Abstract
OBJECTIVE:
Some prenatal factors may program an offspring's blood pressure, but existing evidence is inconclusive and mechanisms remain unclear. We examined the mediating roles of intrauterine and childhood growth in the associations between childhood systolic blood pressure (SBP) and 5 potentially modifiable prenatal factors: maternal smoking during pregnancy; prepregnancy BMI; pregnancy weight gain; chronic hypertension; and preeclampsia-eclampsia.
METHODS:
The sample contained 30 461 mother-child pairs in the Collaborative Perinatal Project. Prenatal data were extracted from obstetric forms, and children's SBP was measured at 7 years of age. Potential mediation by intrauterine growth restriction (IUGR) and childhood growth was examined by the causal step method.
RESULTS:
Heavy maternal smoking during pregnancy was significantly associated with higher offspring SBP (adjusted mean difference versus nonsmoking: 0.73 mm Hg [95% confidence interval (CI): 0.32–1.14]), which attenuated to null (0.13 [95% CI: −0.27–0.54]) after adjustment for changes in BMI from birth to 7 years of age. Prepregnancy overweight-obesity was significantly associated with higher offspring SBP (versus normal weight: 0.89 mm Hg [95% CI: 0.52–1.26]), which also attenuated to null (−0.04 mm Hg [95% CI: −0.40–0.31]) after adjustment for childhood BMI trajectory. Adjustment for BMI trajectory augmented the association between maternal pregnancy weight gain and offspring SBP. Adjustment for childhood weight trajectory similarly changed these associations. However, all these associations were independent of IUGR.
CONCLUSIONS:
Childhood BMI and weight trajectory, but not IUGR, may largely mediate the associations of maternal smoking during pregnancy and prepregnancy BMI with an offspring's SBP.
Keywords: blood pressure, pregnancy, fetal development, infant, small for gestational age, child development, obesity
WHAT'S KNOWN ON THIS SUBJECT:
Inconsistent evidence reveals that some prenatal factors are associated with high blood pressure in later life. The biological mechanisms of these associations are unknown, which is a critical barrier for causal interpretation as well as safe and effective intervention.
WHAT THIS STUDY ADDS:
Maternal heavy smoking during pregnancy, prepregnancy overweight-obesity, chronic hypertension, and preeclampsia-eclampsia are associated with higher offspring systolic blood pressure, which is independent of intrauterine growth restriction. Childhood BMI or weight trajectory may mediate the associations of heavy maternal smoking and prepregnancy BMI with systolic blood pressure.
Ample evidence reveals an inverse association between birth size and blood pressure in children and adults,1 which provides a new perspective for the etiology and prevention of hypertension.2 However, birth size may just be a marker of other genetic and environmental factors that are the true etiologic factors for hypertension.3,4 Therefore, we need to move beyond birth size to examine prenatal exposures that may themselves program offspring blood pressure. In the present analysis, we focused on maternal smoking during pregnancy, prepregnancy BMI, pregnancy weight gain, chronic hypertension, and preeclampsia-eclampsia. These 5 prenatal factors were chosen because (1) they contribute to the majority of low birth weight or intrauterine growth restriction (IUGR) in developed countries,5 and (2) they can potentially be modified or treated by current interventions.3
Some studies have examined the associations between 1 or some of these prenatal factors and offspring blood pressure. But evidence is quite inconsistent6–19 except for maternal chronic hypertension. More importantly, the biological mechanisms through which these prenatal factors are associated with offspring blood pressure remain unclear. Poor knowledge of these mechanisms is a critical barrier for causal interpretation as well as safe and effective intervention. We hypothesized that IUGR and childhood growth might play important roles in these mechanisms. Given the associations between prenatal factors and birth size,5 as well as between birth size and offspring blood pressure,1 the effects of prenatal factors on offspring blood pressure may be partially explained by IUGR. On the other hand, maternal smoking during pregnancy,20 prepregnancy obesity,21 and excessive pregnancy weight gain17,18 are linked to an offspring's risk of overweight and obesity that are well established causal factors for high blood pressure.22 So, childhood growth may also explain some effects of prenatal factors on offspring blood pressure. However, no studies have systematically examined the mediating roles of IUGR and childhood growth in the associations between these prenatal factors and offspring blood pressure.
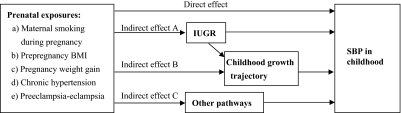
Therefore, we had 2 aims in the current analysis: to (1) simultaneously examine the associations of 5 potentially modifiable prenatal factors with childhood systolic blood pressure (SBP) at 7 years of age; and (2) examine whether any of the observed associations can be mediated by IUGR and/or childhood growth trajectory. Fig 1 shows the theoretical framework for this analysis.
FIGURE 1.
Theoretical framework for the mechanisms in the associations of 5 modifiable prenatal factors on childhood SBP.
METHODS
Data and Sample
We used data from the Collaborative Perinatal Project (CPP), a cohort study conducted at 12 US academic sites.23 Pregnant women were enrolled at prenatal care visits from 1959 to 1965, and ∼58 000 live-born infants were followed and periodically assessed for health status until 8 years of age.
This analysis included CPP singletons with IUGR or normal intrauterine growth. IUGR was defined as small for gestational age (SGA): birth weight < 10th percentile for gestational age within the site-specific CPP cohort. Normal intrauterine growth was defined as appropriate for gestational age (AGA): 10th percentile ≤ birth weight ≤ 90th percentile for gestational age. Considering the possible “U”-shaped association between birth size and blood pressure, as well as unavailability of important maternal determinants (eg, gestational diabetes) for large birth size, we excluded children born large for gestational age. The final sample consisted of 30 461 children for whom there was complete data on 7-year SBP, the 5 prenatal factors of interest, and potential confounders (see below).
Measures of Exposure
Maternal Smoking During Pregnancy
Pregnant women reported their current smoking status at each prenatal care visit. On the basis of the average number of smoked cigarettes per day over pregnancy, we classified maternal smoking status during pregnancy as never smoking, moderate smoking (1–19 cigarettes per day), and heavy smoking (≥20 cigarettes per day). The self-reported smoking during pregnancy was highly concordant with cotinine concentration in both maternal serum (κ = 0.83)24 and cord blood (κ = 0.81) (Melissa A. Clark, PhD, Xiaozhong Wen, MD, PhD, Laura R. Stroud, PhD, unpublished Data, 2009).
Prepregnancy BMI
At registration, pregnant women reported their weight and height before pregnancy. Prepregnancy BMI was calculated as weight (kg)/height (m)2. According to the Institute of Medicine guideline,25 prepregnancy BMI was categorized as underweight (BMI < 19.8), normal weight (19.8 ≤ BMI ≤ 26.0), overweight (26 < BMI ≤ 29.0), and obese (BMI > 29).
Pregnancy Weight Gain
Total pregnancy weight gain was calculated as the difference between prepregnancy weight and measured weight before delivery. According to the Institute of Medicine guideline,25 we classified pregnancy weight gain by prepregnancy weight status: (1) low weight gain (<28, <25, and < 15 lb for underweight, normal weight, and overweight-obese women, respectively); (2) normal weight gain (between 28 and 40, 25–35, and 15–25 lb, respectively); and (3) excessive weight gain (>40, >35, and >25 lb, respectively).
Chronic Hypertension
Chronic hypertension diagnosis was obtained from CPP obstetric forms. Obstetricians diagnosed chronic hypertension on the basis of pregnant women's self-reports and documented evidence (hospital or physician's records of preexisting hypertension before pregnancy, or blood pressure > 140 mm Hg systolic or > 90 mm Hg diastolic before the 24th week of pregnancy, or persistence of elevated blood pressures for at least 6 weeks postpartum).
Preeclampsia-Eclampsia
Diagnosis of preeclampsia and eclampsia also was obtained from CPP obstetric forms. Obstetricians diagnosed these conditions according to the American Committee on Maternal Welfare classification of toxemia.26
Measures of Outcome
At the 7-year follow-up, children's blood pressure was measured by physicians or nurses with a manual sphygmomanometer. Blood pressure was obtained from the right arm with the child at rest in a recumbent position. In this analysis, we focused only on SBP because (1) measuring children's diastolic blood pressure with a manual sphygmomanometer was inaccurate because of difficulty in hearing muffling of the fourth Korotkoff sound,27 and (2) tracking of SBP from childhood to adulthood was much stronger than that of diastolic blood pressure.28
Measures of Mediators
Birth weight was measured immediately after delivery, and birth length was measured within 24 hours after birth. Children's weight and height/length were measured at follow-ups by nurses according to standard protocols.29 In this analysis, we only included growth measures at birth, 1 year, and 7 years of age. The changes in growth measures from birth to 1 year of age and from 1 year to 7 years of age were calculated.
Measures of Confounders
Potential confounders included maternal age at pregnancy, maternal race (white, black, and other), family socioeconomic status, marital status (married versus unmarried) and parity (primiparity versus multiparity), and the child's gender (male versus female) and gestational age. Family socioeconomic status percentile was based on a composite index adapted from the US Census Bureau that averaged percentiles of education and occupation of the household's head, as well as family income.30 Gestational age was defined as the interval between last menstrual period and delivery date.
Statistical Analysis
Multivariable logistic regression was used to estimate adjusted odds ratios for binary outcomes (SGA). Multivariable linear regression was used to estimate adjusted β (mean difference) for continuous outcomes (SBP and growth measures). Regression models were fitted with generalized estimating equations to control for the correlation between multiple siblings, by specifying exchangeable covariance matrix among siblings. We did primary analysis among the total sample and secondary analysis for males and females separately.
On the basis of the causal step method,31 the mediation criteria for this analysis included: (1) the exposure should be significantly associated with the outcome; (2) the exposure is significantly associated with the mediator; (3) the mediator is significantly associated with the outcome after the exposure being controlled; (4) adjustment for the mediator leads to a substantial reduction (eg, 10% or greater) in the estimated coefficient of the exposure; and (5) complete mediation exists if the mediator-adjusted coefficient of the exposure approaches 0.
Accordingly, we examined the mediating roles of IUGR and childhood growth trajectory in associations between prenatal factors and childhood SBP in 4 steps (refer to Fig 1 and Tables 4 and 5). Step I was to examine the overall associations between prenatal factors and childhood SBP. Step II was to examine the associations between prenatal factors and the risk of SGA and childhood growth measures. Step III was to examine the mediation of SGA in the associations between prenatal factors and SBP. Specifically, we fitted 2 models with the same outcome variable (SBP): the basic model (model 1) included prenatal factors and potential confounders; and model 2 included the variables in model 1 and SGA. Step IV was to examine the mediation of childhood growth measures in the associations between prenatal factors and SBP. Model 1 was the same as in step III but within a smaller sample because of missing data on childhood growth measures. Models 2, 3, and 4 included the variables in model 1 and each 1 of 3 variables (at birth, change from birth to 1 year, or change from 1 to 7 years) for childhood growth. Model 5 included both the change variables in models 3 and 4 (from birth to 1 year and 1–7 years) for childhood growth. Model 6 included the childhood growth variables in models 2, 3, and 4 (at birth, from birth to 1 year, and 1–7 years) simultaneously. To distinguish roles of weight, height, and BMI, we fitted 3 separate sets of models in step IV for them.
TABLE 4.
The Mediating Role of SGA in the Associations Between Prenatal Factors and SBP in Children at 7 Years of Age (N = 30 461)
| SBP |
Model 1 |
Model 2 |
||
|---|---|---|---|---|
| Mean | SD | Mean Difference (95% CI)a | Mean Difference (95% CI)a | |
| Maternal smoking during pregnancy | ||||
| Never smoking | 101.5 | 10.2 | Reference | Reference |
| Moderate smoking | 101.7 | 10.1 | 0.26 (0.01–0.50)b | 0.27 (0.03–0.52)b |
| Heavy smoking | 102.7 | 10.2 | 0.72 (0.33–1.11)b | 0.76 (0.37–1.15)b |
| Prepregnancy BMI | ||||
| Underweight (<19.8) | 101.0 | 10.0 | −0.84 (−1.12 to −0.57)b | −0.83 (−1.11 to −0.56)b |
| Normal weight (19.8–26.0) | 101.8 | 10.1 | Reference | Reference |
| Overweight−obesity (>26.0) | 102.7 | 10.5 | 0.91 (0.56–1.26)b | 0.90 (0.55–1.26)b |
| Pregnancy weight gain | ||||
| Low weight gain | 101.8 | 10.2 | 0.22 (−0.05 to 0.48) | 0.24 (−0.03 to 0.50) |
| Normal weight gain | 101.7 | 10.1 | Reference | Reference |
| Excessive weight gain | 101.6 | 10.2 | −0.33 (−0.78 to 0.11) | −0.34 (−0.78 to 0.11) |
| Chronic hypertension | ||||
| No | 101.7 | 10.1 | Reference | Reference |
| Yes | 103.3 | 10.3 | 1.17 (0.53–1.80)b | 1.18 (0.54–1.81)b |
| Preeclampsia-eclampsia | ||||
| No | 101.7 | 10.1 | Reference | Reference |
| Yes | 102.1 | 10.4 | 0.43 (0.11–0.74)b | 0.44 (0.13–0.76)b |
| SGA (vs AGA) | −0.39 (−0.78 to −0.01)b | |||
Adjusted for maternal characteristics (age at pregnancy, race, marital status, family socioeconomic status percentile, and parity) and gestational age and gender of the child.
P < .05.
TABLE 5.
The Mediating Role of Childhood BMI Trajectory in the Associations Between Prenatal Factors and SBP in Children at 7 Years of Age (N = 27 625)
| Independent Variable | Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
|---|---|---|---|---|---|---|
| Mean Difference (95% CI)a | Mean Difference (95% CI)a | Mean Difference (95% CI)a | Mean Difference (95% CI)a | Mean Difference (95% CI)a | Mean Difference (95% CI)a | |
| Maternal smoking during pregnancy | ||||||
| Never smoking | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate smoking | 0.21 (−0.05 to 0.47) | 0.23 (−0.03 to 0.49) | 0.12 (−0.14 to 0.38) | 0.12 (−0.13 to 0.38) | −0.14 (−0.39 to 0.11) | −0.12 (−0.37 to 0.13) |
| Heavy smoking | 0.73 (0.32–1.14)b | 0.78 (0.37–1.20)b | 0.55 (0.13–0.96)b | 0.64 (0.23–1.04)b | 0.13 (−0.27 to 0.54) | 0.28 (−0.12 to 0.68) |
| Prepregnancy BMI | ||||||
| Underweight (<19.8) | −0.85 (−1.14 to −0.56)b | −0.82 (−1.11 to −0.53)b | −0.81 (−1.10 to −0.52)b | −0.56 (−0.85 to −0.27)b | −0.36 (−0.64 to −0.07)b | 0.02 (−0.27 to 0.30) |
| Normal weight (19.8–26.0) | Reference | Reference | Reference | Reference | Reference | Reference |
| Overweight-obesity (>26.0) | 0.89 (0.52–1.26)b | 0.88 (0.51–1.25)b | 0.86 (0.49–1.23)b | 0.48 (0.12–0.85)b | 0.24 (−0.12 to 0.60) | −0.04 (−0.40 to 0.31) |
| Pregnancy weight gain | ||||||
| Low weight gain | 0.18 (−0.10 to 0.46) | 0.22 (−0.06 to 0.50) | 0.14 (−0.14 to 0.41) | 0.18 (−0.09 to 0.46) | 0.08 (−0.19 to 0.35) | 0.40 (0.13–0.67)b |
| Normal weight gain | Reference | Reference | Reference | Reference | Reference | Reference |
| Excessive weight gain | −0.34 (−0.81 to 0.13) | −0.35 (−0.83 to 0.12) | −0.32 (−0.79 to 0.15) | −0.40 (−0.87 to 0.06) | −0.39 (−0.85 to 0.06) | −0.52 (−0.97 to −0.07)b |
| Chronic hypertension | 1.22 (0.55–1.88)b | 1.22 (0.55–1.89)b | 1.26 (0.59–1.93)b | 1.09 (0.43–1.74)b | 1.14 (0.49–1.80)b | 1.21 (0.57–1.85)b |
| Preeclampsia-eclampsia | 0.45 (0.12–0.78)b | 0.48 (0.15–0.81)b | 0.43 (0.11–0.76)b | 0.39 (0.06–0.71)b | 0.32 (0.00–0.64)b | 0.50 (0.19–0.82)b |
| BMI at birth | 0.16 (0.06–0.25)b | 1.39 (1.27–1.51)b | ||||
| Change in BMI from birth to 1 y | 0.34 (0.28–0.41)b | 0.87 (0.80–0.94)b | 1.44 (1.35–1.52)b | |||
| Change in BMI from 1 to 7 y of age | 0.87 (0.80–0.93)b | 1.21 (1.14–1.28)b | 1.46 (1.39–1.54)b |
Adjusted for maternal characteristics (age at pregnancy, race, marital status, family socioeconomic status percentile and parity) and gestational age and gender of the child.
P < .05.
RESULTS
Subject Characteristics
Characteristics of 30 461 mother-child pairs in the analytic sample are shown in Table 1. Among mothers, 41.5% were moderate and 11.9% heavy smokers, 24.4% were underweight before pregnancy, 63.7% had low and 8.9% had excessive pregnancy weight gain, 3.6% had chronic hypertension, and 17.6% had preeclampsia-eclampsia. Half children were boys, 10.3% were SGA, and 89.7% were AGA.
TABLE 1.
Characteristics of 30 461 Pairs of Mothers and Children
| N | % | Mean (SD) | |
|---|---|---|---|
| Maternal characteristic | |||
| Age at pregnancy, y | 24.1 (6.1) | ||
| Race | |||
| White | 13 676 | 44.9 | |
| Black | 15 588 | 51.2 | |
| Others | 1 197 | 3.9 | |
| Marital status | |||
| Unmarried | 7 111 | 23.3 | |
| Married | 23 350 | 76.7 | |
| Family SES percentile | 47 (22) | ||
| Parity | |||
| Primiparity | 8 757 | 28.7 | |
| Multiparity | 21 704 | 71.3 | |
| Smoking during pregnancy | |||
| Never smoking | 14 182 | 46.6 | |
| Moderate smoking | 12 646 | 41.5 | |
| Heavy smoking | 3 633 | 11.9 | |
| Prepregnancy BMI | 22.6 (4.1) | ||
| Underweight (<19.8) | 7 430 | 24.4 | |
| Normal weight (19.8–26.0) | 18 172 | 59.7 | |
| Overweight-obesity (>26.0) | 4 859 | 16.0 | |
| Pregnancy weight gain | |||
| Low weight gain | 19 413 | 63.7 | |
| Normal weight gain | 8 323 | 27.3 | |
| Excessive weight gain | 2 725 | 8.9 | |
| Mean (SD), lb | 21.0 (10.3) | ||
| Chronic hypertension | 1 095 | 3.6 | |
| Preeclampsia-eclampsia | 5 359 | 17.6 | |
| Child characteristic | |||
| Gender | |||
| Male | 15 031 | 49.3 | |
| Female | 15 430 | 50.7 | |
| Gestation, wk | 39.5 (2.8) | ||
| <37 | 3 724 | 12.2 | |
| 37–42 | 23 983 | 78.7 | |
| >42 | 2 754 | 9.0 | |
| Intrauterine growth status | |||
| AGA | 27 316 | 89.7 | |
| SGA | 3 145 | 10.3 | |
| Body size at birth | |||
| Weight, g | 3 072.2 (461.3) | ||
| Lengtha, cm | 49.6 (2.6) | ||
| BMIa, kg/m2 | 12.5 (1.3) | ||
| Body size at 1 y of agea | |||
| Weight, kg | 9.7 (1.2) | ||
| Length, cm | 74.2 (3.4) | ||
| BMI, kg/m2 | 17.6 (1.7) | ||
| Body size at 7 y of agea | |||
| Weight, kg | 23.6 (4.2) | ||
| Height, cm | 121.5 (5.9) | ||
| BMI, kg/m2 | 15.9 (2.0) | ||
| SBP at 7 y, mm Hg | 101.7 (10.2) |
SES indicates socioeconomic status.
The sample size was 27 625 because of missing data on growth measures.
Associations Between Prenatal Factors and Intrauterine and Childhood Growth
Maternal smoking during pregnancy, chronic hypertension, and preeclampsia-eclampsia were significantly associated with higher risk of SGA and small birth size (Table 2). Generally, children's BMI increased from birth to 1 year of age, and then decreased from 1 year to 7 years of age. Both prepregnancy BMI and pregnancy weight gain were positively associated with birth size. Maternal smoking during pregnancy was associated with a greater increase in BMI from birth to 1 year of age, and a smaller decrease (decrease trend among all children with non-, moderate, or heavy maternal smoking) from 1 to 7 years of age (Table 3). In contrast, maternal prepregnancy underweight was associated with smaller increase in BMI from birth to 1 year of age, and greater decrease in BMI from 1 to 7 years of age. Low pregnancy weight gain was associated with greater increase in BMI from birth to 1 year only. Details on childhood weight and height/length growth also are shown in Table 3.
TABLE 2.
Associations Between Prenatal Factors and Intrauterine Growth Restriction and Birth Size
| Prenatal Factors | Small for Gestational Age (N = 30 461) |
Birth Weight, kg (N = 27 625) |
Birth Length, cm (N = 27 625) |
Birth BMI (kg/m2) (N = 27 625) |
|
|---|---|---|---|---|---|
| % | OR (95% CI)a | Mean Difference (95% CI)a | Mean Difference (95% CI)a | Mean Difference (95% CI)a | |
| Maternal smoking during pregnancy | |||||
| Never smoking | 7.4 | Reference | Reference | Reference | Reference |
| Moderate smoking | 11.7 | 1.72 (1.58–1.87)b | −0.09 (−0.10–−0.08)b | −0.49 (−0.55–−0.44)b | −0.14 (−0.17–−0.11)b |
| Heavy smoking | 16.7 | 2.84 (2.53–3.20)b | −0.18 (−0.20–−0.16)b | −0.86 (−0.96–−0.77)b | −0.34 (−0.39–−0.29)b |
| Prepregnancy BMI | |||||
| Underweight (<19.8) | 13.5 | 1.38 (1.27–1.51)b | −0.07 (−0.08–−0.06)b | −0.26 (−0.33–−0.19)b | −0.17 (−0.20–−0.13)b |
| Normal weight (19.8–26.0) | 9.7 | Reference | Reference | Reference | Reference |
| Overweight−obesity (>26.0) | 7.6 | 0.82 (0.72–0.93)b | 0.04 (0.03–0.05)b | 0.18 (0.10–0.26)b | 0.08 (0.04–0.13)b |
| Pregnancy weight gain | |||||
| Low weight gain | 12.3 | 1.84 (1.67,2.03)b | −0.12 (−0.13–−0.11)b | −0.45 (−0.52–−0.39)b | −0.28 (−0.31–−0.24)b |
| Normal weight gain | 7.0 | Reference | Reference | Reference | Reference |
| Excessive weight gain | 6.1 | 0.87 (0.72–1.04) | 0.04 (0.02–0.06)b | 0.09 (−0.02–0.19) | 0.10 (0.04–0.15)b |
| Chronic hypertension | |||||
| No | 10.2 | Reference | Reference | Reference | Reference |
| Yes | 13.0 | 1.30 (1.07–1.57)b | −0.05 (−0.08–−0.02)b | −0.39 (−0.55–−0.23)b | −0.02 (−0.11–0.07) |
| Preeclampsia-eclampsia | |||||
| No | 9.7 | Reference | Reference | Reference | Reference |
| Yes | 13.1 | 1.50 (1.36–1.66)b | −0.04 (−0.05–−0.02)b | −0.05 (−0.13–0.02) | −0.16 (−0.20–−0.11)b |
OR indicates odds ratio.
Adjusted for maternal characteristics (age at pregnancy, race, marital status, family socioeconomic status percentile, and parity) and gestational age and gender of the child.
P < .05.
TABLE 3.
Associations Between Prenatal Factors and Childhood Growth Trajectory (N = 27 625)
| Prenatal Factors | Weight Growth, kg, Mean Difference (95% CI)a |
Height Growth, cm, Mean Difference (95% CI)a |
Change in BMI (kg/m2), Mean Difference (95% CI)a |
|||
|---|---|---|---|---|---|---|
| From Birth to 1 y | From 1 to 7 y | From Birth to 1 y | From 1 to 7 y | From Birth to 1 y | From 1 to 7 y | |
| Maternal smoking during pregnancy | ||||||
| Never smoking | Reference | Reference | Reference | Reference | Reference | Reference |
| Moderate smoking | 0.07 (0.05–0.10)b | 0.32 (0.22–0.42)b | 0.17 (0.08–0.26)b | 0.22 (0.08–0.35)b | 0.26 (0.21–0.31)b | 0.10 (0.05–0.15)b |
| Heavy smoking | 0.12 (0.08–0.17)b | 0.27 (0.12–0.43)b | 0.23 (0.09–0.37)b | 0.02 (−0.19–0.22) | 0.53 (0.45–0.61)b | 0.11 (0.03–0.19)b |
| Prepregnancy BMI | ||||||
| Underweight (<19.8) | −0.12 (−0.15–−0.09)b | −0.90 (−1.00–−0.81)b | 0.11 (0.01–0.21)b | −0.38 (−0.52–−0.23)b | −0.10 (−0.16–−0.04)b | −0.33 (−0.39–−0.28)b |
| Normal weight (19.8–26.0) | Reference | Reference | Reference | Reference | Reference | Reference |
| Overweight-obesity (>26.0) | 0.04 (0.00–0.08)b | 1.02 (0.87–1.18)b | −0.23 (−0.35–−0.11)b | 0.35 (0.17–0.54)b | 0.09 (0.02–0.16)b | 0.47 (0.38–0.55)b |
| Pregnancy weight gain | ||||||
| Low weight gain | −0.05 (−0.08–−0.02)b | −0.32 (−0.43–−0.22)b | 0.12 (0.03–0.21)b | −0.30 (−0.45,−0.16)b | 0.12 (0.07–0.18)b | −0.01 (−0.06–0.05) |
| Normal weight gain | Reference | Reference | Reference | Reference | Reference | Reference |
| Excessive weight gain | 0.00 (−0.05–0.06) | 0.29 (0.10–0.48)b | −0.04 (−0.20–0.12) | 0.24 (0.00–0.48)b | −0.04 (−0.13–0.05) | 0.07 (−0.03–0.17) |
| Chronic hypertension | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | −0.05 (−0.12–0.03) | 0.01 (−0.24–0.26) | 0.30 (0.08–0.51)b | −0.09 (−0.39–0.21) | −0.12 (−0.26–0.01) | 0.15 (0.01–0.28)b |
| Preeclampsia-eclampsia | ||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.00 (−0.04–0.03) | −0.01 (−0.13–0.11) | 0.11 (0.01–0.22)b | −0.07 (−0.23–0.10) | 0.05 (−0.02–0.11) | 0.08 (0.01–0.14)b |
Adjusted for maternal characteristics (age at pregnancy, race, marital status, family socioeconomic status percentile, and parity) and gestational age and gender of the child.
P < .05.
Associations Between Prenatal Factors and SBP
We only reported results for the total sample because secondary analysis by the child's gender (data not shown) did not yield substantial differences in the associations. Children with maternal heavy smoking during pregnancy had higher SBP than those without maternal smoking (adjusted mean difference or β: 0.72 mm Hg [95% confidence interval [CI]: 0.33–1.11]) (model 1 in Table 4). Compared with children of mothers with normal prepregnancy weight, those born to underweight mothers before pregnancy had lower SBP (β: −0.84 mm Hg [95% CI: −1.12 to −0.57]), whereas those of overweight-obese mothers had higher SBP (β: 0.91 mm Hg [95% CI: 0.56–1.26]). Low or excessive pregnancy weight gain was not significantly associated with childhood SBP. Both maternal chronic hypertension (β versus normotension: 1.17 mm Hg [95% CI: 0.53–1.80]) and preeclampsia-eclampsia (β versus absence: 0.43 mm Hg [95% CI: 0.11–0.74]) were significantly associated with higher childhood SBP.
The Potential Mediating Roles of SGA and Childhood Growth Trajectory
SGA was significantly associated with lower SBP (β versus AGA: −0.39 mm Hg [95% CI: −0.78 to −0.01]) (model 2 in Table 4). However, adjustment for SGA did not lead to any substantial changes (<10%) in the associations between 5 prenatal factors and SBP (model 2 vs 1 in Table 4).
Changes in BMI from birth to 1 year and from 1 year to 7 years of age were significantly associated with higher SBP (models 3 and 4 in Table 5). Adjustment for change in BMI from birth to 1 year of age substantially (>10%) reduced the β for maternal heavy smoking (model 3 vs 1 in Table 5). Adjustment for change in BMI from 1 year to 7 years substantially reduced β for both maternal heavy smoking and maternal prepregnancy BMI (model 4 vs 1). Simultaneous adjustment of these 2 changes in model 5 attenuated the β for maternal heavy smoking to null (β: 0.13 [95% CI: −0.27–0.54]). Adjustment for the whole BMI trajectory (at birth and 2 changes) in model 6 further attenuated the β for maternal prepregnancy weight to null (β for underweight versus normal weight: 0.02 [95% CI: −0.27–0.30]) (β for overweight-obesity: −0.04 [95% CI: −0.40–0.31]). Adjustment for the whole BMI trajectory augmented β for maternal pregnancy weight gain versus normal weight gain (β for low weight gain: 0.40 [95% CI: 0.13–0.67]) (β for excessive weight gain: −0.52 [95% CI: −0.97 to −0.07]). Adjustment for BMI trajectory did not change β for chronic hypertension or preeclampsia-eclampsia. Adjustment for weight growth trajectory (data not shown) led to similar changes in β for prenatal factors as adjustment for BMI trajectory. However, adjustment for height/length growth only slightly changed β for prepregnancy weight and pregnancy weight gain (data not shown).
DISCUSSION
Within a national perinatal cohort, we examined whether IUGR and childhood growth trajectory could mediate the associations between 5 potentially modifiable prenatal factors and childhood SBP. Results demonstrated that maternal heavy smoking during pregnancy, prepregnancy overweight, chronic hypertension, and preeclampsia-eclampsia were significantly associated with higher 7-year SBP, independent of IUGR. The associations of heavy maternal smoking during pregnancy and prepregnancy BMI with offspring SBP were attenuated to null after adjustment for childhood weight or BMI trajectory.
Consistent with the literature,5 we found that all 5 prenatal factors of interest were significantly associated with risk of IUGR (measured with SGA). SGA was significantly associated with lower 7-year SBP. However, different from our hypothesis, SGA or birth size could not explain the associations between these prenatal factors and childhood SBP.
Our observed association between heavy maternal smoking during pregnancy and SBP was quite similar to the literature.3 Our novel contribution is that maternal smoking during pregnancy is significantly associated with offspring SBP mainly through childhood weight or BMI trajectory, instead of through intrauterine growth. A previous study also found that the association between maternal smoking during pregnancy and high offspring SBP was independent of birth weight.6 Therefore, intrauterine exposure to maternal smoking may program some intrinsic mechanisms that control postnatal growth, and thus promote weight gain and high BMI in childhood,20 and in turn increase SBP.
We found that prepregnancy overweight-obesity was significantly associated with higher offspring SBP, whereas prepregnancy underweight was associated with lower offspring SBP. These associations diminished to null after adjusting for childhood weight or BMI trajectory, which suggested the possibility of complete mediation. This mediation means that children with overweight or obese mother are more likely to be heavier themselves, and thus have higher blood pressure. Possible mechanisms can be both genetic (eg, energy metabolism and fatness storage) and environmental factors (eg, family diet and physical activity habit).
Different from a recent study,18 our analysis demonstrated that the positive association between pregnancy weight gain and childhood BMI did not automatically lead to positive association between pregnancy weight gain and offspring SBP. Instead, pregnancy weight gain was negatively associated with offspring SBP after adjusting for childhood BMI or weight trajectory. Two distinct mechanisms are possibly involved in this paradox: (1) pregnancy weight gain is positively associated with offspring weight or BMI and in turn positively associated with offspring SBP; and (2) pregnancy weight gain is negatively associated with offspring SBP directly or through other pathways, such as disturbing the normal development of fetal organs related to blood pressure control.32–35
Both maternal chronic hypertension and preeclampsia-eclampsia were significantly associated with high childhood SBP. Our new findings suggested that neither intrauterine nor childhood growth could explain these associations. Other mechanisms must be involved, such as genetic transmission36 and placental-fetal vascular impairment.
Strengths
This is the first analysis to examine the potential mediation of both intrauterine and childhood growth in the associations between 5 potentially modifiable prenatal factors and offspring SBP. Repeated growth measures facilitate the identification for critical growth periods (fetal, infancy, and early childhood) that impact offspring SBP. Adjustment for maternal and family factors can remove substantial confounding. The large national sample of CPP strengthens generalizability of our findings to children born with small or normal body size (large for gestational age was excluded in this analysis).
Limitations
Despite the standard protocol, the single measure of SBP could introduce measurement errors. Self-reported maternal smoking was subject to misclassification, but it was actually highly concordant with serum continine concentration, partially because that smoking during pregnancy was popular and acceptable in the United States in 1960s. We could not distinguish the impacts of different components and rate of maternal pregnancy weight gain on offspring blood pressure. We had no information on pregnancy diet and children's own lifestyle (eg, feeding, diet, salt intake, and physical activities). Despite clear temporality (prenatal exposures affected IUGR/childhood growth, which affected childhood blood pressure), the observed associations can be interpreted as causal pathways only under the assumption of no residual confounding, which is almost impossible for observational studies like ours. Finally, analyzed data were collected approximately 5 decades ago. Since then, the prevalence of maternal smoking has decreased,37 whereas prepregnancy overweight-obesity and excessive pregnancy weight gain38 have increased substantially. However, biological effects of these maternal factors should not change with time substantively. So, new findings from this historical project are still informative for current clinical practice and public health.
CONCLUSION
Our new findings can advance current poor knowledge on mechanisms for fetal programming of SBP. It is not IUGR, but rather childhood weight and BMI trajectory, which may mediate effects of some prenatal factors (eg, maternal smoking during pregnancy and prepregnancy overweight-obesity) on an offspring's SBP. The literature on fetal programming may have overemphasized the role of birth size per se. Evidence from this analysis indicates that, instead of limiting our focus on birth size, monitoring and normalizing childhood weight and BMI trajectory may be more important for long-term cardiovascular health. Parents' and pediatricians' effects of promoting healthy diet and physical activity among children may reduce or even eliminate the elevated risk of hypertension associated with specific prenatal exposures.
ACKNOWLEDGMENTS
This analysis was supported by National Institutes of Health Transdisciplinary Tobacco Use Research Center Award P50 CA084719 by the National Cancer Institute, the National Institute on Drug Abuse, and the Robert Wood Johnson Foundation.
Dr Shenassa was supported by Flight Attendants Medical Research Institute and by grant R40MC03600-01-00 from the Maternal and Child Health Bureau, US Department of Health and Human Services.
We thank Dr Michelle L. Rogers for assistance in data preparation.
Dr Wen contributed to the study hypotheses, planned and conducted the data analysis, and drafted the manuscript; Dr Triche contributed to development of the introduction and discussion sections, result interpretation, revision and proofreading of the manuscript; Dr Hogan supervised the process of data analysis and also contributed to result interpretation and revision of the manuscript; Dr Shenassa contributed to development of the introduction and discussion sections, result interpretation, as well as revision of the manuscript; and Dr Buka was the principle investigator, provided the data, and contributed to the study hypotheses, the data analysis plan, result interpretation, and revision of the manuscript, and also advised Dr Wen.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- IUGR
- intrauterine growth restriction
- SBP
- systolic blood pressure
- CPP
- Collaborative Perinatal Project
- SGA
- small for gestational age
- AGA
- appropriate for gestational age
- CI
- confidence interval
REFERENCES
- 1. Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–831 [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2(12):700–707 [DOI] [PubMed] [Google Scholar]
- 3. Brion MJ, Leary SD, Lawlor DA, et al. Modifiable maternal exposures and offspring blood pressure: a review of epidemiological studies of maternal age, diet, and smoking. Pediatr Res. 2008;63(6):593–598 [DOI] [PubMed] [Google Scholar]
- 4. Gillman MW. Epidemiological challenges in studying the fetal origins of adult disease. Paediatr Perinat Epidemiol. 2005;19(suppl 1):1. [DOI] [PubMed] [Google Scholar]
- 5. Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80(4):502–511 [PubMed] [Google Scholar]
- 6. Blake KV, Gurrin LC, Evans SF, et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev. 2000;57(2):137–147 [DOI] [PubMed] [Google Scholar]
- 7. Morley R, Leeson Payne C, Lister G, et al. Maternal smoking and blood pressure in 7.5- to 8-year-old offspring. Arch Dis Child. 1995;72(2):120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams S, Poulton R. Twins and maternal smoking: ordeals for the fetal origins hypothesis? A cohort study. BMJ. 1999;318(7188):897–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawlor DA, Najman JM, Sterne J, et al. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University study of pregnancy and its outcomes. Circulation. 2004;110(16):2417–2423 [DOI] [PubMed] [Google Scholar]
- 10. Brion MJ, Leary SD, Smith GD, et al. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49(6):1422–1428 [DOI] [PubMed] [Google Scholar]
- 11. Law CM, Barker DJ, Bull AR, et al. Maternal and fetal influences on blood pressure. Arch Dis Child. 1991;66(11):1291–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oken E, Huh SY, Taveras EM, et al. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13(11):2021–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergel E, Haelterman E, Belizan J, et al. Perinatal factors associated with blood pressure during childhood. Am J Epidemiol. 2000;151(6):594–601 [DOI] [PubMed] [Google Scholar]
- 14. Whincup PH, Cook DG, Papacosta O. Do maternal and intrauterine factors influence blood pressure in childhood? Arch Dis Child. 1992;67(12):1423–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Godfrey KM, Forrester T, Barker DJ, et al. Maternal nutritional status in pregnancy and blood pressure in childhood. Br J Obstet Gynaecol. 1994;101(5):398–403 [DOI] [PubMed] [Google Scholar]
- 16. Margetts BM, Rowland MG, Foord FA, et al. The relation of maternal weight to the blood pressures of Gambian children. Int J Epidemiol. 1991;20(4):938–943 [DOI] [PubMed] [Google Scholar]
- 17. Oken E, Taveras EM, Kleinman KP, et al. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mamun AA, O'Callaghan M, Callaway L, et al. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119(13):1720–1727 [DOI] [PubMed] [Google Scholar]
- 19. Phillips DI, Bennett FI, Wilks R, et al. Maternal body composition, offspring blood pressure and the hypothalamic-pituitary-adrenal axis. Paediatr Perinat Epidemiol. 2005;19(4):294–302 [DOI] [PubMed] [Google Scholar]
- 20. von Kries R, Toschke AM, Koletzko B, et al. Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156(10):954–961 [DOI] [PubMed] [Google Scholar]
- 21. Li C, Kaur H, Choi WS, et al. Additive interactions of maternal prepregnancy BMI and breastfeeding on childhood overweight. Obes Res. 2005;13(2):362–371 [DOI] [PubMed] [Google Scholar]
- 22. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252 [DOI] [PubMed] [Google Scholar]
- 23. Niswander KR, Gordon M. The Women and Their Pregnancies: The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke. Washington, DC: US Government Print Office; 1972 [Google Scholar]
- 24. Klebanoff MA, Levine RJ, Clemens JD, et al. Serum cotinine concentration and self-reported smoking during pregnancy. Am J Epidemiol. 1998;148(3):259–262 [DOI] [PubMed] [Google Scholar]
- 25. Institute of Medicine Nutrition During Pregnancy: Report of the Committee on Nutritional Status During Pregnancy and Lactation, Food, and Nutrition Board. Washington, DC: National Academy Press; 1990 [Google Scholar]
- 26. Eastman NJ, Bell ET, Dieckmann WJ, et al. Definition and Classification of Toxemias Brought Up-to-date. Chicago, IL: American Committee on Maternal Welfare; 1952 [Google Scholar]
- 27. Hemachandra AH, Klebanoff MA, Duggan AK, et al. The association between intrauterine growth restriction in the full-term infant and high blood pressure at age 7 years: results from the Collaborative Perinatal Project. Int J Epidemiol. 2006;35(4):871–877 [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falkner F. Office measurement of physical growth. Pediatr Clin North Am. 1961;8:13–18 [DOI] [PubMed] [Google Scholar]
- 30. Myrianthopoulos NC, French KS. An application of the US Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2(3):283–299 [DOI] [PubMed] [Google Scholar]
- 31. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182 [DOI] [PubMed] [Google Scholar]
- 32. Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med. 2008;5(suppl A):S121–S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mañalich R, Reyes L, Herrera M, et al. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58(2):770–773 [DOI] [PubMed] [Google Scholar]
- 34. Martin H, Hu J, Gennser G, et al. Impaired endothelial function and increased carotid stiffness in 9-year-old children with low birthweight. Circulation. 2000;102(22):2739–2744 [DOI] [PubMed] [Google Scholar]
- 35. Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006;572(pt 1):45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palti H, Rothschild E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev. 1989;19(4):263–269 [DOI] [PubMed] [Google Scholar]
- 37. Cnattingius S, Lambe M. Trends in smoking and overweight during pregnancy: prevalence, risks of pregnancy complications, and adverse pregnancy outcomes. Semin Perinatol. 2002;26(4):286–295 [DOI] [PubMed] [Google Scholar]
- 38. Institute of Medicine and National Research Council Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009 [PubMed] [Google Scholar]