Abstract
Panic patients are vulnerable to induction of panic attacks by subthreshold interoceptive stimuli such as intravenous (i.v.) sodium lactate infusions. Facilitation of serotonergic signaling with selective serotonin re-uptake inhibitors (SSRIs) can suppress anxiety and panic-like responses, but the mechanisms involved are not clearly defined. We investigated the effects of i.v. 0.5M sodium lactate or saline, in control and panic-prone rats on c-Fos expression in serotonergic neurons within subdivisions of the midbrain/pontine raphe nuclei. Rats were chronically infused with either the GABA synthesis inhibitor l-allylglycine (l-AG) into the dorsomedial hypothalamus (DMH) to make them panic-prone, or the enantiomer d-allylglycine (d-AG) in controls. Lactate increased c-Fos expression in serotonergic neurons located in the ventrolateral part of the dorsal raphe nucleus (DRVL) and ventrolateral periaqueductal gray (VLPAG) of control, but not panic-prone, rats. The distribution of lactate-sensitive serotonergic neurons in d-AG-treated rats is virtually identical to previously defined pre-sympathomotor serotonergic neurons with multisynaptic projections to peripheral organs mediating “fight-or-flight”-related autonomic and motor responses. We hypothesize that serotonergic neurons within the DRVL/VLPAG region represent a “sympathomotor control system” that normally limits autonomic/behavioral responses to innocuous interoceptive and exteroceptive stimuli, and that dysfunction of this serotonergic system contributes to an anxiety-like state and increases vulnerability to panic in animals and humans.
Keywords: Panic, anxiety, serotonin, 5-HT, raphe, c-Fos, periaqueductal gray, sympathetic, autonomic
Introduction
Panic disorder is a severe anxiety disorder characterized by recurrent panic attacks, consisting of pronounced fear and heightened cardiorespiratory responses (DSM-IV, 1994). The initial pathology in many panic disorder patients appears to be alterations of central neural pathways regulating normal adaptive panic responses, which renders the patients susceptible to unprovoked panic symptoms when exposed to ordinarily mild interoceptive stressors (Vickers and McNally, 2005). In support of this, most panic disorder patients, but not healthy controls, are hyper-responsive to normal interoceptive cues (Hoehn-Saric et al., 2004; Pollock et al., 2006), and are also susceptible to induction of panic attacks by subthreshold interoceptive stimuli such as intravenous (i.v.) 0.5 M sodium lactate infusions or 7.5% CO2 inhalations (Liebowitz et al., 1986; Gorman et al., 1994).
Selective serotonin reuptake inhibitors (SSRIs) are effective in treating panic attacks and have less negative associated side effects (Pohl et al., 1998; Al Haddad et al., 2001) yet little is known about the serotonergic neuronal systems that may contribute to the prevention and attenuation of recurrent panic attacks. The present study utilized a model of anxiety with vulnerability to panic-like responses developed by Shekhar and colleagues, which involves inhibiting γ-amino butyric acid (GABA)-ergic tone in the hypothalamus, in order to assess anxiety-like behavioral responses and i.v. sodium lactate-induced neuronal responses in distinct serotonergic neuronal populations in the midbrain and pons. The hypothalamus is among the central pathways implicated in the development of recurrent panic attacks (Javanmard et al., 1999; Boshuisen et al., 2002) and acutely removing local GABA-mediated inhibition in the dorsomedial hypothalamic (DMH)/perifornical nucleus (PeF) region of rats results in rapid mobilization of behavioral, autonomic and respiratory components of panic attacks [see review (DiMicco et al., 2002)]. In the anxiety model with increased vulnerability to panic developed by Shekhar and colleagues, chronically inhibiting GABA synthesis in the DMH/PeF of rats leads to heightened baseline anxiety-like responses and facilitation of anxiety- and panic-like responses following exposure to mild interoceptive stressors [i.e., i.v. infusions of 0.5 M sodium lactate (Shekhar et al., 1996; Shekhar and Keim, 1997, 2000; Johnson and Shekhar, 2006; Shekhar et al., 2006) or 7.5% CO2 inhalation (Fitz et al., 2003)].
In order to identify specific midbrain/pontine serotonergic neuronal systems potentially involved in regulation of sodium lactate-induced panic-like responses in anxious, panic-prone rats, we studied the effects of i.v. infusions of 0.5 M sodium lactate or saline vehicle in rats with prior infusions of the GABA synthesis inhibitor l-allylglycine (l-AG), or its inactive isomer d-allylglycine (d-AG: controls), into the DMH/PeF. In order to investigate cellular responses, the protein product of the immediate-early gene c-fos was immunostained in midbrain/pontine serotonergic systems. We predicted that anxious, panic-prone rats would have altered cellular responses in a distinct subset of serotonergic neurons in the ventrolateral part of the dorsal raphe nucleus (DRVL)/ventrolateral periaqueductal gray (VLPAG) region following lactate infusions since serotonergic neurons in this region have previously been implicated in the inhibition of stress-induced behavioral and sympathoexcitatory responses to aversive stimuli [see review (Johnson et al., 2004)] and may represent the periventricular serotonergic path proposed to inhibit panic-associated cardiovascular and respiratory responses (Graeff et al., 1997; Deakin, 1998; Johnson et al., 2004; Johnson et al., 2005).
Materials and Methods
Animals and housing conditions
All experiments were conducted on adult male Sprague-Dawley rats (300-350 g), purchased from Harlan Laboratories, that were housed individually in plastic cages under standard environmental conditions (22 °C; 12/12 light/dark cycle; lights on at 7:00 A.M.) for 7-10 days prior to the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80-23) revised 1996 and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
Surgical procedures and osmotic minipump infusions
Prior to surgery, rats were anesthetized by placing them in a closed plexiglass box which was connected to an isoflurane system (MGX Research Machine; Vetamic, Rossville IN); anesthesia was maintained using a nose cone connected to the same system during the surgery. All rats were fitted with femoral arterial catheters for measurement of mean arterial blood pressure (MAP) and heart rate (HR) and with venous catheters for i.v. infusions, as previously described (Shekhar, 1993).
MAP and HR were measured by an arterial line connected to a telemetric probe which contained a pressure transducer [Cat. no. C50-PXT, Data Science International (DSI)]. Windows-based dataquest software (DSI) was used to monitor and record MAP and HR in conscious, freely moving rats.
After 3 days of recovery from surgery, rats were tested for baseline physiological and behavioral responses to lactate (see below). Each rat was then implanted with a T-shaped 26 gauge cannula with the horizontal arm (Cat. no. 3260PG, Plastics One, Ranoake VA) attached to a small Tygon tube containing sterile saline that was in turn connected to an Alzet osmotic minipump (DURECT Corp. cat. no. 2002) containing the appropriate drug solution [i.e., either the inactive isomer d-AG or the active isomer l-AG (an inhibitor of GABA synthesis), Sigma-Aldrich, St. Louis, MO) as described previously (Shekhar et al., 1996). Briefly, cannulae were directed at cardioexcitatory regions of the DMH [see (Johnson and Shekhar, 2006)] based on the following coordinates [from Bregma: −1.2 mm posterior, +2.1 mm lateral, −9.1 mm ventral and adjusted for approaching at a +10 degree angle toward the midline with the stereotaxic incisor bar elevated 5 mm above the interaural line].
Once the cannula was placed at the coordinates targeting the DMH, 50 pmol/100 nl of the GABAA receptor antagonist BMI was injected through the vertical arm of the cannula to ascertain that the tip of the cannula was placed in a cardioexcitatory region (i.e., where BMI elicits ≥ 50 beats/min in HR). The microinjection protocol commenced only after a stable baseline heart rate and blood pressure had been established for ~10 min. Heart rate, MAP and respiratory rate (RR; where applicable) were monitored for 5 min prior to the microinjection and 15 min after. Once it had been established that the cannula placement resulted in cardiovascular responses to BMI, the minipump containing the desired infusion fluid was sutured underneath the skin between the scapulae. The concentration of the l-AG or d-AG solutions was such that 3.5 nmol/0.5 μl/h of the drug was infused into the DMH/PeF region over a 5 day period prior to i.v. infusions of sodium lactate or physiological saline.
Description of sodium lactate infusion
Five days following stereotaxic surgery, rats were randomly assigned to four groups (n = 6 each): 1) d-AG/saline, 2) d-AG/sodium lactate, 3) l-AG/saline, and 4) l-AG/sodium lactate. Rats were given their assigned i.v. infusion (saline or sodium lactate) and HR and MAP were recorded continuously for 5 min prior to infusion (baseline) until 20 min following the onset of sodium lactate infusion. The lactate infusion procedure has been described previously (Shekhar et al., 1996). Briefly, freely moving rats in home cages were given i.v. infusions of either 0.9% saline vehicle or 0.5 M sodium lactate in vehicle (10 ml/kg over 15 min), similar to clinical lactate infusions (Liebowitz et al., 1986).
Cardiovascular responses that are reported are the changes from the minimum baseline value for HR (in beats/min) and MAP (in mm Hg) relative to the maximum HR and MAP during the post-infusion period for each animal. For the duration of the experiment, MAP, HR and RR were recorded continuously in conscious freely moving rats.
Social interaction test
The social interaction (SI) test is a fully validated test of experimental anxiety-like behavior in rats (File, 1980), and the procedure as used in our laboratory has been described previously (Sanders and Shekhar, 1995; Shekhar and Katner, 1995). The apparatus itself consists of a solid wooden box approximately 0.9 m long × 0.9 m wide with walls 0.3 m high with an open roof. A video camera was fixed above the box, and all behavioral tests were videotaped. Rats were tested under low red light (approximately 11 lux), familiar conditions. Briefly, the “experimental” rat and an unfamiliar “partner” rat were placed individually in the center of the box and allowed to habituate to the environment for a 5 min period 24 h prior to each SI test. During the SI test the two rats were placed together in the center of the box and the total duration (sec) of non-aggressive physical contact (grooming, sniffing, crawling over and under, etc.) initiated by the “experimental” rat was quantified. A baseline SI test was performed 72+ h after i.v. catheterization, but prior to osmotic minipump implantation. Another SI test was performed 5 days following infusions of either d-AG or l-AG into the DMH region through osmotic minipumps, immediately following i.v. infusions of either saline or sodium lactate. Videotaped sessions were scored at a later time by (SDF), who was blind to the experimental treatment of each rat.
Perfusion
Ninety min following the initiation of sodium lactate or saline infusions, rats were anesthetized and perfused with 250 ml of ice cold 0.05 M phosphate buffered saline (PBS), then 250 ml of ice cold 4% paraformaldehyde in 0.1 M sodium phosphate buffer containing 1.5% sucrose in preparation for immunohistochemistry. Serial coronal sections (30 μm) were cut using a cryostat to yield six alternate sets of sections. The present analysis used one set for double immunostaining of c-Fos-positive cells and tryptophan hydoxylase- (TPH−) positive neurons in order to analyze cellular responses in midbrain/pontine serotonergic neurons. Alternate sets of sections were used for detailed characterization of c-Fos immunostaining in other brain regions and these results are described elsewhere in two previously published articles. In the first article, two series of DMH/PeF brain sections were immunostained for c-Fos and the NR1 subunit of the NMDA receptor or the GluR2,3 subunit of the AMPA receptor (Johnson and Shekhar, 2006). In the second article, five of the six alternative sets of sections were immunostained [one set each for methyl green (hypothalamus only), GABA (hypothalamus only), c-Fos (entire brain), c-Fos/tyrosine hydroxylase (TH, brainstem only), c-Fos/choline acetyltransferase (ChAT, medulla only) and c-Fos/GAD67 (medulla only)] (Johnson et al., submitted).
Minipump cannula-placement verification
One series of coronal sections was mounted on slides, dried overnight, hydrated, and stained with 1% methyl green (Cat. no. 198080, Sigma) solution in H20. Minipump cannula tip placement was verified at 400X magnification using a Leica DME binocular microscope (Fisher Scientific Ltd.), by an investigator (PLJ), who was blind to the experimental treatment of each rat.
Immunohistochemistry
All brain sections were immunostained with the appropriate primary antibody (i.e., c-Fos/TPH) in a single immunohistochemical run, rather than in batches, with large volume incubations to limit variability in the quality or intensity of immunohistochemical staining among brain sections.
Double immunohistochemistry
Double immunostaining for c-Fos protein and TPH protein was conducted as described previously (Johnson et al., 2005). Immunostaining for c-Fos was done first, followed by immunostaining for TPH using primary antibodies directed against c-Fos [rabbit anti-c-Fos polyclonal, affinity-purified antibody, Cat. no. PC38, Ab-5, Calbiochem; diluted 1:8,000 in PBS containing 0.1% Triton X-100 (PBST)] then TPH (sheep anti-TPH polyclonal affinity-purified antibody, Cat. no. 9260–2505, Biogenesis; diluted 1:12,000 in PBST). For c-Fos and TPH immunostaining, free-floating sections were washed in PBS, incubated in 0.1% H2O2 in PBS for 20 min, washed again and then incubated 14-16 h with primary antibody solution. The following day sections were washed in PBST then incubated 2 h in the appropriate secondary antibody: c-Fos (biotinylated swine anti-rabbit IgG, Cat no. E0353; DakoCytomation, Ltd., Ely, UK, diluted 1:500) or TPH (biotinylated rabbit anti-sheep IgG, Cat. no. BA6000, Vector Laboratories, Peterborough, UK, diluted 1:500). Sections were washed in PBST then incubated 1.5 h in an avidin-biotin complex (Cat no. PK-6100, Vector Laboratories; diluted 1:500). Substrates for chromogen reactions were SG (c-Fos; SK-4700, Vector Laboratories) or 0.01% 3,3′-diaminobenzidine tetrahydrochloride (TPH; DAB, D-5637, Sigma-Aldrich, Poole, UK) in PBS containing 0.003% H2O2, pH 7.4. Substrate reactions were run for 15 min each for c-Fos and TPH.
Methods for cell counting
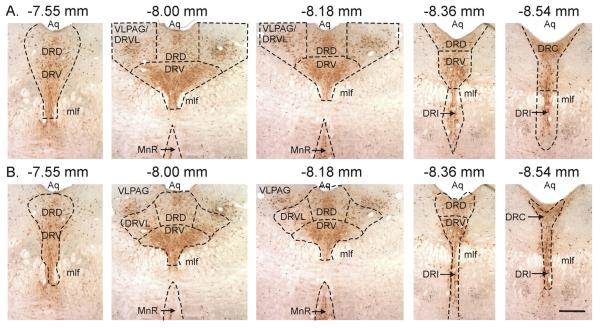
c-Fos immunoreactivity was dark blue/black and confined to the nucleus while TPH immunostaining was a light brown and localized to the cytoplasm. Analysis of the numbers of c-Fos-ir serotonergic neurons (c-Fos-ir/TPH-ir), c-Fos non-serotonergic cells (c-Fos-ir/TPH-immunonegative), and the total number of TPH-ir neurons sampled was conducted using a single section at each of 5 anatomical levels of the midbrain/pons from −7.55 to −8.54 mm Bregma, containing the median (MnR) and dorsal raphe (DR) nuclei. Within the DR, subregional analyses were also conducted (see Fig. 1A). Cell counts were done bilaterally where applicable (e.g., the DRVL/VLPAG region) and the counts were combined for each Bregma level for each rat. Selection of anatomical levels for analysis and identification of subnuclei was conducted with reference to illustrations from a standard stereotaxic rat brain atlas as shown in Fig. 1B (Paxinos and Watson, 1997). The subregional analyses in Fig. 1A were very similar to Paxinos and Watson's subdivisions of the DR, with the exception of the DRVL region. Many of the large multipolar serotonergic neurons in lateral wings of the DR are located in the VLPAG, rather than the DRVL (compare Fig. 1A and 1B). Other regions studied included the dorsal raphe nucleus, caudal part (DRC), dorsal raphe nucleus, dorsal part (DRD), dorsal raphe nucleus, interfascicular part (DRI), dorsal raphe nucleus, ventral part (DRV), and median raphe nucleus (MnR). All cell counts were done at 400X magnification by an observer (PLJ), who was blind to the experimental treatment of each rat.
Figure 1.
Topography of midbrain raphe nuclei studied. A) Areas where cell counts were made compared to B) subregions of the DR according to a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1997). Note in B) how there are substantial numbers of serotonergic neurons which are in the VLPAG rather than in the DR. Scale bar, 400 μm. Abbreviations: Aq, cerebral aqueduct; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; VLPAG, ventrolateral periaqueductal gray.
Photography
Photomicrographs were obtained using a Leica brightfield microscope (DMLB, Leica Mikroskopie and Systeme GmbH, Wetzler, Germany) using N plan 5X, 10X and 40X objective lenses, an Insight digital camera (Diagnostics Instruments Inc., Sterling Heights, MI, USA) and SPOT 3.5.5 for Windows digital imaging software (Silicon Graphics, Mountain View, California, USA). Photographic plates were prepared in CorelDraw 11.633 for Windows (Viglen Ltd.,Wembley, UK).
Statistical analyses
Each dependent variable for in vivo analyses (i.e., the change in SI duration, HR and MAP following i.v. infusions relative to baseline measurements) was analyzed using a two-way ANOVA with repeated measures with l-AG and lactate as between-subjects factors and time as a within-subjects factor. Each dependent variable for cell counts (c-Fos+/TPH+, c-Fos+/TPH−, total TPH+) was analyzed using a two-way ANOVA with repeated measures with lactate and l-AG as between-subjects factors and brain region as a within-subjects factor. In the presence of significant main effects or interactions, post hoc tests were conducted using Fisher's Protected Least Significant Difference (FLSD) tests. All statistical analyses were carried out using SYSTAT 5.02 for Windows (SYSTAT Inc., Evanston, IL), and all graphs were generated using SigmaPlot 2001 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Cannula placement
All cannula placements for osmotic minipump infusions of l-AG or d-AG were included in the present study since they resided in regions of the DMH and posterior hypothalamus (PH) known to be cardioexcitatory (Samuels et al., 2004) and they were confirmed as cardioexcitatory sites by verifying that injection of BMI under anesthesia increased HR by at least 50 beats/min. Although there was some damage at the site of implantation the majority of the tissue in the DMH/PH region was still intact surrounding the infusion site. Also, the amount of tissue damage was similar in the l-AG-infused and the d-AG-infused animals, suggesting that nonspecific injury to the DMH/PH region was not responsible for the lactate response. An illustration of the minipump placements from this study has been published previously in conjunction with a detailed characterization of cellular responses within the DMH/PeF region (Johnson and Shekhar, 2006).
Anxiety-like behavioral and panic-like cardio-respiratory responses
A detailed characterization of the anxiety-like behavioral responses and the panic-like cardio-respiratory responses in these rats has been published previously (Johnson and Shekhar, 2006). Briefly, consistent with previous studies, l-AG- but not d-AG-treated rats, with or without i.v. sodium lactate infusions, displayed shorter SI times, compared to baseline (an anxiogenic effect). A detailed characterization of i.v. sodium lactate-induced panic-like cardio-respiratory responses in l-AG-treated rats has been published elsewhere (Johnson and Shekhar, 2006). Briefly, in agreement with previous studies, i.v. sodium lactate infusions produced tachycardia and increased blood pressure in rats made anxious and panic-prone with prior l-AG infusions into the DMH/PH region but not in d-AG-treated rats.
c-Fos in midbrain and pontine serotonergic neurons
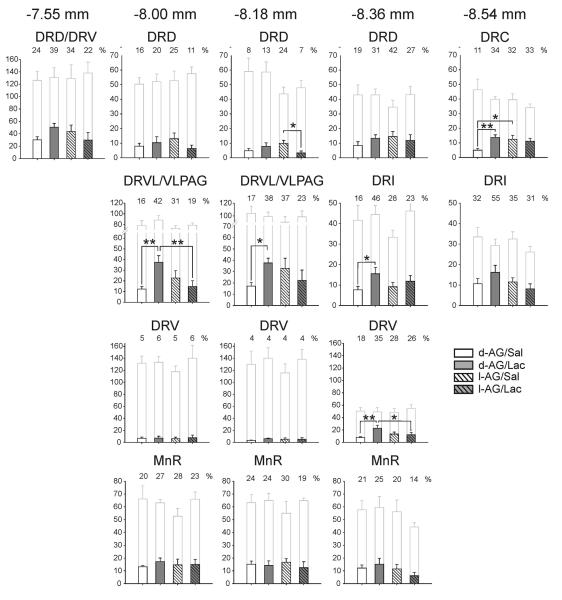
Subregional analysis of the numbers of c-Fos-ir serotonergic neurons within subdivisions of the DR revealed an l-AG × lactate × raphe subregion interaction (Fig. 2; F(15,300) = 3.2, P < 0.001), demonstrating that the effects of treatment were dependent on brain region. No treatment effects were observed on the total number of TPH-ir neurons analyzed in subdivisions of the midbrain and pontine raphe nuclei.
Figure 2.
Disruption of GABAergic tone in the dorsomedial hypothalamus/perifornical nucleus region (DMH/PeF) attenuates lactate-induced responses in subsets of serotonergic neurons in the dorsal raphe nucleus. Graphs illustrate the effects of intra-DMH infusions of either l-allylglycine (l-AG) or its inactive enantiomer, d-allylglycine (d-AG), on subsequent responses to i.v. infusions of sodium lactate (Lac). Bars with gray outlines represent the total number of tryptophan hydroxylase (TPH)-positive neurons sampled. Bars with black outlines represent the number of c-Fos-positive/TPH-positive neurons (see legend located in the lower right of the figure). Numbers above shaded bars represent the percentages of TPH-ir neurons that were also c-Fos-ir. All values represent means ± S.E.M. *P < 0.05, Fisher's Protected Least Significant Difference test, comparing the two groups indicated by black lines above the bars.
Post hoc pairwise comparisons revealed that i.v. lactate infusion increased c-Fos expression in serotonergic neurons within a number of subregions of the midbrain or pontine raphe nuclei studied, but invariably this effect was observed only in d-AG-treated (control) rats (Fig. 2). These increases were particularly evident in the DRVL/VLPAG region at both levels studied (−8.00, −8.18 mm Bregma; Fig. 2, 3A-E, 3E-H). Smaller, but significant, lactate-induced increases in c-Fos expression in serotonergic neurons were observed in other regions including one level of the DRV (Fig. 3A-E, 3I-L) and adjacent DRI studied (−8.36 mm Bregma; Fig. 2) and the caudal pole of the DR (DRC, −8.36 mm Bregma; Fig. 2). There were no effects of i.v. lactate infusion on c-Fos expression in serotonergic neurons in other regions studied, including rostral regions of the DRD and DRV, or any region of the MnR studied. In marked contrast to the effects of lactate in d-AG-treated rats, lactate-induced increases in c-Fos expression in serotonergic neurons were not observed in any subdivision of the raphe nuclei studied in l-AG-treated rats.
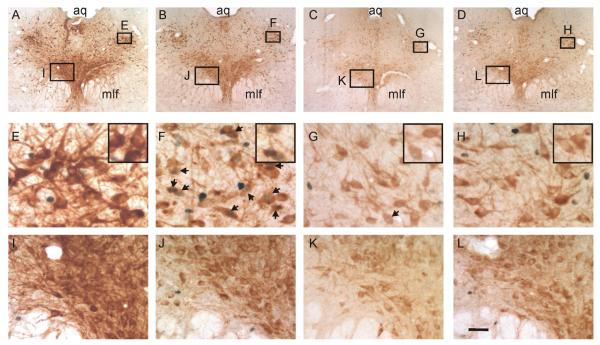
Figure 3.
Disruption of GABAergic tone in the dorsomedial hypothalamus/perifornical nucleus region (DMH/PeF) attenuates lactate-induced responses in serotonergic neurons in the dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG) region. A-D) Low magnification photomicrographs illustrate tryptophan hydroxylase (TPH)/c-Fos dual immunostaining in the dorsal raphe nucleus and VLPAG region at −8.00 mm Bregma. Boxes in A-D indicate regions of the DRVL/VLPAG region (E-H) and dorsal raphe nucleus, ventral part (DRV) (I-L) shown at higher magnification in lower panels. TPH immunostaining (light brown) identifies serotonergic neurons and is localized to the cytoplasm while nuclear c-Fos immunostaining is localized to the nucleus (blue-black). Insets in panels E-H illustrate selected regions of each panel shown at higher magnification. Arrows indicate c-Fos-positive/TPH-positive neurons. Scale bar, A-D, 290 μm; E-L, 30 μm; E-H (insets), 24 μm; I-L, 50 μm. Additional abbreviations: aq, cerebral aqueduct; mlf, medial longitudinal fasciculus.
Regression analyses revealed that the total number of c-Fos/TPH-positive cells in the DRVL/VLPAG region of all rats did not correlate with the HR [−8.00 mm Bregma, r2=0.03, p=0.471; −8.18 mm Bregma, r2=0.005, p=0.782], MAP [−8.00 mm Bregma, r2=0.002, p=0.863; −8.18 mm Bregma, r2=0.01, p=0.725], RR [−8.00 mm Bregma, r2=0.003, p=0.816; −8.18 mm Bregma, r2=0.03, p=0.522] or SI [−8.00 mm Bregma, r2=0.17, p=0.053; −8.18 mm Bregma, r2=0.03, p=0.406] post i.v. infusion.
c-Fos responses in non-serotonergic neurons in the DRVL/VLPAG
The numbers of c-Fos-ir nonserotonergic cells (TPH-immunonegative) were also counted in the DRVL/VLPAG region. Neither treatment (i.e., l-AG or lactate) altered the number of c-Fos+/TPH-immunoreactive cells in the DRVL/VLPAG region at −8.00 mm Bregma (l-AG effect F(1,20) = 0.1, P = 0.798; lactate effect F(1,20) = 3.1, P = 0.095 and l-AG × lactate interaction F(1,20) = 0.9, P = 0.350; data not shown).
Discussion
Disruption of GABA synthesis following chronic infusion of l-AG into the medial hypothalamus resulted in a long-lasting increase in anxiety-like state as measured in the SI test (Johnson and Shekhar, 2006). In addition, l-AG-treated rats with a chronic increase in anxiety-like state, but not d-AG-treated control rats, showed a vulnerability to panic-like cardiorespiratory responses following i.v. infusions of sodium lactate. Vulnerability to panic-like cardiorespiratory responses in l-AG-treated, chronically anxious rats was associated with a lack of c-Fos expression in specific subpopulations of serotonergic neurons with the midbrain/pontine raphe nuclei, a cellular responses that was clearly evident in control (d-AG-treated) rats without lactate-induced panic-like cardiorespiratory responses. Of all the serotonergic cell groups that were analyzed, a distinct cluster of large multipolar serotonergic neurons in the DRVL/VLPAG region had the most robust increase in c-Fos induction following sodium lactate infusions in d-AG-treated rats. The c-Fos induction in serotonergic neurons in the DRVL/VLPAG region did not correlate with any anxiety or panic-like response. This is not surprising as L-AG treatment is likely to both alter (increase) the excitatory drive mediating panic-like responses as well as alter (decrease) the inhibitory drive restraining panic-like responses, and therefore the functional state of serotonergic neurons would not be expected to account entirely for the variability in the response.. Other groups of serotonergic neurons within the caudal part of the DR, including the DRV, DRI, and DRC responded with smaller, but significant, increases in c-Fos expression, that also were observed only in d-AG-treated rats. In contrast, there were no effects of sodium lactate infusion on c-Fos expression in serotonergic neurons in the rostral DR or the MnR of either d-AG or l-AG treated rats.
Serotonergic neurons within the DRVL/VLPAG region are tightly apposed to bilateral penetrating arteries and may provide important inhibitory input to the dorsolateral periaqueductal gray (DLPAG) and rostroventrolateral medulla (RVLM) to prevent or inhibit “fight-or-flight” or panic-like cardiorespiratory responses [for review, see (Johnson et al., 2004)].
In support of this hypothesis, microstimulation of the DLPAG elicits sympathoexcitatory and “fight or flight” responses (Beckett et al., 1992; Beckett and Marsden, 1997; Jacob et al., 2002), whereas chemical stimulation of the VLPAG, which contains serotonergic neurons, leads to an inhibition of sympathoexcitation and freezing behavior (Bago and Dean, 2001; Odeh et al., 2003). Although it has been hypothesized that the VLPAG directly holds the DLPAG in check, the underlying mechanisms are largely unknown (Graeff et al., 1996; Graeff et al., 1997). However, there is evidence suggesting that the DRVL/VLPAG inhibits the DLPAG through a serotonergic mechanism since chemical stimulation of the VLPAG leads to a 15-fold increase in serotonin (5-hydroxytryptamine; 5-HT) in the DLPAG associated with an inhibition of unconditioned fear (Viana et al., 1997). Furthermore, prior microinjections of either 5-HT or a 5-HT1A receptor agonist into the dorsal PAG (DPAG) attenuate fear-related physiological and behavioral responses resulting from microstimulation of the DPAG (Beckett et al., 1992; Beckett and Marsden, 1997; Jacob et al., 2002). Taken together, these data suggest that the serotonin arising from the DRVL/VLPAG region may have panicolytic effects in part via actions within the DLPAG.
Serotonergic neurons in the DRVL/VLPAG region also appear to contribute to inhibition of stress-induced sympathoexcitation through actions in the rostroventrolateral medulla (RVLM), a medullary structure that plays a critical role in increasing blood pressure in response to hypertensive stress (Ross et al., 1984; Yamada et al., 1984). Although a significant proportion of serotonergic innervation in the RVLM arises from medullary sources, the majority of serotonergic projections in the midbrain/pons to the RVLM arise almost exclusively from serotonergic neurons in the DRVL/VLPAG region (Bago et al., 2002), consistent with the finding that lesioning the DR leads to a 35% reduction in concentrations of 5-HT within the RVLM (Underwood et al., 1999). Serotonin appears to act in the RVLM to suppress sympathetic activity, since microinjections of 5-HT or 5-HT1A receptor agonists into the RVLM inhibit pre-existing hypertension (Bago et al., 1999; Bago and Dean, 2001). Overall these findings suggest that serotonergic projections arising from the DRVL/VLPAG region may play a fundamental role in the mechanisms underlying the effects of serotonergic drugs on fear- or panic-associated physiological and behavioral responses. Furthermore, the serotonergic neurons in the DRVL/VLPAG region may be the source of 5-HT within the periventricular path, a serotonergic projection hypothesized by Graeff and colleagues (Graeff et al., 1997) to inhibit or prevent sympathoexcitation and panic in the presence of ordinarily mild interoceptive or exteroceptive stress-related stimuli.
The anatomically specific effects of lactate on subsets of serotonergic neurons within the DR in d-AG-treated rats are most likely due to effects of lactate on circumventricular organs (CVOs) since lactate does not readily cross the blood-brain barrier and CVOs are thought to be critical for the brain to respond to osmotic changes (Hochstenbach and Ciriello, 1996). Furthermore, previous studies have demonstrated the importance of the OVLT in lactate-induced panic-like cardiorespiratory responses in panic-prone rats (i.e., rats with chronic intra-DMH/PeF infusions of l-AG) (Shekhar and Keim, 1997). Although the mechanism through which the lactate signal reaches the serotonergic neurons in the DRVL/VLPAG was not addressed in the present study, there are at least two possible routes. One is that the OVLT relays the signal directly to the DRVL/VLPAG, since the OVLT directly and selectively innervates the DRVL/VLPAG region at that level of the midbrain (Thompson and Swanson, 2003). Another route is via the DMH/PeF region. The DMH/PeF is a major target of CVOs such as the OVLT (Thompson and Swanson, 1998). The DMH/PeF region in turn selectively innervates the DRVL/VLPAG region within the midbrain (Thompson et al., 1996). Based on this, lactate-sensitive neurons in the OVLT (Johnson and Gross, 1993) could relay this signal to the DRVL/VLPAG, either directly or indirectly, via the DMH/PeF.
Based on the present findings, we hypothesize that in control d-AG-treated rats the lactate signal is relayed to the DRVL/VLPAG region as part of an inhibitory feedback mechanism designed to limit panic-like cardiorespiratory responses. Previously we have demonstrated that although the lactate signal is most likely relayed to the DMH/PeF of both control and panic-prone rats, only panic-prone rats had increased cellular responses in the DMH/PeF region (Johnson and Shekhar, 2006) and efferent targets of the DMH/PeF that are involved in central sympathetic responses (Dampney, 1994; Thompson and Swanson, 1998; Fontes et al., 2001). Therefore, it is possible that sodium lactate activates OVLT neurons projecting to the DRVL/VLPAG region in both d-AG- and l-AG-treated rats, but that serotonergic neurons in l-AG-treated rats are desensitized to the afferent signals, lack convergent stimulatory input from other brain regions, or are actively inhibited by local regulatory mechanisms or by afferents from higher brain structures. For example, in primates the DRVL/VLPAG region receives direct input from the frontal cortex (predominantly the orbito-insular region) (Keay and Bandler, 2001). Meanwhile, neuroimaging studies in human patients have shown that the orbitofrontal cortex/anterior insula, together with the anterior cingulate, may represent a nodal point integrating somatic and cognitive symptoms of anxiety disorders (Malizia, 1999). Moreover, the insular cortex is noted as an important viscerosensory structure, responsive to multiple viscerosensory stimuli, including arterial chemoreceptor stimulation, baroreceptor stimulation, gastric mechanoreceptor input, taste, and changes in respiratory activity (Cechetto and Saper, 1987), and therefore is ideally suited to relay interoceptive signals, including multimodal signals derived from sodium lactate infusions, directly to the DRVL/VLPAG region.
Functional topography of serotonergic systems
Another important finding is that serotonergic neurons in the DR had heterogenous responses to l-AG and lactate in different subdivisions, supporting the idea that the DR is not a functionally homogeneous group of serotonergic neurons. Effects of lactate in d-AG-treated rats were observed principally within the DRVL/VLPAG region, but also in the DRC and caudal parts of the DRV and DRI. There were no effects of lactate in the rostral DR or MnR. The idea that serotonergic neurons, mainly in the DRVL/VLPAG region suppress behavioral and autonomic responses to lactate is supported by anatomical studies identifying serotonin neurons in this region as part of a “sympathomotor control center” (Kerman et al., 2006). Briefly, serotonin neurons predominantly within the DRVL/VLPAG region (21 neurons), but also within the DRV (9 neurons) and DRC (3 neurons) regions, were identified as presympathetic-premotor neurons with collateral projections to both the adrenal gland and hindlimb muscle involved in the fight-or-flight response. The pattern of c-Fos activation in the present study is consistent with a selective activation of this subset of serotonergic neurons by lactate, and the hypothesis that under normal conditions these serotonergic neurons limit both the autonomic and behavioral components of panic-like responses. In contrast, failure to activate this subpopulation of serotonergic neurons, as in l-AG-treated rats in the present study, would be expected to leave autonomic and motor responses unchecked.
Clinical relevance: panic and anxiety disorders
The data reported here are consistent with the hypothesis that disinhibition of the DMH/PeF region results in a long-lasting anxiety-like state with vulnerability to panic-like cardiorespiratory responses to sodium lactate. Under normal conditions, activation of serotonergic, pre-sympathomotor regulatory neurons within the DRVL/VLPAG region, resulting in enhanced synaptic availability of serotonin in either the DLPAG or RVLM, serves to prevent or inhibit panic-like responses. Dysregulation of this inhibitory sympathoregulatory system may lead to higher likelihood of panic-like cardiorespiratory responses to otherwise subthreshold interoceptive stimuli, such as infusions of 0.5M sodium lactate. Although it is unknown at this point if treatment with SSRIs will attenuate or block lactate-induced panic-like cardiorespiratory responses in these panic-prone rats, pretreating rats with the antidepressant imipramine attenuates panic-like responses as a result of acutely inhibiting GABAergic activity in the DMH/PeF (Shekhar, 1994). Furthermore, increased tryptophan availability has been linked to reduced onset and severity of panic attacks in humans. Tryptophan is the amino acid precursor of 5-HT and increased availability of tryptophan can lead to increased synthesis and release of central 5-HT (Tagliamonte et al., 1973). Conversely, acute dietary tryptophan depletion in humans results in rapid reductions of central 5-HT and can exaggerate responses to interoceptive stimuli (i.e., hypercarbic gas exposure) in normal humans (Schruers et al., 2000). Furthermore, oral administration of 5-hydroxytryptophan, the precursor to 5-HT, (Schruers et al., 2002) or the SSRI fluvoxamine (Pols et al., 1996) to panic patients attenuates panic-related responses resulting from a single breath of 35% CO2. The ability of SSRIs to increase synaptic availability of 5-HT within the specific serotonergic systems outlined here may explain, in part, the underlying mechanisms through which these drugs have therapeutic value in panic disorder patients (Pohl et al., 1998; Al Haddad et al., 2001). Further characterization of the anatomical and functional properties of central serotonergic systems responsible for inhibition of autonomic and behavioral responses may lead to novel therapeutic strategies for the treatment of panic and anxiety disorders.
Clinical considerations
There are some important similarities in the biological substrates underlying anxiety- and panic-like responses. In the DMH/PeF anxiety model with vulnerability to panic, local chronic l-AG treatment produces increased anxiety at baseline and a vulnerability to panic-like cardiorespiratory responses to i.v. infusions of sodium lactate (Shekhar et al., 1996; Shekhar and Keim, 1997, 2000; Johnson and Shekhar, 2006; Shekhar et al., 2006). In contrast, repeated subthreshold BMI (Sajdyk and Shekhar, 2000) injections into the basolateral amygdaloid complex produces rats that exhibit no baseline increases in anxiety- but panic-like responses (i.e., decreased SI duration and increased cardiovascular responses) after the i.v. lactate challenge (Sajdyk and Shekhar, 2000). We have also dissociated increases in baseline anxiety responses (as measured by the SI test) from vulnerability to lactate-induced cardiorespiratory responses by injecting muscimol, a GABAA receptor agonist, into the bed nucleus of the stria terminalis (BNST) of l-AG-treated rats prior to lactate infusion. Muscimol injections into the BNST restored SI duration to baseline without altering lactate's ability to elicit panic-like cardiorespiratory responses (Johnson et al., unpublished). Thus, vulnerability to panic-like responses to sodium lactate does not always require a baseline increase in anxiety-like state, suggesting that the neural pathways mediating anxiety-like behavioral responses and panic-like cardiorespiratory responses to lactate in vulnerable rats appear to be distinct.
As implied previously, the panic-like cardiorespiratory responses to sodium lactate observed in the present study in l-AG-treated rats appear to be closely related to “fight-or-flight” responses. For instance, although we did not measure behavior in a home cage environment, acutely removing local tonic GABA-mediated inhibition in the DMH/PeF with BMI results in a rapid mobilization of “flight” (as measured by increased locomotion in the home cage environment) in addition to the panic-like autonomic and respiratory components of the fight-or-flight response [see review (DiMicco et al., 2002)].
Technical considerations
Immunostaining the transcription factor c-Fos is a useful tool for detailed mapping of multisynaptic neuronal responses after acute exposure to stress-related stimuli (Kovacs, 1998). Cellular responses can occur independent of c-Fos induction, therefore the absence of c-Fos may not reflect a lack of cellular response to a given treatment (Morgan and Curran, 1989; Chan et al., 1993; Cullinan et al., 1995). Conversely, the presence of c-Fos immunostaining reflects a cellular response, which does not necessarily reflect an increase in neuronal firing rates (Morgan and Curran, 1989). The strengths of using c-Fos as a measure of cellular responses are that it is accepted as a marker of postsynaptic neuronal responses to increased synaptic input (Morgan and Curran, 1986) or receptor signaling (Linden et al., 2003) and is a useful marker of neuronal pathways that are involved in processing these afferent signals.
Conclusions
In intact rats, serotonergic responses in the DRVL/VLPAG region following infusion of sodium lactate may be associated with terminal release of 5-HT at efferent targets such as the DLPAG and/or RVLM where 5-HT is known to inhibit panic-associated cardiovascular responses. This may represent an adaptive mechanism for preventing excessive “fight or flight”/panic-like cardiorespiratory responses when facing an imminent threat. Disruption of GABAergic tone in the DMH/PeF prevents activation of DRVL/VLPAG serotonergic neurons, resulting in hyper-reactivity of cardiorespiratory responses to interoceptive stressors.
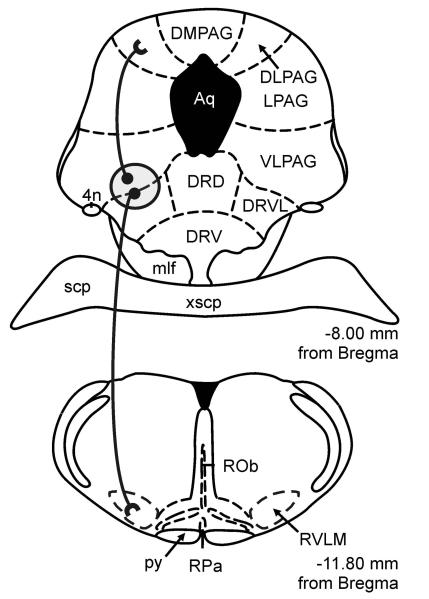
Figure 4.
Proposed periventricular serotonergic path, arising from the DRVL/VLPAG region, as part of a sympathomotor control system. Serotonergic neurons in the DRVL/VLPAG region project, via monosynaptic pathways, to the DLPAG region involved in panic-like behavioral responses to aversive stimuli and to the rostral ventrolateral medulla (RVLM) involved in stress-induced hypertension and sympathetic activation (Johnson et al., 2004). Serotonergic neurons in the DRVL/VLPAG region project, via multisynaptic pathways, to both the adrenal gland and skeletal muscle (Kerman et al., 2006), providing an anatomical substrate for synchronized control of both autonomic and behavioral responses to aversive or panic-inducing stimuli. For functional topography of serotonergic systems, see reviews (Graeff et al., 1997; Lowry, 2002; Johnson et al., 2004). Circle with gray shading indicates the DRVL/VLPAG region containing serotonergic neurons activated by sodium lactate infusions in control rats, but not in anxious, panic-prone rats with inhibition of GABAergic tone in the dorsomedial hypothalamus. Abbreviations: 4n, trochlear nerve; DLPAG, dorsolateral periaqueductal gray; DMPAG, dorsomedial periaqueductal gray; LPAG, lateral periaqueductal gray; py, pyramidal tract; ROb, raphe obscurus nucleus; RPa, raphe pallidus nucleus; RVLM, rostral ventrolateral medulla; scp, superior cerebellar peduncle; xscp, decussation of the superior cerebellar peduncle. For additional abbreviations, see Figure 1 legend.
Acknowledgements
We gratefully acknowledge Stephanie D. Fitz and Amy Dietrich for technical assistance. Supported by the National Institute of Mental Health (RO1 MH52619 and RO1 MH065702 to AS); Anxiety Disorders Association of America (Junior Faculty Research Award to PLJ). CAL was supported by a Wellcome Trust Research Career Development Fellowship (RCDF 068558/Z/02/Z).
References
- Al Haddad MK, Sequeira RP, Nayar U. Neurobiological correlates of panic disorder and agoraphobia. JPostgradMed. 2001;47:55–61. [PubMed] [Google Scholar]
- Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT(1A) receptors in the RVLM. AmJPhysiol RegulIntegrComp Physiol. 2001;280:R976–R984. doi: 10.1152/ajpregu.2001.280.4.R976. [DOI] [PubMed] [Google Scholar]
- Bago M, Sprtel BM, Dean C. Modulation of sympathetic nerve activity by microinjection of the 5-HT1A receptor agonist 8-OH-DPAT into the rostroventrolateral medulla. JAutonNervSyst. 1999;76:127–134. doi: 10.1016/s0165-1838(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Bago M, Marson L, Dean C. Serotonergic projections to the rostroventrolateral medulla from midbrain and raphe nuclei. Brain Res. 2002;945:249–258. doi: 10.1016/s0006-8993(02)02811-1. [DOI] [PubMed] [Google Scholar]
- Beckett S, Marsden CA. The effect of central and systemic injection of the 5-HT1A receptor agonist 8-OHDPAT and the 5-HT1A receptor antagonist WAY100635 on periaqueductal grey-induced defence behaviour. JPsychopharmacol. 1997;11:35–40. doi: 10.1177/026988119701100111. [DOI] [PubMed] [Google Scholar]
- Beckett SR, Lawrence AJ, Marsden CA, Marshall PW. Attenuation of chemically induced defence response by 5-HT1 receptor agonists administered into the periaqueductal gray. Psychopharmacology (Berl) 1992;108:110–114. doi: 10.1007/BF02245294. [DOI] [PubMed] [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AA, den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. BiolPsychiatry. 2002;52:126–135. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ, Sawchenko PE. A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. JNeurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Deakin JF. The role of serotonin in panic, anxiety and depression. IntClinPsychopharmacol. 1998;13(Suppl 4):S1–S5. doi: 10.1097/00004850-199804004-00001. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. PharmacolBiochemBehav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- DSM-IV . Diagnostic and Statistical Manual - Fourth Edn. (DSM - IV) American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Fitz SD, Keim SR, Sajdyk TJ, Shekhar A. Reboxetine, a potent norepinephrine reuptake inhibitor blocks lactate- and CO2-induced panic-like responses in panic-prone rats. Society for Neuroscience. 2003:959.13. [Google Scholar]
- Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. AmJPhysiol Heart CircPhysiol. 2001;280:H2891–H2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Papp LA, Coplan JD, Martinez JM, Lennon S, Goetz RR, Ross D, Klein DF. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. AmJPsychiatry. 1994;151:547–553. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Viana MB, Mora PO. Dual role of 5-HT in defense and anxiety. NeurosciBiobehavRev. 1997;21:791–799. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. PharmacolBiochemBehav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Hochstenbach SL, Ciriello J. Effect of Lesions of forebrain circumventricular organs on c-fos expression in the central nervous system to plasma hypernatrmia. Brain Res. 1996;713:17–28. doi: 10.1016/0006-8993(95)01425-x. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Funderburk F, Kowalski P. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Arch Gen Psychiatry. 2004;61:913–921. doi: 10.1001/archpsyc.61.9.913. [DOI] [PubMed] [Google Scholar]
- Jacob CA, Cabral AH, Almeida LP, Magierek V, Ramos PL, Zanoveli JM, Landeira-Fernandez J, Zangrossi H, Nogueira RL. Chronic imipramine enhances 5-HT(1A) and 5-HT(2) receptors-mediated inhibition of panic-like behavior in the rat dorsal periaqueductal gray. PharmacolBiochemBehav. 2002;72:761–766. doi: 10.1016/s0091-3057(01)00785-7. [DOI] [PubMed] [Google Scholar]
- Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J. Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. BiolPsychiatry. 1999;45:872–882. doi: 10.1016/s0006-3223(98)00348-5. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci. 2006;26:7093–7104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. AnnNYAcadSci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute Hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. JPsychopharmacol. 2005 doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. J Comp Neurol. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. NeurochemInt. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman JM, Fyer A, Dillon D, Levitt M, Klein DF. Possible mechanisms for lactate's induction of panic. AmJPsychiatry. 1986;143:495–502. doi: 10.1176/ajp.143.4.495. [DOI] [PubMed] [Google Scholar]
- Linden AM, Bergeron M, Baez M, Schoepp DD. Systemic administration of the potent mGlu8 receptor agonist (S)-3,4-DCPG induces c-Fos in stress-related brain regions in wild-type, but not mGlu8 receptor knockout mice. Neuropharmacology. 2003;45:473–483. doi: 10.1016/s0028-3908(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. JNeuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13:372–378. doi: 10.1177/026988119901300418. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Odeh F, Antal M, Zagon A. Heterogeneous synaptic inputs from the ventrolateral periaqueductal gray matter to neurons responding to somatosensory stimuli in the rostral ventromedial medulla of rats. Brain Res. 2003;959:287–294. doi: 10.1016/s0006-8993(02)03764-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Pohl RB, Wolkow RM, Clary CM. Sertraline in the treatment of panic disorder: a double-blind multicenter trial. Am J Psychiatry. 1998;155:1189–1195. doi: 10.1176/ajp.155.9.1189. [DOI] [PubMed] [Google Scholar]
- Pollock RA, Carter AS, Amir N, Marks LE. Anxiety sensitivity and auditory perception of heartbeat. Behav Res Ther. 2006 doi: 10.1016/j.brat.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Pols HJ, Hauzer RC, Meijer JA, Verburg K, Griez EJ. Fluvoxamine attenuates panic induced by 35% CO2 challenge. JClinPsychiatry. 1996;57:539–542. doi: 10.4088/jcp.v57n1107. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. JNeurosci. 1984;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. EurJPharmacol. 2000;394:265–273. doi: 10.1016/s0014-2999(00)00128-x. [DOI] [PubMed] [Google Scholar]
- Schruers K, van Diest R, Overbeek T, Griez E. Acute L-5-hydroxytryptophan administration inhibits carbon dioxide-induced panic in panic disorder patients. Psychiatry Res. 2002;113:237–243. doi: 10.1016/s0165-1781(02)00262-7. [DOI] [PubMed] [Google Scholar]
- Schruers K, Klaassen T, Pols H, Overbeek T, Deutz NE, Griez E. Effects of tryptophan depletion on carbon dioxide provoked panic in panic disorder patients. Psychiatry Res. 2000;93:179–187. doi: 10.1016/s0165-1781(00)00117-7. [DOI] [PubMed] [Google Scholar]
- Shekhar A. Effects of treatment with imipramine and clonazepam on an animal model of panic disorder. BiolPsychiatry. 1994;36:748–758. doi: 10.1016/0006-3223(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. JNeurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. LY354740, a potent group II metabotropic glutamate receptor agonist prevents lactate-induced panic-like response in panic-prone rats. Neuropharmacology. 2000;39:1139–1146. doi: 10.1016/s0028-3908(99)00215-4. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. PharmacolBiochemBehav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. AnnNYAcadSci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliamonte A, Biggio G, Vargiu L, Gessa GL. Free tryptophan in serum controls brain tryptophan level and serotonin synthesis. Life SciII. 1973;12:277–287. doi: 10.1016/0024-3205(73)90361-5. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain ResBrain ResRev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. JComp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Arango V, Bakalian MJ, Ruggiero DA, Mann JJ. Dorsal raphe nucleus serotonergic neurons innervate the rostral ventrolateral medulla in rat. Brain Res. 1999;824:45–55. doi: 10.1016/s0006-8993(99)01181-6. [DOI] [PubMed] [Google Scholar]
- Viana MB, Graeff FG, Loschmann PA. Kainate microinjection into the dorsal raphe nucleus induces 5-HT release in the amygdala and periaqueductal gray. PharmacolBiochemBehav. 1997;58:167–172. doi: 10.1016/s0091-3057(96)00451-0. [DOI] [PubMed] [Google Scholar]
- Vickers K, McNally RJ. Respiratory symptoms and panic in the National Comorbidity Survey: a test of Klein's suffocation false alarm theory. Behav Res Ther. 2005;43:1011–1018. doi: 10.1016/j.brat.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Yamada KA, McAllen RM, Loewy AD. GABA antagonists applied to the ventral surface of the medulla oblongata block the baroreceptor reflex. Brain Res. 1984;297:175–180. doi: 10.1016/0006-8993(84)90556-0. [DOI] [PubMed] [Google Scholar]