Abstract
Background:
Extraction temperature influences the total phenolic content (TPC), total flavonoid content (TFC) of medicinal plant extracts to a great extend. TPC and TFC are the principle activity constituents present in the plant. The effects of extraction temperature on TPC, TFC and free radical-scavenging capacity of Gynura divaricata leaf extracts are worth to study.
Materials and Methods:
Folin–Ciocalteu and aluminum chloride colorimetric assay were used to determine the TPC and TFC of Gynura divaricata leaf extracts at different temperatures. The antioxidant and free radical-scavenging activity were measured by 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid (ABTS) and phosphomolybdenum methods.
Results:
TPC and TFC were significantly elevated with increasing extraction temperature (from 40°C to 100°C). However, TPC and TFC were not significantly different (P > 0.05) at the extraction temperatures 90°C and 100°C. Also, the extracts obtained at a higher temperature exhibited a significant free radical-scavenging activity compared with extraction at lower temperatures (P < 0.05). The TPCs (13.95-36.68 mg gallic acid equivalent/g dry material) were highly correlated with DPPH (R2 = 0.9229), ABTS (R2 = 0.9951) free radical-scavenging capacity, and total antioxidant activity (R2 = 0.9872) evaluated by phosphomolybdenum method.
Conclusion:
The TPC and TFC of G. divaricata leaf was significantly influenced by the extraction temperatures, which were the main antioxidant constituents present in the G. divaricata plant.
Keywords: Antioxidant activity, extraction temperature, Gynura divaricata, total phenolic content, total flavonoid content
INTRODUCTION
Reactive free radicals, including superoxide, hydroxyl radical, and peroxyl radical, generally result in degradation of protein, lipid peroxidation, and oxidation of DNA, which have been considered to be linked with many chronic diseases, such as diabetes, cancers, and atherosclerosis.[1,2] Polyphenols are the major antioxidant constituents isolated from many medicinal and edible plants.[3] The extraction process does affect the yield, and to some extent, affects the stability of polyphenols.[4,5]
Gynura divaricata DC. (Compositae) is a traditional Chinese medicinal plant, commonly used for the treatment of bronchitis, pulmonary tuberculosis, pertussis, sore eye, toothache, rheumatic arthralgia, and diabetes in folk medicine.[6] The ethanol extract of G. divaricata has hypoglycemic activity in animal models.[7,8] It has also been reported that many constituents with antiproliferation activity exist in G. divaricata.[9,10] The chemical constituents of G. divaricata leaves include flavonoids, phenolics, cerebrosides, polysaccharide, alkaloids, terpenoids, and sterols.[9–12] Phenolics and flavonoids are the major antioxidant components of Gynura procumbens leaves. A previous report indicated that the concentration of phenolics in the extract is significantly influenced by the extraction temperature; that is, increasing the extraction temperature will decrease the total phenolic content (TPC).[13] However, no literature data are available on the effect of extraction temperature on the TPC, total flavonoid content (TFC), and free radical-scavenging capacity of G. divaricata leaves. Recently, we used single-factor experiments to optimize the extraction conditions of TPC from G. divaricata. An interesting phenomenon was found where the TPC was significantly elevated with increasing the extraction temperature, which was quite different from the same genus plants described in the literature.[13] The present study aims to evaluate the effect of extraction temperature on TPC, TFC, and free radical-scavenging capacity of G. divaricata leaves.
MATERIALS AND METHODS
Chemicals and reagents
Gallic acid (GA), trolox, quercetin, and 1,1-diphenyl-2-picrylhydrazyl (DPPH), and Folin–Ciocalteu’s phenol reagent were purchased from Sigma Chemical Company (St. Louis, MO, USA) and 2,2-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) was obtained from TCI-SU (Tokyo, Japan). Potassium persulfate, aluminum chloride, sodium acetate, and all solvents used were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Visible spectra measurements were done using UV-2450 spectrophotometer (Shimadzu, Japan).
Plant material
The G. divaricata was obtained in 2009 from Guangdong Province, China. A voucher specimen (201001) was deposited at the Department of Chemistry, Nanchang University. The leaves of G. divaricata were dried at 40°C in a hot air oven and finely powdered.
Extraction
Samples of the powered leaf (1 g) were weighed and transferred into conical flasks. The samples were refluxed and extracted for 30 min with 50 mL of 45% aqueous ethanol at different temperatures (40°C, 60°C, 80°C, 90°C, 100°C). Single-factor experiments demonstrated that the 45% aqueous ethanol and 30 min extraction were the optimal extraction conditions (data not shown). The extracts were centrifuged (10000 g, Beckman, USA) and the supernatant was adjusted to a final volume of 50 mL with 45% aqueous ethanol.
Determination of total phenolic and flavonoid content
The TPC in the extracts was determined by using Folin–Ciocalteu’s phenol reagent and external calibration with GA and the results were expressed as mg GA/g dry material.[14] Briefly, 0.2 mL of extract solution in a test tube and 5 mL of Folin–Ciocalteu’s phenol reagent (diluted 10 times) were added and the contents mixed thoroughly. After 4 min, 4 mL of sodium carbonate (7.5% w/v) was added to the mixture. The mixed solution was then immediately diluted to required volume (25 mL) with deionized distilled water and mixed thoroughly. The tubes were allowed to stand for 90 min before absorbance at 760 nm was measured by using UV-2450 spectrophotometer (Shimadzu, Japan). The TPC were calculated by using GA calibration curve. The calibration equation for GA was Y = 0.07411X + 0.0589 (R2 = 0.9977).
The TFC in the extracts were determined by using the colorimetric assay with slight modifications.[15] Briefly, 0.3 mL solution of extracts in 45% ethanol were separately mixed with 8 mL of 10% aluminum chloride, 4 mL of 0.2 M sodium acetate. The mixed solution was then immediately diluted to volume (25 mL) with deionized distilled water and mixed thoroughly and left at room temperature for 30 min. The absorbance of the reaction mixture was measured at 350 nm by using UV-2450 spectrophotometer (Shimadzu, Japan). TFCs were calculated by using kaempferol calibration curve. The calibration equation for kaempferol was Y = 0.04177X + 0.014181 (R2 = 0.9993). The results were expressed as mg kaempferol/g dry material.
DPPH free radical-scavenging capacity
The free radical-scavenging activity of the extracts was evaluated by 1,1,-diphenyl-2-picryl-hydrazil (DPPH) using the method of Sajjad and Patel[16,17] with slight modifications. Briefly, DPPH solution (0.6 mM) was prepared in ethanol and 0.5 mL of this solution was mixed with 0.5 mL of 10-fold diluted extracts (final concentration 0.20 mg/mL dry material). The volume of the solution was adjusted with ethanol to a final volume of 5 mL. After incubation in a dark place for 30 min at room temperature, the absorbance of the mixture was measured at 515 nm against ethanol as blank using UV-2450 spectrophotometer (Shimadzu, Japan). Trolox (0.15 mg/mL) and Quercetin (0.1 mg/mL) were used as positive controls. The activities of the samples were evaluated by comparison with a control (containing 0.5 mL of DPPH solution and 4.5 mL of ethanol). Each sample was measured in triplicate and averaged. The activity was calculated according to the following formula:
where AC is the absorbance value of the control and AS is the absorbance value of the added test samples solution.
ABTS cation free radical-scavenging activity
For ABTS assay, the procedure followed was the method of Dimitrina and Roberta[18,19] with some modifications. ABTS was dissolved in water to make a concentration of 7 mmol/L. ABTS + was produced by reacting the ABTS stock solution with 2.45 mmol/L potassium persulfate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. For the test of samples, the ABTS+ stock solution was diluted with 80% methanol to an absorbance of 0.70 ± 0.02 at 734 nm. After the addition of 4.85 mL of diluted ABTS + to 0.15 mL of 10-fold diluted samples (final concentration 0.06 mg/mL dry material), the absorbance reading was taken 6 min after the initial mixing. Trolox (0.1 mg/mL) and Quercetin (0.05 mg/mL) were used as positive controls. The activities of the samples were evaluated by comparison with a control (containing 4.85 mL of ABTS solution and 0.15 mL of 45% ethanol). Each sample was measured in triplicate and averaged. This activity is given as percentage ABTS + scavenging that is calculated by the following formula:
where AC is the absorbance value of the control and AS is the absorbance value of the added samples test solution.
Evaluation of total antioxidant activity by phosphomolybdenum method
The total antioxidant capacity of the extracts was evaluated according to the method described by Prieto et al.[20] An aliquot of 0.5 mL of samples solution was combined with 4.5 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). In case of blank, 0.5 mL of 45% ethanol was used in place of sample. The tubes were incubated in a boiling water bath at 95°C for 90 min. After the samples were cooled to room temperature, the absorbance of the aqueous solution of each sample was measured at 695 nm against blank in UV-2450 spectrophotometer (Shimadzu, Japan). The total antioxidant activity was expressed as the absorbance of the sample at 695 nm. The higher absorbance value indicated higher antioxidant activity.[21]
Statistical analysis
Results were given as mean ± standard deviation of 3 replicates. Experimental results were analyzed by SPSS version 16.0 (SPSS Inc. Chicago, IL). Differences between means were determined using one-way ANOVA and Duncan’s test. The level of statistical significance was set at P ≤ 0.05.
RESULTS
The effect of extraction temperature on total phenolic and total flavonoid content
Table 1 shows the effect of different extraction temperatures on TPC and TFC of the extracts obtained. Increasing the extraction temperature increased TPC and TFC. However, at temperatures 90°C and above, the values were not significantly different (P > 0.05) with increasing extraction temperature. Although the maximum amount of TPC and TFC was obtained during extraction at 100°C, the values were not significantly different (P > 0.05) from those obtained at 90°C. For the extraction temperatures studied, temperature below 80°C showed a significantly lower total phenolic and total flavonoid contents compared with extractions at 90°C and 100°C. TPC and TFC of all the extracts at different temperatures studied increased in the order: 100°C > 90°C > 80°C > 60°C > 40°C.
Table 1.
Effect of extraction temperature on total phenolic content and total flavonoid content
| Extraction temperature (°C) | TPC (mg GA/g dry material) | TFC (mg Kaempferol/g dry material) |
|---|---|---|
| 40 | 13.95 ± 0.19a | 19.16 ± 0.11a |
| 60 | 21.28 ± 0.37b | 27.04 ± 0.11b |
| 80 | 27.26 ± 0.7c | 35.76 ± 0.11c |
| 90 | 34.30 ± 0.50d | 44.14 ± 0.22d |
| 100 | 36.68 ± 0.62d | 47.52 ± 0.21d |
TPC: Total phenolic content, TFC: Total flavonoid content, GA: Gallic acid, Values are means of 3 replicates ± SD, different letters within the same column indicate significant difference at P < 0.05 by Duncan’s test
The effect of extraction temperature on free radical-scavenging capacity
The results of free radical-scavenging capacity of the extracts obtained at different temperatures by DPPH and ABTS methods are shown in Table 2. All of the extracts demonstrated inhibitory activity against both the DPPH free radical and the ABTS cation free radical. The order of free radical-scavenging capacity of the extracts was 100°C > 90°C > 80°C > 60°C > 40°C. At 100°C, the extracts showed the highest free radical-scavenging capacity; however, the values were not significantly different from those (P > 0.05) obtained at 90°C and those of standard materials.
Table 2.
Effect of extraction temperature on free radical-scavenging capacity
| Extraction temperature (°C) | DPPH (%) | ABTS (%) |
|---|---|---|
| 40 | 46.10 ± 2.36a | 28.65 ± 2.04a |
| 60 | 67.35 ± 3.17b | 38.74 ± 1.50b |
| 80 | 83.67 ± 2.50c | 50.10 ± 0.30c |
| 90 | 88.69 ± 0.50d | 62.91 ± 1.87d |
| 100 | 89.67 ± 0.06d | 68.27 ± 1.36d |
| Trolox | 91.69 ± 0.28d | 67.75 ± 2.88d |
| Quercetion | 90.19 ± 0.17d | 63.62 ± 1.23d |
DPPH: 1,1-diphenyl-2-picrylhydrazyl, ABTS: 2,2-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid, Values are means of 3 replicates ± SD, different letters within the same column indicate significant difference at P < 0.05 by Duncan’s test
The effect of extraction temperature on total antioxidant activity
The phosphomolybdate method has been used routinely to evaluate the total antioxidant capacity of plant extracts.[20,21] In the presence of extracts, Mo (VI) is reduced to Mo (V) and forms a green colored phosphomolybdenum V complex, which shows a maximum absorbance at 695 nm. Figure 1 shows that the antioxidant capacity of the extracts of G. divaricata leaves at different temperatures can be ranked in the order 100°C > 90°C > 80°C > 60°C > 40°C. Extracts at 100°C showed the highest total antioxidant activity; however, the values were not significantly different from those (P > 0.05) obtained at 90°C. This implies that the extracts at 90°C and 100°C have a strong antioxidant ability (P < 0.05) as compared with the extracts obtained at lower temperatures.
Figure 1.
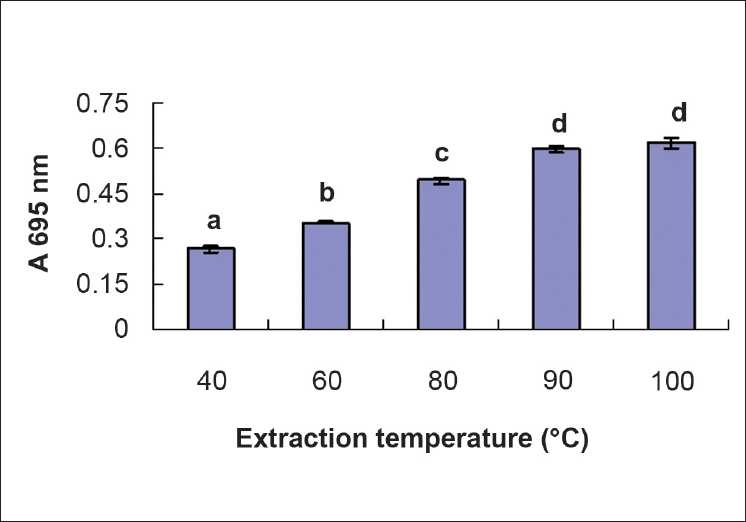
Effect of extraction temperature on total antioxidant activity. Values are means of 3 replicates ± SD; different letters within the same column indicate significant difference at P < 0.05 by Duncan’s test
The correlation between TPC and antioxidant activity
The correlation between TPC and TFC, antioxidant activity, and free radical-scavenging capacity of the extracts obtained at different temperatures is interesting to note. The Pearson correlation analysis [Table 3] revealed that the TPC and TFC showed strong correlations with DPPH radical scavenging capacity, ABTS cation radical scavenging capacity, and total antioxidant activity, with the correlation coefficient (R2) 0.9229, 0.9952, and 0.9872, respectively. This indicates that the antioxidant activity of the extract from G. divaricata leaves is due to its phenolic constituents. These results are in accordance with other reports in the literature, which showed positive strong correlation between antioxidant activities and TPCs.[22] Phytochemical screening showed that kaempferol and its derivatives are the major bioactive components in G. divaricata leaves, and this is in agreement with the study by Chen et al.,[9] and these phytochemicals have been reported to possess high antioxidant and hypoglycemic activity.[23,24]
Table 3.
Correlations between the antioxidant activities and TPC and TFC of extracts of Gynura divaricata leaf
| Antioxidant assays | Correlation R2 |
|
|---|---|---|
| TPC | TFC | |
| DPPH radical scavenging ability | 0.9229 | 0.9145 |
| ABTS radical scavenging ability | 0.9951 | 0.9980 |
| Total antioxidant activity | 0.9872 | 0.9930 |
TPC: Total phenolic content, TFC: Total flavonoid content, DPPH: 1,1-diphenyl-2-picrylhydrazyl, ABTS: 2,2-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid
DISCUSSION
The present study was focused on TPC and TFC, in addition to the antioxidant activity of G. divaricata leaf extracts obtained at different temperatures. The influence of extraction temperature on TPC, TFC, and antioxidant activity of the 45% ethanol extracts was investigated. For all the extraction temperatures surveyed, the highest TPC, TFC, and antioxidant activities were observed in the extracts obtained at 100°C followed by extraction at 90°C. Significant lower levels of TPC and TFC were demonstrated by extractions at temperatures below 80°C. The TPC and TFC of extracts have strong positive correlation with the extraction temperature, with the correlation coefficient (R2) 0.9925 and 0.9928, respectively [Figure 2]. The results of the present study also indicate that there was a significant reduction in the total antioxidant activity and free radical-scavenging capacity of the extracts obtained at temperatures below 80°C compared to those extracted at 90°C or 100°C. This was expected due to the elevation the TPC and TFC of extracts at higher temperatures. Our findings are in contrast to Akowuah et al.’s study[13] on G. procumbens, indicating that TPC and free radical scavenging activities decreased with increasing extraction temperatures. Also our findings implied that extraction temperatures will have different effects on the bioactive components of identical genus plants, although they may share some uniform constituents. It is generally considered that extraction at a high temperature will lead to a degradation of some bioactive compounds, whereas recently some researches have demonstrated that this is not always true, especially with regard to the polyphenols with antioxidant activity. Increasing the extraction temperature has been found to enhance the recovery of phenolic compounds as described in previous reports.[25,26] The mechanism maybe is that increasing extraction temperature promotes solvent extraction by enhancing both diffusion coefficients and the solubility of polyphenol content.[27,28] Also, increasing extraction temperature will contribute to the release of bound polyphenols in plants with the breakdown of cellular constituents of plant cells which leads to increased cell membrane permeability. Moreover, release of these bound polyphenols could further reduce the chances of those polyphenols coagulating with lipoprotein. Thereby enhancing solubility of the polyphenols and inhibiting coagulation with lipoprotein will increase polyphenols yield.[29]
Figure 2.
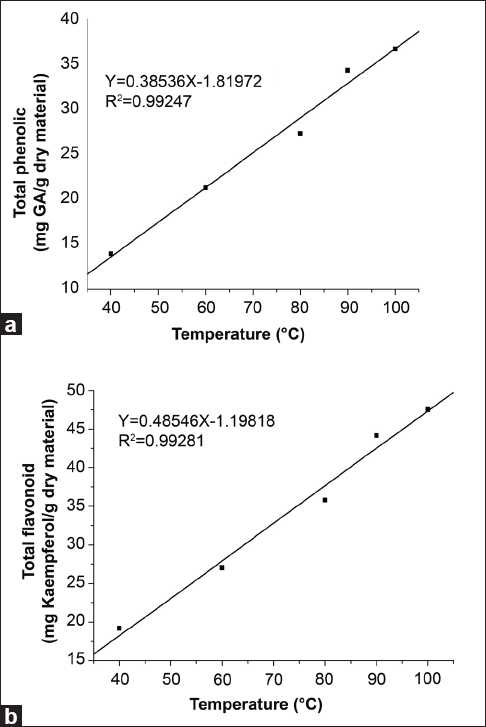
Relationship between (a) extraction temperature and total phenolic contents, (b) extraction temperature and total flavonoid contents
As a dual purpose plant, for its medicinal and edible purpose, G. divaricata was extensively used to treat diabetics in folk medicine. Flavonoids are the bioactive constituents of the plant and are influenced by the decoction temperature; however, there is not enough information about decoction temperature of this medicinal plant. We have herein provided the available information about decoction temperature of the plant; demonstrated the antioxidant activities of the ethanol extract of G. divaricata; and confirmed the significant influence of extraction temperature on the phenolic and flavonoid contents. The results emphasize the extract of G. divaricata has possessed significant antioxidant properties. Therefore, G. divaricata would be a potential source of natural antioxidants and nourishment. The consumption of G. divaricata in the local people might give positive function of health protection against oxidative damages. With the ascertained antioxidant activity of this plant, the separation and identification of the antioxidative components in the ethanol extract and using response–surface methodology (RSM) optimization, the extraction of phenolic compounds should be further investigated.
CONCLUSION
The optimal extraction temperature of G. divaricata leaf, chosen as a comparison between the yield of phenolic components (TPC and TFC) and their antioxidant and free radical-scavenging capacities (DPPH and ABTS), were 45% ethanol for 30 min at 90°C. Significant correlation was found for TPC and TFC with different extraction temperatures. Also TPC and TFC have significant correlation with the total antioxidant activity and free radical-scavenging capacities. This study provides constructive information for further optimization on the extraction of phenolic compounds from G. divaricata using RSM.
Footnotes
Source of Support: This project was supported by the graduate student innovation fund of Jiangxi (No.YC10A019)
Conflict of Interest: None declared
REFERENCES
- 1.Riley Pa. Free-radicals in biology-oxidative stress and the effects of ionizing-radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 2.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–95. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 3.Moon JK, Shibamoto T. Antioxidant assays for plant and food components. J Agric Food Chem. 2009;57:1655–66. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 4.Marete EN, Jacquier JC, O’riordan D. Effects of extraction temperature on the phenolic and parthenolide contents, and colour of aqueous feverfew (Tanacetum parthenium) extracts. Food Chem. 2009;117:226–31. [Google Scholar]
- 5.Heck CI, Schmalko M, De Mejia EG. Effect of growing and drying conditions on the phenolic composition of Mate teas (Ilex paraguariensis) J Agric Food Chem. 2008;56:8394–403. doi: 10.1021/jf801748s. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Li H, Song H, Zhang G. A new cerebroside from Gynura divaricata. Fitoterapia. 2009;80:517–20. doi: 10.1016/j.fitote.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Jiang MH, Hu JZ, Qiu WG, Qian Q, Tan M, Wu K, et al. Hypoglycemic and anti-anoxia effect of polysaccharides and flavonoids in Gynura divaricata (L.) DC. China J Hosp Pharm. 2009;29:1074–6. [Google Scholar]
- 8.Zhang XF, Tan BK. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Med J. 2000;41:9–13. [PubMed] [Google Scholar]
- 9.Chen L, Wang JJ, Song HT, Zhang GG, Qin LP. New cytotoxic cerebroside from Gynura divaricata. Chin Chem Lett. 2009;20:1091–3. [Google Scholar]
- 10.Chen SC, Hong LL, Chang CY, Chen CJ, Hsu MH, Huang YC, et al. Antiproliferative constituents from Gynura divaricata subsp.formosana. Chin Pharm J. 2003;55:109–19. [Google Scholar]
- 11.Chen L, Wang JJ, Zhang GG, Song HT, Qin LP. A new cerebroside from Gynura divaricata. Nat Prod Res. 2009;23:1330–6. doi: 10.1080/14786410902836677. [DOI] [PubMed] [Google Scholar]
- 12.Roeder E, Eckert A, Wiedenfeld H. Pyrrolizidine alkaloids from Gynura divaricata. Planta Med. 1996;62:386. doi: 10.1055/s-2006-957921. [DOI] [PubMed] [Google Scholar]
- 13.Akowuah GA, Mariam A, Chin JH. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn Mag. 2009;4:81–5. [Google Scholar]
- 14.Gutierrez RM, Navarro YT. Antioxidant and hepatoprotective effects of the methanol extract of the leaves of Satureja macrostema. Pharmacogn Mag. 2010;6:125–31. doi: 10.4103/0973-1296.62901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basniwal PK, Suthar M, Rathore GS, Gupta R, Kumar V, Pareek A, et al. In-vitro antioxidant activity of hot aqueous extract of Helicteres isora Linn.fruits. Nat Prod Radiance. 2009;8:483–7. [Google Scholar]
- 16.Sajjad KM, Khanam S, Deepak M, Shivananda BG. Antioxidant activity of a new diarylheptanoid from Zingiberofficinale. Pharmacogn Mag. 2006;2:254–7. [Google Scholar]
- 17.Patel A, Patel A, Patel A, Patel NM. Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea Linn.leaves (Leguminosae) Pharmacogn Res. 2000;2:152–8. doi: 10.4103/0974-8490.65509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheleva-Dimitrova D, Nedialkov P, Kitanov G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn mag. 2010;6:74–8. doi: 10.4103/0973-1296.62889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 20.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 21.Prasad KN, Yang B, Yang SY, Chen YL, Zhao MM, Ashraf M, et al. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009;116:1–7. [Google Scholar]
- 22.Zhao HF, Fan W, Dong JJ, Lu J, Chen J, Shan LJ, et al. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008;107:296–304. [Google Scholar]
- 23.Rosidah , Yam MF, Sadikun A, Asmawi MZ. Antioxidant potential of Gynura procumbens. Pharm Biol. 2008;46:616–25. [Google Scholar]
- 24.Akowuah GA, Sadikun A, Mariam A. Flavonoid identification and hypoglycemic studies of the butanol fraction from Gynura procumbens. Pharm Biol. 2002;40:405–10. [Google Scholar]
- 25.Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, et al. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101:1417–24. [Google Scholar]
- 26.Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–7. [Google Scholar]
- 27.Al-farsi , Lee CY. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008;108:977–85. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Sun BG, Cao YP, Tian YA, Li XH. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–10. [Google Scholar]
- 29.Zhang ZS, Li D, Wang LJ, Ozkan N, Chen XD, Mao ZH, et al. Optimization of ethanol-water extraction of lignans from flaxseed. Sep Purif Technol. 2007;57:17–24. [Google Scholar]


