Abstract
Introduction
Sepsis remains a leading cause of death worldwide. Despite years of extensive research, effective drugs that inhibit the pro-inflammatory effects of lipopolysaccharide (LPS) and improve outcome when added to conventional sepsis treatments are lacking. Eritoran tetrasodium (E5564) is a promising candidate therapy for sepsis belonging to a new class of such drugs which inhibit LPS-induced inflammation by blocking toll-like receptor 4 (TLR4).
Areas covered
This review focuses on the rationale for the use of eritoran tetrasodium in sepsis, as well as on its pharmacokinetics, pharmacodynamics, efficacy and safety. Pre-clinical and clinical studies from a MEDLINE/PubMed literature search in August 2010 with the search terms “eritoran” and “E5564” are discussed.
Expert opinion
Preclinical in vitro and in vivo studies of eritoran tetrasodium indicate it can limit excessive inflammatory mediator release associated with LPS, and improve survival in sepsis models. While early clinical results are promising, its efficacy and safety for treating patients with sepsis is currently under investigation. Even if the ongoing phase III clinical trial enrolling patients with severe sepsis and increased risk of death shows benefit from eritoran, questions remain and confirmatory studies will be necessary to define its clinical usage.
Keywords: Eritoran tetrasodium, E5564, TLR4, LPS, toll like receptor, endotoxin, sepsis, therapy, inflammation
1. Introduction
Stimulation of innate immune and associated inflammatory responses by microbial products is an essential early step in host defense and microbial clearance during invasive bacterial infection. It is hypothesized, however, that in some patients this response can become excessive or poorly controlled, and host inflammatory mediator release itself may then contribute to the organ injury, hypotension and mortality characterizing sepsis and septic shock. A pivotal component of innate immunity is recognition of conserved microbial components, termed pathogen associated molecular patterns (PAMPs), by receptors on host leukocytes. When selectively engaged by PAMPs, these receptors stimulate host intracellular signaling pathways, which in turn activate an array of pro-inflammatory responses (e.g., phagocytosis and release of inflammatory mediators) 1–4.
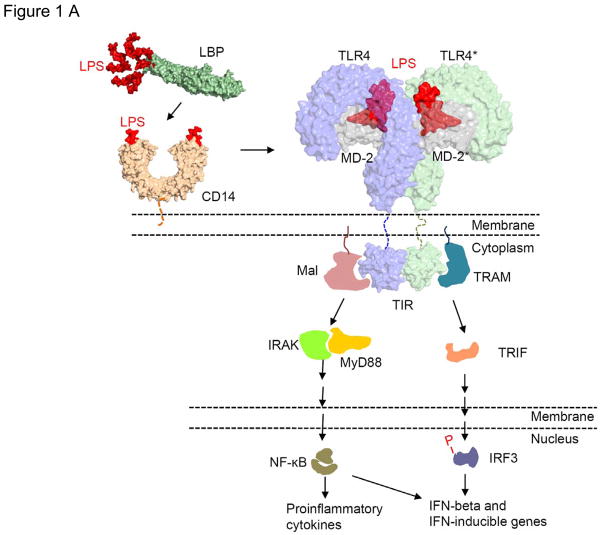
One of the most studied microbial PAMPs is lipopolysaccharide (LPS) or endotoxin, a component of gram-negative bacterial cell walls which is well known for its ability to stimulate pro-inflammatory responses. LPS binds to toll-like receptor 4-myeloid differentiation factor 2 complexes (TLR4-MD2) on host-cell surfaces and promotes their dimerization. This initiates intracellular signaling, including activation of nuclear transduction factors (e.g., nuclear factor kappa B, NF-κB) and the production and release of pro-inflammatory cytokines, chemokines and other molecules (TNF-α, IL-1, IL-6, IL-8, kinins, histamines etc.) (Figure 1A)5.
Figure 1.
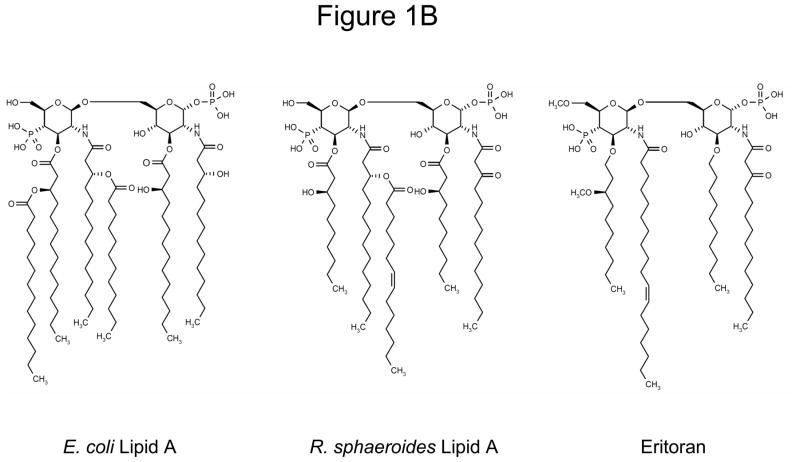
A. LPS signaling through TLR4-MD2 interaction. Molecules are not drawn to scale. Reproduced from [5] with permission from the Nature Publishing Group. B. Comparison of chemical structures of E. coli lipid A, R. sphaeroides lipid A and eritoran. Reproduced, with permission from Thomson Reuters and Rossignol DP, Lynn M: TLR4 antagonists for endotoxemia and beyond Current Opinion in Investigational Drugs (2005) 6(5):496–502. Copyright 2005, Thomson Reuters (Professional) UK Limited (TRPUL).” [66]
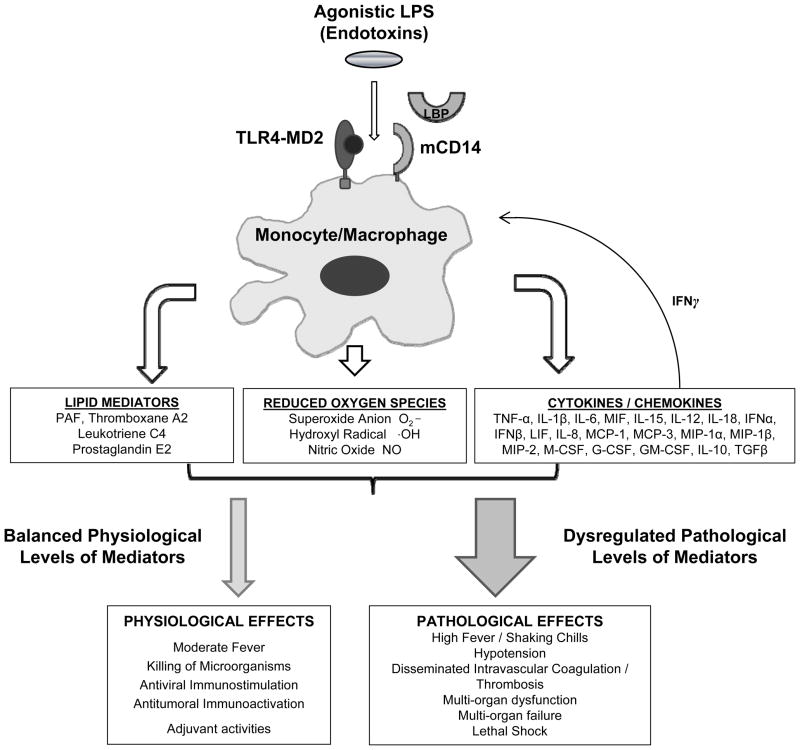
While intact LPS signaling appears important for the clearance of gram-negative bacteria in animal infection models, it is believed also to be associated with the excessive inflammatory response related to sepsis6–9. Thus, despite its potential contribution to innate immunity, LPS remains a logical target for inhibition in the treatment of severe sepsis and septic shock (Figure 2)10. Previous attempts to block LPS signaling clinically included use of monoclonal antibodies against not only LPS, but also associated molecules like CD14 and downstream cytokines like TNF-α. Since TLR4 is the final cell-surface receptor through which LPS mediates its intracellular effects, it may be a more efficacious target.
Figure 2.
Macrophage mediated activation of innate immunity by LPS. Extracellular LPS is transferred to membrane bound CD14 (mCD14) by the action of LPS binding protein (LBP), and then interacts with TLR4-MD2 complex to initiate an intracellular response. In physiological situations, LPS signaling via TLR4-MD2 results in comparably small amounts of cytokine and chemokine mediator release, leading to activation of host defences against invading micro-organisms. If this response is deregulated, unbalanced levels of inflammatory mediators lead to a pathological state with life-threatening results as seen in severe sepsis or septic shock. (Adapted from [14] with permission from Sage Publications.
The ideal TLR4 antagonist should have strong inhibitory effects without any agonist activity. TLR4 antagonists include molecules such as eritoran and its predecessors (e.g., E5531), resatorvid (TAK 242, a small molecule inhibitor of TLR4-CD14 mediated intracellular signaling), and antibodies targeting the TLR4 receptor. Of note, some therapeutic agents such as ketamine, opioids and statins may also non-selectively interfere with TLR410–12.
2. Eritoran tetrasodium
2.1 Overview of Lipid A analogs with TLR4 antagonist actions
LPS is a complex molecule made up of three main parts; the O antigen polysaccharide, the core oligosaccharide and the lipid A region (Figure 1B). While the O antigen and core from different bacterial species vary, the lipid A region, which is also the main toxicophore of LPS, appears to be highly conserved13. Naturally occurring lipid A’s from Rhodobacter capsulatus and sphaeroides lack potent agonist activity, but inhibit the effects of E. coli derived LPS14. Lipid A from R. capsulatus was the basis for the synthesis of E5531, a stable and non-toxic LPS antagonist in endotoxemia models. Difficulties with large scale synthesis and purification of E5531 led to development of the second-generation LPS antagonist E5564 (eritoran tetrasodium)15.
2.2 Introduction to Eritoran tetrasodium
Eritoran tetrasodium is a structural analog of the lipid A from R sphaeroides (RsLA), originally synthesized at the Eisai Research Institute of Boston (Andover, MA)15. Eritoran competitively binds to TLR4-MD2 and inhibits LPS from initiating an inflammatory response without significant intrinsic agonistic effects. It blocked NF-κB activation, and TNF-α and IL-6 production following LPS stimulation in vitro and in vivo, both in animal and human models of endotoxemia16. Eritoran conferred a survival benefit in animal models of bacterial sepsis. Phase I and II studies showed eritoran could inhibit endotoxin challenge induced cytokine production. A phase II study in septic patients showed possible survival benefits in subgroups. Other investigators are studying its use for treating various other potentially TLR4 mediated conditions, e.g., chronic LPS-mediated airway disease,17 liver disease,18, 19 contact lens-associated corneal inflammation,20 myocardial and renal ischemia-reperfusion injury21, 22.
2.3 Chemistry
The chemical name of eritoran is α-D-Glucopyranose,3-O-decyl-2-deoxy-6-O-[2-deoxy-3-O-(3R)-3-methoxydecyl]-6-O-methyl-2-[[(11Z)-1-oxo-11-octadecenyl]amino-4-O-phosphono-β-D-glucopyranosyl]-2-[(1,3-dioxotetradecyl)amino]-1-(dihydrogen phosphate), tetrasodium salt (Box 1)23. It is a synthetic glycolipid dimer containing an ether linkage, derived from naturally occurring RsLA15. Compared to its predecessor E5531, eritoran is more potent in its anti-endotoxin effects, longer acting (due to less inactivation by plasma lipoproteins) and easier to manufacture. No LPS-like agonist activity has been observed in humans, dogs, rats and mice treated with eritoran, although it has been noted in equine whole blood15, 24. Eritoran does not directly interact with TLR4, but competes with LPS in binding to the hydrophobic pocket in MD-2 and deters dimerization of TLR4-MD2 complexes, thus inhibiting intracellular signaling5, 25, 26. While LPS interaction with TLR4-MD-2 on the cell surface occurs through a series of steps involving other molecules such as LPS binding protein (LBP), sCD14 and mCD14, eritoran interacts with TLR4-MD2 directly16.
2.4 Pharmacodynamics
The pharmacodynamic effects and half-life of eritoran are dose dependent, and much shorter than its pharmacokinetic half-life, as the drug rapidly becomes inactive in whole blood and serum, possibly due to binding with HDL24, 27. Eritoran’s duration of action can be extended by using higher doses and by continuous or intermittent infusions28. The 50% inhibitory concentration (IC50) necessary to block LPS-induced responses varies with the timing of administration of eritoran in relation to LPS, as well as dose and bacterial type of LPS challenge (Table 1)16. The concentration of eritoran that completely abolishes cytokine production in response to LPS also varies with individual cytokines. Although similar doses of eritoran (unadjusted for weight) produced somewhat higher drug levels in females compared to males in a phase I study, this did not appear to produce significantly different pharmacodynamic activity or safety profiles28.
Table 1.
Mean inhibitory concentrations (IC50) of eritoran required to inhibit TNF-α production by 50% in whole human blood incubated with LPS (10 ng/mL, except for S. enteritidis, which was 1ng/mL) from various bacterial strains or with whole killed bacteria (dry weight 100ng/mL). Adapted from Reference 16 with permission from the American Society of Pharmacology and Experimental Therapeutics.
| Agonist used to stimulate TNF-α production | Amount of TNF-α released (Mean ± SE, pg/mL) | IC50 (mean ± SE) of Eritoran (nM) |
|---|---|---|
| Bacterial Source of LPS | ||
| S. minnesota | 2793 ± 99 | 12.4 ± 5.1 |
| S. marcescens | 3128 ± 91 | 10.3 ± 6.2 |
| S. typhimurium | 3091 ±182 | 9.4 ± 6.7 |
| K. pneumoniae | 2868 ± 104 | 8.5 ± 5.0 |
| S. minnesota R595 | 1578 ± 284 | 7.6 ± 2.9 |
| S. enteritidis | 2279 ± 184 | 2.6 ± 0.47 |
| E. coli | 1142 ±155 | 1.6 ± 0.3 |
| P. aeruginosa | 2027 ± 185 | 1.0 ± 0.21 |
| Type of whole killed bacteria | ||
| E. aerogenes (ATCC) | 2165 ± 299 | 1.5 ± 0.7 |
| E. aerogenes (clinical isolate) | 2558 ± 389 | 1.2 ± 0.5 |
| Whole E. coli | 3172 ± 413 | 0.65 ± 0.32 |
2.5 Pharmacokinetics and metabolism
Intravenous eritoran appears to have linear pharmacokinetics. Maximum concentrations (Cmax) of the drug in serum are achieved in a dose dependent fashion at the end of infusion, as determined by the area under the plasma concentration versus time curve (AUC). The volume of distribution at steady state (Vdss) is relatively small – about 40–55mL/kg - even after longer, higher dose infusions, indicating that eritoran is not widely distributed to other body tissues. Plasma clearance is slow (around 1mL/h/kg) and inversely proportional to the dose, and its elimination half-life is consequently relatively long (t1/2 ~ 50h). Eritoran pharmacokinetics do not differ significantly between men and women after adjustment for body weight27–29.
Since eritoran and its predecessor E5531 gradually become inactive upon binding to high density lipoprotein (HDL) in plasma, drug distribution within plasma lipoproteins has been the subject of some study29, 30. Binding to lipoproteins occurs quickly (within 5 min), without significant subsequent redistribution between various lipoprotein fractions. Drug dose or total cholesterol levels do not significantly affect distribution of eritoran in plasma proteins; > 60% of the drug was recovered from the HDL fraction, ~15% from low density lipoprotein (LDL) and < 5% from very low density lipoprotein (VLDL) in subjects with either high or low cholesterol levels.30 However, partitioning of the drug within the lipoprotein fractions was dependent on the relative size and content of these fractions. Eritoran has an affinity for HDL, and more drug was recovered from HDL in those subjects who had higher HDL levels, and likewise from the non-HDL compartments. Within the HDL compartment, it preferentially associates with HDL3, which contains more protein, compared to HDL2. Eritoran is highly soluble in the lipid portion of the non-HDL fractions (LDL and triglyceride-rich lipoprotein, TRL), and drug binding to HDL is influenced by LDL and TRL lipid mass and surface area, but not their surface charges29, 30. LBP does not alter the distribution and binding of eritoran to plasma lipoproteins31.Since septic patients can have alterations in plasma lipoprotein content, these findings may have to be considered during clinical use of eritoran30.
Based on rat studies, shortly after infusion most of the drug is found in plasma, but this decreases over time as it is taken up by the liver and to a lesser extent by the adrenal gland, bone marrow, lymph nodes and spleen. Overall, total blood clearance of the drug is much smaller than hepatic blood flow. It is converted to its inactive metabolites by dephosphorylation. These metabolites are eliminated slowly from the body mainly through feces32. Eritoran formulated in small micelles (8 nm) resulted in increased AUC0–72h and drug concentration at 5 min, and decreased clearance than large micelles (27 nm) in rabbits33.
Since eritoran is mainly metabolized hepatically, the effects of liver disease on eritoran pharmacokinetics were studied in volunteers with Child Pugh class A and B liver disease34. Six doses (total 30mg) of eritoran were infused every 12 h and blood was collected at multiple time points for analysis. There were no measurable differences between healthy and hepatically-impaired subjects in terms of eritoran pharmacokinetics, suggesting that dose adjustment would be unnecessary when using the drug in patients with liver disease. However, since eritoran is hepatically metabolized, its use in patients with various forms of hepatic dysfunction or in those requiring other therapies that alter hepatic metabolism may still require adjustment.
2.6 Efficacy
2.6.1 Pre-clinical studies
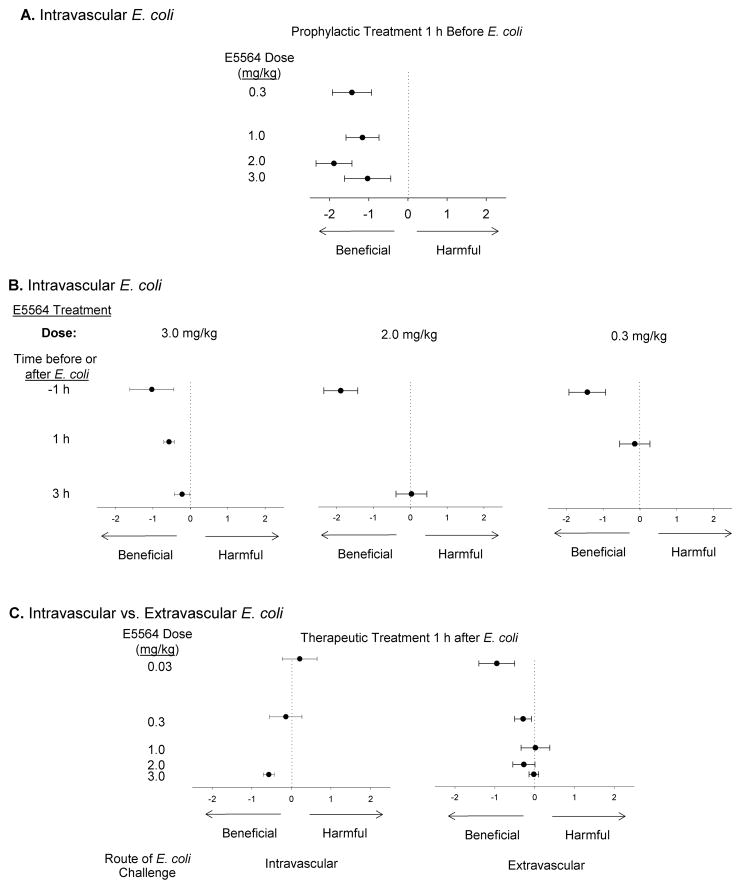
Using whole blood or monocytes from humans as well as blood or macrophages from mice, rats or guinea pigs, eritoran has been shown to inhibit LPS stimulated cytokine (IL-1β, TNF-α, IL-6 and IL-8) production in ex vivo assays16. Furthermore, in vivo studies have demonstrated the efficacy of eritoran in blocking responses to intravenous LPS in BCG-primed mice, guinea pigs and rats, as well as in dogs and humans. Few studies in animals investigated the effect of eritoran on LPS or bacteria induced mortality (outlined in Table 2 and 3). Indeed, the only study we know of that evaluated eritoran in an immunocompetent animal model employing a live bacterial challenge and concurrent antibiotic treatment (i.e., simulating important conditions encountered clinically) was performed by our group35. We tested the effects of varying doses of eritoran given at differing times before or after intravascular (IV) or intrabronchial (IB) E. coli challenge; results suggested that effective dosing with eritoran was dependent on both the timing of treatment and the route of infection (Figure 3).
Table 2.
Summary of published animal studies assessing the survival effects of eritoran in various models of sepsis †
| Author/Date | Model/Challenge | Treatment | N | Dose | Mortality | Duration |
|---|---|---|---|---|---|---|
| Mullarkey et al, 200316 | BCG primed mice challenged with IV LPS (100mcg/kg) | Placebo | 10 | 0 mg/kg | 90% | 72h |
| Eritoran | 10 | 6.25mcg/kg | 70% | 72h | ||
| Eritoran | 10 | 12.5mcg/kg | 60% | 72h | ||
| Eritoran | 10 | 25mcg/kg | *10% | 72h | ||
| Eritoran | 10 | 50mcg/kg | *10% | 72h | ||
| Eritoran | 10 | 100mcg/kg | *0% | 72h | ||
| Mullarkey et al, 200316 | BCG primed mice challenged with IP E. coli bacteria | Placebo | 20 | 0 mg/kg | 90% | 72h |
| Lmfx alone | 20 | 30mg/kg | 65% | 72h | ||
| Eritoran alone | 20 | 5mg/kg | 75% | 72h | ||
| Lmfx + Eritoran | 20 | As above | *20% | 72h | ||
| Kitazawa et al, 200918 | Male Wistar rats challenged with IP GalN (500mg/kg) and LPS (50mcg/kg) to induce acute liver failure | Placebo | 60 | 0 mg/kg | 92% | 24 h |
| Eritoran | 16 | 3 mg/kg | 57% | 24 h | ||
One study (Reference 35), not listed in this table, is described in detail elsewhere in the text and in Figure 3.
Significantly different compared to controls. IV, intravenous; IP, intraperitoneal; IB, intrabronchial; LPS, lipopolysaccharide; BCG, Bacille Calmette-Guerin; Cftx, Ceftriaxone; Lmfx; Latamoxef (antibiotic); GalN, D-galactosamine.
Table 3.
Summary of published pre-clinical in vivo studies investigating the characteristics (chemical, pharmacokinetic or pharmacodynamic) or the anti-inflammatory effects of eritoran
| Study Author (Date) | Animal Model | Purpose of Study | In vivo Challenge | Dose of Eritoran | Description of Outcome |
|---|---|---|---|---|---|
| Kaneko et al. (1999)63 | Sprague-Dawley rats, Beagles | Develop an assay for plasma eritoran levels to allow evaluation of PK/PD in rat/dog plasma | NA | 0.3mg/kg | Validation of HPLC method for measuring eritoran levels (range 30ng-20mcg/mL) and PK profiles in rat and dog plasma |
| Suganuma et al. (2000)67 | Beagles | Determine the effect (and PK/PD) of eritoran in dogs challenged with lipopolysaccharide (LPS) | LPS, 300ng/kg at 20h and 24h post infusion | 0.03 and 0.1 mg/kg/h x 24h | Presence of drug in plasma (by measured drug levels) does not correlate with drug activity to block LPS induced changes (no PK/PD correlation) |
| Kaneko et al. (2003)32 | Sprague-Dawley rats | Determine PK and disposition of eritoran in rats | NA | 0.1, 0.3, 0.5 or 1mg/kg | Eritoran is mainly taken up by the liver, dephosphorylated and excreted in feces |
| Kaneko et al. (2004)31 | C57/Bl6 female mice, LBP KO mice | Determine whether LPS binding protein (LBP) participates in PK of eritoran | NA | 0.5 mg/kg | LBP binds eritoran similar to LPS, but is not necessary for redistribution or clearance of drug from plasma |
| Savov et al. (2005)17 | C3HeB/FeJ mice | Test efficacy of intratracheal eritoran pretreatment in preventing chronic LPS induced airway disease | LPS 4.7mcg/m2 aerosol | 10, 100mcg; 50mcg tiw for 5 wks | Eritoran attenuated physiologic and biologic effects of acute and chronic LPS |
| Zhou et al. (2005)64 | C57/Bl6 mice | Determine the effects of eritoran in LPS and oleic acid (OA) mediated acute lung injury (ALI) | 20mcg/g | 4mcg/g | Pre-treatment with eritoran attenuated synergistic injurious effects of LPS and OA on the lungs |
| Shimamoto et al. (2006)21 | C57/Bl6 mice | Determine effect of eritoran on myocardial infarction (MI) and reperfusion injury after LAD occlusion | Left anterior descending artery (LAD) occlusion | 5mg/kg | Eritoran attenuated infarct size, JNK phosphorylation, NF-κB activation and myocardial inflammatory gene expression |
| Wasan et al. (2008)33 | Female rabbits | Determine influence of plasma lipids and eritoran micelle size on PK and ex vivo activity | NA | 0.5mg/kg | Small micelles cleared slower and had more PD activity than large ones; diet or lipid levels did not affect eritoran PK/PD |
| Sun et al. (2009)20 | C57/Bl6 mice | Determine effect of eritoran in contact lens associated corneal infiltrates | 40 mcg LPS or killed P. aeruginosa suspended in 0.3% tobramycin in PBS | 1–2 mcL of a 1.1mg/mL solution | Eritoran pre- and post-treatment attenuated corneal inflammation induced by LPS or killed bacteria. |
| Kitazawa et al. (2009)19 | Wistar rats | Determine the effect of eritoran on D-galactosamine (GalN) induced acute liver failure in rats | GalN, 1g/kg intraperitoneal | 3mg/kg Eritoran iv | Eritoran reduced liver injury secondary to GalN |
| Ma et al. (2009)65 | Sprague-Dawley rats | Determine if the pathogenesis of subarchnoid hemorrhage (SAH) is TLR4 mediated | Surgically induced SAH | 20mcg in 20mcL | Eritoran decreased NF-κB activity, inflammatory cytokine levels and neurological dysfunction secondary to experimental SAH |
| Liu et al. (2010) 22 | Fisher rats | Determine if eritoran inhibits inflammatory effects of ischemia-reperfusion in kidneys | Surgical clamping of renal artery and vein | 5mg/kg iv | Eritoran decreased mortality, renal function decline, renal tubular injury and inflammatory gene expression secondary to ischemia-reperfusion |
PK, pharmacokinetics; PD, pharmacodynamics; NA, not applicable; HPLC, high performance liquid chromatography; JNK, c-Jun NH2-terminal kinase; NF-κB, nuclear factor kappa B.
Figure 3.
Effect of eritoran (E5564) on the hazard ratio for death (mean ± SE) in E. coli challenged rats with varying drug doses, times of treatment and routes of infection. Sprague-Dawley rats (n = 1550) were challenged with intravascular (IV), intrabronchial (IB) or intraperitoneal (IP) E. coli (designed to produce 60–70% mortality in controls), and treated with eritoran (0.03 to 3mg/kg IV bolus, followed by a 24h infusion of 10% of this dose/h) or placebo at 1h prior to, or 1 or 3h after infection. A. Effect of increasing doses of E5564 administered 1 h before IV challenge (p = 0.0001, for all doses combined). B. Effect of delaying treatment in IV challenged rats, for different doses of eritoran. Across all treatment doses, eritoran was less beneficial when delayed (p = 0.004, for loss of beneficial effect for delayed [1 or 3 h] vs. early [−1 h] treatment). C. Effect of increasing doses of eritoran administered 1 h after IV or extravascular (IP or IB) challenge. An inverse pattern was found: increasing doses of eritoran were more beneficial for intravascular challenge, but less beneficial for extravascular challenge. (Reproduced from [35] with permission of Oxford University Press).
2.6.2 Phase 1 clinical studies
Phase I studies in humans established that eritoran effectively inhibited cytokine production and clinical symptoms in response to LPS (Table 4)27–29, 34, 36, 37. This inhibitory activity was dose dependent and diminished faster than plasma clearance of the drug. The loss of activity over time could be attenuated by higher doses administered more frequently; drug activity was retained for up to 72h after infusion with the highest dose tested in humans (252mg infused over 72h). Additionally, intermittent doses could replace continuous intravenous infusions. The only notable side effect of eritoran in these studies was a dose-dependent incidence of phlebitis.
Table 4.
Summary of phase I clinical trials of eritoran*
| Study (Author/date) | Purpose | Dose of eritoran administered | Mean plasma eritoran levels | LPS challenge used | Outcome/Results | Adverse reactions noted** |
|---|---|---|---|---|---|---|
| Wong et al, 200327 | Determine the safety/tolerability of ascending single doses of eritoran in healthy male volunteers (monitored for 120 h after drug infusion), and to determine the in vivo PK and ex vivo PD activity of the drug. | 350, 1000, 2000 or 3500 mcg given over 30 minutes (total n = 29, including placebo) | 115, 365, 722 and 1214 ng/mL, respectively | 0, 1 or 10 ng/mL added to 0.4 mL of whole blood ex vivo | Eritoran shown to be safe at doses administered, with dose-dependent increase in Cmax and AUC, with maximum concentration just after infusion completion. Eritoran had a small Vdss (41–54 mL/kg) and slow clearance from plasma (t1/2 42–51 h). Ex vivo antagonist activity against LPS maintained up to 8h post infusion with higher doses. | Headache, injection site reaction or hemorrhage, pharyngitis |
| Lynn et al, 200336 | Assess the ability of eritoran to block the effects of LPS in vivo in healthy male volunteers | 50, 100 or 250 mcg given over 30 minutes (total n = 24, including placebo) | 4 ng/kg, administered intravenously 15 minutes after starting eritoran infusion | Eritoran limited LPS stimulated changes in vital signs and increases in cytokine with no significant increase in adverse effects. | Headache | |
| Rossignol et al, 200429 | Determine the safety, PK, PD and lipid distribution profile of eritoran during 72 h infusions of various doses of the drug in healthy male volunteers | 500, 2000 or 3500 mcg/h for 72 h (total n = 23, including placebo) | 4, 18 or 41 mcg/mL, respectively | 0, 0.1, 1 or 10 ng/mL added to 0.4 mL of whole blood ex vivo | Eritoran safely tolerated and noted to have small Vdss (46–50 mL/kg) and long t1/2 (50–62 h). Ex vivo response to LPS inhibited by >85% up to 72 h after end of infusion, dependant on dose of eritoran and LPS. Majority of drug bound to HDL; eritoran inactivated by HDL, but not by LDL, VLDL or albumin. | Phlebitis, rhinitis, headache |
| Lynn et al, 200437 | Assess the in vivo PD activity of eritoran after 4 and 72 h infusions in healthy male volunteers with various lipid profiles challenged with LPS | 3.5mg/h × 72h (252 mg); 3mg/h × 4h (12 mg); or 0.5mg/h × 4h (2mg) (total n = 38, including placebo) | Cmax 46, 34, 41, 4 and 1 mcg/mL, respectively | 4ng/kg at end of infusion, or 48 h or 72 h after completing the 72 h infusion, or 8 h after completing the 4 h infusion. | Higher doses (252 mg and 12 mg) of eritoran completetely blocked LPS effects (up to 72h and 8h, respectively) after dosing. Lowest dose (2mg) ameliorated but did not completely inhibit LPS effects 8 h post-dose. Lipid profile did not affect eritoran efficacy. | Phlebitis† with 72 h infusion, but not with the 4 h infusion |
| Rossignol et al, 200828 | Assess safety and the PD and PK profiles of eritoran in healthy male and female volunteers with intermittent (twice daily) infusions of eritoran | 11, 33, 77 or 105 mg in divided loading and maintenance doses every 12 h (n = 33, including placebo) | 1345 – 14,816 ng/mL | 0, 0.05, 1 or 10ng/mL added to whole blood ex vivo | Eritoran was safely tolerated. Efficacy in blocking LPS induced cytokine production ex vivo was dependent on the dose of both eritoran and LPS. | Injection site pain † and inflammation, elevated liver enzymes, flushing, conjunctivitis |
| Liang et al, 200334 | Assess the PK profile of eritoran in subjects with hepatic disease compared to healthy volunteers | 3mg/h × 4h, 3mg/h × 2h, then 1.5mg/h × 2h every 12h (total n = 48) | 3698 – 4183 ng/mL | NA | PK parameters in subjects with liver disease were comparable to those in healthy volunteers; measured eritoran levels in these volunteers were independent of hepatic function. | None |
PK, pharmacokinetics; PD, pharmacodynamics; Cmax, maximum concentration; LPS, lipopolysaccharide; HDL, high density lipoprotein; LDL, low density lipoprotein; VLDL, very low density lipoprotein; TRL; triglyceride rich lipoprotein; AUC, area under the curve; Vdss, volume of distribution at steady state; t1/2, elimination half life; NA, not applicable. Pharmacokinetic parameter values provided in the text are ranges or approximations from the phase I clinical studies above.
Of note, one other phase I study testing eritoran treatment over 14 days in leukemia patients prior to and during bone marrow engraftment after a myeloablative bone marrow transplant regimen was posted on clinicaltrials.gov in September 2008. However, it was reported terminated two months later due to what was described as a “business decision (resources)” (NCT00756912).
2.6.3 Phase II study in cardiac surgery
A randomized, placebo-controlled, double blind, ascending dose trial of eritoran in patients undergoing coronary artery bypass graft and/or cardiac valvular surgery on cardiopulmonary bypass was performed in nine US hospitals between July 2003 and April 200438. Patients received a 4h infusion of one of the following treatments starting 1h prior to surgery: placebo (n = 78) or eritoran 2 mg (n = 24), 12 mg (n = 26) or 28 mg (n = 24). Only one death occurred within 28 days in this trial, and this was in the 28 mg eritoran group. No significant differences were noted in outcomes (laboratory and postoperative parameters, serious adverse events, hospital and ICU length of stay and 28 day all-cause mortality). While eritoran did not appear to cause toxicity, it also did not reduce markers of inflammation i.e., fever, or serum IL-6 or CRP levels. Potentially, this lack of anti-inflammatory effect could have been related to inadequate dosing of eritoran or to significantly greater LPS exposure in cardiac surgery patients compared to human endotoxemia models. It is also possible that inflammation in this instance is not driven through the TLR4 pathway, thus making eritoran use ineffective.
2.6.4 Phase II study in sepsis
A single phase II, prospective, randomized, double-blind, placebo controlled, ascending dose, multi-center study testing the safety and tolerability of eritoran in patients with severe sepsis has been completed39. Stratified by the Acute Physiology and Chronic Health Evaluation II (APACHE-II) score, septic patients with a 20–80% predicted risk of mortality were randomized to placebo (n = 96), or eritoran; 45mg (n = 103) or 105mg (n = 101). Eritoran did not significantly reduce overall mortality at 28 days (32% with 45mg, 26.6% with 105mg vs. 33.3% with placebo; p values non-significant). The higher dose was noted to produce a trend towards decreased mortality (33% vs. 56% with placebo, p = 0.105) in a prospectively defined subgroup with the highest risk of death (APACHE II score > 28). However, there also appeared to be a trend towards increased mortality with the higher eritoran dose compared to placebo (12% vs. 0%, p = 0.083) in the subgroup with the lowest risk of death (APACHE II score < 21) (Figure 4). Of note, benefit to high risk, but harm to low risk groups has been noted with other anti-inflammatory agents in sepsis 40. Interestingly, eritoran did not significantly alter cytokine (IL-6) levels in this study. This absence of inhibition could be secondary to inadequate drug levels; however, the investigators report that median drug levels achieved with both doses (2206 ng/mL and 4338 ng/mL, respectively) would have been sufficient to completely block the amount of LPS usually observed in patients with severe sepsis (mean endotoxin levels ~ 0.6ng/mL in the study by Opal et al.)41.
Figure 4.
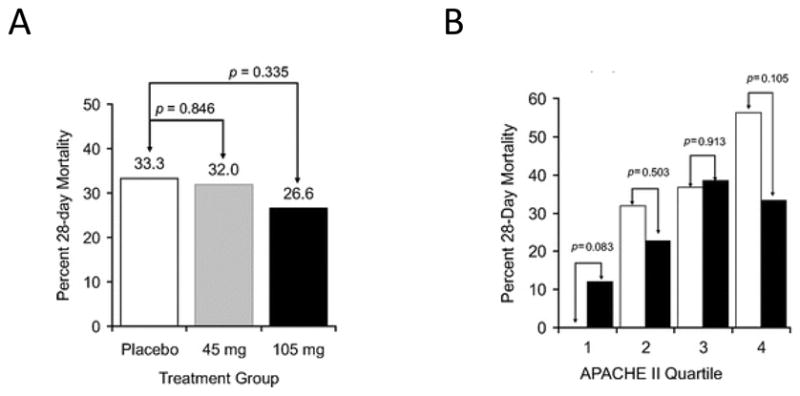
A. All-cause mortality at day 28 by treatment groups in the modified intention-to-treat population (total n = 293) from the Tidswell et al. study. Although mortality was lower in the eritoran 105mg group, it was not statistically different from placebo. B. All cause 28-day mortality in pre-specified subgroups defined by APACHE II quartiles treated with eritoran 105mg. Quartile I corresponds to an APACHE II score < 21, (n = 25, eritoran and n = 23, placebo); quartile 2, score 21–24 (n = 22, for both eritoran and placebo); quartile 3, score 25–28 (n = 26, eritoran and n = 19, placebo); and quartile 4, score > 28 (n = 21, eritoran and n = 32, placebo). Mortality in the treated group was lower than placebo for the 4th quartile with the highest APACHE II scores, but higher than placebo in the lowest quartile, with APACHE II < 21. Reproduced from [39]; with permission of Wolters Kluwer Health
2.6.5 Phase III studies in sepsis
An international multi-center trial in adults with severe sepsis using 105 mg of intravenous eritoran has been completed and its results are under analysis. Sponsored by Eisai Inc., it is known as the ACCESS trial (A Controlled Comparison of Eritoran and Placebo in Patients with Severe Sepsis). Based on the phase II trial results described above, with eritoran showing possible benefit in high-risk patients, but potentially the opposite in low risk patients, this trial was designed to only enroll septic patients with a high predicted risk of death, i.e., with APACHE II scores 21 to 37. (ClinicalTrials.gov identifier: NCT00334828).
2.7 Safety and tolerability
In dogs, eritoran was noted to be safe when infused intermittently to achieve steady state plasma levels of 20–40 mcg/mL for 14 days24. A dose and time dependent increase in the incidence of phlebitis at the site of intravenous infusion was noted in phase I human studies, although this appeared to have diminished with intermittent infusions26, 27, 38. Patients who got eritoran in the phase II sepsis study demonstrated a higher rate of phlebitis, but this did not reach statistical significance (3% vs. 0% for placebo, p = 0.21) 39. In those patients who received one or more doses through a peripheral vein instead of a central venous catheter, the incidence was higher (5.7 – 6.7%). There was also increased acute renal failure associated with eritoran use (p = 0.05). Compared to placebo, the higher dose group had more episodes of elevated creatinine (p = 0.03) and transaminases (p = 0.086). Although not statistically significant, patients who received 105mg of eritoran had a higher incidence of atrial fibrillation (p = 0.18) compared to placebo.
3. Conclusion
Based on 20 years of comprehensive and rigorous scientific research, eritoran tetrasodium appears to be a potentially effective therapy for the inhibition of LPS in severe sepsis and septic shock. Currently, clinical evidence is being gathered for its efficacy and results of a phase III trial will be available soon. If found effective in this phase III trial (and a confirmatory study), it will be one of the few strategies shown to successfully change outcomes in septic patients in recent times42. However, for several reasons, if eritoran fails to improve outcome in this trial, questions must be raised regarding the anti-LPS approach for the therapy of sepsis. First, the science underlying the development of eritoran for sepsis was of high quality and its specificity for inhibiting LPS-associated inflammation was well documented. Second, the Phase III trial investigators have taken into account lessons learnt from previously conducted clinical trials testing anti-inflammatory agents in sepsis. Notably, based in part on trends seen in the Phase II trial, the Phase III trial has specifically targeted septic patients with a higher risk of death in whom an excessive and deregulated inflammatory response is likely to contribute to a worse outcome.
4. Expert opinion
The last two decades have seen a variety of approaches targeting LPS-related inflammation to improve outcomes in sepsis43. Several trials of an anti-LPS antibody failed to show consistent clinical efficacy, perhaps because these therapies did not completely block the effects of LPS, due to low specificity or affinity for toxin40, 44–47. Anti-CD14 antibodies may have been unsuccessful because of the difficulty in saturating all CD14 molecules48. Multiple anti-inflammatory therapies for sepsis have also shown disappointing effects clinically, perhaps because these trials were underpowered40.
Besides true lack of drug efficacy, it is possible these therapies were tested using inadequately designed studies. If anti-inflammatory therapies benefit only certain subgroups of septic patients, proving their efficacy using the heterogeneous populations recruited in most clinical sepsis studies would be difficult. In septic patients in whom the inflammatory response is protective, and who are unlikely to die from it, depressing inflammation with drugs might actually cause harm. However, in those in whom inflammation is excessive and itself causes injury, treatment with anti-inflammatory agents could prove beneficial and reduce mortality. Including both these populations in a clinical study without accounting for underlying risk of death could make it difficult, if not impossible, to determine the therapy’s true merit.
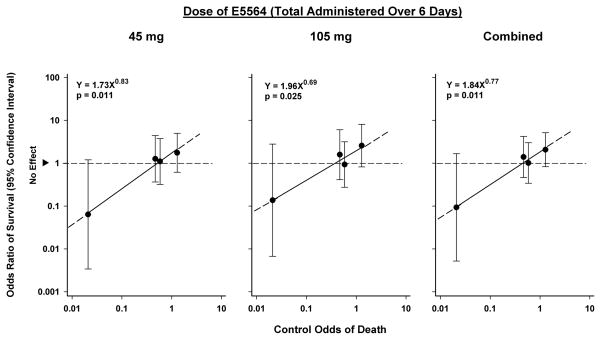
That a relationship exists between risk of death and efficacy of anti-inflammatory agents is suggested by the results of trials of Lenercept, IL-1ra, afelimomab, rhAPC and corticosteroids49–54. Meta-regression analysis of clinical trials of these therapies found that as sepsis-associated risk of death increased, so did the benefit of these therapies. However, these therapies were not beneficial but potentially harmful in patients with a low risk of death55, 56. A similar relationship may exist between the severity of sepsis and the efficacy of eritoran as noted in the phase II clinical trial (Figure 5)57.
Figure 5.
Relationship between severity of illness and efficacy of eritoran in septic patients randomized to eritoran vs. placebo (Tidswell et al). Patients were divided into quartiles based on their APACHE II score. Odds ratios of survival for each quartile of patients receiving eritoran were plotted against control odds of death calculated from observed mortality in the corresponding quartile of the placebo arm. The odds ratios of survival are shown as circles with 95% confidence intervals shown by the vertical lines. The regression lines in this figure show that the effect of both low (45mg) and high (105mg) dose eritoran, either separately (panels A and B, respectively), or combined (Panel C) were similar, and appear directly related to control odds of death. Both doses were most beneficial in the APACHE II quartile with the highest control mortality rate, but this benefit declined as control mortality rate decreased, and was no longer evident in the quartile with the lowest mortality rate. The correlation between effect and control odds was high (r ≥ 0.97 for each), and the slope of each regression line was significant (p ≤ 0.025). (Reproduced from [57] with permission of Wolters Kluwer Health).
Well-designed clinical trials accounting for the underlying risk of death may lead us closer to finding effective sepsis therapies. The Phase III clinical investigators using eritoran appear to have taken underlying severity of sepsis into consideration; they have recruited only patients at a high risk of death, denoted by APACHE II scores of 21–37. However, the APACHE II score was not designed to predict individual patient mortality, but rather to gauge the severity of illness of an ICU population. The APACHE II score is subject to variation based on patient case mix, location (e.g. emergency room vs. intensive care unit) as well as the time it is calculated. Although the score is increasingly used to help determine who should be treated (or randomized), its use as such has not been prospectively validated. Other methods to risk-stratify patients similarly require validation58.
Difficulties also lie in the inherent differences between pre-clinical and clinical trials of therapies for sepsis. In pre-clinical and phase I clinical studies, efficacy of eritoran was demonstrated by its ability to block LPS-induced changes in cytokine levels or clinical signs and symptoms. With the exception of a few animal studies, most pre-clinical studies were performed in the artificial setting of an LPS challenge rather than live bacterial infection. LPS levels can be elevated in both gram-positive and gram-negative infections, but it is not the only pathogenic molecule that stimulates an immune response (and inflammation) in sepsis. Moreover, LPS levels can be undetectable in both gram-negative and gram-positive infections using very sensitive assays (down to the micromolar range)59. Eritoran is specific for TLR4-MD2, and may not block other pathways. In fact, ex vivo studies showed that eritoran inhibited induction of TNF-α in human blood when incubated with gram-negative bacteria (E. coli), but not gram-positive bacteria (S. aureus) 16 which may signal intracellular responses via TLR2. About a quarter of all patients in the Tidswell study were infected with gram-negative bacteria alone, a third with gram-positive bacteria alone and a tenth with mixed organisms, but elevated endotoxin levels were detected at baseline in ~70% of patients. However, exploratory analyses showed that type of pathogen, or indeed endotoxin levels, did not appear to influence outcome39.
Furthermore, inhibition of TLR4 may have as yet unknown deleterious effects. While TLR4 deficient mice are resistant to LPS, they are simultaneously extremely susceptible to gram negative infections. TLR4 signaling is an important part of the innate immune response, and inhibition of this response may hinder timely recognition and clearance of infectious pathogens, resulting in possible harm. It has been reported that TLR4 mutations which render the receptors non-functional are associated with an increased sepsis associated mortality and susceptibility to gram negative infections60–62.
Yet other variables deserving attention are the timing and dosing of eritoran. In the majority of pre-clinical and phase 1 clinical studies eritoran was administered either before or at the time of LPS/bacterial challenge. However, one rat study which attempted to simulate clinical conditions (eritoran administered 1 or 3 h after a live bacterial challenge) found that efficacy diminished with delayed administration35. This loss in efficacy could partly be overcome by using a higher dose of the drug for intravascular infection but, notably, lowering the dose appeared more effective for extravascular infection (Figure 3). Thus, eritoran may have to be administered early in sepsis to show benefit, and its dosing may have to be altered based on the source of underlying infection. Optimizing such delivery clinically may be challenging.
Given the dearth of new therapeutic options for sepsis over the past three decades, success with eritoran would be welcomed by clinicians. However, many questions still remain, and will hopefully be answered by the results of the phase III clinical trial. Even if this trial shows eritoran to be effective, past experience in the field of sepsis suggests that confirmation of its benefit will be necessary42. Furthermore, if there is a potential for the therapy to adversely affect outcome in certain subgroups (e.g., septic patients with a lower risk of death), it will be important to clearly define how patients can be reliably identified for treatment during broad clinical application. Finally, failure of eritoran in well-conducted sepsis trials must raise fundamental questions about the importance of circulating LPS as a therapeutic target in sepsis. 63–66
Footnotes
Declaration of Interest
The authors declare intramural funding from the National Institutes of Health
References
- 1.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000 Aug 17;406(6797):782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001 Aug;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003 Jan 22;85(2):85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Park BS, Song DH, Kim HM, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009 Apr 30;458(7242):1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 6.Golenbock DT, Leggett JE, Rasmussen P, et al. Lipid X protects mice against fatal Escherichia coli infection. Infect Immun. 1988 Apr;56(4):779–84. doi: 10.1128/iai.56.4.779-784.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golenbock DT, Will JA, Raetz CR, et al. Lipid X ameliorates pulmonary hypertension and protects sheep from death due to endotoxin. Infect Immun. 1987 Oct;55(10):2471–6. doi: 10.1128/iai.55.10.2471-2476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000 Aug 21;192(4):565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey KJ, Lowry SF, Cerami A. Cachectin/TNF mediates the pathophysiological effects of bacterial endotoxin/lipopolysaccharide (LPS) Prog Clin Biol Res. 1988;272:77–88. [PubMed] [Google Scholar]
- 10.Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm. 2010;2010:568396. doi: 10.1155/2010/568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice TW, Wheeler AP, Bernard GR, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med. 2010 Aug;38(8):1685–94. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 12.Roger T, Froidevaux C, Le Roy D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2348–52. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994 Feb;8(2):217–25. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 14.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7(3):167–202. [PubMed] [Google Scholar]
- 15.Hawkins LD, Christ WJ, Rossignol DP. Inhibition of endotoxin response by synthetic TLR4 antagonists. Curr Top Med Chem. 2004;4(11):1147–71. doi: 10.2174/1568026043388123. [DOI] [PubMed] [Google Scholar]
- 16.Mullarkey M, Rose JR, Bristol J, et al. Inhibition of endotoxin response by e5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther. 2003 Mar;304(3):1093–102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 17.Savov JD, Brass DM, Lawson BL, et al. Toll-like receptor 4 antagonist (E5564) prevents the chronic airway response to inhaled lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2005 Aug;289(2):L329–37. doi: 10.1152/ajplung.00014.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kitazawa T, Tsujimoto T, Kawaratani H, et al. Salvage effect of E5564, Toll-like receptor 4 antagonist on d-galactosamine and lipopolysaccharide-induced acute liver failure in rats. J Gastroenterol Hepatol. 2010 May;25(5):1009–12. doi: 10.1111/j.1440-1746.2009.06145.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitazawa T, Tsujimoto T, Kawaratani H, et al. Therapeutic approach to regulate innate immune response by Toll-like receptor 4 antagonist E5564 in rats with D-galactosamine-induced acute severe liver injury. J Gastroenterol Hepatol. 2009 Jun;24(6):1089–94. doi: 10.1111/j.1440-1746.2008.05770.x. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1247–54. doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimamoto A, Chong AJ, Yada M, et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006 Jul 4;114(1 Suppl):I270–4. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Gu M, Xu D, et al. Protective effects of Toll-like receptor 4 inhibitor eritoran on renal ischemia-reperfusion injury. Transplant Proc. 2010 Jun;42(5):1539–44. doi: 10.1016/j.transproceed.2010.03.133. [DOI] [PubMed] [Google Scholar]
- 23.Ondiveeran HK, Fox-Robichaud A. Drug evaluation: E-5564. IDrugs. 2004 Jun;7(6):582–90. [PubMed] [Google Scholar]
- 24.Rossignol DP, Lynn M. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J Endotoxin Res. 2002;8(6):483–8. doi: 10.1179/096805102125001127. [DOI] [PubMed] [Google Scholar]
- 25.Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007 Sep 7;130(5):906–17. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Visintin A, Halmen KA, Latz E, et al. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J Immunol. 2005 Nov 15;175(10):6465–72. doi: 10.4049/jimmunol.175.10.6465. [DOI] [PubMed] [Google Scholar]
- 27.Wong YN, Rossignol D, Rose JR, et al. Safety, pharmacokinetics, and pharmacodynamics of E5564, a lipid A antagonist, during an ascending single-dose clinical study. J Clin Pharmacol. 2003 Jul;43(7):735–42. [PubMed] [Google Scholar]
- 28.Rossignol DP, Wong N, Noveck R, et al. Continuous pharmacodynamic activity of eritoran tetrasodium, a TLR4 antagonist, during intermittent intravenous infusion into normal volunteers. Innate Immun. 2008 Dec;14(6):383–94. doi: 10.1177/1753425908099173. [DOI] [PubMed] [Google Scholar]
- 29.Rossignol DP, Wasan KM, Choo E, et al. Safety, pharmacokinetics, pharmacodynamics, and plasma lipoprotein distribution of eritoran (E5564) during continuous intravenous infusion into healthy volunteers. Antimicrob Agents Chemother. 2004 Sep;48(9):3233–40. doi: 10.1128/AAC.48.9.3233-3240.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasan KM, Sivak O, Cote RA, et al. Association of the endotoxin antagonist E5564 with high-density lipoproteins in vitro: dependence on low-density and triglyceride-rich lipoprotein concentrations. Antimicrob Agents Chemother. 2003 Sep;47(9):2796–803. doi: 10.1128/AAC.47.9.2796-2803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko K, Ueda R, Kawata T, et al. LPS binding protein does not participate in the pharmacokinetics of E5564. J Endotoxin Res. 2004;10(3):185–94. doi: 10.1179/096805104225004860. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko K, Ueda R, Nemoto H, et al. Disposition of a synthetic analogue of lipid A (E5564) in rats. Xenobiotica. 2003 Mar;33(3):323–39. doi: 10.1080/0049825021000058097. [DOI] [PubMed] [Google Scholar]
- 33.Wasan KM, Risovic V, Sivak O, et al. Influence of plasma cholesterol and triglyceride concentrations and eritoran (E5564) micelle size on its plasma pharmacokinetics and ex vivo activity following single intravenous bolus dose into healthy female rabbits. Pharm Res. 2008 Jan;25(1):176–82. doi: 10.1007/s11095-007-9428-8. [DOI] [PubMed] [Google Scholar]
- 34.Liang E, Wong YN, Allen I, et al. Pharmacokinetics of E5564, a lipopolysaccharide antagonist, in patients with impaired hepatic function. J Clin Pharmacol. 2003 Dec;43(12):1361–9. doi: 10.1177/0091270003258653. [DOI] [PubMed] [Google Scholar]
- 35.Solomon SB, Cui X, Gerstenberger E, et al. Effective dosing of lipid A analogue E5564 in rats depends on the timing of treatment and the route of Escherichia coli infection. J Infect Dis. 2006 Mar 1;193(5):634–44. doi: 10.1086/500147. [DOI] [PubMed] [Google Scholar]
- 36.Lynn M, Rossignol DP, Wheeler JL, et al. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J Infect Dis. 2003 Feb 15;187(4):631–9. doi: 10.1086/367990. [DOI] [PubMed] [Google Scholar]
- 37.Lynn M, Wong YN, Wheeler JL, et al. Extended in vivo pharmacodynamic activity of E5564 in normal volunteers with experimental endotoxemia [corrected] J Pharmacol Exp Ther. 2004 Jan;308(1):175–81. doi: 10.1124/jpet.103.056531. [DOI] [PubMed] [Google Scholar]
- 38.Bennett-Guerrero E, Grocott HP, Levy JH, et al. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2007 Feb;104(2):378–83. doi: 10.1213/01.ane.0000253501.07183.2a. [DOI] [PubMed] [Google Scholar]
- 39.Tidswell M, Tillis W, Larosa SP, et al. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med. 2010 Jan;38(1):72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- 40.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000 Jul;9(7):1651–63. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 41.Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. Journal of Infectious Diseases. 1999 Nov;180(5):1584–89. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 42.Sweeney DA, Danner RL, Eichacker PQ, et al. Once is not enough: clinical trials in sepsis. Intensive Care Med. 2008 Nov;34(11):1955–60. doi: 10.1007/s00134-008-1274-6. [DOI] [PubMed] [Google Scholar]
- 43.Manocha S, Feinstein D, Kumar A. Novel therapies for sepsis: antiendotoxin therapies. Expert Opin Investig Drugs. 2002 Dec;11(12):1795–812. doi: 10.1517/13543784.11.12.1795. [DOI] [PubMed] [Google Scholar]
- 44.Angus DC, Birmingham MC, Balk RA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA. 2000 Apr 5;283(13):1723–30. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 45.Bone RC, Balk RA, Fein AM, et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. The E5 Sepsis Study Group. Crit Care Med. 1995 Jun;23(6):994–1006. doi: 10.1097/00003246-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Greenberg RN, Wilson KM, Kunz AY, et al. Observations using antiendotoxin antibody (E5) as adjuvant therapy in humans with suspected, serious, gram-negative sepsis. Crit Care Med. 1992 Jun;20(6):730–5. doi: 10.1097/00003246-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Harkonen S, Scannon P, Mischak RP, et al. Phase I study of a murine monoclonal anti-lipid A antibody in bacteremic and nonbacteremic patients. Antimicrob Agents Chemother. 1988 May;32(5):710–6. doi: 10.1128/aac.32.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinhart K, Gluck T, Ligtenberg J, et al. CD14 receptor occupancy in severe sepsis: results of a phase I clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14) Crit Care Med. 2004 May;32(5):1100–8. doi: 10.1097/01.ccm.0000124870.42312.c4. [DOI] [PubMed] [Google Scholar]
- 49.Panacek EA, Marshall JC, Albertson TE, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab′)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004 Nov;32(11):2173–82. doi: 10.1097/01.ccm.0000145229.59014.6c. [DOI] [PubMed] [Google Scholar]
- 50.Abraham E, Glauser MP, Butler T, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45–2081 Study Group. JAMA. 1997 May 21;277(19):1531–8. [PubMed] [Google Scholar]
- 51.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994 Jun 15;271(23):1836–43. [PubMed] [Google Scholar]
- 52.Ziegler EJ, Fisher CJ, Jr, Sprung CL, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–36. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 53.Abraham E, Laterre PF, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005 Sep 29;353(13):1332–41. doi: 10.1056/NEJMoa050935. [DOI] [PubMed] [Google Scholar]
- 54.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 55.Eichacker PQ, Parent C, Kalil A, et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002 Nov 1;166(9):1197–205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 56.Minneci PC, Deans KJ, Eichacker PQ, et al. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009 Apr;15(4):308–18. doi: 10.1111/j.1469-0691.2009.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barochia AV, Cui X, Natanson C, et al. Risk of death and the efficacy of eritoran tetrasodium (E5564): design considerations for clinical trials of anti-inflammatory agents in sepsis. Crit Care Med. 2010 Jan;38(1):306–8. doi: 10.1097/CCM.0b013e3181b77fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Opal SM, Scannon PJ, Vincent JL, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999 Nov;180(5):1584–9. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 60.Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002 Nov 15;186(10):1522–5. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 61.Child NJ, Yang IA, Pulletz MC, et al. Polymorphisms in Toll-like receptor 4 and the systemic inflammatory response syndrome. Biochem Soc Trans. 2003 Jun;31(Pt 3):652–3. doi: 10.1042/bst0310652. [DOI] [PubMed] [Google Scholar]
- 62.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998 Dec 11;282(5396):2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 63.Kaneko K, Ueda R, Kikuchi K, et al. Quantitative determination of a potent lipopolysaccharide antagonist, E5564, in rat and dog plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1999 Dec 24;736(1–2):67–75. doi: 10.1016/s0378-4347(99)00438-7. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Z, Kozlowski J, Schuster DP. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am J Respir Crit Care Med. 2005 Aug 1;172(3):344–51. doi: 10.1164/rccm.200503-343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma CX, Yin WN, Cai BW, et al. Activation of TLR4/NF-kappaB signaling pathway in early brain injury after subarachnoid hemorrhage. Neurol Res. 2009 Aug 21; doi: 10.1179/016164109X12445616596283. [DOI] [PubMed] [Google Scholar]
- 66.Rossignol DP, Lynn M. TLR4 antagonists for endotoxemia and beyond. Curr Opin Investig Drugs. 2005 May;6(5):496–502. [PubMed] [Google Scholar]
- 67.Suganuma A, Rossignol DP, Kaneko K, et al. Clinical Pharmacology of E5564, a lipid A antagonist, in a canine lipopolysaccharide challenge model. J Endotoxin Res. 2000;6:115. [Google Scholar]