Abstract
The human body is continuously exposed to small organic molecules containing one or more basic nitrogen atoms. Many of these are endogenous (i.e., neurotransmitters, polyamines and biogenic amines), while others are exogenously supplied in the form of drugs, foods and pollutants. It is well-known that many amines have a strong propensity to specifically and substantially accumulate in highly acidic intracellular compartments, such as lysosomes, through a mechanism referred to as ion trapping. It is also known that cells have acquired the unique ability to sense and respond to amine accumulation in lysosomes in an effort to prevent potential negative consequences associated with hyperaccumulation. We describe here methods that are used to evaluate the dynamics of amine accumulation in, and egress from, lysosomes. Moreover, we highlight specific proteins that are thought to play important roles in these pathways. A theoretical model describing lysosomal amine dynamics is described and shown to adequately fit experimental kinetic data. The implications of this research in understanding and treating disease are discussed.
For over a century, scientists have studied the interactions between low-molecular-weight dyes and cells and tissues. Initially, it was serendipitously discovered that certain dye molecules preferentially associated with specific subcellular structures/compartments. These so-called ‘vital stains’, together with advances in cell imaging, provided a basis for diagnosing and understanding disease and later became crucial in basic scientific research, providing investigators tools with which to study basic aspects of cell structure and function.
In this article, we will focus our attention on the accumulation and egress pathways for small-molecular-weight amine-containing molecules that specifically localize within lysosomes. Lysosomes are found in virtually all mammalian cells and contain various hydrolytic enzymes that function in the digestion and recycling of cellular materials (i.e., proteins, lipids and nucleic acids) [1–5]. These hydrolytic enzymes have optimal activity at low pH (4–5), which is maintained through the activity of the vacuolar H+ ATPase (V-ATPase), which is located on the limiting membrane and functions in pumping protons from the cell cytosol to the luminal space [6,7]. The steep intracellular pH gradient that exists between the lysosomal lumen and the cell cytosol is responsible for driving the hyperaccumulation of many small-molecular-weight amine-containing molecules through a process referred to as ion trapping or pH partitioning [2]. Molecules that specifically accumulate within lysosomes are collectively referred to as lysosomotropic agents.
There is currently a great deal of interest in studying and utilizing molecules with lysosomotropic properties. Many highly fluorescent amine-containing molecules have been specifically designed for the purpose of labeling acidic organelles, such as lysosomes, to study properties including their morphology, pH and intracellular trafficking (see commercially available LysoSensor™ and LysoTracker™ dyes from Invitrogen). In addition, and perhaps more importantly, there is much recent interest in understanding the uptake and subsequent trafficking of amines in cells, for therapeutic reasons.
In the past, it was thought that if a drug were to become sufficiently concentrated within target-containing cells, one could anticipate adequate drug–drug target interactions and thus a therapeutic response. Drug accumulation in cells is, unquestionably, a necessary stage; however, it may not always represent the final stage in drug delivery. Considering the highly compartmentalized nature of mammalian cells, it is likely that the drug and drug target could fail to ‘find’ one another in the intracellular environment. Much is known about drug target localization. Drug targets are not typically evenly dispersed throughout the cellular organelles and compartments; rather, they are often confined to discrete subcellular locations. If drugs tend to localize exclusively within nondrug target-containing compartments, one can anticipate either no effects or significant side effects, resulting from off-target interactions. Conversely, if drugs can specifically localize within the compartment housing the intended target, they can be expected to have improved potency and reduced potential for side effects.
It is well-known that basic amines are ubiquitously found in therapeutic drug molecules; over half of all commercially available drugs contain at least one basic amine and many of these molecules are thought to have some degree of lysosomotropic potential. Consequently, understanding amine accumulation and egress from lysosomes has numerous therapeutic implications.
In some cases, it is therapeutically advantageous to have drugs with lysosomotropic properties. The antimalarial drug chloroquine is perhaps the most well-known example. Chloroquine exerts its toxic effects while in the lysosomes of the malaria parasite [8,9]. It has also been shown that some antipsychotic and antidepressant drugs extensively accumulate in lysosomes [10–13], and it is thought that this accumulation could contribute to the mechanism of action of these agents through inhibition of acid sphingomyelinase [14,15]. We have also recently shown that lysosomotropic properties of anticancer agents can be beneficial in enhancing drug selectivity towards tumor cells with defective pH regulation [16]. Conversely, in some instances, the accumulation of basic drugs in lysosomes has been associated with negative side effects. For example, the development of drug-induced phospholipidosis has been associated with lysosomal sequestration potential [17–20].
In this article, we will describe the mechanisms for weak base accumulation in lysosomes and highlight work that has been carried out to establish structure–localization-type relationships. We will also describe how cells have evolved an impressive mechanism to cope with exposure to lysosomotropic amines by creating so-called ‘amine-induced vacuoles’. Likewise, we will review recent literature and present a new kinetic model that attempts to describe the mechanism by which cells facilitate the egress of lysosomotropic amines from this compartment. Finally, we will summarize some of the techniques that have been developed and utilized to study the concentration of amine-containing compounds in lysosomes, specifically highlighting the advantages and disadvantages associated with each.
Experimental procedures
Reagents & cell culture
[3H]-dextran (MW 70,000) was obtained from American Radiolabeled Chemicals (St Louis, MO, USA). Iron-conjugated dextran was prepared as described elsewhere [21]. Dextran (MW 64,000–76,000) was purchased from Sigma-Aldrich (St Louis, MO, USA). All other reagents were obtained from Sigma-Aldrich, unless otherwise stated. All cells and experiments were maintained at 37°C in a humidified 5% CO2 atmosphere and grown in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
Kinetic data analysis
The kinetics of lysosomal [3H]-dextran release from normal human foreskin fibroblasts (CRL-2076, Coriell) and human foreskin fibroblasts with nonfunctional Niemann–Pick C1 (NPC1; denoted NPC1−/−, GM03123, Coriell) were obtained from previously published work [22]. Modeling of kinetic data was performed using SAAMII, version 1.1 (Seattle, WA, USA) for Microsoft Windows. Separate simulations were set up to model [3H]-dextran release from normal and NPC1−/− cells and solutions were obtained simultaneously for both models.
Experimental estimation of intracellular cargo trafficking rate constants
In order to achieve the best fit possible, we experimentally estimated rate constants described in our theoretical model. Data from previously analyzed fluorescence resonance energy transfer (FRET)-based, late endosome–lysosome fusion assays for quantitation of hybrid organelle formation rates were utilized for this purpose [23]. Data from these experiments were used to obtain rate constants for late endosome to hybrid organelle trafficking as well as lysosome to hybrid organelle trafficking. All other rate constants were adjusted iteratively by the SAAMSII software.
Experimental determination of steady-state concentrations of dextran in intracellular compartments
The steady-state amounts of [3H]-dextran localized in each of the cellular compartments necessary for modeling purposes were determined. Initially, 25 μCi/ml of [3H]-dextran was added to cells grown in culture and incubated for 6 h. The [3H]-dextran in each of the subcellular compartments was estimated by biochemically purifying the indicated organelles and measuring the radioactivity associated with that fraction. The isolation of specified endocytic organelles was achieved using an adaptation of a previously described magnetic chromatography-based approach for isolating lysosomes [24]. Briefly, iron-conjugated dextran particles are localized in specified endocytic compartments using appropriate pulse-chase parameters. The organelles containing the iron-conjugated dextran are subsequently purified by passing a cell lysate over a magnetic column. Endocytic compartments enriched in early and late endosomes were obtained by pulsing cells with 2 mg/ml iron-conjugated dextran for 15 min. Iron-conjugated dextran was specifically localized to lysosomes by using a 15-min pulse, followed by a 6-h chase. Western blot analysis was performed on these two samples using the following organelle-specific antibodies: EEA1 (early endosomes), MPR (late endosomes) and LAMP1 (lysosomes). Densitometry was then performed on the Western blot to determine the relative abundance of the specified organelles in each of the isolated fractions.
Bright and co-workers have published a study on the dynamics of lysosomes and hybrid organelles, and they estimated that, at any given time, approximately 15% of lysosomes are fused with late endosomes forming hybrid organelles [25]. We, therefore, used this estimate; we subtracted 15% of the lysosomal fraction and assigned this number to hybrid organelles.
Mechanisms for weak base accumulation in lysosomes
Lysosomotropism refers to the propensity of any molecule to specifically and significantly associate with lysosomes; however, it is important to note that this term does not imply a mechanism for the observed compartmentalization. Generally speaking, there are at least four unique mechanistic pathways that can account for lysosomal accumulation: passive diffusion, endocytic uptake, autophagy and/or a lysosomal transmembrane transport system. Of these, it is clear that passive diffusion-mediated delivery allows for the greatest magnitude of lysosomal accumulation [2]. Consequently, we will limit our following discussion to this pathway.
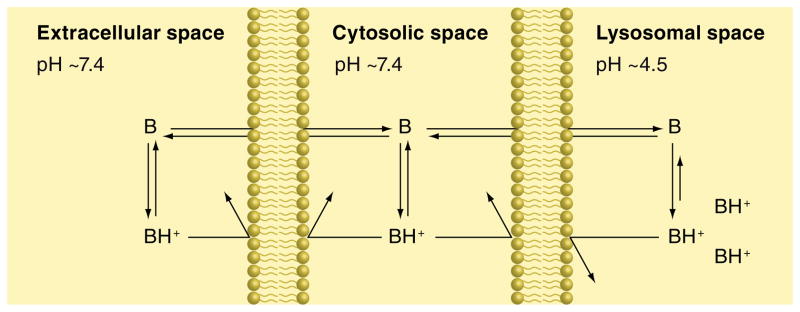
Lysosomotropic behavior is most often observed with molecules that are weakly basic with pKa values near or above neutrality [2,26]. Importantly, the molecule must be relatively membrane permeable when it is unionized and membrane impermeable when it exists in its ionized conjugate acid state. Under these constraints, such molecules can readily permeate into the near-neutral cell cytosol and exist to a significant degree as the membrane-permeable, unionized base. The free base can subsequently partition across organelle lipid bilayers down its concentration gradient. When the base encounters the low pH of lysosomes (4–5), the percentage of the molecule now existing as the free base is virtually zero, which subsequently drives additional free base to diffuse into the lysosomal lumen from the cytosol. At steady state, the concentration of free base inside the lysosomes will be equivalent to the concentration in the cytosol. However, due to differences in the ionization state of the amine in the two compartments, the total concentration of the amine will be several orders of magnitude greater in lysosomes, compared with the cytosol. This behavior has been referred to as pH partitioning or ion trapping (Figure 1). It is important to note that such ion-trapping behavior will be observed for any organelle that has an acidic luminal pH relative to the cell cytosol (i.e., Golgi apparatus, early and late endosomes). For the sake of simplicity, we will use the term ‘lysosomes’ to describe any such acidic organelle.
Figure 1. pH partitioning mechanism of accumulation for weakly basic amine-containing compounds.
There are three compartments separated by the presence of cellular membranes composed of a phospholipid bilayer for which the unionized weakly basic compound (B) is permeable and able to diffuse and therefore capable of attaining equivalent concentrations in the three compartments at steady state. By contrast, the ionized form of the compound (BH+) is unable to permeate through the lipid bilayer and becomes trapped within whichever compartment it resides. The relative concentration of B and BH+ in each compartment is dictated by the pH of the medium within that compartment as well as the pKa of the compound. The decrease in pH encountered within the lysosomal space compared with the cytosolic and extracellular spaces causes a shift in the ionic equilibrium favoring the membrane-impermeable ionized species, thus leading to an overall increase in compound accumulation within the lysosomal compartment.
In a landmark commentary on lysosomotropism, Christian de Duve has thoroughly outlined the theoretical basis supporting this phenomenon and has illustrated how both the pH differential existing between the lysosomes and cytosol as well as the pKa of the molecule can be expected to influence the steady-state concentration ratios for amines in relevant cellular compartments [2]. At the time of this publication, analytical techniques were not sophisticated enough to allow for experimental evaluation of these theoretical predictions. More recently, the concentrations of various amines in lysosomes have been estimated using a variety of techniques (see discussion later). It is important to note that, even though lysosomotropic amines have been shown to be highly concentrated in lysosomes (relative to the cell cytosol), the total amount of amine that is associated with lysosomes may not account for the total amount of amine contained within the cell. This is because lysosomes constitute such a small fraction of the total cell volume. For example, we have previously shown that approximately 40% of a model lysosomotropic amine is associated with lysosomes [24].
Interestingly, we and others have shown that lysosomotropic amines may achieve significantly higher lysosomal accumulation than pure theoretical models would predict [24,27,28]. For example, a weakly basic amine with a pKa value of 8 should theoretically achieve a lysosome-to-cytosol concentration ratio of approximately 40 in a cell with cytosol and lysosomal pH determined as 7.4 and 5.1, respectively. We have shown that experimentally determined concentration ratios were 3–15-times greater than would be theoretically predicted. The precise reasons for higher-than-predicted lysosomal accumulation have not been fully elucidated, however, there are several possibilities. For relatively hydrophobic amines, it is possible that they may reach concentrations in lysosomes that exceed their solubility. This is potentially plausible, considering that concentrations in lysosomes can reach millimolar levels, even with relatively low incubation concentrations [2]. Related to this, the amine could also form complexes with lysosomal contents, both soluble and membrane-associated.
In order for extensive amine accumulation to occur, the lysosomal pH must remain low through a rapid influx of protons; otherwise the accumulating amine would cause lysosomal pH to increase, thus destroying the driving force behind sequestration. The V-ATPase is a multi-subunit proton pump that is responsible for pumping protons into the lumen of lysosomes and, therefore, maintaining the low pH. As expected, inhibiting the V-ATPase with concanamycin A results in a severely diminished sequestration capacity of common lysosomotropic amines such as LysoTracker Red (LTR; Figure 2). Moreover, the rate of amine uptake into lysosomes has been shown to correlate with the efficiency of the V-ATPase in proton transfer into lysosomes [29]. Additionally, transporter-mediated uptake of various amine-containing compounds into acidic vesicles has also been shown to be dependent on the pH gradient generated by the V-ATPase. A well-established example is the loading of biogenic amines into secretory vesicles via vesicular monoamine transporters (VMATs), as well as the lysosomal uptake of quaternary amine compounds through an organic cation/H+-exchange transporter [30,31]. Interestingly, the P-glycoprotein (P-gp) has also been found to reside on intracellular vesicles and may play an important role in the lysosomal sequestration of select xenobiotics [32]. There is much recent interest in correlating structure and physicochemical properties of weakly basic amines with their propensity to be sequestered in lysosomes, as was discussed in the Introduction. In his early commentary, de Duve had made some key predictions in this regard that are still extremely valid today [2]. Briefly, he suggested that the pKa and the permeability characteristics of the amine were key determinants in lysosomotropic potential. Specifically, amines with pKa values near 8 that are freely membrane permeable when unionized and virtually membrane impermeable when ionized will exhibit optimal lysosomal sequestration. For a more comprehensive review of physical and structural properties that have been shown to correlate with lysosomotropic behavior, the reader is directed to a recent review written on this topic [33]. More recently, first principle-based modeling methods have been developed that correlate physicochemical properties of weakly basic amines with subcellular localization, including lysosomal sequestration [34,35]. The current limitation of these approaches appears to be the relatively small set of molecules for which there is reliable, quantitative information regarding their intracellular distribution. With the generation of more data, information from such simulations will certainly aid medicinal chemists of the future in the design of drugs with optimal intracellular distribution.
Figure 2. Maintenance of the lysosomal pH gradient is required for accumulation of a weakly basic amine.
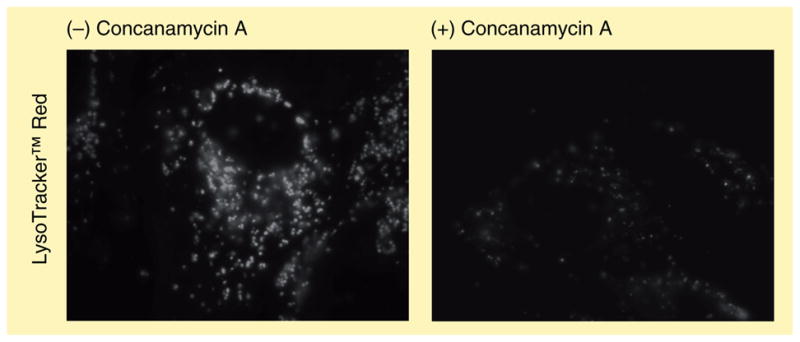
Human fibroblasts were exposed to 100 nM of the weakly basic lysosomal probe LysoTracker™ Red with or without concomitant exposure to 100 nM of the vacuolar ATPase inhibitor concanamycin A. Inhibition of vacuolar ATPase is known to significantly elevate the intralysosomal pH and thus dissipate the pH gradient existing between the lysosomal and cytosolic lumens, thereby decreasing lysosomal accumulation of the probe.
Amine-induced vacuolization
Since the 1940s, various researchers have documented the formation of giant cytoplasmic vesicles originating in cultured cells exposed to relatively high concentrations of small-molecular-weight amines [36,37]. Yang and co-workers further demonstrated this phenomenon by studying the amine-induced vacuolization caused by anesthetics such as procaine [38]. Using phase-contrast microscopy, the authors demonstrated that the vacuolization was exceptionally extensive (vacuoles could occupy a third of the total cell volume) and yet completely reversible.
Today, it is well established that this vacuolization results from extensive ion-trapping-based accumulation of lysosomotropic amines in lysosomes, as discussed previously. This is supported by the finding that disruption of the lysosomal V-ATPase inhibits formation of the vacuoles. Examples of amine-induced vacuoles formed in human foreskin fibroblasts treated with the lysosomotropic amines neutral red (NR) and chloroquine are shown in Figure 3.
Figure 3. Amine-induced vacuolization in human cells.
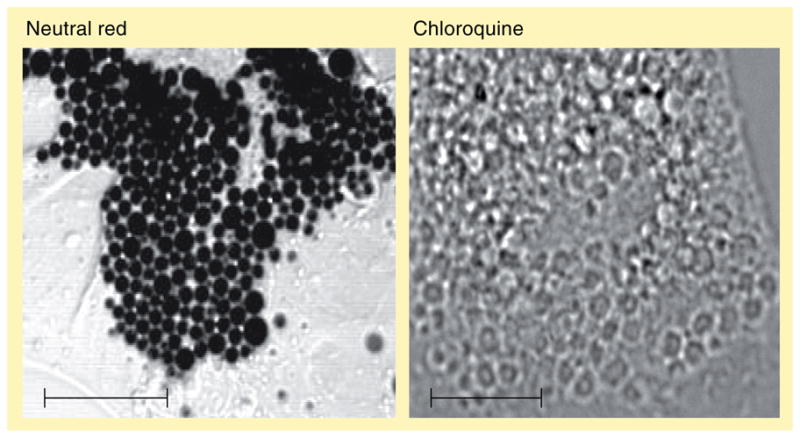
Human fibroblasts treated with 70-μM neutral red or 100-μM chloroquine for 6 h show extensive vacuolization using light transmission microscopy or phase-contrast microscopy, respectively.
Scale bars represent 10 μm.
The precise reason that cells have evolved with such a profound capacity to form these vacuoles is not currently understood. However, it is reasonable to speculate that vacuolization serves an important role in relieving osmotic pressure that would otherwise steadily increase as the concentration of a lysosomotropic amine increased in lysosomes. This is consistent with early work by Finnen and co-workers, who demonstrated that increasing the osmotic pressure in the cell-culture medium reduces procaine-induced vacuolization [39]. Without such an expansion in the volume of the amine-containing compartment, it is likely that the influx of water would eventually result in catastrophic bursting of lysosomes. This would likely be detrimental to cell viability since it has been previously shown that the release of lysosomal enzymes into the cell cytosol is a potent initiator of apoptosis [40,41].
Recently, several groups have begun to investigate the substrate specificity for amine-induced vacuolization in a variety of cell types [42,43]. Prior to this, Ohkuma and Poole had systematically evaluated the kinetics of uptake and vacuolization potential for a series of weakly basic amines [29]. It was noted that all amines that caused vacuolization had predicted lysosomotropic properties, but not all amines with lysosomotropic properties efficiently induced vacuolization. The authors were able to establish a clear correlation between the concentrations of bases that accumulate in cells and the degree of vacuolization. Consistent with this, Morissette and co-workers have also provided evidence suggesting that amine uptake into cells appears to be the limiting factor in vacuolization [42]. The authors demonstrated that different cell types exhibited varying degrees of vacuolization using the same amine. Specifically, the authors’ work suggests that the degree of vacuolization between different cell lines may correlate with the activity of tertiary amine/H+ antiport systems at the plasma membrane.
The origins of amine-induced vacuoles have also been recently examined, and there are some inconsistencies that can be found in the literature. Michalik and co-workers found that amine-induced vacuoles co-localized with endocytosed Lucifer yellow, a well-established tracer of fluid-phase endocytosis [44], whereas Morissette and colleagues did not observe this in their work. Based on co-localization of amine-induced vacuoles with a Golgi-specific fluorescent C5 ceramide, the latter group suggests that the vacuoles are of Golgi origin. We have recently performed immunofluorescence analysis on human foreskin fibroblasts and found that amine-induced vacuoles co-localize with the late-endosomal- and lysosomal-resident proteins, namely the mannose 6-phosphate receptor and LAMP-1, respectively. We did not observe any co-localization of vacuoles with the Golgi-specific protein GM130 or the early-endosome-specific protein EEA1 (Figure 4) [22].
Figure 4. Amine-induced vacuolization stems from late endosomal–lysosomal fusion.
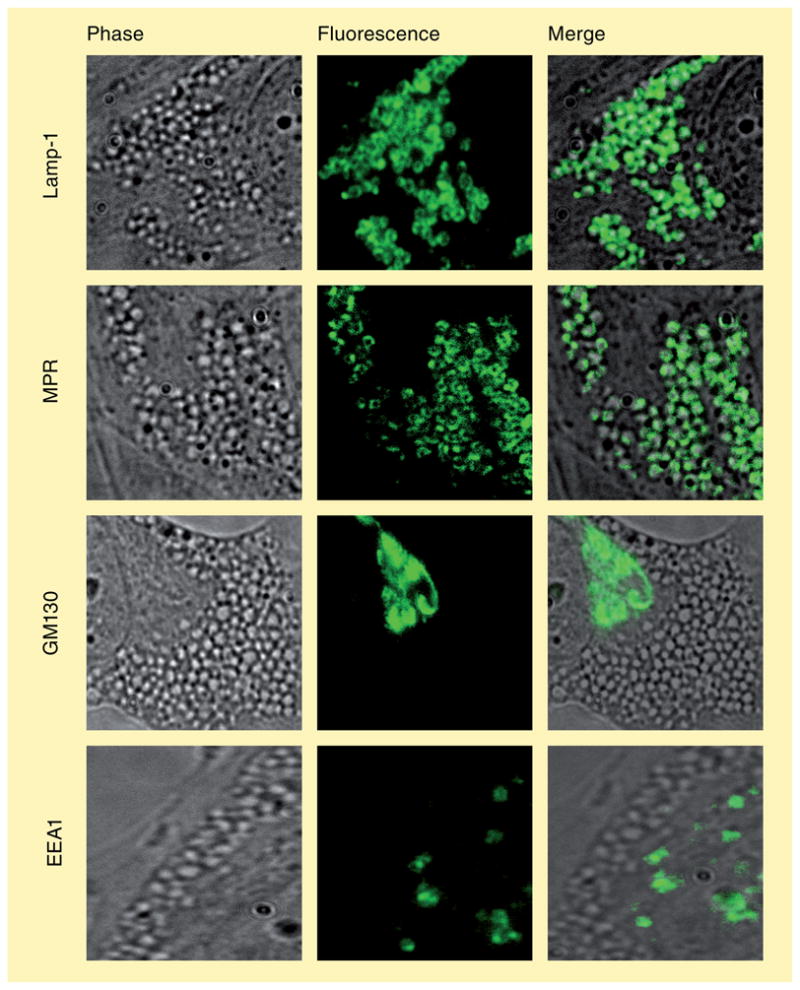
(A) Phase-contrast, (B) immunofluorescence and (C) merged images of normal fibroblasts after treatment with neutral red to induce vacuolization. Merged images confirm vacuoles consist of lysosomes (Lamp-1) and late endosomes (MPR), but not of Golgi (GM130) or early endosomes (EEA1). This is consistent with the hypothesis that amine-induced vacuoles represent enlarged hybrid organelles.
There is ample evidence to suggest that amine-induced vacuoles form through the coalescence (fusion) of smaller organelles/vesicles, either homotypically or heterotypically. The evidence for this is based on the findings of our laboratory and others, which demonstrate the requirement of intact microtubule networks for vacuolization [22,44]. In contrast to this model, one could potentially envision a scenario in which individual amine-containing compartments become swollen through expansion of the limiting organelle membrane due to the forces created by the build-up of osmotic pressure in the luminal domain. In earlier work, Solheim and colleagues evaluated this mechanistic possibility [45]; they evaluated the size of amine-induced vacuoles and compared them with the concentration of amine remaining in the cells (under sink conditions). Importantly, the authors clearly demonstrated that the large amine-induced vacuoles persist for longer than the time required for all of the amine to be cleared from the cell.
Are there proteins that function to ‘sense’ the hyperaccumulation of amines in lysosomes and subsequently facilitate their fusion with other compartments to create amine-induced vacuoles? As previously stated, our immunofluorescence-based evaluations are consistent with amine-induced vacuoles having both lysosomal and late-endosomal resident proteins (Figure 4). Interestingly, we have shown that incubation of cells with lysosomotropic amines dramatically enhances the co-localization of these organelle-specific proteins [22]. Luzio and co-workers have shown that lysosomes traffic in a retrograde manner and fuse with late endosomes to create so-called hybrid organelles, which are much greater in size than lysosomes [46]. The hybrid organelles subsequently reform lysosomes to avoid their consumption. We hypothesized that amine-induced vacuoles are merely greatly expanded hybrid organelles, which is consistent with our immunofluorescence evaluations. We have recently developed a FRET-based assay that allows us to quantitatively measure the retrograde fusion of lysosomes with late endosomes [23]. Using this assay, we have shown that the lysosomal membrane protein NPC1 is required for efficient hybrid organelle formation (mutations in NPC1 lead to 95% of cases of Niemann–Pick type-C disease, a fatal neurodegenerative lysosomal-storage disorder) [22,23]. Moreover, we have demonstrated that NPC1 is required for amine-induced vacuole formation. To illustrate this, fibroblasts that harbor functional mutations in NPC1 were obtained from patients with Niemann–Pick type C disease. We have shown that without functional NPC1, cells fail to form amine-induced vacuoles under conditions in which they readily do so in normal fibroblasts. Upon treatment with NR, normal cells form large vacuoles, while those with nonfunctional NPC1 do not (Figure 5). Likewise, we could rescue the vacuolization phenotype in NPC1-deficient cells by introducing a green fluorescent protein (GFP)-tagged functional version of NPC1 (NPC1–GFP). Interestingly, the NPC1–GFP was exclusively localized to the newly formed amine-induced vacuoles.
Figure 5. The size of hybrid organelles is decreased in cells with nonfunctional NPC1.
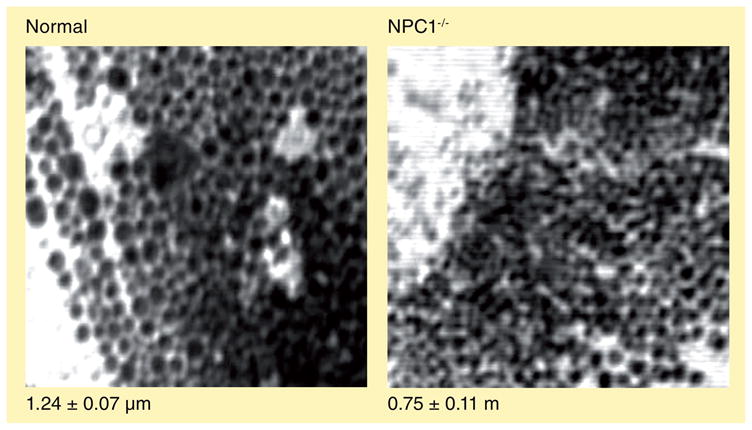
Normal and NPC1−/− cells were treated with 70-μM neutral red for 6 h. Phase-contrast images were acquired using confocal microscopy with an argon laser at 458 nm. Sizing was performed using ImageJ™ software. NPC1−/− cells have impaired late endosome–lysosome fusion and, therefore, exhibit smaller hybrid organelles compared with normal fibroblasts.
The previously described studies illustrate that NPC1 is required for amine-induced vacuole formation; however, they do not necessarily demonstrate that the function of NPC1 is to sense amine accumulation in lysosomes and subsequently facilitate hybrid organelle formation. To test this hypothesis, we evaluated hybrid organelle formation rates in normal fibroblasts, with and without the addition of amines. Interestingly, we have shown that certain lysosomotropic amines can significantly stimulate hybrid organelle formation rates. The amines were also shown to specifically stimulate NPC1 (or its pathway), since the same amines had no impact on hybrid organelle formation rates in cells without functional NPC1 [22].
Collectively, these results support the notion that amine-induced vacuoles are expanded late endosomal/lysosomal hybrid organelles and that NPC1 may play an important role in sensing amine accumulation in lysosomes and, subsequently, facilitating the retrograde fusion with the late endosomes. More work is necessary to elucidate the precise mechanism by which the amines seemingly serve to stimulate NPC1 function in this event.
Mechanism of egress for entrapped amines from lysosomes
There are essentially two mechanisms by which amines can exit lysosomes. First, it is possible that amines can directly cross the lysosomal membrane back into the cytosol. The second possibility involves some form of retroendocytic vesicle-mediated transport pathway. These two pathways will be addressed separately later.
We and others have shown that, when lysosomotropic amines are removed from the cell culture medium, the amine is cleared from the cell extremely efficiently. Additionally, in cells treated with LTR or NR, we have shown that most of the molecules are released from cells 12 h after the amine was removed from the culture medium [22,44]. If we assume that the ionized form of the base has very limited membrane permeability, it is unlikely that simple passive diffusion of amines out of lysosomes can account for significant release in the aforementioned timeframe. Amines that hyperaccumulate in lysosomes typically have pKa values near or above neutrality. Consequently, only a minute fraction of the base would exist in its unionized form and, thus, be available for diffusion out. There is also the possibility that an amine transporter could be present on the limiting membrane of lysosomes that could function in translocating the protonated amine directly into the cytosol. Davies and coworkers have reported that the lysosomal protein NPC1 (discussed previously) functions as a transmembrane pump for amines [47]. The authors demonstrated that NPC1 has significant sequence homology with a family of prokaryotic RND permeases that function in this regard. The authors presented data that acriflavine (a combination of tertiary and quaternary ammonium compounds) is exported from lysosomes through the action of this protein; however, since this original work, we and others have provided evidence that suggest that NPC1 may not function as a transmembrane pump [48,49]. As will be discussed later, we have evidence that suggests NPC1 does indeed play an important role in the release of amines from lysosomes, but through a mechanism that does not involve transmembrane efflux.
Although one cannot definitively state that some degree of passive diffusion does not contribute to amine release from lysosomes, a number of observations suggest that it is not a major contributor. We have isolated amine-containing lysosomes from cells and have shown that the majority of the amine remains associated with the lysosomes for at least several hours [50]. If the release of amines from lysosomes requires some intracellular trafficking events, it should be dramatically reduced when microtubule-depolymerizing agents are added to the cells. Indeed, we and others have shown that the addition of the microtubule-depolymerizing agent nocodazole significantly interferes with the efficient clearance of the lysosomotropic anticancer agent daunorubicin from lysosomes [49,51]. Interestingly, it has been shown that NR release from lysosomes occurs very rapidly in normal skin fibroblasts (~12 h); however, when the fibroblasts are treated with 2.5-mM procaine, the NR levels in cells remain virtually unchanged for 24 h [44]. It may be that procaine interferes with organelle fusion processes, although this has not been firmly established. Interestingly, procaine is structurally similar to other cationic amphiphilic drugs (CADs) that have been shown to interfere with lysosomal/late endosomal trafficking events. The best known example of a CAD that interferes with this trafficking is U18666A. A recent review on this molecule suggests that it may intercalate into membranes and have a negative influence on the proper functioning of membrane proteins that play important roles in intracellular trafficking [52].
Recently, Chen and co-workers have shown that multivesicular bodies (MVBs) play an important role in the egress of the weakly basic drug doxorubicin from K562 cells [53]. MVBs are found within late endocytic compartments and have been shown to fuse with the plasma membrane to release small shed vesicles, referred to as ‘exosomes’ [54]. Since MVBs have an acidic intralumenal environment (similar to lysosomes), it is likely that weakly basic molecules accumulate within them according to an ion-trapping-based mechanism. Interestingly, the authors have shown that the introduction of a dominant negative vacuolar sorting protein 4a, a protein normally required for efficient MVB formation, significantly decreased doxorubicin egress.
We have recently evaluated the role of the protein NPC1 in the vesicle-mediated release of amines from lysosomes. Using normal human fibroblasts, we have shown that both LTR and NR are completely released from lysosomes 12 h after incubation in amine-free media. Cells with functional mutations in NPC1, as well as normal cells treated with NPC1 siRNA, retained well over 50% of their amine content following incubation in amine-free media after a 12-h period. These results strongly suggest that NPC1 plays an important role in the cellular egress of amines trapped in lysosomes. If one functional role of NPC1 is aiding in the clearance of amines from lysosomes, this NPC1-mediated clearance pathway should be responsive to the presence of amines. To test this, we specifically localized [3H]-dextran polymers into lysosomes and monitored the kinetics of release in the presence or absence of lysosomotropic amines. We found that certain lysosomotropic amines significantly stimulated release of the lysosomal cargo. Importantly, the stimulatory effect was shown to be specific to NPC1 or its pathway, since these amines had no influence on the release of dextran from cells in the absence of functional NPC1 [22].
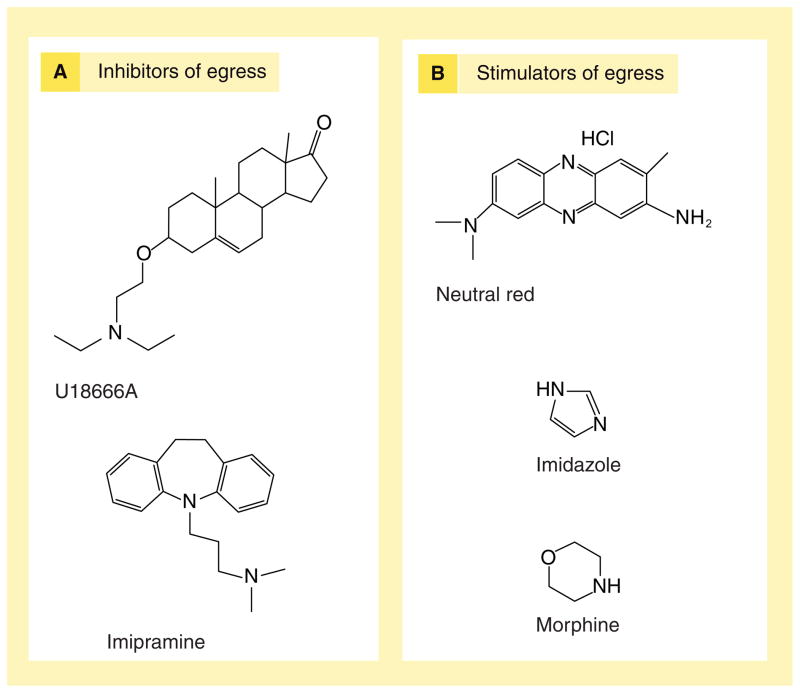
It is important to emphasize that not all lysosomotropic amines stimulate NPC1-mediated egress of lysosomal cargo. As previously mentioned, amphiphilic amines tended to inhibit NPC1-mediated egress of lysosomal cargo, potentially due to their propensity to intercalate into lysosomal membranes and disrupt NPC1 function (as previously described in the case of U18666A) [52]. Amines that had stimulatory effects on NPC1 were not considered amphiphilic. It is possible that these amines accumulate in lysosomes and favorably modify the tertiary structure of NPC1, but this remains poorly understood. Examples of amines shown to stimulate or inhibit NPC1-mediated retroendocytic trafficking are given in Figure 6.
Figure 6. Amines can influence lysosomal egress pathways.
(A) Inhibitors of egress are, for the most part, amphiphilic molecules; possibly interacting with lysosomal membranes to inhibit retrograde trafficking. (B) Stimulators are capable of stimulating retrograde trafficking in a Niemann–Pick C1-dependent manner.
Modeling lysosomal cargo release in the retroendocytic trafficking pathway
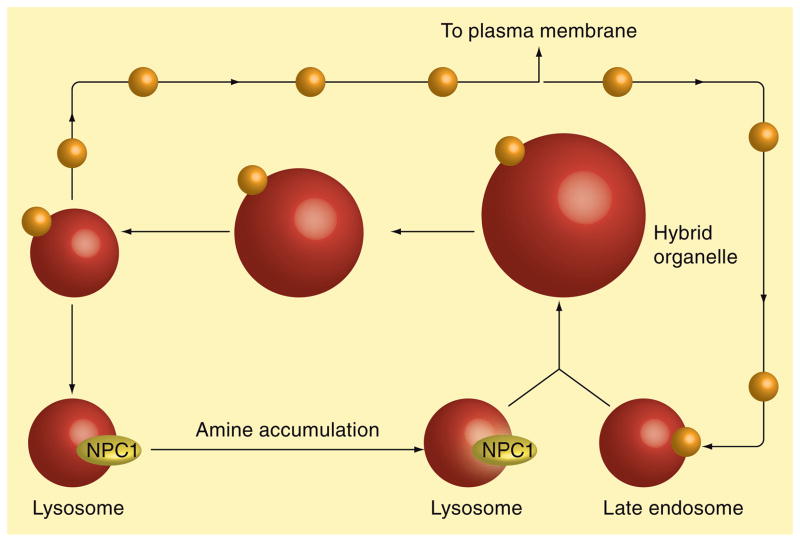
In the preceding two sections, we have described a functional role for NPC1 in both the formation of amine-induced vacuoles as well as vesicle-mediated egress of amines from cells. Based on these observations, we have arrived at a theoretical model to describe a mechanism by which amines entrapped in lysosomes are trafficked out of cells, according to a NPC1-mediated pathway (Figure 7). We propose that lysosomotropic amines enter into lysosomes via an ion-trapping mechanism. If the amines are not amphiphilic they have been shown to stimulate NPC1-mediated fusion of lysosomes with late endosomes to create expanded hybrid organelles. The hybrid organelle must subsequently undergo fission to release membrane proteins, allowing them to condense their contents and reform into dense lysosomal compartments [46]. It is during this lysosome reformation that we propose the formation of transport vesicles containing lysosomal amines that are destined for release from the cell, either directly fusing with the plasma membrane or perhaps indirectly through some intermediate compartment such as the Golgi apparatus. The steady-state size and abundance of hybrid organelles should be dictated by the relative rates of formation and depletion. Under normal conditions (i.e., no amine treatment), the relative abundance of hybrid organelles is quite minimal, as evidenced by very little co-localization of lysosomal and late endosomal resident proteins using immunofluorescence analysis [22]. Under these conditions, it is likely that the rate of hybrid organelle formation is slow relative to its depletion. We have previously shown that amines can stimulate the NPC1-mediated formation of hybrid organelles [22]. It is possible that, in the presence of amines, the rate of hybrid organelle formation becomes fast relative to their rate of depletion. Under these circumstances, the resultant hybrid organelles would be highly abundant and visibly expanded. This is consistent with the ability of certain amines to induce the formation of enlarged vacuoles. When the amine is removed from the cell-culture medium, the stimulatory effect on hybrid organelle formation subsides and the amine is subsequently trafficked out of the hybrid organelle, presumably through transport vesicles formed during fission events.
Figure 7. Hypothetical model depicting the NPC1-mediated trafficking of amines out of cells.
Lysosomotropic amines enter into lysosomes via an ion-trapping mechanism. Certain amines can stimulate NPC1-mediated lysosome fusion with late endosomes to create expanded hybrid organelles. Hybrid organelles subsequently undergo fission, allowing them to condense their contents and reform dense lysosomal compartments. It is during this lysosome reformation that transport vesicles form containing lysosomal amines that are destined for release from the cell.
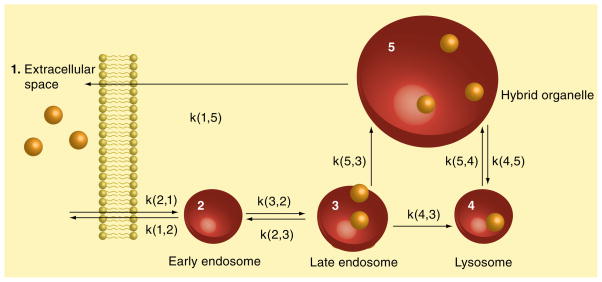
As part of this review, we have chosen to utilize kinetic data on both hybrid organelle formation as well as the release of lysosomal cargo from cells in order to test whether our data could be described by our proposed model for retroendocytic trafficking of lysosomal cargo. A detailed kinetic scheme depicting these pathways for retroendocytic transport is shown in Figure 8.
Figure 8. Kinetic model showing amine-trafficking pathways in cells.
Rate constants are written so the first number in the parenthesis is the cargo destination compartment while the second number is the source compartment.
The model consists of five compartments: cargo is initially endocytosed from the extracellular space (compartment 1) into early endosomes (compartment 2), followed by trafficking into late endosomes (compartment 3). Vesicle-mediated trafficking continues from late endosomes to lysosomes (compartment 4). In a retrograde fashion, lysosomes can fuse with late endosomes to form hybrid organelles (compartment 5), which subsequently traffic cargo to the extracellular space (compartment 1).
In order to optimize the reliability of information provided by a modeling algorithm, it is important to experimentally determine as many of the rate constants and initial conditions as possible. Using our FRET-based hybrid organelle-formation assay, we were able to specifically measure fusion rates associated with hybrid organelle formation (see k(5,3) and k(5,4) of Figure 8). Initially we assigned k(5,3) = k(5,4) in both models because the rate of hybrid organelle formation depends on late endosomal–lysosomal fusion (i.e., the kinetic rates for these fusion event would be identical). The concentrations of [3H]-dextran in each of the compartments at steady state were experimentally determined and used as initial conditions for the model depicted in Figure 8. Final initial conditions used were as follows (in percentage total dextran per compartment):
Extracellular space: 0%
Early endosomes: 0.55%
Late endosomes: 4.33%
Lysosomes: 80.87%
Hybrid organelles: 14.25%
Utilizing the values for our kinetic data and initial conditions discussed above, we utilized the SAAM1.1 for Windows to establish a fit to the model shown in Figure 8. This program runs iterations from a nonlinear least-squares algorithm to arrive at solutions for unknown parameters. As shown in Figure 9, the program arrived at solutions for unknown rate constants in the model that led to a reasonable fit to the dextran secretion data.
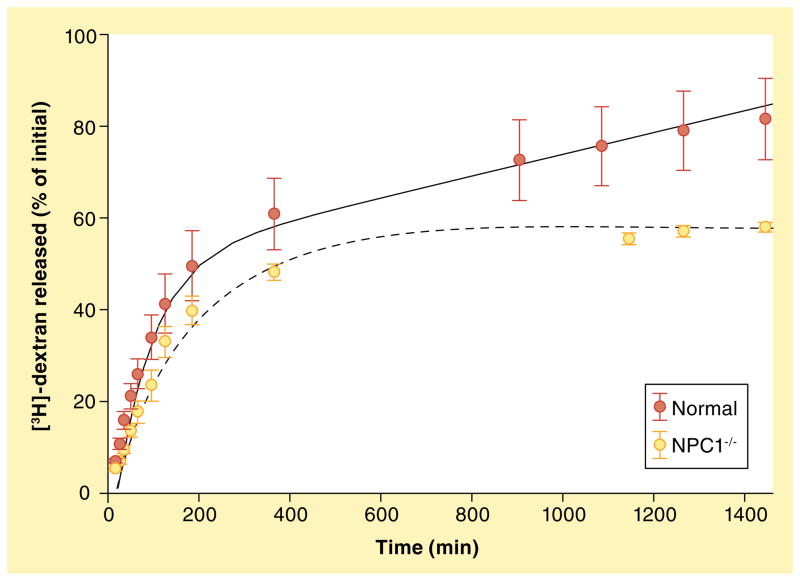
Figure 9. Radioactive dextran release is impaired in cells with nonfunctional NPC1.
[3H]-dextran release was performed as described in ‘Experimental procedures’. Normal cells release approximately 80% of their dextran over 24 h, while cells with nonfunctional NPC1 release approximately 55%. The trend lines are the fits from SAAM modeling, the solid line represents normal dextran release, while the dotted line represents NPC1−/− release.
Considering the important role of the NPC1 protein in retroendocytic trafficking of lysosomal cargo (including amines), we utilized the same modeling protocol using experimental data obtained from cells with functional mutations in the NPC1 protein (NPC1−/−). The initial conditions previously described were identical in this model. Using our FRET assay, we determined that k(5,3) and k(5,4) were threefold higher for normal cells than for the NPC1−/− cells and, therefore, k(5,3) and k(5,4) were defined as being equal to 0.33 × k(5,3) and k(5,4) from normal cells. Again, our model was found to fit the experimental data very closely (Figure 9).
To evaluate the relevance of our model, we compared our results to previously published data on endocytic trafficking. Blomhoff and others modeled fluid-phase endocytosis using 125I-poly(vinyl-pyrrolidone) as a marker [55]. They noted that a high percentage of the endocytosed marker was released into the medium from early endosomes. In our model, over 60% of the dextran taken up by cells was released into the extracellular space from early endosomes. Neufeld and co-workers performed a similar model describing the difference in late-endosomal efflux between normal cells and cells deficient in NPC1 [56]. They observed impaired rates of efflux of [14C]-sucrose from late endosomal compartments to the extracellular space for NPC-diseased cells, relative to normal cells (~50%). It is important to note that, in their model, aside from the early endosomes, the only pathway for egress of cargo was through the late endosomes. In our model, however, egress occurs through late endosomal–lysosomal fusion and subsequent fission steps from hybrid organelles. We observed a significant impairment in these steps in NPC1−/− cells when compared with normal cells (see steps (5,3) and (5,4) in Table 1).
Table 1.
Trafficking values from SAAM fit of normal cell dextran release.
| Trafficking step* | Normal‡ | NPC1−/−‡ |
|---|---|---|
| 1,2 | 0.119 | 0.002 |
| 2,1 | 0.223 | 0.165 |
| 3,2 | 7.969 | 0.164 |
| 2,3 | 7.866 | 0.002 |
| 4,3 | 0.060 | 0.162 |
| 5,3 | 0.043 | 0.000 |
| 4,5 | 1.673 | 0.883 |
| 5,4 | 1.733 | 1.045 |
| 1,5 | 0.103 | 0.163 |
Destination compartment, source compartment at time = 1440 min.
Units of trafficking are in percentages of total dextran/min.
Overall, our theoretical model for retroendocytic transport fits experimentally determined data from both normal and NPC1−/− cells; however, these results cannot exclude other mechanistic pathways as being possible.
Tools to evaluate lysosomal drug accumulation
There are a number of techniques and approaches that have been developed over the years in an attempt to arrive at some quantitative indication of lysosomal sequestration for basic amines in cultured cells. Here, we will briefly review some of the approaches that have been more recently described and/or utilized. For a more extensive review of these approaches, the reader is referred to our recent review on the subject [33].
Although not a new approach, fluorescence microscopy is undoubtedly the most commonly utilized tool for analyzing lysosomotropic properties of amines with suitable fluorescence properties. The advantages of fluorescence microscopy are that it is relatively easy and can be performed in live cells; however, there are also a number of drawbacks associated with this approach. Whether or not this technique is quantitative is certainly debatable; however, no technique is without limitations. The problems with quantitation of the fluorescent molecule typically arises from a lack of information regarding the microenvironment of the molecule and/or whether it binds to other molecules in the cell, all of which can change fluorescence emission intensity and/or the fluorescent profile itself [57]. More recently, the use of microspectrofluorometry has allowed researchers to overcome some problems associated with conventional fluorescence microscopy. Using microspectrofluorometry, it is possible to observe spectral shifts of fluorophores that may have occurred. This technique has been used to estimate the concentration of fluorescent drugs in living cells and has yielded reasonable estimates [57,58]. However, fluorescence quenching, caused by intracellular binding events or through self-association, seemingly remain a potential problem with quantitation using this approach.
A unique analytical approach for evaluating the accumulation of fluorescent compounds into acidic organelles, such as lysosomes, was described by Chen and co-workers [59]. They utilized capillary electrophoresis with laser-induced fluorescence and dual-channel detection to identify lysosomes containing fluorescent nanospheres that were preloaded into lysosomes using an endocytic uptake-based pulse-chase technique. Selecting organelles possessing both nanosphere and doxorubicin fluorescence allowed them to specifically determine the content of the drug in lysosomes, without potential interference from other organelles that the drug could be associated with. The authors were able to identify heterogeneity among lysosomes in their doxorubicin content, an observation not readily achievable using alternative techniques.
More recently, an indirect approach for evaluating lysosomal sequestration was described by Lemieux and colleagues, which takes advantage of the simplicity of monitoring fluorescence in whole cells and is useful for assessing accumulation of nonfluorescent compounds [60]. In this approach, the cells are incubated with a low concentration of a fluorescent lysosomal vital stain LTR and then test analytes are subsequently incubated with the cells containing LTR. If LTR fluorescence is visually diminished, the test analyte is deemed to have accumulated into the lysosomes to an extent that raised the pH sufficiently to disrupt the retention of LTR in the lysosomes. The disadvantage of this approach is that different amines may disrupt lysosomal pH and, therefore, LTR retention, by different mechanisms. Some amines may raise lysosomal pH by accumulating at a greater rate than the lysosomal V-ATPase can supply protons. Alternatively, other amines may influence lysosomal pH through inhibition of the V-ATPase activity, which may be the case for more amphiphilic amines [27,61].
We have recently described an approach for quantitating amines in lysosomes that employs a magnetic chromatography-based approach for isolating lysosomes from cells [24]. Briefly, iron-conjugated dextran polymers are specifically localized within lysosomes prior to incubation with the amine. The dextran-containing lysosomes can be rapidly purified from cells by passing a cell homogenate over a magnetic column. The amine is subsequently extracted from the isolated lysosomes and quantitated using conventional analytical tools (i.e., HPLC–MS/MS). The advantage of this approach is that it does not require that the test amine possess any fluorescence properties. The disadvantage is the potential for diffusion of the amine from the lysosomes during purification.
Each of the existing approaches for assessing lysosomotropic properties has distinct advantages and disadvantages. Although we have learned a great deal from the aforementioned assays, it is clear that improved techniques are required, as will be discussed in our following concluding remarks.
Future perspective
It is interesting to consider how research directed at understanding mechanisms relating to lysosomal amine accumulation and regulation will likely evolve over the next 5–10 years. It is also important to consider how such information will be utilized in both understanding and treating disease. Currently, the major goal of this research is focused toward the design of new, more effective medicines that possess optimized intracellular distribution. The rate-determining step in achieving this goal is the lack of large, reliable, quantitative data sets that correlate drug structure and physicochemical properties with intracellular localization. The absence of optimal assays to measure intracellular drug distribution is slowing progress in this area. Existing assays have, for the most part, been accompanied by significant limitations, which include low throughput and/or limited structural diversity. It is anticipated that new approaches will be developed in the near future that will be amenable to high-throughput formats and allow analysis of libraries of structurally diverse drug and drug-like molecules. With extensive structure–localization relationship information, medicinal chemists can begin to incorporate desired properties into drugs, in order to maximize interactions with respective cognate drug target molecules.
In addition to the previously described benefits to drug development, it is anticipated that this research will have a profound impact on our understanding of the pathology of certain diseases. Cells are known to contain high concentrations of endogenous amine-containing molecules that may also be substrates for lysosomal sequestration (i.e., neurotransmitters, polyamines and other biogenic amines). The dynamics of endogenous amine accumulation in, and egress from, lysosomes may represent an important component in maintaining normal homeostatic concentrations of these amines and/or their metabolites in specific subcellular compartments. Specifically, various lysosomal-storage diseases (LSDs), such as Niemann-Pick type-C disease, have been shown to be associated with the hyperaccumulation of lipid cargo in lysosomes [62,63]. Our recent work has shown that the normal egress of amines is also compromised in NPC-disease cells [22]. It is possible that the clinical manifestations of various LSDs may be, at least partially, caused by loss of intracellular homeostasis of certain endogenous amines.
Recently, work investigating the effects of the lysosomotropic polyamine 3-aminopropanal (3-AP) on lysosomes has been conducted by our laboratory and others [22,64,65]. The sequestration of 3-AP in lysosomes can lead to lysosomal rupture, a process preceding apoptosis, which leads to significant neurotoxicity [66,67]. Interestingly, cells with nonfunctional NPC1 have recently been shown to be more susceptible to the toxic effects of 3-AP, consistent with the increased residence of 3-AP in lysosomes of NPC1-defective cells [22]. These studies illustrate the importance of amine homeostasis, especially in the regulation of endogenous molecules, such as polyamines.
It is anticipated that future work will be aimed at evaluating the intracellular distribution of other endogenous amines and how intracellular levels are disturbed in diseases that influence normal accumulation and egress pathways. Such information may provide substantial insight into the etiology of diseases and rational strategies to treat them.
- Lysosome
Membrane-bound subcellular organelle characterized by its low luminal pH and the presence of digestive enzymes
- Vacuolar H+ ATPase
Transmembrane protein responsible for maintenance of a low luminal pH within lysosomes
- pH partitioning
Primary pathway for accumulation of weakly basic amine-containing compounds in the acidified lysosome
- Lysosomotropism
Propensity of a compound to accumulate within the lysosome
- Niemann Pick type-C disease
An autosomal recessive lysosomal-storage disease that leads to severe neurodegeneration and death
Footnotes
Financial & competing interests disclosure
This work was supported in whole by National Institutes of Health Grant RO1 CA106655. Stephen Goldman is supported by the PhRMA Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Coffey JW, de Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968;243:3255–3263. [PubMed] [Google Scholar]
- 2▪▪.de Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Lysosomotropic agents. Biochem Pharmacol. 1974;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. Comprehensive review into lysosomotropism, including discussions on lysosomes, pH partitioning, drug accumulation and disease states. [DOI] [PubMed] [Google Scholar]
- 3.Fowler S, de Duve C. Digestive activity of lysosomes. 3 The digestion of lipids by extracts of rat liver lysosomes. J Biol Chem. 1969;244(2):471–481. [PubMed] [Google Scholar]
- 4.Aronson NN, Jr, de Duve C. Digestive activity of lysosomes. II. The digestion of macromolecular carbohydrates by extracts of rat liver lysosomes. J Biol Chem. 1968;243(17):4564–4573. [PubMed] [Google Scholar]
- 5.de Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Forgac M. A novel mechanism for regulation of vacuolar acidification. J Biol Chem. 1992;267(28):19769–19772. [PubMed] [Google Scholar]
- 7.eisow M. Lysosome proton pump identified. Nature. 1982;298(5874):515–516. doi: 10.1038/298515a0. [DOI] [PubMed] [Google Scholar]
- 8.Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972;235(5332):50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Krugliak M, Ginsburg H. The fate of ferriprotorphyrin IX in malaria-infected erythrocytes in conjunction with the mode of action of antimalarial drugs. Mol Biochem Parasitol. 1999;99(1):129–141. doi: 10.1016/s0166-6851(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 10.Daniel WA, Wojcikowski J. Contribution of lysosomal trapping to the total tissue uptake of psychotropic drugs. Pharmacol Toxicol. 1997;80(2):62–68. doi: 10.1111/j.1600-0773.1997.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 11.Daniel WA, Wojcikowski J. Interactions between promazine and antidepressants at the level of cellular distribution. Pharmacol Toxicol. 1997;81(6):259–264. [PubMed] [Google Scholar]
- 12.Daniel WA, Wojcikowski J. Lysosomal trapping as an important mechanism involved in the cellular distribution of perazine and in pharmacokinetic interaction with antidepressants. Eur Neuropsychopharmacol. 1999;9(6):483–491. doi: 10.1016/s0924-977x(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 13.Daniel WA, Wojcikowski J. The role of lysosomes in the cellular distribution of thioridazine and potential drug interactions. Toxicol Appl Pharmacol. 1999;158(2):115–124. doi: 10.1006/taap.1999.8688. [DOI] [PubMed] [Google Scholar]
- 14.Kornhuber J, Medlin A, Bleich S, et al. High activity of acid sphingomyelinase in major depression. J Neural Transm. 2005;112(11):1583–1590. doi: 10.1007/s00702-005-0374-5. [DOI] [PubMed] [Google Scholar]
- 15.Kornhuber J, Tripal P, Reichel M, et al. Identification of new functional inhibitors of acid sphingomyelinase using a structure–property–activity relation model. J Med Chem. 2008;51(2):219–237. doi: 10.1021/jm070524a. [DOI] [PubMed] [Google Scholar]
- 16.Duvvuri M, Konkar S, Hong KH, Blagg BSJ, Krise JP. A new approach for enhancing differential selectivity of drugs to cancer cells. ACS Chem Biol. 2006;1(5):309–315. doi: 10.1021/cb6001202. [DOI] [PubMed] [Google Scholar]
- 17.Anderson N, Borlak J. Drug-induced phospholipidosis. FEBS Lett. 2006;580(23):5533–5540. doi: 10.1016/j.febslet.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 18.Reasor MJ, Hastings KL, Ulrich RG. Drug-induced phospholipidosis: issues and future directions. Expert Opin Drug Saf. 2006;5(4):567–583. doi: 10.1517/14740338.5.4.567. [DOI] [PubMed] [Google Scholar]
- 19.Kaloyanides GJ. Drug–phospholipid interactions: role in aminoglycoside nephrotoxicity. Ren Fail. 1992;14(3):351–357. doi: 10.3109/08860229209106642. [DOI] [PubMed] [Google Scholar]
- 20.Tulkens PM. Nephrotoxicity of aminoglycoside antibiotics. Toxicol Lett. 1989;46(1–3):107–123. doi: 10.1016/0378-4274(89)90121-5. [DOI] [PubMed] [Google Scholar]
- 21.Diettrich O, Mills K, Johnson AW, Hasilik A, Winchester BG. Application of magnetic chromatography to the isolation of lysosomes from fibroblasts of patients with lysosomal storage disorders. FEBS Lett. 1998;441(3):369–372. doi: 10.1016/s0014-5793(98)01578-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann AM, Krise JP. Niemann–Pick c1 functions in regulating lysosomal amine content. J Biol Chem. 2008;283(36):24584–24593. doi: 10.1074/jbc.M803715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann AM, Goldman SDB, Krise JP. A fluorescence resonance energy transfer-based approach for investigating late endosome–lysosome retrograde fusion events. Anal Biochem. 2009;386(1):91–97. doi: 10.1016/j.ab.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duvvuri M, Krise JP. A novel assay reveals that weakly basic model compounds concentrate in lysosomes to an extent greater than ph-partitioning theory would predict. Mol Pharm. 2005;2(6):440–448. doi: 10.1021/mp050043s. [DOI] [PubMed] [Google Scholar]
- 25.Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are reformed from the resultant hybrid organelles. J Cell Sci. 1997;110(17):2027–2040. doi: 10.1242/jcs.110.17.2027. [DOI] [PubMed] [Google Scholar]
- 26.Andrew CL, Klemm AR, Lloyd JB. Lysosome membrane permeability to amines. Biochim Biophys Acta. 1997;1330(1):71–82. doi: 10.1016/s0005-2736(97)00145-4. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki J, Yokogawa K, Ichimura F, Ohkuma S. Uptake of imipramine in rat liver lysosomes in vitro and its inhibition by basic drugs. J Pharmacol Exp Ther. 2000;294(3):1088–1098. [PubMed] [Google Scholar]
- 28.Cramb G. Selective lysosomal uptake and accumulation of the β-adrenergic antagonist propranolol in cultured and isolated cell systems. Biochem Pharmacol. 1986;35(8):1365–1372. doi: 10.1016/0006-2952(86)90283-2. [DOI] [PubMed] [Google Scholar]
- 29▪.Ohkuma S, Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981;90(3):656–664. doi: 10.1083/jcb.90.3.656. Complete study on vacuolization, including structure–activity information as well as the effects of weak bases on cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moseley RH, Van Dyke RW. Organic cation transport by rat liver lysosomes. Am J Physiol. 1995;268(3 Pt 1):G480–G486. doi: 10.1152/ajpgi.1995.268.3.G480. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhry FA, Edwards RH, Fonnum F. Vesicular neurotransmitter transporters as targets for endogenous and exogenous toxic substances. Annu Rev Pharmacol Toxicol. 2008;48:277–301. doi: 10.1146/annurev.pharmtox.46.120604.141146. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro AB, Fox K, Lee P, Yang YD, Ling V. Functional intracellular p-glycoprotein. Int J Cancer. 1998;76(6):857–864. doi: 10.1002/(sici)1097-0215(19980610)76:6<857::aid-ijc15>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J Pharm Sci. 2007;96(4):729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 34.Trapp S, Rosania GR, Horobin RW, Kornhuber J. Quantitative modeling of selective lysosomal targeting for drug design. Eur Biophys J. 2008;37(8):1317–1328. doi: 10.1007/s00249-008-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Zheng N, Rosania GR. Simulation-based cheminformatic analysis of organelle-targeted molecules: lysosomotropic monobasic amines. J Comput Aided Mol Des. 2008;22(9):629–645. doi: 10.1007/s10822-008-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lettre H, Albrecht B. Zur wirkung von aminen auf in vitro gezuechtete zellen. Z Physiol Chem. 1943;279:206–208. [Google Scholar]
- 37.Pomerat CM, Emerson GA. Induction of aqueous vacuolization in cells grown in tissue culture by various agents. Proc Trans Texas Acad Sci. 28(91):1944–1945. [Google Scholar]
- 38.Yang WC, Strasser FF, Pomerat CM. Mechanism of drug-induced vacuolization in tissue culture. Exp Cell Res. 1965;38:495–506. doi: 10.1016/0014-4827(65)90373-3. [DOI] [PubMed] [Google Scholar]
- 39.Finnin BC, Reed BL, Ruffin NE. The effects of osmotic pressure on procaine-induced vacuolation in cell culture. J Pharm Pharmacol. 1969;21(2):114–117. doi: 10.1111/j.2042-7158.1969.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 40.Yuan XM, Li W, Brunk UT, Dalen H, Chang YH, Sevanian A. Lysosomal destabilization during macrophage damage induced by cholesterol oxidation products. Free Radic Biol Med. 2000;28(2):208–218. doi: 10.1016/s0891-5849(99)00220-8. [DOI] [PubMed] [Google Scholar]
- 41.Brunk UT, Dalen H, Roberg K, Hellquist HB. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med. 1997;23(4):616–626. doi: 10.1016/s0891-5849(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 42.Morissette G, Moreau EC, Gaudreault R, Marceau F. Massive cell vacuolization induced by organic amines such as procainamide. J Pharmacol Exp Ther. 2004;310(1):395–406. doi: 10.1124/jpet.104.066084. [DOI] [PubMed] [Google Scholar]
- 43.Iveson GP, Bird SI, Lloyd JB. Passive diffusion of non-electrolytes across the lysosome membrane. Biochem J. 1989;261(2):451–456. doi: 10.1042/bj2610451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michalik M, Pierzchalska M, Pabianczyk-Kulka A, Korohoda W. Procaine-induced enhancement of fluid-phase endocytosis and inhibition of exocytosis in human skin fibroblasts. Eur J Pharmacol. 2003;475(1–3):1–10. doi: 10.1016/s0014-2999(03)02000-4. [DOI] [PubMed] [Google Scholar]
- 45.Solheim AE, Seglen PO. Structural and physical changes in lysosomes from isolated rat hepatocytes treated with methylamine. Biochim Biophys Acta. 1983;763(3):284–291. doi: 10.1016/0167-4889(83)90136-2. [DOI] [PubMed] [Google Scholar]
- 46.Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Piper RC. Lysosome–endosome fusion and lysosome biogenesis. J Cell Sci. 2000;113(9):1515–1524. doi: 10.1242/jcs.113.9.1515. [DOI] [PubMed] [Google Scholar]
- 47.Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of niemann-pick c1 protein. Science. 2000;290(5500):2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- 48.Passeggio J, Liscum L. Flux of fatty acids through npc1 lysosomes. J Biol Chem. 2005;280(11):10333–10339. doi: 10.1074/jbc.M413657200. [DOI] [PubMed] [Google Scholar]
- 49.Gong Y, Duvvuri M, Duncan MB, Liu J, Krise JP. Niemann-pick C1 protein facilitates the efflux of the anticancer drug daunorubicin from cells according to a novel vesicle-mediated pathway. J Pharmacol Exp Ther. 2006;316(1):242–247. doi: 10.1124/jpet.105.089482. [DOI] [PubMed] [Google Scholar]
- 50.Duvvuri M, Feng W, Mathis A, Krise JP. A cell fractionation approach for the quantitative analysis of subcellular drug disposition. Pharm Res. 2004;21(1):26–32. doi: 10.1023/b:pham.0000012148.12516.3f. [DOI] [PubMed] [Google Scholar]
- 51.Seidel A, Hasmann M, Loser R, et al. Intracellular localization, vesicular accumulation and kinetics of daunorubicin in sensitive and multidrug-resistant gastric carcinoma EPG85-257 cells. Virchows Arch. 1995;426(3):249–256. doi: 10.1007/BF00191362. [DOI] [PubMed] [Google Scholar]
- 52.Cenedella RJ. Cholesterol synthesis inhibitor u18666a and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44(6):477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 53.Chen VY, Posada MM, Blazer LL, Zhao T, Rosania GR. The role of the vps4a-exosome pathway in the intrinsic egress route of a DNA-binding anticancer drug. Pharm Res. 2006;23(8):1687–1695. doi: 10.1007/s11095-006-9043-0. [DOI] [PubMed] [Google Scholar]
- 54.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63(15):4331–4337. [PubMed] [Google Scholar]
- 55.Blomhoff R, Nenseter MS, Green MH, Berg T. A multicompartmental model of fluid-phase endocytosis in rabbit liver parenchymal cells. Biochem J. 1989;262(2):605–610. doi: 10.1042/bj2620605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56▪.Neufeld EB, Wastney M, Patel S, et al. The Niemann–Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem. 1999;274(14):9627–9635. doi: 10.1074/jbc.274.14.9627. Contains valuable information on immunolocalization of NPC1 and proposes a model on endocytic trafficking. [DOI] [PubMed] [Google Scholar]
- 57.Millot JM, Rasoanaivo TD, Morjani H, Manfait M. Role of the aclacinomycin A – doxorubicin association in reversal of doxorubicin resistance in K562 tumour cells. Br J Cancer. 1989;60(5):678–684. doi: 10.1038/bjc.1989.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gigli M, Rasoanaivo TW, Millot JM, et al. Correlation between growth inhibition and intranuclear doxorubicin and 4′-deoxy-4′-iododoxorubicin quantitated in living K562 cells by microspectrofluorometry. Cancer Res. 1989;49(3):560–564. [PubMed] [Google Scholar]
- 59.Chen Y, Walsh RJ, Arriaga EA. Selective determination of the doxorubicin content of individual acidic organelles in impure subcellular fractions. Anal Chem. 2005;77(8):2281–2287. doi: 10.1021/ac0480996. [DOI] [PubMed] [Google Scholar]
- 60.Lemieux B, Percival MD, Falgueyret JP. Quantitation of the lysosomotropic character of cationic amphiphilic drugs using the fluorescent basic amine red dnd-99. Anal Biochem. 2004;327(2):247–251. doi: 10.1016/j.ab.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Yokogawa K, Ishizaki J, Ohkuma S, Miyamoto K. Influence of lipophilicity and lysosomal accumulation on tissue distribution kinetics of basic drugs: a physiologically based pharmacokinetic model. Methods Find Exp Clin Pharmacol. 2002;24(2):81–93. doi: 10.1358/mf.2002.24.2.677131. [DOI] [PubMed] [Google Scholar]
- 62.Pentchev PG, Comly ME, Kruth HS, et al. Group C niemann-pick disease: faulty regulation of low-density lipoprotein uptake and cholesterol storage in cultured fibroblasts. FASEB J. 1987;1(1):40–45. doi: 10.1096/fasebj.1.1.3609608. [DOI] [PubMed] [Google Scholar]
- 63.Liscum L, Ruggiero RM, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann–Pick type C fibroblasts. J Cell Biol. 1989;108(5):1625–1636. doi: 10.1083/jcb.108.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wood PL, Khan MA, Kulow SR, Mahmood SA, Moskal JR. Neurotoxicity of reactive aldehydes: the concept of “aldehyde load” as demonstrated by neuroprotection with hydroxylamines. Brain Res. 2006;1095(1):190–199. doi: 10.1016/j.brainres.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 65.Wood PL, Khan MA, Moskal JR. The concept of “aldehyde load” in neurodegenerative mechanisms: cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line. Brain Res. 2007;1145:150–156. doi: 10.1016/j.brainres.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Ivanova S, Botchkina GI, Al-Abed Y, et al. Cerebral ischemia enhances polyamine oxidation: identification of enzymatically formed 3-aminopropanal as an endogenous mediator of neuronal and glial cell death. J Exp Med. 1998;188(2):327–340. doi: 10.1084/jem.188.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W, Yuan XM, Ivanova S, Tracey KJ, Eaton JW, Brunk UT. 3-aminopropanal, formed during cerebral ischaemia, is a potent lysosomotropic neurotoxin. Biochem J. 2003;371(2):429–436. doi: 10.1042/BJ20021520. [DOI] [PMC free article] [PubMed] [Google Scholar]