Abstract
Background
Postmenopausal estrogen deficiency and alcohol abuse are known risk factors for osteoporosis. Previous studies of the combined effect of alcohol and ovariectomy on bone loss using chronic alcohol-feeding models have not demonstrated additional alcohol-induced bone loss in ovariectomized (OVX) animals. Binge alcohol treatment causes rapid bone loss in male rats. We hypothesized that binge alcohol would cause additional bone loss in OVX rats.
Methods
Ninety-six adult (400 g) female Sprague–Dawley rats (48 sham-operated and 48 OVX, pair fed) were randomly divided into 4 treatment groups: (a) saline-treated, (b) binge alcohol-treated (3 g/kg alcohol as a 20% weight to volume alcohol/saline solution, intraperitoneal (IP), 3 times per week), (c) parathyroid hormone (PTH)-treated (80 µg/kg, SC, 5 d/wk), and (d) binge alcohol plus PTH. Rats were treated for either 2 or 4 weeks. Following treatment periods, blood was collected for alcohol concentration (BAC) measurements; lumbar vertebrae were removed for bone mineral density (BMD) levels, trabecular microarchitecture assessment, and vertebral compressive strength analysis.
Results
Peak binge BACs averaged 300 mg/dL. Alcohol and OVX decreased cancellous BMD: alcohol and OVX treatment in combination caused additional cancellous BMD loss and significant cortical BMD reductions. Compressive strength was also decreased by OVX and alcohol. Combination treatment resulted in further declines in bone strength. Micro-CT analysis revealed a significant effect of combined OVX and alcohol treatment resulting in decreased trabecular bone volume/total volume (BV/TV). Intermittent PTH administration compensated for losses of BMD, compressive strength, and restored BV/TV deficits caused by OVX, alcohol, or their combination.
Conclusions
Bone loss following OVX can be significantly increased by concurrent binge alcohol treatment. The effects of alcohol and OVX are compensated by concurrent intermittent treatment with PTH. These results suggest that postmenopausal women who abuse alcohol may place their skeleton at additional risk for osteoporotic fracture.
Keywords: Binge Alcohol, Osteoporosis, Parathyroid Hormone, Rat
Despite decades of increased public awareness and medical intervention, osteoporosis remains a major health issue in the United States and abroad (Johnell and Kanis, 2004; Melton, 2003). While dietary factors and hormonal status are widely recognized for their importance to bone health, alcohol abuse is also an important risk factor for the development of osteoporosis (Olszynski et al., 2004). Alcoholism and alcohol abuse affect at least 14 million Americans (Grant et al., 2004). While previous investigators have reported on the increased prevalence of osteopenia and osteoporosis in male alcoholics (Bilke et al., 1985; Crilly et al., 1988; Diamond et al., 1989), little is known about the effects of alcohol abuse on bone health in women, although it has been demonstrated epidemiologically that women are more susceptible than men to alcohol-related organ injury and disease (Sato et al., 2001). The effects of moderate alcohol consumption on bone health in females may also be in question; whereas human epidemiological studies have shown some beneficial effects of moderate drinking on bone density (Angus et al., 1988; Felson et al., 1995; Laitinen et al., 1991), a recent study in rodents found that even moderate alcohol consumption caused decreased bone turnover, which could lead to detrimental effects on female skeletal health (Turner et al., 2001b).
Animal models of both alcohol-induced bone loss and postmenopausal osteoporosis are important tools in the study of pathologic bone loss. Alcohol-induced bone loss in rats has been demonstrated in both long-term feeding models (Sampson, 1998; Turner et al., 1998; Wezeman et al., 2000) and binge exposure models (Callaci et al., 2004). The ovariectomized (OVX) rat has been extensively utilized as a model of postmenopausal osteoporosis. (Kalu, 1991). While alcohol abuse and menopause are recognized as important risk factors for the development of osteoporosis, previous investigations have examined only the effects of moderate alcohol intake on bone loss in OVX rats. These studies found that alcohol either did not accelerate bone loss (Kidder and Turner, 1998) or did not cause further decreases in bone mineral density (Sampson and Shipley, 1997) in OVX rats. Moderate alcohol exposure was actually shown to reduce bone resorption in another OVX model (Fanti et al., 1997). Although none of these studies reported additional alcohol-induced bone loss in OVX animals, bone loss in the OVX state is not maximal as demonstrated by the increased bone loss observed in studies of both whole-body skeletal unloading during spaceflight and hind limb immobilization studies performed in OVX animals (Cavolina et al., 1997; Westerlind et al., 1997). These unloading studies suggest that the post-OVX skeleton remains vulnerable to further bone loss. Furthermore, as previously noted, the alcohol studies in OVX animals that have been performed used moderate levels of alcohol exposure and did not examine the effects of higher blood alcohol concentrations (BACs) that can occur with alcoholic or binge drinking, suggesting that the OVX skeleton may be vulnerable to further alcohol-induced bone loss.
Binge alcohol drinking is a pattern of alcohol consumption resulting in highly intoxicating BACs (Witt, 1994) coupled with repeated withdrawal phases (Lundqvist et al., 1995). There are over 13 million alcoholics and problem drinkers in the United States; between 2.7 and 4.5 million adult women fit this description (Blumenthal, 1998; Straussner and Attia, 2002). Binge drinking is common among alcoholics; alcoholic binge drinkers can achieve extremely high BACs (Penland et al., 2001). Although high BACs are common among binge drinking alcoholics, binge alcohol consumption is not limited to this population. Social binge drinking of high alcohol content beverages by nonalcoholics is a commonly observed phenomenon in many cultures (Kuntsche et al., 2004; Makela et al., 2001) and in college age and older adults in the United States (Dawson et al., 2004; Miller et al., 2004). A recent national survey found that approximately 25% of men and 20% of women ages 18 to 30 reported at least 1 binge drinking episode (6 or more drinks per occasion) in the 30 days preceding the study (Grossberg et al., 2004). Of these young adults reporting a history of binge drinking behavior, 12% of the men and 6% of the women reported that they actually engaged in binge drinking behavior 3 to 4 times in this same 30-day period, and 13% of men and 2.5% of women admitted engaging in binge drinking 5 or more times during the study period. Other estimates of adult female binge drinking show that 13.5% of US women aged 35 to 54 and 4.3% of women age 55 and over reported consuming alcohol in a binge pattern up to 7 times per year (Naimi et al., 2003). Despite the prevalence of binge drinking behavior, the effects of binge drinking and high BACs on bone health parameters had not been examined until recent studies in our laboratory (Callaci et al., 2004). Only one previous study attempted to examine the effects of binge drinking on bone in an actively growing rat model. This investigation administered alcohol at levels (43 to 106 mg/dL) that might be considered to be more representative of moderate drinking than true binge alcohol exposure and showed an unexpected beneficial effect on skeletal growth and BMD with a 2 d/wk alcohol binge (Sampson et al., 1999).
The current study used intraperitoneal (IP) injection to administer alcohol to adult female OVX rats in a binge-like pattern, mimicking the high BACs seen in alcoholics and social binge drinkers. As our laboratory has previously shown that this binge alcohol treatment induces aggressive bone loss in male rats (Callaci et al., 2004) we hypothesized that binge-like alcohol treatment would also increase bone loss in OVX rats and that bone loss because of alcohol, OVX, or the combination of both treatments would be compensated by intermittent parathyroid hormone (PTH) treatment. Parathyroid hormone given as a daily injection has an anabolic effect on bone that is recognized as a viable alternative treatment for osteoporosis. Parathyroid hormone has been shown to increase bone mass in rats (Vahle et al., 2002; Wronski et al., 1993) and human osteoporotics (Lane, 2004) and may improve trabecular architecture by thickening existing trabeculae (Lane et al., 2003).
MATERIALS AND METHODS
Binge Alcohol Model
The Loyola University Institutional Animal Care and Use Committee (IACUC) approved the animal study used in this investigation. A model was developed to examine the effects of binge alcohol exposure on additional bone loss in osteopenic-OVX rats. Ninety-six adult (400 g) female Sprague–Dawley rats (Harlan Corp., Indianapolis, IN) were divided into 2 groups of 48 animals that underwent either bilateral ovariectomy or sham surgery. One month postsurgery these rats were randomly divided into 4 treatment groups: (a) vehicle-injected, (b) binge alcohol-treated (3 g/kg, IP: 3 times/wk), (c) PTH-treated (80 µg/kg, s.c., 5 d/wk: PTH 1–34, Bachem Inc., Torrance, CA), and (d) binge alcohol plus PTH. Alcohol-treated rats were given the binge alcohol treatment on 3 consecutive days each week and received no alcohol treatment on the remaining 4 days of that week. Rats were treated with alcohol and/or PTH for 2 and 4 weeks. The time course of ovariectomy-induced bone loss in the rat varies by anatomical region with significant loss of trabecular bone volume (BV) occurring in the proximal tibia and femoral neck (Li et al., 1997; Wronski et al., 1988) much earlier than in the lumbar spine (Wronski et al., 1989), so experimental treatments (alcohol/PTH) were begun 1 month after surgery to coincide with measurable vertebral cancellous bone loss. The PTH dosing regimen used in this study has previously been shown to increase bone mass and strength in rats (Wronski et al., 1993) and was used in a previous study of the effects of PTH on bone formation during chronic alcohol exposure (Turner et al., 2001a). Animals were housed in pairs, with both paired animals assigned to the same treatment group. Animals were weighed twice weekly throughout the study period. Alcohol administration was by a single daily IP injection of a 20% (v/v) ethanol/saline solution at a dose of 3 g/kg. This dose was chosen to achieve peak BACs of approximately 300 mg/dL (Nation et al., 1993). Alcohol injections were given starting at 9:00 am, 3 d/wk. No injections were given on the remaining 4 days each week to mimic a binge alcohol-drinking pattern. Six rats from each treatment group were rendered unconscious by CO2 inhalation and killed by decapitation from 1 to 3 hours after the final alcohol injection of each treatment period (2 or 4 weeks). Blood was collected at time of death for blood alcohol analysis. Lumbar spine was removed and segments separated, cleaned and stored in 70% alcohol for subsequent analysis.
Blood Alcohol Determination
Blood alcohol concentrations were determined by NAD+ reduction assay (Sigma Chemical Company, St. Louis, MO) on blood samples collected from experimental animals at the time of death. Peak BAC was measured in 4 rats that were not included in any of the experimental protocol groups. These animals were killed by decapitation 60 minutes after a singe IP injection of 20% (v/v) ethanol/saline solution. The timing of peak BAC after IP injection was estimated based on a previous study that evaluated the pharmacokinetic profile of a 3 g/kg IP alcohol injection in rats (Nation et al., 1993).
BMD Measurements
Cancellous and cortical BMD of each third lumbar vertebra (L3) was determined using quantitative computed tomography (QCT). Lumbar vertebral bodies were placed in a Norland Stratec bone densitometer (Orthometrix Inc., White Plains, NY). The bones were positioned uniformly on a support so that the instrument-scanning plane was perpendicular to the longitudinal axis of the bone. Scout views were obtained to determine the midpoint of the vertebral body. Two measurements at a resolution of 70 µm/voxel were performed 1 mm apart at this point. The cancellous area of the bone was defined as 45% of the total bone cross-sectional area at the midpoint of the vertebral body. The instrument was set to use the threshold contour mode (soft-tissue threshold set at 220 mg/cm3) and a concentric peel algorithm (55% peel-off). Scans were made at 50 kV and 0.3 mA. Data were stored electronically.
Three-Dimensional Trabecular Microarchitectural Analysis
Three-dimensional analysis of trabecular architecture of each L3 vertebral body was performed on a Micro-CT 40 instrument (Scanco Medical AG, Bassersdorf, Switzerland). Full scans of each specimen were obtained at a resolution of 16 × 16 × 16 µm/voxel. A reconstructed three-dimensional image was used to specify the volume of interest, which was defined as a rectangular box of 75 × 50 × 40 voxels. The box was uniformly placed 0.5 mm from, and parallel to, the cranial end plate. To ensure that only trabecular bone was analyzed, a margin of at least 50 voxels was left between the volume of analysis and the cortex. The volume of interest was analyzed for bone volume/total volume (BV/TV) (Laib et al., 2001).
Biomechanical Structural Properties of Lumbar Vertebrae
Compressive strength tests were performed on the L3 vertebral body from each rat using an Instron materials testing machine (Model 1122, Canton, MA). The vertebral endplates were potted in bone cement using a previously described method that resulted in two parallel loading surfaces necessary to perform a uniform compression test on individual rodent vertebrae (Steinke et al., 1999). The specimens were prepared so that the posterior elements of the vertebra did not contact the loading platforms. All compression tests were performed in the displacement-control mode at a crosshead speed of 0.5 mm/min to eliminate any strain rate effects. A 50-kg load cell was used to monitor the compressive load and a precision sensor was used to measure the axial deformation of the specimen. The load–deformation data were analyzed to obtain the compressive strength, defined as the maximum load sustained before failure.
Statistical Analysis
Statistical analysis was performed using SPSS v.10.0.5 software (SPSS Inc, Chicago, IL). At both time points of the study, the data was normalized to the average of the sham saline group and the percent change was computed for every animal. The data from the non-PTH treatment groups were analyzed by 1-way ANOVA followed by Tukey’s multiple comparisons procedure, if appropriate. Data from PTH and each respective non-PTH group were compared by the Student’s t test. Significance for all statistical analyses was noted at p<0.05.
RESULTS
General Observations
Ovariectomized and sham-operated rats were matched by weight and pair fed for the duration of the experiment. All animals gained weight during the protocol. The only significant factor affecting weight gain during the protocol was OVX (p<0.5); no significant differences in weight gain were observed with other (alcohol or PTH) treatment(s). There was no effect of treatment on vertebral body size; cross-sectional area measurements of each vertebral body obtained during pQCT BMD determinations were equivalent across all treatment groups (data not shown). Peak BACs were measured in this study using 4 rats not part of the overall experiment by giving a single IP injection of alcohol at 3 g/kg and measuring BAC 1 hour later. In these rats peak BAC averaged 303 ± 40 mg/dL. Serum alcohol concentrations at the time of death of rats included in the study measured 285 ± 60 mg/dL in 2-week alcohol binge animals and 245 ± 30 mg/dL in 4-week alcohol binge animals. A once daily treatment regimen was chosen to avoid alcohol withdrawal symptoms that can occur when high doses of alcohol are administered twice daily (Penland et al., 2001). All alcohol-treated animals were monitored throughout the study period and no obvious symptoms of alcohol withdrawal were observed during the 4-day period each week when alcohol was not administered. Rats exhibited a short (approximately 1 to 2 hours) period of acute alcohol intoxication immediately following each IP injection. Necropsy performed on each animal revealed no obvious internal injury from IP injections; abdominal organs (including liver) of alcohol-treated animals appeared normal by gross inspection.
Vertebral BMD
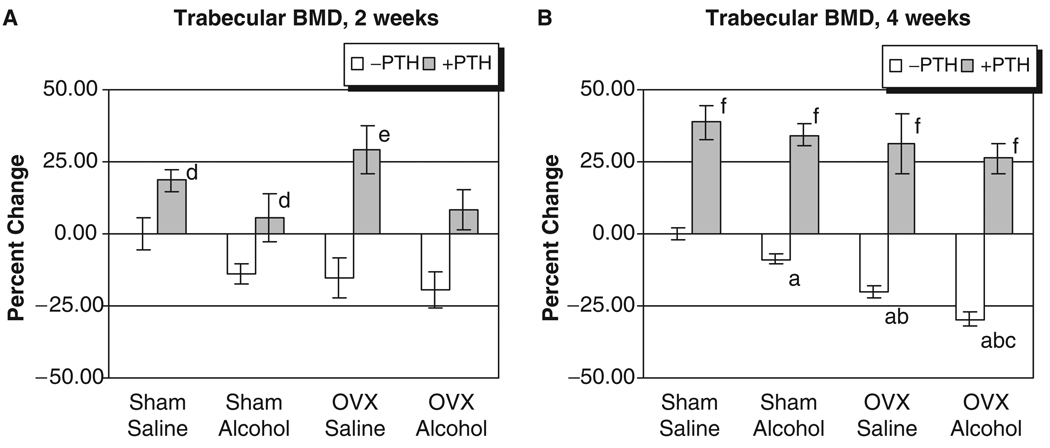
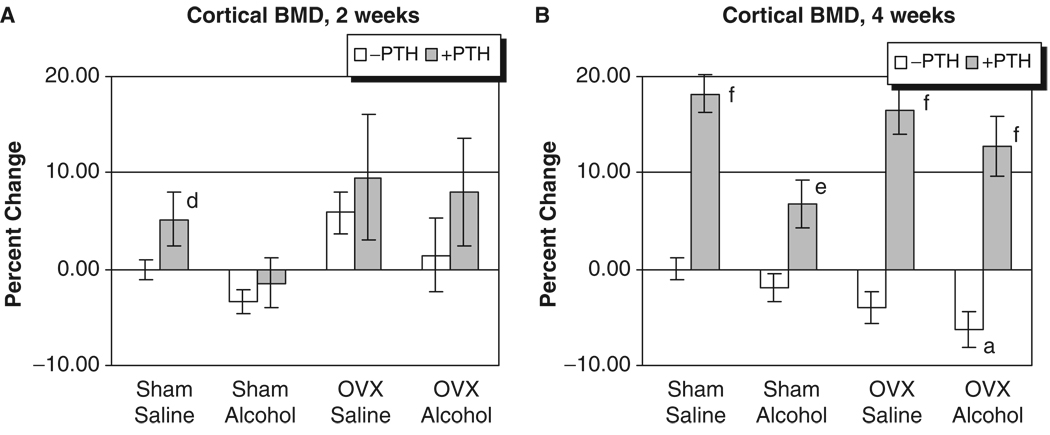
The L3 of each rat was analyzed for trabecular BMD after 2 and 4 weeks of treatment. As seen in Figs. 1A and 2A, no significant effects of alcohol or OVX treatment were detected on cortical or trabecular BMD values at the 2-week time point. However, Fig. 1B illustrates that OVX and binge alcohol treatment had significant effects on vertebral trabecular BMD values after 4 weeks of treatment, with alcohol decreasing BMD by almost 10%, and OVX causing a 20% decrease in BMD (p<0.05). Binge alcohol treatment of OVX rats caused an even greater significant decrease in vertebral trabecular BMD, with values decreasing by almost 30% below sham–saline control levels (p<0.05). This decrease in trabecular BMD observed in the OVX–alcohol group was also significantly lower than BMD values of rats from the alcohol or OVX treatment groups (p<0.05). Parathyroid hormone treatment significantly increased vertebral trabecular BMD values in all treatment groups after 4 weeks (p<0.001). With respect to cortical BMD values, only OVX–alcohol combined treatment caused a significant decrease in this parameter (p<0.05) (Fig. 2B). Parathyroid hormone treatment also significantly increased vertebral cortical BMD in all treatment groups at 4 weeks (p<0.01).
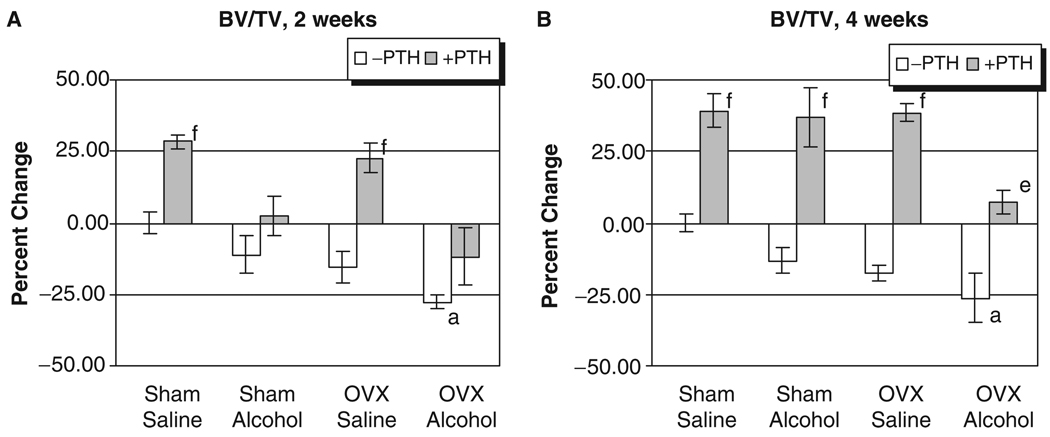
Fig. 1.
Rat vertebral trabecular bone mineral density analysis. (A) Two weeks of treatment. (B) Four weeks of treatment: ap<0.05 compared with sham–saline group; bp<0.05 compared with sham alcohol group; cp<0.05 compared with ovriectomized–saline group, 1-way ANOVA and Tukey’s multiple comparison procedure; dp<0.05; ep<0.01; fp<0.001 compared with its respective non-PTH group, Student’s t test.
Fig. 2.
Rat vertebral cortical bone mineral density analysis. (A) Two weeks of treatment. (B) Four weeks of treatment: ap<0.05 compared with sham–saline group, 1-way ANOVA and Tukey’s multiple comparison procedure; dp<0.05; ep<0.01; fp<0.001 compared with its respective non-PTH group; Student’s t-test.
Vertebral Compressive Strength
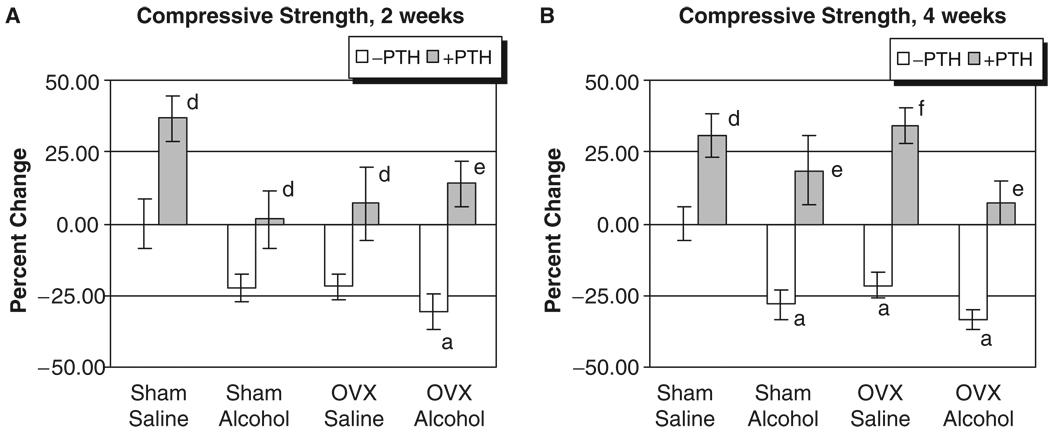
The L3 of each rat was also analyzed for strength by determining the maximal compressive load in Newtons (N) each bone could sustain before structural failure. Vertebrae from alcohol-treated rats and OVX animals had decreased bone strength at both 2 and 4 weeks (Fig. 3A and B). Compressive strengths of lumbar vertebrae had significantly decreased after 2 weeks of treatment only in the OVX–alcohol group (31% below control, p<0.05). At 4 weeks, compressive strength in the OVX–alcohol group was 32.5% below sham–saline control levels (p<0.05), which was also 12% lower than the matched OVX–saline group. The size of vertebral bodies as determined by cross-sectional area measurement obtained during QCT was equivalent across treatment groups, suggesting that the decreased vertebral compressive strength values observed after combined OVX–alcohol treatment were the result of altered biomechanical properties of the vertebrae and not because of treatment-induced differences in vertebral body area. Parathyroid hormone treatment caused significant gains in compressive strength in all groups (p<0.05).
Fig. 3.
Rat vertebral compressive strength analysis. (A) Two weeks of treatment. (B) Four weeks of treatment: ap<0.05 compared with sham–saline group, 1-way ANOVA and Tukey’s multiple comparison procedure; dp<0.05; ep<0.01; fp<0.001 compared with its respective non-parathyroid hormone group, Student’s t test.
Trabecular Architecture
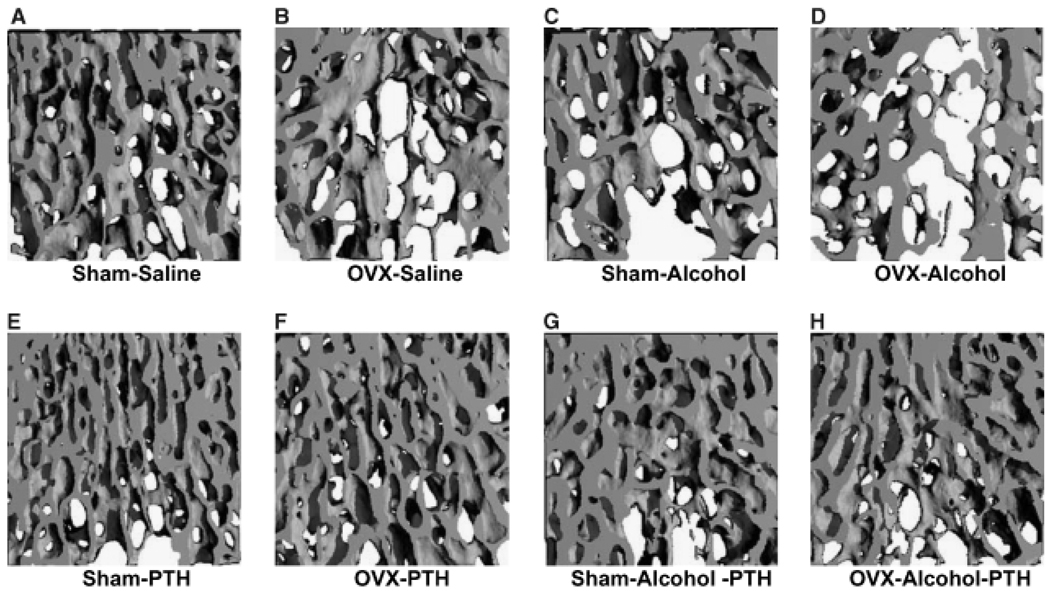
The L3 of each rat was also analyzed for the effect of treatment on three-dimensional trabecular micro architecture. Figure 4 shows representative three-dimensional reconstructed micro-CT images of vertebral trabecular bone from each treatment group. Figs. 4A through 4D show vertebral trabecular structure of sham, OVX, alcohol and OVX, and alcohol-treated animals respectively, illustrating the additive effects of binge alcohol-treatment and OVX on bone loss. Figs. 4E through 4H show the effect of intermittent PTH treatment on vertebral trabecular structure of sham, OVX alcohol-treated, and OVX + alcohol-treated animals, illustrating the effect of PTH to compensate for bone loss in each group. Quantification of three-dimensional trabecular structure (Figs. 5A and 5B) revealed significant effects of combined binge alcohol and OVX treatment on BV/TV with values decreasing approximately 27% at both 2 and 4 weeks, which was 9% below matched OVX–saline values (p<0.05). Binge alcohol and ovariectomy as independent treatments did decrease BV/TV but not to significant levels. At 4 weeks, PTH treatment significantly increased BV/TV in all treatment groups (p<0.01).
Fig. 4.
Visualization of rat vertebral trabecular architecture using 3-dimensional micro-computed tomography. All specimens depicted after 4 weeks of treatment. (A–D) No parathyroid hormone (PTH) treatment. (E–H) With PTH treatment.
Fig. 5.
Rat vertebral BV/TV analysis. (A) Two weeks of treatment. (B) Four weeks of treatment: ap<0.05 compared with sham–saline group, 1-way ANOVA and Tukey’s multiple comparison procedure; ep<0.01; fp<0.001 compared with its respective non-parathyroid hormone group, Student’s t test.
DISCUSSION
In this study, we used a binge alcohol treatment paradigm that was previously shown in our laboratory to cause aggressive bone loss in male rats (Callaci et al., 2004) to test the hypothesis that alcohol given in a pattern consistent with binge drinking would cause additional vertebral bone loss in OVX rats, compared with the vertebral osteopenia expected in OVX animals. We further hypothesized that intermittent PTH treatment would compensate for the alcohol and ovariectomy-induced bone loss predicted in our model. We found that binge alcohol treatment caused additional bone loss in the lumbar spine of OVX rats, as measured by the significant declines in lumbar BMD, compressive strength and BV/TV observed during combined treatment. Taken together, we submit that these data strongly suggests that binge alcohol treatment, which raises BACs to levels observed during human binge drinking episodes, causes biologically relevant additional bone loss in the lumbar vertebrae of OVX rats. Furthermore, we found that concurrent PTH treatment not only compensated for alcohol and ovariectomy-induced bone loss, but was also able to enhance vertebral bone mass despite the actions of 2 bone damaging factors.
Previous studies of the effect of alcohol on bone in OVX animals failed to observe any significant additional bone loss above that expected in OVX animals. These studies, however, examined only the effects of low to moderate levels of alcohol exposure on bone loss in OVX animals. One study found that OVX rats chronically fed a diet with alcohol contributing 13 or 35% of total caloric intake showed no accelerated bone loss above control OVX animals (Kidder and Turner, 1998). Other investigations found that low levels of alcohol did not affect BMD in OVX rats (Sampson and Shipley, 1997) and that moderately high alcohol levels suppressed bone resorption in OVX animals, which the authors speculated might even lead to decreased bone loss in OVX rats (Fanti et al., 1997).
Although these studies provide important information about the effects of alcohol treatment and OVX on bone health, they do not address the question of whether alcohol given at higher doses or in a different pattern of administration can accelerate bone loss above that already occurring after ovariectomy. Binge drinking by alcoholics can produce high peak BACs in the range of 300 to over 800 mg/dL (Penland et al., 2001), which are well above the blood alcohol levels attained in rat alcohol feeding studies (Sampson, 1998; Wezeman et al., 2000). In addition, social binge drinking of high alcohol content beverages is common in many cultures (Makela et al., 2001; Miller et al., 2004), making an examination of the consequences of binge drinking and high blood alcohol levels relevant to the study of alcohol-induced osteopenia. Finally, the pattern of alcohol administration and peak BAC attained have also been shown to be more important factors than total alcohol intake for causing alcohol-induced tissue injury in other organ systems such as the brain (Lundqvist et al., 1995). The current study addresses these issues by administering alcohol to OVX rats once daily by IP injection, controlling dose and duration of exposure in an attempt to more closely mimic the pattern and level of alcohol consumption of alcoholics and social binge drinkers. We asked the question of whether this treatment paradigm would cause additional vertebral bone loss in the rat 1 month postovariectomy. We found that a higher dose alcohol binge treatment did indeed increase vertebral bone loss in OVX animals. Our data support previous studies showing that ovariectomy-induced bone loss in rats is not maximal and is also increased by other bone damaging regimens such as hind limb unloading or functional unloading of the entire skeleton during spaceflight (Cavolina et al., 1997; Westerlind et al., 1997). Our investigation also supports a related hypothesis derived from a study on disuse osteoporosis where the authors determined that alcohol-induced bone loss was also not maximal and could be increased by hind limb immobilization (Hefferan et al., 2003).
The rapidity of bone loss in this and prior alcohol studies (Callaci et al., 2004; Hefferan et al., 2003) suggests that alcohol-induced bone loss can be attributed at least in part to increased bone resorption. Our current data also suggest that the rapid bone loss observed after ovariectomy represents only a portion of the total resorption capability of the skeleton. As mentioned previously, the time course of lumbar spine osteopenia after OVX occurs later than in the femoral neck and proximal tibia (Li et al., 1997; Wronski et al., 1988, 1989). We show an additional insult of binge alcohol treatment can accelerate the time course of bone loss in lumbar vertebra. Although this study focused on the effects of treatment on vertebral trabecular BMD and architecture and observed that OVX and binge alcohol treatment have pronounced effects on the cancellous compartment of the vertebral body, more conservative but significant bone loss was observed in the vertebral cortex as well. Relative contributions of the trabecular and cortical components of individual rat vertebra to its biomechanical properties have been studied, finding that trabecular microstructure makes a significant contribution to bone strength in intact vertebrae, whereas in vertebral bone from OVX animals exhibiting deteriorated trabecular structure, biomechanical strength is more dependent on the cortical compartment (Ito et al., 2002). The trabecular compartment of vertebrae from OVX–alcohol-treated rats exhibited deterioration in the form of decreased BMD and BV/TV. Based on the conclusions of Ito et al. (2002), the compressive strength of these compromised vertebrae may have been disproportionately supported by the cortical compartment that suffered less damage from alcohol and OVX treatment. Had binge alcohol treatment been extended past 4 weeks, we would anticipate further damage to cortical bone and a concurrent drop in the biomechanical strength of vertebrae, significantly below that of matched OVX–saline animals.
Parathyroid hormone treatment was able to compensate for the effects of alcohol, OVX, and combined alcohol plus OVX treatment on bone loss, bringing parameters back to, and in some cases exceeding, control levels. For example, alcohol-treated OVX animals lost almost 29% of lumbar trabecular BMD at 4 weeks; concurrent PTH treatment not only reversed this loss but also increased lumbar trabecular BMD 26% above control values (Fig. 1B). These data suggest that the bone building capacity of PTH to increase bone mass and strength can override the bone damaging actions of alcohol and OVX. A previous study examining the effects of PTH on bone loss in a rat model of chronic alcohol abuse suggested that alcohol affected bone mass primarily by reducing indices of bone formation and found that PTH-induced bone formation was blunted but not inhibited by alcohol (Turner et al., 2001a). The current study does not show a blunting effect of alcohol on PTH activity on bone, suggesting that bone formation was minimally affected by our alcohol treatment. This observation, taken together with previous data from our laboratory showing that alcohol has substantial stimulatory effects on bone resorption, may suggest that the effects of PTH on binge alcohol-induced bone loss are different than those observed by Turner et al. Alternate statistical analysis of our data revealed no significant interactions between alcohol and OVX treatment on bone loss parameters. This analysis indicates that these 2 variables had independent, additive effects on bone loss. This finding is in agreement with a recent study that also found independent, additive effects of combined alcohol and reduced weight bearing on tibial cortical bone loss (Hefferan et al., 2003). Based on this observation, it is intriguing to speculate that bone loss due to alcohol exposure and ovariectomy-induced estrogen depletion may occur through distinct pathways. Studies along this line may increase our understanding of bone loss and lead to novel mechanism-based therapeutics for osteoporosis which target commonality between bone loss due to multiple intrinsic or extrinsic factors.
In conclusion, this study found that alcohol given in a pattern and level reflective of binge drinking causes additional significant vertebral bone loss in the ovariectomy-compromised skeleton. Concurrent treatment with PTH compensates for both alcohol- and ovariectomy-induced bone loss. The observation that bone loss because of estrogen deficiency is not maximal and can be increased by a lifestyle factor such as excessive patterned drinking suggests that postmenopausal women who engage in excessive or binge drinking behavior may be at greater risk for osteoporotic fractures and resulting disability.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants AA12576 to FHW and AA13527 (Loyola University Training Grant). The authors thank Dr. James Sinacore for his assistance with statistical analysis of the data.
REFERENCES
- Angus RM, Sambrook PN, Pocock NA. Dietary intake and bone mineral density. Osteoporosis Int. 1988;1:265–277. [PubMed] [Google Scholar]
- Bilke DD, Genant HK, Cann C, Recker RR, Halloran BP, Strewler GJ. Bone disease in alcohol abuse. Ann Intern Med. 1985;103:42–48. doi: 10.7326/0003-4819-103-1-42. [DOI] [PubMed] [Google Scholar]
- Blumenthal SJ. Women and substance abuse: a new national focus. In: Wetherington CL, Roman AB, editors. Drug Addiction Research and the Health of Women. Bethesda, MD: USDHHS, NIH/NIDA; 1998. pp. 13–32. [Google Scholar]
- Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH. The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcohol Clin Exp Res. 2004;28:182–191. doi: 10.1097/01.ALC.0000108661.41560.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavolina JM, Evans GL, Harris SA, Zhang M, Westerlind KC, Turner RT. The effects of orbital spaceflight on bone histomorphometry and messenger ribonucleic acid levels for bone matrix proteins and skeletal signaling peptides in ovariectomized growing rats. Endocrinology. 1997;8:1567–1576. doi: 10.1210/endo.138.4.5040. [DOI] [PubMed] [Google Scholar]
- Crilly RG, Anderson C, Hogan D, Delaquerriere-Richardson L. Bone histomorphometry, bone mass, and related parameters in alcoholic males. Calcif Tissue Int. 1988;43:269–276. doi: 10.1007/BF02556634. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Another look at heavy episodic drinking and alcohol use disorders among college and noncollege youth. J Stud Alcohol. 2004;65:477–488. doi: 10.15288/jsa.2004.65.477. [DOI] [PubMed] [Google Scholar]
- Diamond T, Stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. Am J Med. 1989;86:282–288. doi: 10.1016/0002-9343(89)90297-0. [DOI] [PubMed] [Google Scholar]
- Fanti P, Monier-Faugere MC, Geng Z, Cohen D, Malluche HH. Moderately high consumption of ethanol suppresses bone resorption in ovariectomized but not in sexually intact adult female rats. Alcohol Clin Exp Res. 1997;21:1150–1154. doi: 10.1111/j.1530-0277.1997.tb04266.x. [DOI] [PubMed] [Google Scholar]
- Felson DT, Zhang Y, Hannan MT, Kannal WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485–492. doi: 10.1093/oxfordjournals.aje.a117664. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: united States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grossberg PM, Brown DD, Fleming MF. Brief physician advice for high-risk drinking among young adults. Ann Fam Med. 2004;2:474–480. doi: 10.1370/afm.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefferan TE, Kennedy AM, Evans GL, Turner RT. Disuse exaggerates the detrimental effects of alcohol on cortical bone. Alcohol Clin Exp Res. 2003;27:111–117. doi: 10.1097/01.ALC.0000047348.23797.6E. [DOI] [PubMed] [Google Scholar]
- Ito M, Nishida A, Koga A, Ikeda S, Shiraishi A, Uetani M, Hayashi K, Nakamura T. Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone. 2002;31:351–358. doi: 10.1016/s8756-3282(02)00830-x. [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporosis Int. 2004;15:897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Kidder LS, Turner RT. Dietary ethanol does not accelerate bone loss in ovariectomized rats. Alcohol Clin Exp Res. 1998;229:2159–2164. [PubMed] [Google Scholar]
- Kuntsche E, Rehm J, Gmel G. Characteristics of binge drinkers in Europe. Soc Sci Med. 2004;59:113–127. doi: 10.1016/j.socscimed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Laib A, Kumer JL, Majumdar S, Lane NE. The temporal changes of trabecular architecture in ovariectomized rats assessed by microct. Osteoporosis Int. 2001;12:936–941. doi: 10.1007/s001980170022. [DOI] [PubMed] [Google Scholar]
- Laitinen K, Valimaki M, Keto P. Bone mineral density measured by dual-energy X-ray absorptiometry in Finnish women. Calcif Tissues Int. 1991;48:224–231. doi: 10.1007/BF02556372. [DOI] [PubMed] [Google Scholar]
- Lane NE, Yao W, Kinney JH, Modin G, Balooch M, Wronski TJ. Both hpth (1–34) and bfgf increase trabecular bone mass in osteopenic rats but they have different effects on trabecular bone architecture. J Bone Miner Res. 2003;18:2105–2115. doi: 10.1359/jbmr.2003.18.12.2105. [DOI] [PubMed] [Google Scholar]
- Lane NE. Parathyroid hormone: evolving therapeutic concepts. Curr Opin Rheumatol. 2004;16:457–456. doi: 10.1097/01.bor.0000129721.98691.cb. [DOI] [PubMed] [Google Scholar]
- Li M, Shen Y, Wronski TJ. Time course of femoral neck osteopenia in ovariectomized rats. Bone. 1997;20:55–61. doi: 10.1016/s8756-3282(96)00317-1. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Alling C, Knoth R, Volk B. Intermittent ethanol exposure of adult rats: hippocampal cell loss after one month of treatment. Alcoholism. 1995;30:737–748. [PubMed] [Google Scholar]
- Makela P, Fonager K, Hibell B, Nordlund S, Sabroe S, Simpura J. Episodic heavy drinking in four Nordic countries: a comparative survey. Addiction. 2001;96:1575–1588. doi: 10.1080/09652140120080714. [DOI] [PubMed] [Google Scholar]
- Melton LJ., III Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res. 2003;18:1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- Miller JW, Gfroerer JC, Brewer RD, Naimi TS, Mokdad A, Giles WH. Prevalence of adult binge drinking: a comparison of two national surveys. Am J Prev Med. 2004;27:197–204. doi: 10.1016/j.amepre.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289:70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- Nation JR, Burkey RT, Grover CA. Lead/ethanol interactions II: phamacokinetics. Alcoholism. 1993;10:363–367. doi: 10.1016/0741-8329(93)90021-f. [DOI] [PubMed] [Google Scholar]
- Olszynski WP, Shawn Davison K, Adachi JD, Brown JP, Cummings SR, Hanley DA, Harris SP, Hodsman AB, Kendler D, McClung MR, Miller PD, Yuen CK. Osteoporosis in men: epidemiology, diagnosis, prevention, and treatment. Clin Ther. 2004;26:5–28. doi: 10.1016/s0149-2918(04)90002-1. [DOI] [PubMed] [Google Scholar]
- Penland S, Hoplight B, Obernier J, Crews FT. Effects of nicotine on ethanol dependence and brain damage. Alcoholism. 2001;24:45–54. doi: 10.1016/s0741-8329(01)00142-2. [DOI] [PubMed] [Google Scholar]
- Sampson HW, Shipley D. Moderate alcohol consumption does not augment bone density in ovariectomized rats. Alcohol Clin Exp Res. 1997;21:1165–1168. [PubMed] [Google Scholar]
- Sampson HW, Gallager S, Lange J, Chondra W, Hogan HA. Binge drinking and bone metabolism in a young actively growing rat model. Alcohol Clin Exp Res. 1999;23:1228–1231. doi: 10.1111/j.1530-0277.1999.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Sampson HW. Effect of alcohol consumption on adult and aged bone: a histomorphometric study of the rat animal model. Alcohol Clin Exp Res. 1998;22:2029–2034. [PubMed] [Google Scholar]
- Sato N, Lindros KO, Baraona E, Ikejima K, Mezey, Jarvelainen HA, Ramchandani VA. Sex differences in alcohol-related organ injury. Alcohol Clin Exp Res. 2001;25:40S–45S. doi: 10.1097/00000374-200105051-00007. [DOI] [PubMed] [Google Scholar]
- Steinke B, Patwardhan AG, Havey R, King D. Human growth hormone transgene expression increases the biomechanical structural properties of mouse vertebrae. Spine. 1999;24:1–4. doi: 10.1097/00007632-199901010-00002. [DOI] [PubMed] [Google Scholar]
- Straussner SLA, Attia P. Women’s addiction and treatment through a historical lens. In: Straussner SLA, Brown S, editors. The Handbook of Addiction Treatment for Women. San Francisco, CA: Jossey-Bass/Wiley; 2002. pp. 3–25. [Google Scholar]
- Turner RT, Evans GL, Zhang M, Sibonga JD. Effects of parathyroid hormone on bone formation in a rat model for chronic alcohol abuse. Alcohol Clin Exp Res. 2001a;25:667–671. [PubMed] [Google Scholar]
- Turner RT, Kidder LS, Kennedy A, Evans GL, Sibonga JD. Moderate alcohol consumption suppresses bone turnover in adult female rats. J Bone Miner Res. 2001b;16:589–594. doi: 10.1359/jbmr.2001.16.3.589. [DOI] [PubMed] [Google Scholar]
- Turner RT, Wronski TJ, Zhang M, Kidder LS, Bloomfield SA, Sibonga JD. Effects of ethanol on gene expression in rat bone: transient dose-dependent changes in mRNA levels for bone matrix proteins, skeletal growth factors and cytokines are followed by reductions in bone formation. Alcohol Clin Exp Res. 1998;22:1591–1599. doi: 10.1111/j.1530-0277.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, Turner RT. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci U S A. 1997;94:4199–4204. doi: 10.1073/pnas.94.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wezeman FH, Emanuele MA, Moskal SF, Steiner J, Lapaglia N. Alendronate administration and skeletal response during chronic alcohol intake in the adolescent male rat. J Bone Miner Res. 2000;15:2033–2041. doi: 10.1359/jbmr.2000.15.10.2033. [DOI] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988;43:179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Dann LM, Horner SL. Time course of vertebral osteopenia in ovariectomized rats. Bone. 1989;10:295–301. doi: 10.1016/8756-3282(89)90067-7. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Yen CF, Qi H, Dann LM. Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology. 1993;132:823–831. doi: 10.1210/endo.132.2.8425497. [DOI] [PubMed] [Google Scholar]