yale Pediatric Obesity Clinic.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00409422).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- HOMA-IR
- homeostasis model assessment of insulin resistance
REFERENCES
- 1. Summerbell CD, Ashton V, Campbell KJ, Edmunds L, Kelly S, Waters E. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2003;(3):CD001872. [DOI] [PubMed] [Google Scholar]
- 2. Savoye M, Berry D, Dziura J, et al. Anthropometric and psychosocial changes in obese adolescents enrolled in a Weight Management Program. J Am Diet Assoc. 2005;105(3):364–370 [DOI] [PubMed] [Google Scholar]
- 3. Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297(24):2697–2704 [DOI] [PubMed] [Google Scholar]
- 4. Shaw M, Savoye M, Cali A, Dziura J, Tamborlane WV, Caprio S. Effect of a successful intensive lifestyle program on insulin sensitivity and glucose tolerance in obese youth. Diabetes Care. 2009;32(1):45–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitlock EP, O'Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125(2). Available at: www.pediatrics.org/cgi/content/full/125/2/e396 [DOI] [PubMed] [Google Scholar]
- 6. Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1):CD001872. [DOI] [PubMed] [Google Scholar]
- 7. Epstein LH, McCurley J, Wing. RR, Valoski A. Five-year follow-up of family-based behavioral treatments for childhood obesity. J Consult Clin Psychol. 1990;58(5):661–664 [DOI] [PubMed] [Google Scholar]
- 8. Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990;264(19):2519–2523 [PubMed] [Google Scholar]
- 9. Epstein LH, Valoski AM, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373–383 [DOI] [PubMed] [Google Scholar]
- 10. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27 [PubMed] [Google Scholar]
- 11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419 [DOI] [PubMed] [Google Scholar]
- 12. Allison PD. Missing Data. Thousand Oaks, CA: Sage; 2001 [Google Scholar]
- 13. Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78 [Google Scholar]
- 14. Braet C, Tanghe A, Decaluwe V, Moens E, Rosseel Y. Inpatient treatment for children with obesity: weight loss, psychological well-being, and eating behavior. J Pediatr Psychol. 2004;29(7):519–529 [DOI] [PubMed] [Google Scholar]
- 15. Rolland-Cachera MF, Thibault H, Souberbielle JC, et al. Massive obesity in adolescents: dietary interventions and behaviours associated with weight regain at 2 y follow-up. Int J Obes Relat Metab Disord. 2004;28(4):514–519 [DOI] [PubMed] [Google Scholar]
- 16. Weyhreter H, Tannhauser S, Muche R, et al. Evaluation of an outpatient treatment program for obese children and adolescents [in German]. Klin Padiatr. 2003;215(2):57–64 [DOI] [PubMed] [Google Scholar]
- 17. Williamson DA, Martin PD, White MA, et al. Efficacy of an internet-based behavioral weight loss program for overweight adolescent African-American girls. Eat Weight Disord. 2005;10(3):193–203 [DOI] [PubMed] [Google Scholar]
- 18. Williamson DA, Walden HM, White MA, et al. Two-year internet-based randomized controlled trial for weight loss in African-American girls. Obesity (Silver Spring). 2006;14(7):1231–1243 [DOI] [PubMed] [Google Scholar]
- 19. Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362(9388):951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrison JA, Glueck CJ, Horn PS, et al. Pre-teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18–19 y: a 10-y prospective study of black and white girls. Am J Clin Nutr. 2008;88(3):778–788 [DOI] [PubMed] [Google Scholar]
- 21. Nguyen QM, Srinivasan SR, Xu JH, et al. Utility of childhood glucose homeostasis variables in predicting adult diabetes and related cardiometabolic risk factors: the Bogalusa Heart Study. Diabetes Care. 2010;33(3):670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]