Abstract
The Ca2+-calmodulin-activated Ser/Thr protein phosphatase calcineurin and the downstream transcriptional effectors of calcineurin, nuclear factor of activated T cells, have been implicated in the hypertrophic response of the myocardium. Recently, the calcineurin inhibitory agents cyclosporine A and FK506 have been extensively used to evaluate the importance of this signaling pathway in rodent models of cardiac hypertrophy. However, pharmacologic approaches have rendered equivocal results necessitating more specific or genetic-based inhibitory strategies. In this regard, we have generated Tg mice expressing the calcineurin inhibitory domains of Cain/Cabin-1 and A-kinase anchoring protein 79 specifically in the heart. ΔCain and ΔA-kinase-anchoring protein Tg mice demonstrated reduced cardiac calcineurin activity and reduced hypertrophy in response to catecholamine infusion or pressure overload. In a second approach, adenoviral-mediated gene transfer of ΔCain was performed in the adult rat myocardium to evaluate the effectiveness of an acute intervention and any potential species dependency. ΔCain adenoviral gene transfer inhibited cardiac calcineurin activity and reduced hypertrophy in response to pressure overload without reducing aortic pressure. These results provide genetic evidence implicating calcineurin as an important mediator of the cardiac hypertrophic response in vivo.
Cardiac hypertrophy is broadly defined as an adaptive enlargement of the myocardium characterized by the growth of individual cardiac myocytes rather than an increase in cell number. Whereas cardiac hypertrophy is a beneficial response that temporarily augments output, sustained hypertrophy often becomes maladaptive and is a leading predictor of future heart failure (1, 2). To understand the molecular mechanisms that underlie adaptive and maladaptive cardiac hypertrophy, investigation has centered around a characterization of the intracellular signal transduction pathways that promote cardiac myocyte growth (3, 4).
One such intracellular signaling pathway involves the calcium-calmodulin, Ser/Thr protein phosphatase calcineurin (PP2B). Sustained elevations in intracellular calcium concentration, in association with calmodulin, directly activate calcineurin phosphatase activity leading to the dephosphorylation and nuclear translocation of a family of transcription factors known as nuclear factor of activated T cells (5, 6).
A role for calcineurin and nuclear factor of activated T cells as regulators of cardiac hypertrophy was recently identified (7). Transgenic (Tg) mice expressing an activated calcineurin or a constitutively nuclear nuclear factor of activated T cells c4 factor in the heart demonstrated profound hypertrophy that rapidly progressed to heart failure (7). In vitro, adenoviral-mediated gene transfer of activated calcineurin also promoted hypertrophic growth of neonatal cardiac myocytes (8). Treatment of cultured cardiomyocytes with the calcineurin inhibitory agents cyclosporine A (CsA) or FK506 blocked agonist-induced hypertrophy, suggesting a necessary role for calcineurin in the hypertrophic response (7). In vivo, three separate mouse models of hypertrophic cardiomyopathy were largely rescued by the administration of CsA or FK506, further implicating this signaling pathway in the heart (9). CsA and FK506 have also been reported to attenuate/prevent pressure overload-induced cardiac hypertrophy in most (9–18) but not all in vivo studies (19–22).
Although the main biological effects of CsA and FK506 are attributed to calcineurin inhibition, each agent has multiple intracellular targets that are independent of calcineurin (23–25). To block calcineurin activity without using pharmacologic agents, we generated cardiac-restricted Tg mice expressing the calcineurin inhibitory protein domains from Cain/Cabin-1 and A-kinase-anchoring protein 79 (AKAP79) (26–28). ΔCain- and ΔAKAP-expressing Tg mice each demonstrated inhibited calcineurin activity and attenuated cardiac hypertrophy in response to physiologic hypertrophic stimuli. In a separate approach, adult wild-type (WT) rats subjected to acute myocardial adenoviral gene delivery of ΔCain demonstrated attenuated hypertrophy in response to pressure overload stimulation. Collectively, these results provide genetic evidence that calcineurin is an important regulator of catecholamine- or pressure overload-induced adaptive growth of the heart in vivo.
Materials and Methods
Generation of Tg Mice.
A 582-bp cDNA fragment encoding the calcineurin inhibitory domain of Cain/Cabin-1 (amino acids 1989–2182) (29) was subcloned with a 5′ Flag epitope into the murine α-myosin heavy chain promoter expression vector (gift from Jeffrey Robbins' Children's Hospital, Cincinnati, OH). Similarly, a cDNA fragment corresponding to amino acids 60–358 of AKAP79 (gift from John Scott, Vollum Institute, Portland, OR) was also subcloned into the murine α-myosin heavy chain promoter expression vector. The injection fragment was gel purified and reconstituted in 2 mM Tris⋅HCl (pH 7.5)/20 μM EDTA and injected into fertilized eggs from FVB/N mice (2–5 ng/ml), and transferred to the oviducts of pseudopregnant recipients.
Western and Northern Blotting.
Western and Northern blots were performed as described in detail (12, 29). Western blot membranes were incubated with a 1:5,000 dilution of anti-Flag mAb (Sigma) followed by an anti-mouse alkaline phosphatase-conjugated secondary antibody to allow visualization by chemiluminescence (ECF kit; Amersham International). For Northern blots, 10 μg of total RNA was electrophoresed and blotted to nylon membranes. All images were scanned and quantified by using a Storm 860 PhosphorImager (Molecular Dynamics).
Calcineurin Activity Assay.
The calcineurin phosphatase assay was performed as described (12). Phosphatase activity was measured from cardiac protein extracts as the dephosphorylation rate of a synthetic [32P]ATP-labeled phosphopeptide substrate (R-II peptide; Peninsula Laboratories) in the presence of 0.5 mM CaCl2/1.0 μM calmodulin/1.0 μM okadaic acid. Calcineurin-specific activity was blocked with the addition of 500 μM calcineurin autoinhibitory peptide (Calbiochem), and subtracted from the unblocked reaction to calculate total enzymatic activity.
Animal Models.
Eight-week-old nonTg, ΔCain, or ΔAKAP mice were anesthetized with isoflurane and their abdominal aortas were exposed through a midline abdominal incision. A 6–0 silk suture was tied around the abdominal aorta immediately below the celiac trunk and a blunted 25-gauge needle, after which the needle was removed to create a defined constriction.
Alzet miniosmotic pumps (no. 2002; Alza) containing either isoproterenol (60 mg/kg/day) or PBS (control) were surgically inserted dorsally and s.c. in 8-week-old mice under isoflurane anesthesia for an additional 2 weeks.
Adenoviral delivery in rats and their subsequent aortic banding were performed as described (30–33). Echocardiography was performed by using a Hewlett–Packard Sonos 5500 Ultrasound System equipped with a 15-MHz transducer.
Statistical Analysis.
The results are presented as means ± SEM. Statistical analyses were performed by using instat 3.0 software (GraphPad, San Diego) and ANOVA followed by Bonferroni's post-test when appropriate.
Results
Generation of ΔCain and ΔAKAP79 TG Mice.
CsA and FK506 have been shown to mediate calcineurin-independent biological effects, suggesting additional mechanisms whereby cardiac hypertrophy might be influenced by these agents (23–25). To more directly investigate the role of calcineurin as a regulator of cardiac hypertrophy, we generated heart-specific Tg mice expressing the calcineurin inhibitory domains from Cain or AKAP79. The calcineurin inhibitory domain of Cain has a consensus sequence that is also conserved in the calcineurin inhibitory domains of AKAP79 and FK506-binding protein 12 (Fig. 1A). Flag epitope containing cDNA fragments encoding the inhibitory domains from either Cain or AKAP79 were cloned into the α-myosin heavy chain promoter construct for generation of Tg mice (Fig. 1B).
Figure 1.
(A) The noncompetitive calcineurin inhibitory domain from Cain contains a putative motif that is conserved in AKAP79 and FKBP12. (B) The 194-aa Flag-tagged Cain peptide (21 kDa) and the 299-aa Flag-tagged AKAP79 peptide (40 kDa) were placed under control of the 5.5-kb murine α-myosin heavy chain promoter to drive transgene expression in the mouse heart. (C) Western blot analysis by using anti-Flag antibody of heart extracts from ΔCain and ΔAKAP TG mice. Positive controls consisted of extracts from neonatal ventricular cardiomyocytes infected with adenovirus encoding each protein domain.
Four Cain chimeric founders were originally generated, of which two failed to transmit the transgene into the germ line, one died prematurely with dilated cardiomyopathy, and one successfully passed the transgene into the germ line, creating an established line for subsequent analyses. The successful ΔCain founder line demonstrated low transgene copy number (one to three transgene inserts) which was associated with low but detectable levels of protein expression by anti-Flag Western blotting of heart protein extracts (Fig. 1C). Because the biologic effects of Cain might also include calcineurin-independent actions, the calcineurin inhibitory domain of AKAP79 was also used to generate Tg mice (one to three transgene inserts; Fig. 1C).
Low-Expressing ΔCain and ΔAKAP Tg Mice Have Normal Cardiac Phenotypes.
Analysis of high-expressing ΔCain or ΔAKAP Tg founder lines demonstrated diminished myofibrillar cross-sectional area and thin ventricular walls, suggesting an inhibition of “normal” developmental hypertrophy (see Discussion). However, lines expressing relatively low levels of either ΔCain or ΔAKAP had no discernable defects in heart maturation or adult heart function. Echocardiographic analysis of low-expressing double-copy (+/+) lines demonstrated normal left ventricular posterior wall and septal thicknesses at 8 weeks of age (Table 1). In addition, left ventricular end-diastolic or end-systolic chamber dimensions were comparable to WT FVB/N littermates and ΔCain and ΔAKAP mice had normal fractional shortening (Table 1).
Table 1.
Echocardiographic analysis of cardiac dimensions and function in ΔCain and ΔAKAP Tg mice at 8 weeks of age
| Cardiac dimension | WT | ΔCain | ΔAKAP |
|---|---|---|---|
| Number | 6 | 6 | 6 |
| SEPth-diastole, mm | 0.59 ± 0.03 | 0.62 ± 0.03 | 0.63 ± 0.03 |
| SEPth-systole, mm | 0.84 ± 0.04 | 0.91 ± 0.03 | 0.88 ± 0.06 |
| LVth wall-diastole, mm | 0.62 ± 0.04 | 0.63 ± 0.04 | 0.62 ± 0.01 |
| LVth wall-systole, mm | 0.94 ± 0.03 | 0.98 ± 0.04 | 0.92 ± 0.04 |
| LVEDD, mm | 3.27 ± 0.06 | 3.35 ± 0.08 | 3.18 ± 0.10 |
| LVESD, mm | 1.80 ± 0.05 | 1.81 ± 0.06 | 1.64 ± 0.04 |
| Fractional shortening, % | 45.0 ± 2 | 46 ± 1 | 48 ± 2 |
| LV mass, mg | 47 ± 4 | 50 ± 3 | 46 ± 2 |
Double-copy ΔCain or ΔAKAP mice and strain-matched (FVB/N) control mice at 8 weeks were each measured in triplicate by echocardiography (six mice in each group). LVth, left ventricular posterior wall thickness; SEPth, septal thickness; LV, left ventricle; LVEDD, left ventricular end-diastolic dimension; and LVESD, left ventricular end-systolic dimension. Fractional shortening was calculated as (LVEDD − LVESD)/LVEDD × 100.
ΔCain and ΔAKAP Inhibit Isoproterenol (Iso)-Induced Cardiac Hypertrophy in Vivo.
To investigate the importance of calcineurin activation as a mediator of cardiac hypertrophy, low-expressing ΔCain and ΔAKAP Tg lines were subjected to iso minipump infusion over 14 days. The data demonstrate comparable heart-to-body weight ratios between WT, ΔCain, and ΔAKAP Tg mice infused with saline over 14 days (Fig. 2A). However, WT FVB/N mice demonstrated a 24% increase in heart-to-body weight ratio in response to isoinfusion whereas single (+/0) or double-copy (+/+) ΔCain and ΔAKAP Tg mice each demonstrated an ≈50% reduction in hypertrophy (P < 0.05) (Fig. 2A).
Figure 2.
(A) Quantitation of heart-to-body weight ratios after isoinfusion (60 mg/kg/day) over 14 days demonstrates a significant attenuation of cardiac hypertrophy in ΔCain and ΔAKAP Tg mice containing either single- (+/0) or double- (+/+) copy transgene integrations. (B) Similarly, abdominal aortic banding-induced cardiac hypertrophy was significantly attenuated in both single- or double-copy ΔCain and ΔAKAP Tg mice over 14 days. (C and D) Isoproterenol infusion and aortic banding stimulated a significant increase in cardiac calcineurin activity as measured by RII peptide dephosphorylation in WT mice (n = 3, each), which was reduced in isoinfused ΔCain (n = 3) or aortic-banded ΔAKAP Tg mice (n = 3). *, P < 0.05 vs. nonTG-iso or banded; †, P < 0.05 vs. nonTG; and #, P < 0.05 vs. AKAP (+/0).
Cardiac calcineurin enzymatic activity was analyzed from saline and isoinfused WT and ΔCain Tg mice. The results show a nearly fivefold increase in cardiac calcineurin activity in WT isoinfused mice compared with saline mice (P < 0.05) (Fig. 2C). In contrast, ΔCain Tg mice failed to show an induction in cardiac calcineurin activity in response to isoinfusion (P < 0.05) (Fig. 2C).
ΔCain and ΔAKAP Inhibit Pressure Overload Cardiac Hypertrophy in Vivo.
To further investigate the importance of calcineurin activation in a different model of cardiac hypertrophy, low-expressing ΔCain and ΔAKAP Tg mice were subjected to abdominal aortic constriction to induce pressure overload. Induction of pressure overload stimulated an increase in heart-to-body weight ratio in WT FVB/N mice over 14 days (Fig. 2B). However, single- and double-copy ΔCain Tg mice showed a 68% and 79% reduction in pressure overload-induced cardiac hypertrophy, respectively, whereas single- and double-copy ΔAKAP Tg mice showed a 25% and 38% reduction, respectively, when compared with WT FVB/N-banded mice (Fig. 2B). In response to aortic banding, WT FVB/N mice showed a greater than twofold increase in cardiac calcineurin activity, which was significantly blunted in double-copy ΔAKAP Tg mice (Fig. 2D). Collectively, these data demonstrate that genetic inhibition of calcineurin activity in the heart reduces the magnitude of load-induced hypertrophy.
Histological analysis of aortic-banded and isoinfused WT mice demonstrated a qualitative increase in heart size that was significantly less prominent in ΔCain Tg mice (Fig. 3A). Microscopic analysis of hematoxylin and eosin-, and trichrome-stained cardiac histological sections failed to identify any signs of significant pathology in any group at 14 days (data not shown). Hearts from aortic-banded ΔCain mice were also analyzed for mRNA levels of hypertrophy-related marker genes by Northern blot analysis. WT FVB/N mice showed a significant increase in atrial natriuretic factor, skeletal α-actin, and myosin light chain 2a, whereas this increase was attenuated in the hearts of aortic-banded ΔCain Tg mice (Fig. 3B).
Figure 3.
(A) Gross morphology from histological cross section of the hearts from the indicated groups. Aortic banding and isoinfusion induced a noticeable hypertrophy response that was blunted in ΔCain Tg mice. (B) Northern blots of atrial natriuretic factor, skeletal α-actin, and myosin light chain 2a showed increased cardiac mRNA levels in WT-banded mice compared with sham WT or sham Cain Tg mice. However, Cain-banded mice demonstrated a qualitative decrease in marker expression compared with WT-banded mice. Glyceraldehyde-3-phosphate dehydrogenase mRNA levels are shown as a loading control.
Adenoviral Delivery of ΔCain Attenuates Pressure Overload Hypertrophy in the Rat.
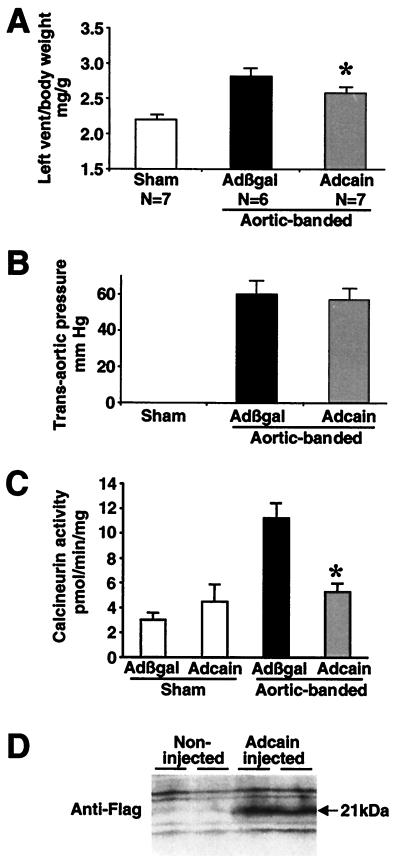
To address differences in animal models and the timing of calcineurin inhibition as potential variables, adult rats were acutely infected with a ΔCain-expressing adenovirus by aortic-coronary perfusion followed by aortic banding. Hajjar et al. (30) demonstrated an in vivo method of acute gene transfer by using recombinant adenovirus whereby 50–70% of cardiac myocytes are uniformly infected throughout the entire myocardium, without signs of a inflammation over 7 days. Since this description, multiple reports have used this technique to alter the function and hypertrophic characteristics of the myocardium (31–34). In the present study, adult rat hearts were infected with Adcain or Adβgal (control) and subjected to aortic banding for 7 days. Significant cardiac hypertrophy was observed in Adβgal-infected rats whereas Adcain infection resulted in a 40% decrease in left ventricular hypertrophy (P < 0.05) (Fig. 4A). Importantly, measurement of trans-aortic pressure gradient induced by aortic banding showed no difference between Adβgal- and Adcain-infected groups (Fig. 4B). Furthermore, aortic-banded control rats (Adβgal) demonstrated a nearly threefold increase in cardiac calcineurin activity, whereas Adcain aortic-banded rats showed no increase in cardiac calcineurin activity (P < 0.05) (Fig. 4C). To verify expression of the adenoviral-encoded ΔCain protein, two Adcain-injected rat hearts were processed and subjected to Western blotting with Flag antibody. The data demonstrate significant viral-mediated expression of the ΔCain protein domain 7 days after cardiac injection (Fig. 4D). These results indicate that acute delivery of the ΔCain-expressing adenovirus generates significant protein expression in the heart that is associated with inhibition of calcineurin activity and a reduction in pressure overload-induced hypertrophy.
Figure 4.
(A) Rat hearts were infected with adenoviruses expressing either β-galactosidase (Adβgal) or the ΔCain transgene (Adcain) by aortic-coronary perfusion and subjected to transverse aortic constriction for 7 days. Adcain-infected rats showed a significant attenuation of hypertrophy compared with Adβgal infection. (B) Adβgal- and Adcain-infected cohorts obtained a similar trans-aortic pressure gradient of ≈60 mmHg. (C) Measurement of RII-peptide dephosphorylation rates showed a significant increase in cardiac calcineurin activity after aortic banding in control rats, which was normalized in Adcain-infected and -banded rats. (D) Western blotting for the Flag epitope demonstrated significant Cain protein expression 7 days after in vivo infection. *, P < 0.05 vs. Adβgal-banded.
Discussion
Within the last 2 years, controversy has emerged concerning the effectiveness of calcineurin inhibitory drugs (CsA and FK506) as antihypertrophic agents. In the present study, we generated several genetic approaches to further evaluate the role of calcineurin as a regulator of the cardiac hypertrophic response in vivo. Tg mice expressing the calcineurin inhibitory domain of either Cain or AKAP79 each demonstrated partial reduction in catecholamine- and pressure overload-induced cardiac hypertrophy. Adenoviral-mediated gene delivery of ΔCain in adult WT rat hearts also attenuated load-induced hypertrophy in vivo. These divergent approaches strongly implicate calcineurin as an important regulator whereby cardiomyocytes respond to pathophysiologic stimuli.
Reduction of Cardiac Hypertrophy with ΔCain and ΔAKAP.
Tg mice expressing either ΔCain and ΔAKAP in the heart demonstrated a significant reduction in cardiac calcineurin activity and load- or catecholamine-induced cardiac hypertrophy. However, doubling ΔCain or ΔAKAP transgene copy number did not further reduce cardiac hypertrophy, except in ΔAKAP double-copy mice subjected to isoinfusion (Fig. 2A). This lack of further reduction in double-copy Tg mice suggests that the effects of ΔCain or ΔAKAP are largely saturating at single-copy levels. Comparison between ΔCain or ΔAKAP hypertrophic responses also suggested that ΔCain was a slightly better inhibitor than ΔAKAP, although this comparison is difficult to interpret because relative protein levels may differ between ΔCain or ΔAKAP Tg mice. It remains possible that ΔCain is a better calcineurin inhibitor than ΔAKAP, or that ΔCain inhibits other intracellular factors which further reduces hypertrophy (see below). Analysis of Adcain-infected rats showed less reduction of load-induced cardiac hypertrophy compared with aortic-banded ΔCain Tg mice. However, such a comparison is complicated by the species difference, the difference in the surgical procedure, and the difference in time course (7 vs. 14 days).
The degree to which the calcineurin enzymatic assay accurately reflects endogenous calcineurin activity remains uncertain. Indeed, the calcineurin assay measures activity from homogenized and processed protein extracts under saturating calcium and calmodulin concentrations, any point at which associated positive or negative regulatory factors could be lost.
ΔCain and ΔAKAP as Calcineurin Inhibitors.
We previously characterized the effectiveness of recombinant ΔCain- and ΔAKAP-expressing adenovirus to antagonize phenylephrine or angiotensin II-induced hypertrophy of cultured neonatal cardiomyocytes (29). The calcineurin-docking domain of AKAP79, which is distinct from the protein kinases C- and A-docking domains, acts as a potent noncompetitive inhibitor of calcineurin activity (28). Cain/Cabin-1 is a 230-kDa protein highly expressed in the brain that contains a potent noncompetitive calcineurin inhibitory domain in the extreme C terminus (26, 27). Neither AKAP79 nor Cain are expressed at significant levels in the heart, suggesting that they are not physiologic regulators of cardiac calcineurin activity. More recently, the calcineurin inhibitory genes MCIP1 and MCIP2 (DSCR1 and ZAKI-4) were identified and shown to be highly expressed in the heart and skeletal muscle, potentially representing physiologic regulators of calcineurin activity in striated muscle (35, 36). Even though AKAP79 and Cain are unlikely to regulate calcineurin in the heart, the current Tg approach reduced endogenous activity in the heart in much the same way a drug like CsA would be used, yet without the systemic side effects.
Cabin-1/Cain has recently been implicated as a direct negative regulator of the transcription factor myocyte enhancer factor 2 (MEF2; ref. 37), which itself might regulate cardiac hypertrophy (38, 39). Indeed, the MEF2 inhibitory domain of Cain is coincident with the C-terminal calcineurin inhibitory domain (37). Consistent with this report, we determined that the 194-aa inhibitory domain of Cain used for transgenesis also inhibited MEF2 transcriptional responses in cultured cells (data not shown). However, the calcineurin inhibitory domain of AKAP79 did not block MEF2 transcriptional activity, suggesting that AKAP79 does not influence MEF2 function (data not shown). In any event, roughly comparable inhibition of cardiac hypertrophy was observed in ΔCain and ΔAKAP mice, suggesting a primary role for calcineurin in the hypertrophic response.
Role for Calcineurin in the Hypertrophic Response.
At ≈1 week of postnatal development, cardiomyocytes permanently exit the cell cycle so that all subsequent growth of the rodent heart occurs through hypertrophy of individual myocytes (40). Developmental hypertrophy is likely mediated by many of the same intracellular signaling pathways that are implicated in adult pathological hypertrophy. Consistent with this hypothesis, we observed that high copy number ΔCain or ΔAKAP Tg were not viable beyond 2 weeks of life because of ineffective developmental hypertrophy and subsequent ventricular dilation (data not shown). In contrast, low copy number ΔCain and ΔAKAP Tg mice were viable and overtly normal, suggesting that robust inhibition of calcineurin in the heart is not well tolerated during early postnatal development.
The results of the present study support an increasing body of literature consisting of 13 reports at present which demonstrate a partial or complete inhibition of cardiac hypertrophy or heart failure by using cyclosporine and/or FK506 (9–18, 41–43). However, such reports remain controversial for a number of reasons. First, four studies failed to identify a significant decrease in cardiac hypertrophy in rodent models of pressure overload treated with CsA or FK506 (19–22). Second, CsA and FK506 are known to have additional biological effects, separate from calcineurin inhibition (23–25). Third, chronic CsA therapy induces renal toxicity leading to hypertension and secondary cardiac hypertrophy in humans (44).
Another confounding issue is the dosage of CsA or FK506 that is required to show an effect on cardiac hypertrophy. Indeed, 5- to 10-fold higher levels of CsA are required to inhibit cardiac calcineurin activity and attenuate the hypertrophic response compared with immunosuppressive doses (12). These findings are likely related to the higher calcineurin protein content in cardiomyocytes relative to T or B cells (5).
A final and perhaps more important consideration is related to the multifactorial nature of the cardiac hypertrophic response itself. Indeed, we have identified two Tg mouse models of hypertrophic cardiomyopathy that are insensitive to CsA [nuclear factor of activated T cells c4 or mutant RXRα-overexpressing Tg mice (9, 41)]. In this study, ΔCain and ΔAKAP Tg mice and Adcain-infected rats did not show “complete” blockade in cardiac hypertrophy or in the expression of molecular markers of hypertrophy. Such observations underscore the multifactorial nature of the cardiac hypertrophic response and suggest molecular heterogeneity in the process singularly referred to as cardiac hypertrophy. Nevertheless, the results of the present study implicate calcineurin as an important mediator of pathophysiologic hypertrophy and suggest the development of new approaches to selectively inhibit calcineurin activity in the heart.
Acknowledgments
This work was supported by National Institutes of Health Grants HL69562, HL62927, HL52318, and a Pew Scholar Award (to J.D.M.), and HL50361, HL57623, a Doris Duke Award, and an American Federation of Aging Research Grant (to R.J.H.).
Abbreviations
- AKAP
A-kinase-anchoring protein
- CsA
cyclosporine A
- Tg
transgenic
- iso
isoproterenol
- WT
wild type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 2947.
References
- 1.Levy D, Garrison R J, Savage D D, Kannel W B, Castelli W P. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Ho K K, Levy D, Kannel W B, Pinsky J L. J Am Coll Cardiol. 1993;22:6–13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 3.Olson E N, Molkentin J D. Circ Res. 1999;84:623–632. doi: 10.1161/01.res.84.6.623. [DOI] [PubMed] [Google Scholar]
- 4.Hunter J J, Chien K R. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree G R. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 6.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 7.Molkentin J D, Lu J R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Windt L J, Lim H W, Taigen T, Wencker D, Condorelli G, Dorn G W, II, Kitsis R N, Molkentin J D. Circ Res. 2000;86:255–263. doi: 10.1161/01.res.86.3.255. [DOI] [PubMed] [Google Scholar]
- 9.Sussman M A, Lim H W, Gude N, Taigen T, Olson E N, Robbins J, Colbert M C, Gualberto A, Wieczorek D F, Molkentin J D. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 10.Meguro T, Hong C, Asai K, Takagi G, McKinsey T A, Olson E N, Vatner S F. Circ Res. 1999;84:735–740. doi: 10.1161/01.res.84.6.735. [DOI] [PubMed] [Google Scholar]
- 11.Shimoyama M, Hayashi D, Takimoto E, Zou Y, Oka T, Uozumi H, Kudoh S, Shibasaki F, Yazaki Y, Nagai R, et al. Circulation. 1999;100:2449–2454. doi: 10.1161/01.cir.100.24.2449. [DOI] [PubMed] [Google Scholar]
- 12.Lim H W, De Windt L J, Steinberg L, Taigen T, Witt S A, Kimball T R, Molkentin J D. Circulation. 2000;101:2431–2437. doi: 10.1161/01.cir.101.20.2431. [DOI] [PubMed] [Google Scholar]
- 13.Øie E B, Reidar O P F, Clausen H. Am J Heart Circ Physiol. 2000;278:2115–2123. [Google Scholar]
- 14.Hill J A, Karimi M, Kutschke W, Davisson R L, Zimmerman K, Wang Z, Kerber R E, Weiss R M. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 15.Eto Y, Yonekura K, Sonoda M, Arai N, Sata M, Sugiura S, Takenaka K, Gualberto A, Hixon M L, Wagner M W, et al. Circulation. 2000;101:2134–2137. doi: 10.1161/01.cir.101.18.2134. [DOI] [PubMed] [Google Scholar]
- 16.Shimoyama M, Hayashi D, Zou Y, Takimoto E, Mizukami M, Monzen K, Kudoh S, Hiroi Y, Yakaki Y, Nagai R, et al. Circulation. 2000;102:1996–2004. doi: 10.1161/01.cir.102.16.1996. [DOI] [PubMed] [Google Scholar]
- 17.Sakata Y, Masuyama T, Yamamoto K, Nishikawa N, Yamamoto H, Kondo H, Ono K, Otsu K, Kuzuya T, Miwa T, et al. Circulation. 2000;102:2269–2275. doi: 10.1161/01.cir.102.18.2269. [DOI] [PubMed] [Google Scholar]
- 18.Murat A, Pellieux C, Brunner H R, Pedrazzini T. J Biol Chem. 2000;275:40867–40873. doi: 10.1074/jbc.M008071200. [DOI] [PubMed] [Google Scholar]
- 19.Ding B, Price R L, Borg T K, Weinberg E O, Halloran P F, Lorell B H. Circ Res. 1999;84:729–734. doi: 10.1161/01.res.84.6.729. [DOI] [PubMed] [Google Scholar]
- 20.Luo Z, Shyu K G, Gualberto A, Walsh K. Nat Med. 1998;10:1092–1093. doi: 10.1038/2578. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Kowal R C, Rusnak F, Sikkink R A, Olson E N, Victor R G. Circ Res. 1999;84:722–728. doi: 10.1161/01.res.84.6.722. [DOI] [PubMed] [Google Scholar]
- 22.Müller J G, Nemoto S, Laser M, Carabello B A, Menick D R. Science. 1998;282:1007. [Google Scholar]
- 23.duBell W H, Gaa S T, Lederer W J, Rogers T B. Am J Heart Circ Physiol. 1998;275:2041–2052. [Google Scholar]
- 24.Kaibori M, Okumura T, Ito S, Oda M, Inoue T, Kamiyama Y. Transplant Proc. 1999;31:804–805. doi: 10.1016/s0041-1345(98)01778-3. [DOI] [PubMed] [Google Scholar]
- 25.Meyer S, Kohler N G, Joly A. FEBS Lett. 1997;413:354–358. doi: 10.1016/s0014-5793(97)00930-7. [DOI] [PubMed] [Google Scholar]
- 26.Lai M M, Burnett P E, Wolosker H, Blackshaw S, Snyder S H. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Youn H D, Loh C, Stolow M, He W, Liu J O. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 28.Coghlan V M, Perrino B A, Howard M, Langeberg L K, Hicks J B, Gallatin W M, Scott J D. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 29.Taigen T, De Windt L J, Lim H W, Molkentin J D. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajjar R J, Schmidt U, Matsui T, Guerrero J L, Lee K H, Gwathmey J K, Dec G W, Semigran M J, Rosenzweig A. Proc Natl Acad Sci USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choukroun G, Hajjar R, Fry S, del Monte F, Haq S, Guerrero L, Picard M, Rosenzweig A, Force T. J Clin Invest. 1999;104:391–398. doi: 10.1172/JCI6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto M I, del Monte F, Schmidt U, DiSalvo T S, Kang Z B, Matsui T, Guerrero J L, Gwathmey J K, Rosenzweig A, Hajjar R J. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt U, del Monte F, Miyamoto M I, Matsui T, Gwathmey J K, Rosenzweig A, Hajjar R J. Circulation. 2000;101:790–796. doi: 10.1161/01.cir.101.7.790. [DOI] [PubMed] [Google Scholar]
- 34.Maurice J P, Hata J A, Shah A S, White D C, McDonald P H, Dolber P C, Wilson K H, Lefkowitz R J, Glower D D, Koch W J. J Clin Invest. 1999;104:21–29. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothermel B, Vega R B, Yang J, Wu H, Bassel-Duby R, Williams R S. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 36.Fuentes J J, Genesca L, Kingsbury T J, Cunningham K W, Perez-Riba M, Estivill X, de la Luna S. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- 37.Youn H D, Sun L, Prywes R, Liu J O. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 38.Passier R, Zeng H, Frey N, Naya F J, Nicol R L, McKinsey T A, Overbeek P, Richardson J A, Grant S R, Olson E N. J Clin Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolodziejczyk S M, Wang L, Balazsi K, DeRepentigny Y, Kothary R, Megeney L A. Curr Biol. 1999;9:1203–1206. doi: 10.1016/S0960-9822(00)80027-5. [DOI] [PubMed] [Google Scholar]
- 40.Claycomb W C. Biochem J. 1977;168:599–601. doi: 10.1042/bj1680599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim H W, De Windt L J, Mante J, Kimball T R, Witt S A, Sussman M A, Molkentin J D. J Mol Cell Cardiol. 2000;32:697–709. doi: 10.1006/jmcc.2000.1113. [DOI] [PubMed] [Google Scholar]
- 42.Mende U, Kagen A, Cohen A, Aramburu J, Schoen F J, Neer E J. Proc Natl Acad Sci USA. 1998;95:13893–13898. doi: 10.1073/pnas.95.23.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mervaala F, Muller D N, Park J K, Dechend R, Schmidt F, Fiebeler A, Bieringer M, Breu V, Ganten D, Haller H, et al. Hypertension. 2000;35:360–366. doi: 10.1161/01.hyp.35.1.360. [DOI] [PubMed] [Google Scholar]
- 44.Ventura H O, Malik F S, Mehra M R, Stapleton D D, Smart F W. Curr Opin Cardiol. 1997;12:375–381. [PubMed] [Google Scholar]