Proinflammatory and Profibrotic Mediators: Principal Effectors of Leiomyoma Development as a Fibrotic Disorder
Nasser Chegini, Ph.D1
The publisher regrets that Figure 1 in the above article in Seminars in Reproductive Medicine, Volume 28, Number 3, 2010, p. 182, was poorly reproduced.
Figure 1.
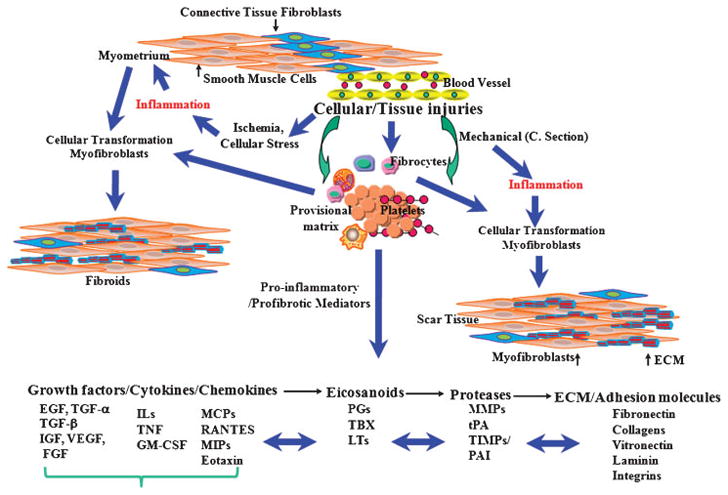
Schematic diagram illustrating an overview of cellular injury and inflammatory-mediated steps in development of leiomyoma as compared with uterine scar formation caused by mechanical injury (i.e., myomectomy and cesarean delivery). Cellular/tissue injuries and generation of an inflammatory response, either at a small scale (microenvironment) or an extended area (myomectomy/cesarean delivery), result in individual and combined regulatory interactions among several proinflammatory and profibrotic mediators. These mediators, including cytokines, chemokines, growth factors, eicosanoids, proteases, and extracellular matrix (ECM), activate to cause myoblasts and resident fibroblasts to differentiate into myofibroblastic phenotype. In addition, possible participation of fibrocytes, which are derived from bone marrow and through circulation reside at the site of inflammation/injury, as well as the transformation of vascular endothelial cells into mesenchymal cells, exist to transform into myofibroblastic phenotype. Collectively, myofibroblasts are highly responsive to the actions of many of the previously mentioned mediators, including profibrotic cytokines; they produce and deposit various components of ECM that are essential for tissue repair process. Continuous inflammation, excess myofibroblastic transformation, and production of a large quantity of ECM, with concurrently reduced degradation, represent a pathway that leads to development of either leiomyoma and/or scar tissue formation.
EGF, endothelial growth factor; FGF, fibroblast growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IGF, insulinlike growth factor; IL, interleukin; LT, leukotriene; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; MMP, metalloproteinase; PAI, plasminogen activator inhibitor; PG, prostaglandin; RANTES, regulated upon activation, normal T-cell expressed and secreted; TBX, thromboxane; TGF, transforming growth factor; TNF, tumor necrosis factor; TIMP, tissue inhibitor of metalloproteinase; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor.
The high resolution figure is reproduced on the following page.
1Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, University of Florida, Gainesville, Florida.
Address for correspondence and reprint requests: Nasser Chegini, Ph.D., Professor, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, University of Florida, Gainesville, FL, 32610 (cheginin@ufl.edu).
Ethical Controversies in Reproductive Medicine; Guest Editor, Mark V. Sauer, M.D.


