Abstract
Objective
To investigate whether occupational exposure to polycyclic aromatic hydrocarbons and certain plastic monomers increased renal cell carcinomas (RCC) risk.
Methods
Unconditional logistic regression was used to calculate RCC risk in relation to exposure.
Results
No association between RCC risk and having ever been occupationally exposed to any polycyclic aromatic hydrocarbons or plastics was observed. Duration of exposure and average exposure also showed no association with risk. Suggestive positive associations between RCC risk and cumulative exposure to styrene (P-trend = 0.02) and acrylonitrile (P-trend = 0.06) were found. Cumulative exposure to petroleum/gasoline engine emissions was inversely associated with risk (P-trend = 0.02).
Conclusions
Results indicate a possible association between occupational styrene and acrylonitrile exposure and RCC risk. Additional studies are needed to replicate findings, as this is the first time these associations have been reported and they may be due to chance.
Polycyclic aromatic hydrocarbons (PAHs) are a group of chemical compounds that are naturally found in fossil fuels and formed as by-products during incomplete combustion of organic material, such as coal, oil, wood, garbage, gas, tobacco, and charbroiled meat.1,2 Polycyclic aromatic hydrocarbons exist exclusively as complex mixtures and have been used in the production of plastics, dyes, medicines, and pesticides.1 Several types of PAHs such as benzo[a]pyrene and benzo[a]anthracene are known or suspected human carcinogens.1 Over a few hundred PAH compounds have been characterized; some have been identified as carcinogens, mutagens, and teratogens.1--4 Furthermore, the International Agency for Research on Cancer has determined that several complex mixtures containing PAHs (eg, coal tars, soot, diesel engine exhaust) are carcinogenic or probably carcinogenic to humans.5
Numerous industries such as those involved in transportation, coal gasification, aluminum, rubber, plastic, and coke production, and iron and steel foundries,1,2,6--8 are responsible for emitting significant amounts of PAH-containing particles into the environment and are, therefore, a source of occupational exposure for considerable groups of workers.2,6 Polycyclic aromatic hydrocarbons released into the environment have been found in at least 600 of 1430 US National Priorities List sites identified by the Environmental Protection Agency.1 Epidemiological studies have shown increased risks of lung, skin, and bladder cancer associated with occupational PAH exposure.2,6 Inconsistent results have been reported for occupational studies examining the relationship between kidney cancer risk and workers who may have high levels of PAH exposure, such as asphalt workers, printers, machinists, and mechanics.6,9--12
The conflicting results reported for kidney cancer risk in occupational PAH exposure studies warrant additional large-scale studies with expert occupational assessment. Once absorbed in the body, PAHs are capable of entering any tissue that contains fat due to their lipophilic nature.1 Typically, however, PAHs are stored in the kidneys, liver, and fat and are bio-activated through renal and hepatic metabolic pathways.1,13,14 In the current study, we sought to investigate the association between renal cell carcinoma (RCC) risk and occupational exposure to PAHs and plastic monomers, as PAHs are occasionally used in the production of rubber and plastic materials. This investigation was carried out in a large, multicenter, renal case--control study with expert retrospective occupational assessment in Central and Eastern Europe, an area with historically heavy industrial exposures and one of the highest rates of RCC in the world.15
Methods
Details of the Central and Eastern European Renal Cell Carcinoma (CEERCC) study have been previously reported.16 Briefly, the CEERCC study is a hospital-based case--control study that was conducted in seven centers across four Central and Eastern European countries. Between August 1999 and January 2003, newly diagnosed and histologically confirmed RCC (IDC-O-2 code 64) cases between 20 and 88 years of age who were living in the study areas for at least 1 year were recruited for participation. Controls were frequency-matched to cases on age, sex, and place of residence, from patients admitted to participating hospitals for diagnoses unrelated to smoking or urological disorders (with the exception of benign prostatic hyperplasia) between August 1998 and March 2003. No single disease made up greater than 20% of the control group. Some controls also were recruited from an earlier study of lung and head and neck cancers.17,18 Overall, 1097 RCC cases and 1476 controls were included in the study. Response rates for participation across study centers ranged from 90% to 99% for cases and from 90% to 96% for controls. The institutional review boards of all participating centers and organizations approved the study, and all subjects provided written informed consent.
Cases and controls were interviewed with the same questionnaires. During hospitalization or within 3 months of diagnosis for cases, participants were administered a standardized questionnaire by trained interviewers for information on demographic characteristics, medical histories, and lifestyle factors. Lifetime occupational information for jobs held for 12 or more months' duration was also collected during interviews through the use of a general occupational questionnaire. Data collected on all jobs included title, tasks, working environment, time spent on each task, and type of employer, as well as year of beginning and ending employment. To improve precision of the assessment, specialized occupational questionnaires were also used for specific jobs or industries likely to entail exposure to known or suspected occupational carcinogens. These specific jobs/industries included toolmaker or machinist, motor vehicle mechanic, auto body repairer, miner/quarryman, woodworker, painter, welder, insulation worker, meat worker or farmer, and the iron, steel, coke, foundry, glass, tannery, chemical, and rubber industries. The specialized questionnaires covered information regarding (1) possible exposures to agents of interest, such as acrylonitrile, styrene, acrylics, etc; (2) hours per week of exposure; (3) source of exposure; and (4) a description of agent use. Details on the questionnaires have been reported previously.19
All occupational questionnaires were reviewed by local occupational health experts or industrial hygienists who were trained by the study's lead industrial hygienist. For every job in each subject's work history, the experts evaluated the frequency, intensity, and confidence of exposure to PAHs and plastics, on the basis of the general occupational questionnaire, the specialized questionnaires, and their own experience in industrial hygiene and knowledge about historical working conditions at the specific plants in their study area while blinded to case--control status. Estimates were specific to the date of exposure. Frequency of exposure was assessed as less than 5%, 5%--30%, and more than 30% of total working time in a 40-hour workweek. To compute across jobs that had different frequencies of exposure, frequency weights were assigned, corresponding to the midpoint of the ranges (0.025, 0.175, and 0.65, respectively). Intensity of exposure was assessed as low, medium, and high, based on agent-specific categories anchored to measurement data and jobs. For each agent considered present, the assessors also noted the degree of their confidence that the job would entail exposure to the agent, categorized as possible (<40%), probable (40%--90%), or definite (>90%).
Exposure metrics analyzed included (1) ever exposure, (2) duration of exposure in years, (3) cumulative exposure, calculated as the product of duration of employment in each exposed job multiplied by the midpoint of the frequency category and by the intensity weight of the job, summed across all of the subject's jobs, and (4) average exposure, calculated by dividing cumulative exposure by the number of years exposed.
Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to estimate RCC risk by occupational exposure using unconditional logistic regression adjusting for sex, age, center, smoking status, self-reported hypertension (yes/no), body mass index (BMI), and family history of cancer, unless otherwise noted. Correlation analyses (Spearman) were conducted to identify agents or groups of agents that were associated with suspected PAH exposures. All coexposures with an r2 > 0.65 were used as adjustment variables. Analyses included all subjects and then only included jobs for subjects in which the likelihood of exposure was high (ie, high confidence [≥40%] of exposure [cases, N = 1061; and controls, N = 1437]). Analyses were additionally modeled to account for a 20-year lag between exposure and diagnosis to restrict analyses to subjects with a sufficient latency period from exposure to disease. Lastly, since a number of studies have shown cytochrome P450 enzymes (CYP1A1 and CYP1B1) and glutathione-S-transferases (GSTs) genes to modify the association between PAH exposure and renal cancer risk,20,21 we also evaluated whether these genetic polymorphisisms (CYP1A1 [rs1048943 and rs4646903], CYP1B1 [rs1056836 and rs1800440], GSTM1 and GSTT1) modified RCC risk. Information regarding genotyping methods and analyses of these genes has been previously reported.22,23 All analyses were conducted in STATA 9.0 (STATA Corporation, College Station, TX).
Results
A description of study participants and known RCC risk factors is provided in Table 1. Study participants were comparable in age; however, cases were more likely to be female, to have a first-degree relative with cancer, and have excess BMI (>30 kg/m2) and hypertension. Although cases were less likely to have been smokers, the inverse association with smoking was no longer observed after adjustment for sex, age, self-reported hypertension, BMI, and study center.16
TABLE 1. General Characteristics of Participants in the Central and Eastern European Renal Cell Carcinoma Study.
| Variables | Cases | Controls | P | ||
|---|---|---|---|---|---|
| N | % * | N | %* | ||
| Participants | 1097 | 42.6 | 1476 | 57.4 | |
| Sex | |||||
| Male | 648 | 59.1 | 952 | 64.5 | |
| Female | 449 | 40.9 | 524 | 35.5 | 0.01 |
| Age at interview, yr | |||||
| <45 | 86 | 7.8 | 122 | 11.1 | |
| 45--54 | 278 | 25.3 | 379 | 34.5 | |
| 55--64 | 335 | 30.5 | 460 | 41.9 | |
| 65--74 | 353 | 32.2 | 452 | 41.2 | |
| 75+ | 45 | 4.1 | 63 | 5.7 | 0.50 |
| Center | |||||
| Romania-Bucharest | 95 | 8.7 | 160 | 10.8 | |
| Poland-Lodz | 99 | 8.7 | 198 | 13.4 | |
| Russia-Moscow | 317 | 28.9 | 463 | 31.4 | |
| Czech Republic† | 586 | 53.4 | 655 | 44.4 | <0.001 |
| Body mass index at interview | |||||
| <25 | 327 | 29.8 | 532 | 36.0 | |
| 25--29.9 | 476 | 43.4 | 620 | 42.0 | |
| 30+ | 294 | 26.8 | 324 | 22.0 | <0.001 |
| Tobacco status | |||||
| Never | 510 | 46.5 | 599 | 40.6 | |
| Ever | 584 | 53.2 | 874 | 59.2 | 0.003 |
| Hypertension | |||||
| No | 600 | 54.7 | 906 | 61.4 | |
| Yes | 496 | 45.2 | 569 | 38.6 | 0.001 |
| Familial history of cancer | |||||
| No first-degree relative with cancer | 733 | 66.8 | 1074 | 72.8 | |
| First-degree relative with cancer | 364 | 33.2 | 402 | 27.2 | 0.001 |
Because of missing values, some categories do not sum to 100%.
Brno, Olomouc, Prague, Ceske-Budejovice.
Significant associations were not observed between RCC risk and having ever been occupationally exposed to any agents estimated to contain PAHs or to the three plastic monomers (Table 2). Similarly, analyses by duration of exposure and average exposure revealed no significant association (data not shown). However, when cumulative exposure was examined (Fig. 1), suggestive positive trends were observed for occupational exposure to styrene (exposure below the median: OR = 0.6, 95% CI = 0.2--1.7; exposure at or above the median: OR = 6.7, 95% CI = 1.8--24.3; P-trend = 0.02), and acrylonitrile (exposure below the median: OR = 1.6, 95% CI = 0.4--6.4; exposure at or above the median: OR = 4.3, 95% CI = 0.9--22.1; P-trend = 0.06) and RCC risk. Cumulative exposure to petroleum/gasoline engine emissions (exposure below the median: OR = 1.0, 95% CI = 0.7--1.4; exposure at or above the median: OR = 0.6, 95% CI = 0.4--0.9; P-trend = 0.02) was inversely associated with risk.
TABLE 2. Occupational Exposure to PAHs and Plastics in Relation to RCC Risk.
| Exposure | Cases | Controls | OR (95% CI) |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| PAHs | ||||||
| All PAHs | ||||||
| Unexposed | 808 | 97.8 | 1156 | 97.5 | 1.0 | |
| Exposed | 18 | 2.2 | 30 | 2.5 | 0.8 | (0.4--1.4) |
| Coal combustion fumes | ||||||
| Unexposed | 805 | 97.5 | 1146 | 96.6 | 1.0 | |
| Exposed | 21 | 2.5 | 40 | 3.4 | 0.8 | (0.5--1.4) |
| Coke combustion fumes* | ||||||
| Unexposed | 803 | 97.2 | 1152 | 97.1 | 1.0 | |
| Exposed | 23 | 2.8 | 34 | 2.9 | 1.1 | (0.6--2.2) |
| Wood combustion fumes | ||||||
| Unexposed | 817 | 98.9 | 1175 | 99.1 | 1.0 | |
| Exposed | 9 | 1.1 | 11 | 0.9 | 1.2 | (0.5--3.0) |
| Petroleum oil combustion fumes | ||||||
| Unexposed | 816 | 98.8 | 1160 | 97.8 | 1.0 | |
| Exposed | 10 | 1.2 | 26 | 2.2 | 0.7 | (0.3--1.4) |
| Petroleum/gasoline engine emissions | ||||||
| Unexposed | 717 | 86.8 | 975 | 82.2 | 1.0 | |
| Exposed | 109 | 13.2 | 211 | 17.8 | 0.8 | (0.6--1.0) |
| Diesel/kerosene engine emissions | ||||||
| Unexposed | 647 | 78.3 | 919 | 77.5 | 1.0 | |
| Exposed | 179 | 21.7 | 267 | 22.5 | 1.0 | (0.8--1.2) |
| Plastics pyrolysis products | ||||||
| Unexposed | 753 | 91.2 | 1074 | 90.6 | 1.0 | |
| Exposed | 73 | 8.8 | 112 | 9.4 | 0.9 | (0.6--1.2) |
| Lubricating oil mist | ||||||
| Unexposed | 569 | 68.9 | 837 | 70.6 | 1.0 | |
| Exposed | 257 | 31.1 | 349 | 29.4 | 1.0 | (0.8--1.3) |
| Cutting fluids mist | ||||||
| Unexposed | 746 | 90.3 | 1032 | 87.0 | 1.0 | |
| Exposed | 80 | 9.7 | 154 | 13.0 | 0.8 | (0.6--1.1) |
| Other mineral oils mist | ||||||
| Unexposed | 681 | 82.4 | 1010 | 85.2 | 1.0 | |
| Exposed | 145 | 17.6 | 176 | 14.8 | 1.1 | (0.8--1.4) |
| Asphalt bitumen fumes | ||||||
| Unexposed | 805 | 97.5 | 1154 | 97.3 | 1.0 | |
| Exposed | 21 | 2.5 | 32 | 2.7 | 1.0 | (0.5--1.7) |
| Coal tar and pitch fumes | ||||||
| Unexposed | 809 | 97.9 | 1163 | 98.1 | 1.0 | |
| Exposed | 17 | 2.1 | 23 | 1.9 | 0.9 | (0.5--1.8) |
| Plastics | ||||||
| Vinyl chloride | ||||||
| Unexposed | 816 | 98.8 | 1167 | 98.4 | 1.0 | |
| Exposed | 10 | 1.2 | 19 | 1.6 | 0.9 | (0.4--1.9) |
| Acrylonitrile | ||||||
| Unexposed | 816 | 98.8 | 1180 | 99.5 | 1.0 | |
| Exposed | 10 | 1.2 | 6 | 0.5 | 2.5 | (0.9--7.1) |
| Styrene | ||||||
| Unexposed | 809 | 97.9 | 1172 | 98.8 | 1.0 | |
| Exposed | 17 | 2.1 | 14 | 1.2 | 1.7 | (0.8--3.6) |
PAH, polycyclic aromatic hydrocarbon; RCC, renal cell carcinomas.
Adjusted for age, sex, study center, body mass index, self-reported hypertension (no, yes), smoking status (never, ever), and family history of cancer (no, yes).
Also adjusted for occupational coke dust exposure. RCC risk for occupational exposure to creosote fumes not shown due to small number of exposed subjects (N = 4 cases, N = 4 controls).
FIGURE 1. RCC risk by occupational cumulative exposure to PAHs and Plastics.
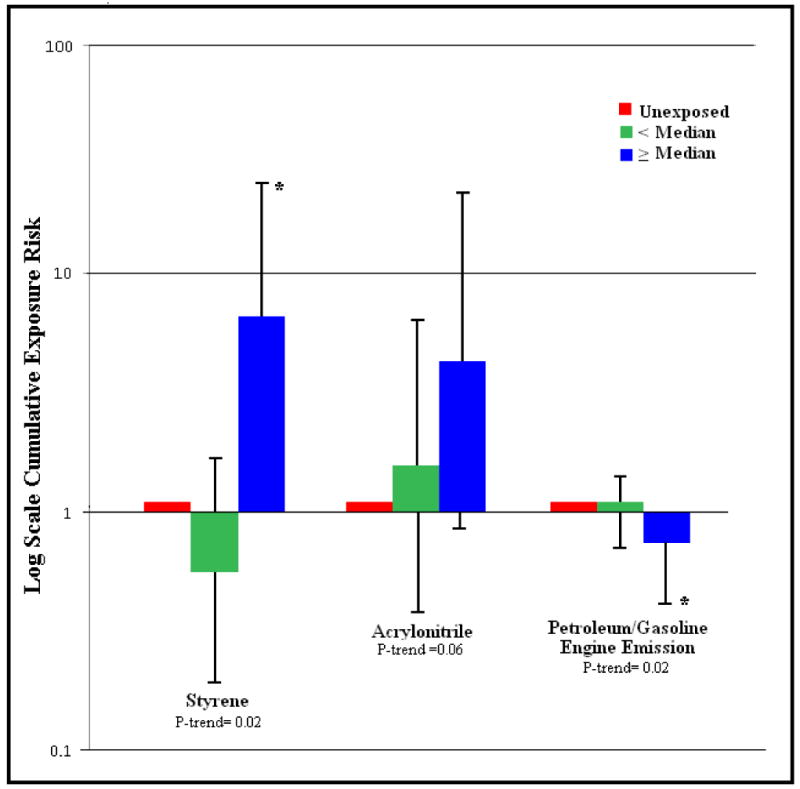
Renal cell carcinomas risk by occupational cumulative exposure to polycyclic aromatic hydrocarbons and plastics cumulative exposure for (A) styrene (exposure below the median: OR = 0.6, 95% CI = 0.2--1.7; exposure at or above the median: OR = 6.7, 95% CI = 1.8--24.3; P-trend = 0.02), (B) acrylonitrile (exposure below the median: OR = 1.6, 95% CI = 0.4--6.4; exposure at or above the median: OR = 4.3, 95% CI = 0.9--22.1; P-trend = 0.06), and C) petroleum/gasoline engine emissions (exposure below the median: OR = 1.0, 95% CI = 0.7--1.4; exposure at or above the median: OR = 0.6, 95% CI = 0.4--0.9; P-trend = 0.02).
* P-value <0.05
Stratified analyses by sex, BMI, self-reported hypertension, and smoking status revealed no further insight in the results (data not shown). The inclusion of a 20-year lag in exposure did not modify results nor did analyses restricted to exposures with a high level of confidence (data not shown). Likewise, neither cytochrome P450 enzymes (rs1048943, rs4646903, rs1056836, and rs1800440) nor GST genes (GSTM1 and GSTT1) were shown to modify associations (data not shown).
Discussion
This study was initially conducted to evaluate RCC risk and occupational exposure to PAHs and plastics. No associations were observed in this study with ever versus never exposure to any of the PAH or plastics exposure metrics evaluated. However, suggestive positive associations between RCC risk and cumulative exposure to styrene and acrylonitrile were observed. In contrast, petroleum/gasoline engine emission was inversely associated with risk.
Findings from epidemiological studies of occupational PAH exposure and kidney cancer risk have been inconsistent.6,24,25 Similar to the results of our study, no association between occupational exposure to PAH and kidney cancer incidence or mortality risk was reported in a recently published retrospective cohort study of aerospace workers.24 However, an indication of increased kidney cancer risk among heavily PAH-exposed male aluminum plant workers with a lag time of 30 years was reported in a large, multicentered, Norwegian study.25 This cohort may have had much higher PAH exposure levels than those estimated in the current study. A quantitative review of eight cohort studies conducted on workers from PAH-related occupations published between 1997 and 2005 revealed only limited evidence of an association between kidney cancer risk and PAH exposure. Standardized mortality ratios (SMR)/standardized incidence ratios across these studies ranged from 0.5 to 1.9 and pooled analyses revealed no significant findings.6 Most of these studies were underpowered to conclude statistically meaningful results, and exposure misclassification was particularly likely in the studies that used cruder assessment methods. Exposure levels may have differed across the studies. In addition, the individual types of PAHs and their amounts vary from source to source, likely resulting in further dilution of observed risks.
Scientific literature suggests that the association between PAH exposures and kidney cancer risk is biologically plausible, yet this association has not been supported by previous epidemiological studies or by the current study. Polycyclic aromatic hydrocarbons are absorbed internally through ingestion, inhalation, and dermal contact. Once absorbed, PAHs readily enter the lymphatic system where they circulate in the blood.1 Because of their lipophilic nature, PAHs are capable of entering any tissue in the body that contains adipose.1,2,13 As aforementioned, PAHs are generally stored in the kidneys, liver, and fat and are bio-activated through hepatic and renal metabolic pathways.1,13,14 Within the kidneys, cytochrome P450 enzymes, CYP1A1 and CYP1B1, mediate the oxidative metabolism of PAHs to reactive intermediates that bind covalently to DNA, forming adducts.20 These PAH-DNA adducts can lead to DNA replication errors and thus renal carcinogenesis. Furthermore, GSTs may influence the level of carcinogenic-DNA adducts formed, given their involvement in detoxifying reactive intermediates produced by cytochrome P450 enzymes.21 In our study, variants in neither cytochrome P450 enzymes nor GST genes were shown to modify the association between RCC risk and occupational exposure to PAHs.
The inverse association with petroleum/gasoline engine emissions in our study was unexpected and is suspected to be caused by chance, as gasoline exposure has been shown to consistently increase kidney cancer risk in both animal and human studies.26 Petroleum/gasoline emissions are also known to contain benzene, a known human carcinogen.27 Although not hypothesized a priori, the suggestive positive association observed between RCC risk and cumulative exposure to acrylonitrile and styrene was an interesting finding and requires additional follow-up. Classified as a probable human carcinogen (2A) by the International Agency for Research on Cancer, acrylonitrile exposure has been shown to negatively affect kidney function in human and animal studies, although evidence for renal carcinogenicity has been weak.28 Similarly, reports from animal studies indicate adverse kidney effects following exposure to styrene, although animal cancer studies have been inconsistent and provide limited evidence of carcinogenicity.29 Yet, because associations with other exposure metrics were not observed and because the number of exposed subjects was relatively small (N = 16 and 31, respectively), it is possible that our results could be due to chance.
In the current study, occupational styrene exposure was observed primarily among styrene manufacture operators, tank cleaners and tank operators of copolymers manufacturers, auto body repairmen who utilized polyester resins, and plastic boat manufacturers who processed unsaturated polyesters. A recent cohort study of 5204 American workers exposed to styrene in the reinforced plastic boat--building industry reported a borderline threefold increase risk for kidney cancer mortality among workers with high levels of exposure.30 Similarly, elevated kidney cancer mortality (SMR = 1.75, 95% CI = 0.98--2.89) was found among styrene-exposed workers in an earlier cohort of 15908 reinforced plastic industry workers.31 However, increased associations between kidney cancer and styrene exposure, or occupations likely to entail styrene exposure, have not been observed in all studies.32--34 Moreover, the occupational groups held by participants exposed to acrylonitrile in our study included manufacturers of acrylonitrile or acrylic fibers, manufacturers of plastic shoes that processed polymers, and workers who cut acrylic fabric. Previous studies examining cancer risk in relation to acrylonitrile exposure have generally been null with regards to kidney cancer.28,35,36 However, the vast majority of studies were well underpowered to conclude statistically meaningful results.
Strengths of our study include high participation rates, inclusion of only histologically confirmed cancers, use of job-specific questionnaire modules to collect individual-specific exposure information, and the expert-based exposure assessment. The large sample size of this study provided sufficient statistical power to detect relatively small associations between exposure and risk; however, power for stratified analyses was limited. The carcinogenic effect of PAHs depends not only on the duration and level of the exposure but also on the specific chemical composition of the PAH, because the composition can influence the toxicokinetics and toxicodynamics of the PAHs and ultimately the biological effect. Unfortunately, individual measurements of PAH dose were not collected in this study and, therefore, we had to rely on retrospective recall by study participants of their occupational history and other risk factors. However, given that controls were also hospital-based patients, any bias in recall would likely be nondifferential with respect to exposure, which would tend to mitigate risk estimates. While we were able to control for known RCC risk factors, like hypertension, smoking, and BMI, other potential exposures such as diet (ie, charbroiled meats) and nonoccupational PAH exposures (ie, air pollution) were not considered. Additional limitations of our study include the possibility of nondifferential, inaccurate, or incomplete recall of all occupational histories, and the possibility of nondifferential exposure misclassification, which could have biased results toward the null. The use of hospital-based controls could also be a concern since this population may not represent the general nondiseased reference population. However, we attempted to address this issue by recruiting controls with a wide range of diagnoses. Lastly, we cannot ignore the possibility of chance findings.
In summary, our study found no association between RCC risk and ever exposure to occupational PAHs and plastics among subjects in Central and Eastern Europe. Indication of increased renal cancer risk associated with acrylonitrile and styrene exposure was observed, but these findings require replication in other populations. Additional studies with detailed occupational history information and sufficient power are needed to confirm these results.
Acknowledgments
This study was funded by National Institutes of Health.
References
- 1.Agency for Toxic Substances and Disease Registry Toxicological Profile for Polycyclic Aromatic Hydrocarbons. [October 7, 2010]; http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf. [PubMed]
- 2.Jacob J, Seidel A. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:31–47. doi: 10.1016/s0378-4347(01)00467-4. [DOI] [PubMed] [Google Scholar]
- 3.Rajput N, Khemani LD, Lakhani A. Polycyclic aromatic hydrocarbons in ambient air at Agra: distribution and toxicity assessment. J Environ Sci Eng. 2008;50:111–114. [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer Polynuclear aromatic compounds, part 1, chemical, environmental and experimental data. IARC Monogr Eval Carcinog Risk Chem Hum. 1983;32:1–453. [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks Humans. Lyon: IARC; [October 7, 2010]. http://monographs.iarc.fr. [PMC free article] [PubMed] [Google Scholar]
- 6.Bosetti C, Boffetta P, La Vecchia C. Occupational exposures to polycyclic aromatic hydrocarbons, and respiratory and urinary tract cancers: a quantitative review to 2005. Ann Oncol. 2007;18:431–446. doi: 10.1093/annonc/mdl172. [DOI] [PubMed] [Google Scholar]
- 7.Li CT, Zhuang HK, Hsieh LT, Lee WJ, Tsao MC. PAH emission from the incineration of three plastic wastes. Environ Int. 2001;27:61–67. doi: 10.1016/s0160-4120(01)00056-3. [DOI] [PubMed] [Google Scholar]
- 8.Fustinoni S, Campo L, Cirla AM, et al. Biological monitoring in the molding of plastics and rubbers. G Ital Med Lav Ergon. 2007;29(suppl):284–286. [PubMed] [Google Scholar]
- 9.Parent ME, Hua Y, Siemiatycki J. Occupational risk factors for renal cell carcinoma in Montreal. Am J Ind Med. 2000;38:609–618. doi: 10.1002/1097-0274(200012)38:6<609::aid-ajim1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. Occupational risk factors for renal-cell carcinoma in Denmark. Scand J Work Environ Health. 1994;20:160–165. doi: 10.5271/sjweh.1413. [DOI] [PubMed] [Google Scholar]
- 11.McCredie M, Stewart JH. Risk factors for kidney cancer in New South Wales. IV. Occupation. Br J Ind Med. 1993;50:349–354. doi: 10.1136/oem.50.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin JK, Malker HS, Stone BJ, et al. Occupational risks for renal cancer in Sweden. Br J Ind Med. 1987;44:119–123. doi: 10.1136/oem.44.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Word Health Organization Chapter 5.9: Polycyclic aromatic hydrocarbon (PAH) [October 7, 2010];2000 http://test.cp.euro.who.int/document/aiq/5_9pah.pdf.
- 14.Obana H, Hori S, Kashimoto T, Kunita N. Polycyclic aromatic hydrocarbons in human fat and liver. Bull Environ Contam Toxicol. 1981;27:23–27. doi: 10.1007/BF01610981. [DOI] [PubMed] [Google Scholar]
- 15.International Agency for Research on Cancer GLOBACAN. [October 7, 2010]; http://www-dep.iarc.fr.
- 16.van der Hel O, Brennan P, Moore LE, et al. Tobacco smoking, body mass index, hypertension, and kidney cancer in Central and Eastern Europe. Br J Cancer. 2008;99:1912–1915. doi: 10.1038/sj.bjc.6604761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scelo G, Constantinescu V, Csiki I, et al. Occupational exposure to vinyl chloride, acrylonitrile and styrene and lung cancer risk. Cancer Causes Control. 2004;15:445–452. doi: 10.1023/B:CACO.0000036444.11655.be. [DOI] [PubMed] [Google Scholar]
- 18.Hashibe M, Boffetta P, Zaridze D, et al. Contribution of tobacco and alcohol to the high rates of squamous cell carcinoma of the supraglottis and glottis in Central Europe. Am J Epidemiol. 2007;165:814–820. doi: 10.1093/aje/kwk066. [DOI] [PubMed] [Google Scholar]
- 19.Moore LE, Boffetta P, Karami S, et al. Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res. 2010;70:6527–6536. doi: 10.1158/0008-5472.CAN-09-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falahatpisheh M, Kerzee J, Metz R, Donnelly K, Ramos K. Inducible cytochrome P450 activities in renal glomerular mesangial cells: biochemical basis for antagonistic interactions among nephrocarcinogenic polycyclic aromatic hydrocarbons. J Carcinog. 2004;3:12. doi: 10.1186/1477-3163-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butkiewicz D, Grzybowska E, Phillips DH, Hemminki K, Chorazy M. Polymorphisms of the GSTP1 and GSTM1 genes and PAH-DNA adducts in human mononuclear white blood cells. Environ Mol Mutagen. 2000;35:99–105. [PubMed] [Google Scholar]
- 22.Karami S, Boffetta P, Rothman N, et al. Renal cell carcinoma, occupational pesticide exposure and modification by glutathione S-transferase polymorphisms. Carcinogenesis. 2008;29:1567–1571. doi: 10.1093/carcin/bgn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karami S, Brennan P, Rosenberg PS, et al. Analysis of SNPs and haplotypes in vitamin D pathway genes and renal cancer risk. PLoS One. 2009;4:e7013. doi: 10.1371/journal.pone.0007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Krishnadasan A, Kennedy N, Morgenstern H, Ritz B. Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am J Ind Med. 2005;48:249–258. doi: 10.1002/ajim.20216. [DOI] [PubMed] [Google Scholar]
- 25.Romundstad P, Andersen A, Haldorsen T. Cancer incidence among workers in six Norwegian aluminum plants. Scand J Work Environ Health. 2000;26:461–469. doi: 10.5271/sjweh.569. [DOI] [PubMed] [Google Scholar]
- 26.Dement JM, Hensley L, Gitelman A. Carcinogenicity of gasoline: a review of epidemiological evidence. Ann N Y Acad Sci. 1997;837:53–76. doi: 10.1111/j.1749-6632.1997.tb56864.x. [DOI] [PubMed] [Google Scholar]
- 27.Agency for Toxic Substances & Diseases Registry Benzene. [October 7, 2010]; http://www.atsdr.cdc.gov/tfacts3.html.
- 28.US Environmental Protection Agency [October 7, 2010];Acrylonitrile Fact Sheet: Support Document (CAS No 107--13-1) http://www.epa.gov/chemfact/acry-sd.txt.
- 29.US Environmental Protection Agency Styrene. [October 7, 2010]; http://www.epa.gov/ttn/atw/hlthef/styrene.html. [PubMed]
- 30.Ruder AM, Ward EM, Dong M, Okun AH, Davis-King K. Mortality patterns among workers exposed to styrene in the reinforced plastic boatbuilding industry: an update. Am J Ind Med. 2004;45:165–176. doi: 10.1002/ajim.10349. [DOI] [PubMed] [Google Scholar]
- 31.Wong O, Trent LS, Whorton MD. An updated cohort mortality study of workers exposed to styrene in the reinforced plastics and composites industry. Occup Environ Med. 1994;51:386–396. doi: 10.1136/oem.51.6.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathiakumar N, Delzell E. A follow-up study of mortality among women in the North American synthetic rubber industry. J Occup Environ Med. 2009;51:1314–1325. doi: 10.1097/JOM.0b013e3181bd8972. [DOI] [PubMed] [Google Scholar]
- 33.Gérin M, Siemiatycki J, Désy M, Krewski D. Associations between several sites of cancer and occupational exposure to benzene, toluene, xylene, and styrene: results of a case--control study in Montreal. Am J Ind Med. 1998;34:144–156. doi: 10.1002/(sici)1097-0274(199808)34:2<144::aid-ajim7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 34.Sathiakumar N, Graff J, Macaluso M, Maldonado G, Matthews R, Delzell E. An updated study of mortality among North American synthetic rubber industry workers. Occup Environ Med. 2005;62:822–829. doi: 10.1136/oem.2004.018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blair A, Stewart PA, Zaebst DD, et al. Mortality of industrial workers exposed to acrylonitrile. Scand J Work Environ Health. 1998;24(suppl 2):25–41. [PubMed] [Google Scholar]
- 36.Swaen GM, Bloemen LJ, Twisk J, et al. Mortality update of workers exposed to acrylonitrile in The Netherlands. J Occup Environ Med. 2004;46:691–698. doi: 10.1097/01.jom.0000128161.17144.27. [DOI] [PubMed] [Google Scholar]


