Abstract
This study describes postnatal critical period changes in cellular and laminar expression of Dab-1, a gene shown to play a role in controlling neuronal positioning during embryonic brain development, in cat visual cortex and the effects of dark rearing (DR). At 1 week, there is dense cellular staining which is uniform across cortical layers and very light neuropil staining. At the peak of the critical period (5 weeks), dense cell staining is largely restricted to large pyramidal cells of deep layer III and layer V, there is faint cell body staining throughout all cortical layers, neuropil staining is markedly increased and uniform in layers III to VI. This dramatic change in laminar and cellular labeling is independent of visual input, since immunostaining is similar in 5 week DR cats. By 10 weeks, the mature laminar and cellular staining pattern is established and the major subsequent change is a further reduction in the density of cellular staining in all cortical layers. Neuropil staining is pronounced and uniform across cortical layers. These developmental changes are altered by DR. Quantification by cell counts indicated that age and DR interact such that differences in cellular expression are opposite in direction between 5 week old and 20 week old cats. This bidirectional regulation of cellular expression is the same in all cortical laminae. The bidirectional regulation of cellular expression matches the effects of age and DR on physiological plasticity during the critical period as assessed by ocular dominance shifts in response to monocular deprivation.
Keywords: Disabled-1, differential display PCR, Neuronal Plasticity, Development
1. Introduction
Dab-1 is the mammalian homologue of the disabled-1 gene in Drosophila. It forms part of the Reelin/Dab-1 signaling pathway that controls neuronal positioning during embryonic brain development, directing deep-to-superficial migration of cerebral and cerebellar cortical neurons (see Rice and Curran, 2001; Tissir and Goffinet, 2003 for reviews). The Reelin/Dab-1 pathway is integral during development of numerous parts of the nervous system (Andrade et al., 2007; Bock et al., 2003; Frotscher et al., 2009; Stolt et al., 2005; Yabut et al., 2007). Dab-1 acts downstream of Reelin as an intracellular, adaptor protein that is phosphorylated when the Reelin ligand binds to very low density lipoprotein receptor and Apolipoprotein E Receptor-2 (VLDLR and ApoER2) receptors on migrating neurons (Howell et al., 1997a; Rice et al., 2001). Recent research suggests that Dab-1 is important as more than just an adapter protein activating Src kinases but also as a structural scaffold for numerous signaling complexes (see Feng and Cooper, 2009 for review).
Mutants lacking the Dab-1 gene are viable, and appear behaviorally normal until post-natal day 10, at which time they develop a characteristic ataxia; however, they do survive into adulthood (Howell et al., 1997b). Morphologically, Dab-1 mutants show an abnormal cortical development that produces an inversion of cortical lamina, as found in reeler mice (a mutation of Reelin), through disruption of neuronal migration and altered lamination patterns in cortex and cerebellum (Deguchi et al., 2003; Howell et al., 1997b; Pesold et al., 1999; Rice et al., 1998). The Reelin/Dab-1 signaling pathway continues to be expressed in the postnatal and adult brain, where evolving evidence indicates it plays a role in synaptic plasticity. Signaling through this pathway has been in implicated in synaptic plasticity related processes including long term potentiation (LTP), dendritic maturation, and changes in NMDA and AMPA receptor currents and subunit composition (see Herz and Chen, 2006; Levenson et al., 2008; Rogers and Weeber, 2008 for reviews).
The classic model of synaptic plasticity is the postnatal critical period for development of visual cortex, which is guided by visual experience during early postnatal life. The clearest demonstration of such environmental effects on visual cortical development is rearing cats with monocular deprivation, a condition that leads to dramatic anatomical and physiological abnormalities (see Daw, 2006 for review). In normal development, sensitivity to monocular deprivation is limited to a “critical period,” which in cats begins several weeks after birth, peaks at about 5 weeks, gradually declines to very low levels at 20 weeks and disappears at about 1 year of age (Daw, 2006). Rearing in total darkness extends this critical period and prolongs neuronal plasticity far beyond its normal age limits (Cynader and Mitchell, 1980; Mower et al., 1981). Electrophysiological results indicate that the effect of dark rearing is to slow the entire time course of the critical period, such that at young ages (5 weeks) normal cats are more plastic than dark reared cats, while at later ages (20 weeks) dark reared cats are more plastic (Beaver et al., 2001; Mower, 1991). Thus, a stringent criterion for identifying candidate genes that are important for plasticity in visual cortex is that their level of expression shows differences between normal and dark reared cats that are of opposite direction in young versus older animals. We have applied this criterion in a differential display PCR screening of normal and dark reared cats at 5 and 20 weeks to identify candidate genes for critical period neuroplasticity. Dab-1 was one of the genes identified as showing differences in mRNA and protein expression between normal and dark reared cats and mice that were of opposite direction in young versus older animals. Dab-1 was elevated in normal cats at the peak of the critical period (5 weeks) and elevated in DR cats at the nadir (20 weeks) of the critical period (Yang et al., 2006).
The present study was undertaken to describe the cellular and laminar pattern of Dab-1 protein expression in visual cortex, to determine normal postnatal changes in those expression patterns and to determine if dark rearing alters cellular and laminar expression patterns of this candidate plasticity gene product.
2. Results
2.1 Confirmation of antibody specificity
A rabbit polyclonal antibody against amino acids 400–555 of the C’ terminal of Dab-1 (Rockland Immunochemicals, Inc., Gilbertsville, PA) was used for immunohistochemistry. Mouse Dab-1 shows high homology across a wide range of species in which the sequence is known, including avian, marsupial, rodent, equine, bovine, panda, and numerous primates (NCBI Blastx 2.2.24+, Altschul et al., 1997). In cats, the only available sequence is the untranslated 3′ end (Yang et al., 2006) which is 90% homologous to mouse. A Blast search with this cat sequence shows unique matches with human Dab-1 on chromosome 1 and with mouse Dab-1 on chromosome 4.
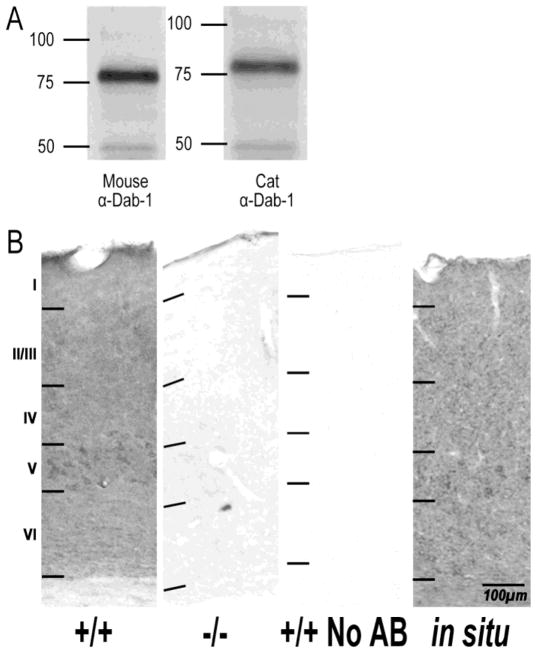
Figure 1 shows comparisons of immunostaining in wild-type mouse tissue, Dab-1 knockout mutant mouse tissue, and wild-type mouse tissue incubated without primary antibody. Western blot analysis indicated that this Dab-1 antibody intensely labeled an appropriate sized band at approximately 80kDa in both cats and mice. Immunopositive labeling was found solely in the wild-type tissue exposed to the Dab-1 antibody. The immunohistochemical staining pattern across cortical laminae was comparable to in situ mRNA labeling of Dab-1 mRNA of the same tissue. In cats, immunostaining in visual cortex (eg. Figure 2) was also eliminated when the primary antibody was omitted (not shown). These negative and positive controls confirm the specificity of the antibody for Dab-1.
Figure 1.
Antibody specificity testing of a commercial anti-Dab-1 antibody. A. Western blots showing labeling of an appropriately sized band (approximately 80kDa) in mouse and cat visual cortex. B. Immunohistochemical tests of the Dab-1 antibody confirmed its specificity. Visual cortical sections from four week old mice were stained for comparison including, from left to right, wild-type, Dab-1 knockout (negative control), wild type with no primary antibody (negative control) and an in-situ stained section of wild-type showing Dab-1 mRNA expression (positive control). Scale bar indicates magnification.
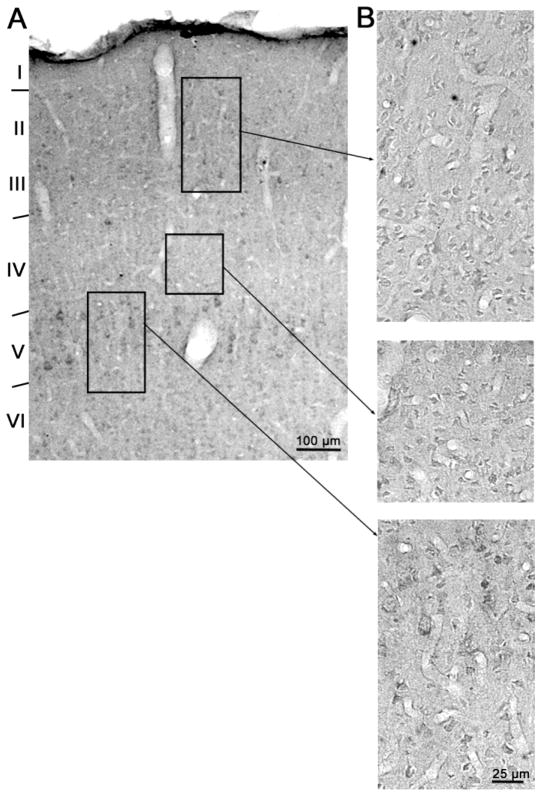
Figure 2.
A: Low magnification photomicrograph of normal 20 week old cat visual cortex immunolabeled for Dab-1. B: Higher magnification examples of sections of same image showing detail in layers II/III (top), layer IV (middle) and layers V/VI (bottom) as represented by boxes on low power image. While cellular staining is not apparent in all layers at low power (especially in layer IV) it becomes clearly evident when magnified for cellular quantification. Scale bars indicate magnification.
2.2 Dab-1 Laminar and Cellular Expression in Mature Visual Cortex
It is helpful in describing Dab-1 cellular and laminar expression in visual cortex to begin with consideration of the end product of postnatal critical period development. Figure 2 shows low and selected high magnification micrographs of a normally reared 20 week old cat. In low magnification inspection, the most pronounced immunolabel is the relatively intense staining of the large pyramidal cells of layers III and V and neuropil staining which is rather uniform across cortical layers. Against this background, layer IV appears to have diminished overall staining compared to extragranular layers. However, higher magnification inspection reveals immunopositive cellular labeling across all cortical lamina with lightly but definitively stained cells prevalent in granular and extragranular layers.
2.3 Development of Dab-1 Expression during the Visual Cortical Critical Period
Figure 3 shows development of Dab-1 expression in normally reared cat visual cortex throughout the critical period from postnatal week 1 to adult. To interpret the immunohistochemical laminar staining pattern at 1 week, it is necessary to recognize the immature cortical layering at this age. We followed the laminar designations of Luskin and Shatz (1985a). In neonates (1 week), layers V and VI are identifiable and a significant number of cells in layer VI are remnants of the embryonic subplate. At this age, cells in the superficial cortical plate (CP) are destined for deep layer III in the adult and cells in the deep cortical plate are destined to become layer IV. The remaining cells that will form layers II and the rest of layer III are densely packed in the compact zone (CZ). Layer I, which corresponds to the embryonic marginal zone (MZ) is also identifiable. At 1 week of age, there is prominent Dab-1 immunopositive cell staining across all layers which is most dense below the CZ (future layers II and upper III). Neuropil staining is faint across all layers and particularly light in the CZ and layer I.
Figure 3.

Low magnification photomicrographs showing Dab-1 immunoreactivity in cat visual cortex during postnatal development. The panel shows representative images of coronal sections of area 17 in normally reared 1, 5, 10, 20 week and adult cats. Cortical layers were identified and labeled following the designations of Luskin and Shatz (Luskin and Shatz, 1985a; Luskin and Shatz, 1985b) through cresyl violet staining of adjacent sections to those processed for Dab-1 immunohistochemistry. Scale bar indicates magnification.
As the cortex continues to develop postnatally, there are dramatic laminar differences in Dab-1 expression. By five weeks of age, there is a marked reduction in cellular label and a marked increase in neuropil labeling. Neuropil staining intensifies in all layers at this age but remains lightest in layers I and II. At 10 weeks of age, there is an increase in neuropil staining in layers I and II and this general laminar staining pattern is qualitatively maintained at subsequent ages. At 20 weeks, there is a continued decrease in cellular label and neuropil staining persists. In the adult, neuropil staining is densest in the superficial layers.
There were differences across ages in the distribution of Dab-1 within individual cells when viewed at higher magnification as shown in Figure 4. In 1 week old animals, staining in the soma included the nucleus, cytoplasm, cell membrane and apical dendrite. At 5 weeks, staining of the nucleus was much less evident. By 20 weeks, within individual cells, staining is most dense on the membrane and in the cytoplasm and nuclei is pale.
Figure 4.
High magnification photomicrographs showing comparison of immunohistochemical cellular labeling of cell somas at postnatal weeks 1, 5 and 20. Cytoplasmic staining is fairly consistent across all ages. Nuclear labeling changes from darkly labeled at one week, lighter by five weeks and faintly labeled compared to cytoplasmic at twenty weeks postnatally. Scale bar indicates magnification.
For quantification of Dab-1 immunolabeling, we initially attempted quantification by densitometric comparison of small regions in different cortical layers, a measure of combined cell body and neuropil staining. This procedure proved to be insufficiently sensitive to detect laminar differences due to age and dark rearing. For example, in 5, 10 and 20 week cats, densitometric readings comparing superficial layers (II, III), layer IV and deep layers (V,VI) were comparable in value. Moreover, these data are not readily interpreted because the laminar position of the cell body whose process contains the reaction product seen in the neuropil cannot be determined. We therefore turned to cell counts for quantification. A cell was considered as immunopositive if it showed the avidin-biotin reaction product, had a definable soma and an evident nucleus. No distinction was made between darkly and lightly stained cell bodies. The cell counts reliably discriminated superficial layers (II/III), layer IV and deep layers (V/VI) across age and rearing condition (see below). This quantification procedure has the advantage that a straightforward criterion is applied which helps minimize bias and criterion drift which can be introduced when cells are sorted by perceived staining intensity. It also provides a means to correct for changes in total cell density due to age and dark rearing by correcting against total cell counts made on adjacent cresyl violet stained sections (see Experimental Procedure,4.3). The disadvantage with this approach is that it does not account for neuropil staining or intense versus light cell body staining and consequently underestimates total Dab-1 expression. Dab-1 counts were expressed as a ratio to total cresyl violet cell counts to provide an index of relative proportion of Dab-1 neurons.
The top of Figure 5 shows the results of quantification of Dab-1 cellular expression indicating a marked (approximately 3 fold) progressive reduction in Dab-1 cellular expression across all layers as a function of age. The bottom of Figure 5 shows the age trend for total cellular Dab-1 expression by combining all layers. The observed developmental decline in Dab-1 cellular staining across ages was shown to be statistically significant by two way analysis of variance (F[2,18]=41.319, p<0.001). Analysis of laminar staining patterns throughout development also revealed significant differences (F[2,18]=8.143, p=0.003) among cortical laminae. Layer IV showed the highest relative proportion of Dab-1 neurons, layers II/III the lowest level and layers V, VI were intermediate. There was no statistically significant age x layer interaction, indicating that the decline in relative Dab-1 expression due to age was similar in all cortical layers.
Figure 5.
Line graphs showing quantification of Dab-1 immunohistochemistry in postnatal development. Top: Dab-1 cellular expression in superficial layers (II/III), layer IV, and deep layers (V/VI) corrected against total cell counts from adjacent cresyl violet stained sections. Bottom: Combined results of all cortical lamina. Individual counts as described above from each layer in each animal at each age were averaged together to determine data points. Each data point is the mean and standard error of three cats.
2.4 Effect of Dark Rearing on the Development on Dab-1 Expression
Figure 6 shows differences in Dab-1 immunohistochemical labeling between normal and dark reared cats at the peak (5 weeks) and nadir (20 weeks) of the critical period. While the overall qualitative laminar staining patterns are similar across age and rearing conditions, quantification revealed significant differences due to dark rearing as shown in Figure 7. Analysis of variance (three way) revealed a significant interaction between age and rearing condition (F[1,24]=10.881, P=0.003) indicating that the effect of age on relative Dab-1 expression is altered by dark rearing. Relative Dab-1 expression shows bidirectional regulation by age and dark rearing. The differences in cellular expression between normal and dark reared cats are opposite in direction between 5 week and 20 week animals. There was no significant age x dark rearing x cortical layer interaction, indicating that the effects of age and dark rearing were similar in all visual cortical laminae. Layer IV showed the highest cellular expression, layers II/III the lowest and layers V/VI were intermediate in expression in all age/rearing conditions.
Figure 6.
Low power photomicrographs showing Dab-1 immunoreactivity in cat visual cortex. The panel shows representative images of coronal sections of area 17 in normal and dark reared cats at the peak (5 weeks) and nadir (20 weeks) of the critical period. Cortical laminae are indicated and were determined by adjacent sections stained with cresyl violet. Scale bar indicates magnification.
Figure 7.
Line graphs showing quantification of Dab-1 immunohistochemisty in cortical lamina of cat visual cortex comparing normal and dark reared cats at the peak (5 weeks) and nadir (20 weeks) of the critical period. Top: Dab-1 cellular expression in superficial layers (II/III), layer IV, and deep layers (V/VI) corrected against total cell counts from adjacent cresyl violet stained sections. Bottom: Combined results of all cortical lamina. Individual counts as described above from each layer in each animal at each age were averaged together to determine data points. Each data point is the mean and standard error of three cats.
3. Discussion
3.1 Dab-1 Expression and the Visual Cortical Critical Period
The present results are the first to describe the laminar and cellular expression of the products of a newly recognized candidate neuroplasticity gene, Dab-1, in the classic model of postnatal neuronal plasticity, the critical period in visual cortex. The most dramatic laminar changes in Dab-1 expression occurred between 1 and 5 weeks of age, a period encompassing normal eye opening and the onset of the critical period. At 1 week, there is dense cellular staining which is rather uniform across the immature cortical layers and only light neuropil staining. At the peak of the critical period (5 weeks), dense cell staining is largely restricted to the large pyramidal cells of deep layer III and layer V. There is faint cell body staining throughout the remaining cortical layers and neuropil staining is markedly increased and rather uniform in layers III to VI. This dramatic change in laminar and cellular labeling intensity is a maturational process that is independent of visual input, since the laminar pattern is similar in normal and dark reared cats at 5 weeks. By 10 weeks, the mature laminar and cellular staining pattern is established and the major subsequent change is a further reduction in the density of cellular staining which is similar in all cortical layers. These developmental changes are altered by dark rearing. Age and dark rearing interact such that the differences in cellular expression between normal and dark reared cats are opposite in direction between young versus older animals. This bidirectional regulation of cellular expression, however, is the same in all cortical laminae. Thus, after 5 weeks, age and dark rearing appear to alter the levels of expression within cells but not laminar patterns of expression.
The bidirectional regulation of cellular expression by age and dark rearing matches the effects of age and dark rearing on physiological plasticity during the critical period as assessed by ocular dominance shifts that occur in response to monocular deprivation. Physiological plasticity is greater in normal than dark reared cats at 5 weeks and greater in dark reared than normal cats at 20 weeks (Beaver et al., 2001; Mower, 1991). This pattern of bidirectional regulation of cellular expression is also the same as that found by western blot analysis of total Dab-1 expression (Yang et al., 2006), however of much lower magnitude. In western blot analysis, the elevation of Dab-1 protein expression at 5 weeks and in dark reared cats at 20 weeks averaged 2.5 fold, whereas, in cell count analysis the differences in superficial, layer IV and deep layers averaged only 1.4 fold. This difference in magnitude is undoubtedly related to the fact that the cell counts do not account for neuropil or more intense cellular labeling and therefore underestimate total Dab-1 expression. For comparison of absolute changes in level of expression due to age and dark rearing, the western blot results should be considered definitive.
There are laminar differences in physiological plasticity of visual cortex. The effects of MD were classically thought to result from anatomical reorganization of thalamocortical input to ocular dominance columns in layer IV (Hubel and Wiesel, 1972). It is now clear that there is an extragranular mechanism for critical period plasticity. Studies of susceptibility to MD in dark reared cats (Mower et al., 1985) and of residual plasticity in visual cortex of more mature normally reared cats (Daw et al., 1992) indicated dramatic ocular dominance changes in the extragranular layers without significant alterations in layer IV. Our cellular quantification results, however, did not detect laminar differences: the age and dark rearing effects on the level of cellular expression were comparable in all layers. Perhaps, differences among laminae in physiological plasticity can be explained by changes in absolute level of expression that are similar across cortical layers. For example, differential thresholds for Dab-1 signaling in different cortical layers could be sufficient to activate plasticity mechanisms in some but not other cortical layers in response to age and dark rearing. The contribution of Dab-1 in the neuropil, which is greatest in extragranular layers, also cannot be overlooked.
3.2 Reelin/Dab-1 Signaling in Neuronal Development and Plasticity
Several lines of functional evidence, primarily from postnatal and adult hippocampus, directly implicate the Reelin/Dab-1 pathway in synaptic plasticity. Interference with Reelin/Dab-1 signaling reduces long-term potentiation (LTP) and perfusion of Reelin enhances LTP in the hippocampus (Beffert et al., 2005; Weeber et al., 2002). Reelin/Dab-1 signaling promotes dendritic maturation of hippocampal and cortical neurons as well as dendritic spine development (Chameau et al., 2009; Niu et al., 2004; Niu et al., 2008; Pujadas et al., 2010). Developmentally, changes in levels of exogenous Reelin affect normal changes in NMDA and AMPA receptor currents (Chen et al., 2005; Qiu et al., 2006; Sinagra et al., 2005). Reelin/Dab-1 signaling also alters the relative contribution of NR2A and NR2B receptor subunits to NMDA receptor composition (Campo et al., 2009; Groc et al., 2007). A developmental switch from immature to mature NMDA receptors, which is due to a change from NR2B to NR2A subunit dominance, is a widely accepted mechanism for hippocampal LTP, LTD and visual cortical neuroplasticity (see Lau and Zukin, 2007; Yashiro and Philpot, 2008 for reviews). A balance of excitation and inhibition is also an important mechanism for visual cortical plasticity. Inhibitory mechanisms may suppress excitatory ones to contribute to the close of the critical period (Kirkwood and Bear, 1994). However, for critical period onset a threshold of inhibition must be exceeded for plasticity to occur (Hensch and Fagiolini, 2005). Double label immunohistochemical studies indicate that Reelin is expressed predominately in GABA interneurons ((Alcantara et al., 1998; Pesold et al., 1998a; Pesold et al., 1998b; Pesold et al., 1999) and Dab-1 predominately in pyramidal cells (Pesold et al., 1999) of young and adult neocortex. This segregation of Reelin and Dab-1 expression to inhibitory and excitatory neurons respectively, positions the Reelin/Dab-1 pathway to mediate excitatory inhibitory interactions believed to control visual cortical plasticity.
Growing evidence indicates a role of the Reelin/Dab-1 pathway in critical period neuroplasticity of visual cortex. The present results strengthen that evidence by showing that the effects of age and dark rearing on cellular expression of Dab-1 in visual cortex are the same as their effects on physiologically determined neuroplasticity in response to monocular deprivation.
4. Experimental Procedure
4.1 Animals
A total of 21 cats were used for this study. They were reared in a normal 12 light/dark cycle until 1, 5, 10, 20 weeks or adult (n = 3 at each age) or in complete darkness from birth to 5 or 20 weeks of age (n = 3 at each age). Cats were purchased from Liberty Research, Inc (Waverly, NY). They were raised in the Research Resource Center, University of Louisville. Dab-1 knockout and wild type mice were used to test antibody specificity. Heterozygous animals were purchased from The Jackson Laboratory (Bar Harbour, Maine), bred in the Research Resource Center, University of Louisville and genotyped by PCR using supplied primers.
Cats and mice were sacrificed by an overdose of sodium pentobarbital (75mg/kg i.p.). Perfusion was performed intracardially through the left ventricle with 37°C PBS (0.9% NaCl in 0.1 M phosphate buffer, pH=7.4) until fluid from right atrium ran clear indicating complete flushing of blood from the systemic circuit. This was immediately followed by perfusion of 4% paraformaldehyde in PBS at 4°C for approximately 20 minutes. Brains were removed and blocked into cerebral hemispheres divided into anterior and posterior regions, cerebellar hemispheres and brainstem structures. The tissues were post-fixed in 4% paraformaldehyde in PBS overnight at 4°C. Finally, the tissues were cryoprotected by immersion in 30% sucrose in PBS at 4°C until they sank. The blocked sections were frozen in crushed dry ice and stored at −80°C until use. All procedures conformed to the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
4.2 Staining Procedure: Immunohistochemistry and in-situ hybridization
Free floating 40μm sections were used for this study and all steps were performed on a shaker table at room temperature unless otherwise specified. Adjacent sections of each sample were stained in different concentrations of primary antibody (1:4000 and 1:8000) to verify staining patterns within individual sample runs. Sections from all ages and rearing conditions were run simultaneously in the same solutions to ensure comparability between animals in each run. Triplicate runs were performed to ensure repeatability.
The immunohistochemical protocol began with a series of three, 5 minute washes in PBS. Sections were blocked with 10% normal goat serum (NGS) in PBS with 0.1% Triton X-100 for 60 min. The immunohistochemical reaction itself followed this sequence of steps: incubation in primary antibody in PBS with 1% NGS and 0.1% Triton X-100 at 4°C overnight; treatment with 4% NGS in PBS for 45 min.; incubation in biotinylated secondary antibody (1:200 per Vector ABC Kit) for 45 min.; three 15 min. washes with PBS; incubation in ABC Reagent (per Vector ABC Kit) for one hour; three 15 min. washes with PBS; and finally the DAB reaction (0.05% diaminobenzedine-4 HCl with 0.01% H2O2 in 0.17M Tris-HCl) per DAB kit (Vector Laboratories, Burlingame, CA) for 3–5 min or until clearly visible brown reaction product appeared. Once staining was complete, sections were washed three times with PBS for 15 minutes.
The stained sections were mounted onto subbed Superfrost/Plus slides (Fisher Scientific) and air-dried overnight. The sections were then dehydrated through a sequential series of alcohols for 3 minutes each, (50%, 70%, 95%, 100%), cleared in xylenes (3 min × 2), and coverslipped with Permount (Fisher Scientific).
The sections adjacent to those labeled by immunohistochemistry were stained with cresyl violet for laminar analysis and cellular quantification as follows. Sections were mounted onto subbed Superfrost/Plus microscope slides and, air-dried overnight. Sections were cleared in xylenes for 3 min. and were then rehydrated through a sequential alcohol series for 3 min. each (100%, 95%, and 70%). Sections were then rinsed with ddH2O for 3 min.; immersed in warm Cresyl violet (1g Cresyl fast violet dissolved in 1000 ml ddH2O with 10 ml 10% acetic acid, warmed to 45°C) for 30 sec.; briefly rinsed in ddH2O and differentiated in 70% alcohol (with a few drops of glacial acetic acid) for 1 min. The sections were then dehydrated through a sequential series alcohols for 3 minutes each (50%, 70%, 95%, and 100%), and cleared in xylenes (3 min × 2) before being coverslipped with Permount.
Western blot, for antibody specificity testing, was performed as previously described (Guo et al., 1997) using the same primary antibody as immunohistochemistry and visualized with enhanced chemiluminescence (ThermoScientific, Rockford, IL). In-situ hybridization (ISH), for antibody specificity testing, was performed as described (Liu et al., 2008). cDNA templates for ISH were obtained by reverse transcription- PCR of postnatal brain tissues. Several Dab-1 probes were designed. Two of them worked well, one against nucleotides 349–1142, and a second against nucleotides 1906–2666.
4.3 Immunohistochemistry Quantification and Analysis
The slides were photographed using a computerized system (SPOT software, v2.2, SPOT Diagnostic Instruments, Inc.) to obtain both high and low magnification images of the visual cortex. Qualitative analysis was based on visual observation of laminar staining differences within and between samples of different ages and rearing conditions. Quantitative analysis was conducted through blind manual cell counts in different cortical lamina using AlphaView software (version 2.0.1.1, Alpha Innotech Corporation) as described below.
In order to obtain unbiased results, digital images of immunostained sections and their adjacent cresyl violet stained sections were formatted for software analysis (same file size and creation date) and placed into folders on the computer desktop. A single copy of the coding key identifying each sample section was printed and, in the absence of the primary author, each file was renamed with a random file name (numbers for immunolabeled and letters for cresyl violet sections) and recorded on the code sheet. This sheet was kept secured until quantification was complete.
Each unknown picture file was opened into the counting software. Two identical size counting area boxes were created for each layer examined (II/III, IV and V/VI) which were maintained across all samples analyzed. The boxes were placed within representative regions of the layers to be analyzed at a lower magnification to maintain accurate positioning within each layer. Once all boxes were positioned within an individual sample, magnification was increased in each box to accurately identify immunopositive cells. A cell was counted if it showed the avidin-biotin reaction product, had a definable soma and an evident nucleus. Cells intersecting the left and bottom margins of the counting box were not counted but those intersecting the top and right margins were included. Through the use of the software, a manual mouse-click on each cell marked it as being counted, preventing it from being double-counted, and it was added to the running count maintained by the computer. Upon completion, the individual box cell count was recorded and reset each time for each box. This procedure was repeated on adjacent Dab-1 immunolabeled sections from the same animals resulting in four sample boxes from each lamina for each age and rearing condition. Only after all samples had been counted and recorded were the codes revealed identifying which cell counts related to which ages and rearing conditions for data analysis.
In order to correct for changes in total cell density due to age and/or dark-rearing, Dab-1 cell counts were corrected by total cell counts as described previously (Guo et al., 1997; Mower et al., 1988)). Counting procedures for cresyl violet sections were the same as those described for immunohistochemistry. Dab-1 to total cell count ratios were determined from the same layers in adjacent sections.
Acknowledgments
This work was supported by NIH R01 EY016724 (GDM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–99. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade N, Komnenovic V, Blake SM, Jossin Y, Howell B, Goffinet A, Schneider WJ, Nimpf J. ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc Natl Acad Sci U S A. 2007;104:8508–13. doi: 10.1073/pnas.0611391104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Daw NW. Layer differences in the effect of monocular vision in light- and dark-reared kittens. Vis Neurosci. 2001;18:811–20. doi: 10.1017/s0952523801185147. [DOI] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–79. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, Herz J. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–9. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One. 2009;4:e5505. doi: 10.1371/journal.pone.0005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2009;106:7227–32. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–16. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–40. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Daw NW, Fox K, Sato H, Czepita D. Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol. 1992;67:197–202. doi: 10.1152/jn.1992.67.1.197. [DOI] [PubMed] [Google Scholar]
- Daw NW. Visual Development. Springer Science+Business Media, Inc; New York, NY: 2006. [Google Scholar]
- Deguchi K, Inoue K, Avila WE, Lopez-Terrada D, Antalffy BA, Quattrocchi CC, Sheldon M, Mikoshiba K, D’Arcangelo G, Armstrong DL. Reelin and disabled-1 expression in developing and mature human cortical neurons. J Neuropathol Exp Neurol. 2003;62:676–84. doi: 10.1093/jnen/62.6.676. [DOI] [PubMed] [Google Scholar]
- Feng L, Cooper JA. Dual functions of Dab1 during brain development. Mol Cell Biol. 2009;29:324–32. doi: 10.1128/MCB.00663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Chai X, Bock HH, Haas CA, Forster E, Zhao S. Role of Reelin in the development and maintenance of cortical lamination. J Neural Transm. 2009;116:1451–5. doi: 10.1007/s00702-009-0228-7. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci. 2007;27:10165–75. doi: 10.1523/JNEUROSCI.1772-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Kaplan IV, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of the cat visual cortex. Brain Res Dev Brain Res. 1997;103:127–41. doi: 10.1016/s0165-3806(97)81789-0. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–24. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997a;16:121–32. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997b;389:733–7. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146:421–50. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–45. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Qiu S, Weeber EJ. The role of reelin in adult synaptic function and the genetic and epigenetic regulation of the reelin gene. Biochim Biophys Acta. 2008;1779:422–31. doi: 10.1016/j.bbagrm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li H, Hu X, Yu L, Liu H, Han R, Colella R, Mower GD, Chen Y, Qiu M. Control of precerebellar neuron development by Olig3 bHLH transcription factor. J Neurosci. 2008;28:10124–33. doi: 10.1523/JNEUROSCI.3769-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Neurogenesis of the cat’s primary visual cortex. J Comp Neurol. 1985a;242:611–31. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Studies of the earliest generated cells of the cat’s visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985b;5:1062–75. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Res. 1981;220:255–67. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Mower GD, Caplan CJ, Christen WG, Duffy FH. Dark rearing prolongs physiological but not anatomical plasticity of the cat visual cortex. J Comp Neurol. 1985;235:448–66. doi: 10.1002/cne.902350404. [DOI] [PubMed] [Google Scholar]
- Mower GD, Rustad R, White WF. Quantitative comparisons of gamma-aminobutyric acid neurons and receptors in the visual cortex of normal and dark-reared cats. J Comp Neurol. 1988;272:293–302. doi: 10.1002/cne.902720211. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58:151–8. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10339–48. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998a;95:3221–6. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Pisu MG, Impagnatiello F, Uzunov DP, Caruncho HJ. Simultaneous detection of glutamic acid decarboxylase and reelin mRNA in adult rat neurons using in situ hybridization and immunofluorescence. Brain Res Brain Res Protoc. 1998b;3:155–60. doi: 10.1016/s1385-299x(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ. Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci U S A. 1999;96:3217–22. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martinez A, Delgado-Garcia JM, Soriano E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–49. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–55. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DS, Sheldon M, D’Arcangelo G, Nakajima K, Goldowitz D, Curran T. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125:3719–29. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci. 2001;24:1005–39. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Rice DS, Nusinowitz S, Azimi AM, Martinez A, Soriano E, Curran T. The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron. 2001;31:929–41. doi: 10.1016/s0896-6273(01)00436-6. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Weeber EJ. Reelin and apoE actions on signal transduction, synaptic function and memory formation. Neuron Glia Biol. 2008;4:259–70. doi: 10.1017/S1740925X09990184. [DOI] [PubMed] [Google Scholar]
- Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P. Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci. 2005;25:6127–36. doi: 10.1523/JNEUROSCI.1757-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt PC, Chen Y, Liu P, Bock HH, Blacklow SC, Herz J. Phosphoinositide binding by the disabled-1 PTB domain is necessary for membrane localization and Reelin signal transduction. J Biol Chem. 2005;280:9671–7. doi: 10.1074/jbc.M413356200. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–52. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- Yabut O, Renfro A, Niu S, Swann JW, Marin O, D’Arcangelo G. Abnormal laminar position and dendrite development of interneurons in the reeler forebrain. Brain Res. 2007;1140:75–83. doi: 10.1016/j.brainres.2005.09.070. [DOI] [PubMed] [Google Scholar]
- Yang CB, Zheng YT, Kiser PJ, Mower GD. Identification of disabled-1 as a candidate gene for critical period neuroplasticity in cat and mouse visual cortex. Eur J Neurosci. 2006;23:2804–8. doi: 10.1111/j.1460-9568.2006.04799.x. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–94. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]