Abstract
CXCR3 is a chemokine receptor that is highly expressed on effector T cells and plays an important role in T cell trafficking and function. CXCR3 is rapidly induced on naïve cells following activation and preferentially remains highly expressed on Th1-type CD4+ T cells and effector CD8+ T cells. CXCR3 is activated by three interferon-inducible ligands CXCL9 (MIG), CXCL10 (IP-10) and CXCL11 (I-TAC). Early studies demonstrated a role for CXCR3 in the trafficking of Th1 and CD8 T cells to peripheral sites of Th1-type inflammation and the establishment on Th1 amplification loop mediated by IFNγ and the IFNγ-inducible CXCR3 ligands. More recent studies have also suggested that CXCR3 plays a role in the migration of T cells in the microenvironment of the peripheral tissue and lymphoid compartment, facilitating the interaction of T cells with antigen presenting cells leading to the generation of effector and memory cells.
Introduction
During the course of an immune response distinct functional subsets of effector and regulatory T cells are generated in the lymphoid compartment following the differentiation of naïve T cells under the influence of specific cytokines. CD4+ effector subsets include type-1 helper (Th1), Th2 and Th17, which are characterized by the production of different inflammatory cytokines to drive immunity and T regulatory cells (Tregs), which counterbalance these responses. Similar to Th1 cells that produce large amounts of interferon-gamma (IFNγ), CD8+ T cells can differentiate to become cytotoxic lymphocytes (CTLs), which secrete IFNγ along with other effector molecules. Once differentiated, these T cell subsets upregulate chemokine receptors, which guide them out of the lymphoid compartment and into sites of inflammation or infection to deliver an adaptive immune response. Effector T cell subsets differ profoundly in their migratory properties [1]. The induction of Th1 and CTL cells is strongly linked to the upregulation of the chemokine receptor CXCR3. CXCR3 binds three chemokines CXCL9 (also known as MIG, monokine induced by gamma-interferon), CXCL10 (IP-10, interferon-induced protein of 10kDa), CXCL11 (I-TAC, interferon inducible T cell alpha chemoattractant) to induce migration of activated T cells in vitro and in vivo [2–6]. CXCR3 and its ligands are undoubtedly an inflammatory chemokine system, capable of coordinating T cell responses in the inflamed periphery. In addition, this system may also play a role in the generation of both inflammatory and suppressive T cell responses.
While this review will focus exclusively on the role of CXCR3 on effector CD4+ and CD8+ T cells, it is also worth noting that CXCR3 is also highly expressed on innate lymphocytes, such as NK cells and NKT cells where CXCR3 is thought to participate in the localization of these first line defenders at sites of infection and inflammation [7, 8]. Further, CXCR3 is expressed on plasmacytoid dendritic cells (DCs) and subsets of B cells where it may play a role in the migration of these cells in the inflamed lymph node (LN) [9, 10].
CXCR3 – the inflammatory T cell chemokine receptor
Early studies found that T cells recovered from inflamed peripheral tissue in human autoimmune disease were highly enriched in CXCR3 surface expression relative to T cells found in the blood [7, 11, 12]. In addition, CXCR3 ligands were also found to be highly expressed in these same diseased tissues where CXCR3 positive T cells had accumulated (Table 1). These observations indicated a specific role for CXCR3 and its ligands in the recruitment of T cells into these otherwise restricted sites. While absent on naïve T cells, effector and memory T cells highly express CXCR3 [13]. This upregulation of CXCR3 occurs rapidly following DC-induced T cell activation, prior to T cell proliferation in an antigen-specific manner [14, 15]. The expression of CXCR3, along with CCR5 and CXCR6, discriminates between different effector T cell populations. Specifically, naturally occurring IFNγ-producing Th1 cells are almost exclusively CXCR3 positive [14, 16]. In addition, in vitro Th1-polarized T cells highly upregulate CXCR3 [17]. The tight correlation between CXCR3 expression and Th1 differentiation lead to the hypothesis, subsequently verified in mouse models, that CXCR3 and its ligands regulate the migration of Th1 cells into sites of Th1-driven inflammation (Table 1) [2, 3, 15, 18].
Table 1.
CXCR3 and its ligands in human disease and murine disease models
| Human Disease | [ref] | Mouse Models | CXCR3 | CXCL9 | CXCL10 | [ref] |
|---|---|---|---|---|---|---|
| Autoimmmune | ||||||
| Psoriasis | [78–80] | |||||
| Sarcoid | [81–83] | |||||
| Rheumatoid Arthritis | [7, 10, 84–86] | Adjuvant-induced arthritis | √ | √ | [34, 35] | |
| Asthma | [87–89] | Allergic Pulmonary Inflammation | √ | √ | √ | [90, 91] |
| Atherosclerosis | [92] | ApoE−/−, LDLR−/− | √ | √ | [63, 93] | |
| Multiple Sclerosis | [94–96] | EAE | √ | √ | [61, 97–101] | |
| IBD | [102] | IL-10−/−, RBhi transfer | √ | √ | [103] | |
| Idiopathic Pulmonary Fibrosis | [104] | Bleomycin-induced injury | √ | √ | [105–107] | |
| Type I Diabetes Mellitus | [108, 109] | Insulitis | √ | √ | √ | [36, 110] |
| SLE | [111–113] | MRL/lpr | √ | √ | √ | [37] |
| Cigarette smoke injury/COPD | [114, 115] | Cigarette smoke injury | √ | [116, 117] | ||
| Myocarditis | √ | √ | [25, 118] | |||
| Transplantation | ||||||
| Heart Transplant | [119, 120] | Orthotopic Heart Transplant | √ | √ | √ | [44, 121–125] |
| Lung Transplant | [126] | Orthotopic Tracheal Transplant | √ | √ | √ | [43, 127] |
| GVH | [128] | GVH | √ | √ | [129] | |
| Small Bowel | √ | [130] | ||||
| Infections | ||||||
| Leprosy | [131] | |||||
| Tuberculosis | [132, 133] | |||||
| Influenza | √ | [134, 135] | ||||
| Toxoplasma gondii | √ | √ | [18, 136] | |||
| Malaria (P. falciparium) | [137] | Malaria (P. berghei) | √ | √ | √ | [26, 27] |
| Dengue | [138] | Dengue | √ | √ | [139] | |
| Hepatitis B and C | [140–142] | Mouse Hepatitis Virus | √ | [68, 143] | ||
| Herpes Simplex | [144] | Herpes Simplex | √ | √ | √ | [29–31, 145] |
| HIV-1 | [146–148] | |||||
| Leishmania | [149] | Leishmania | √ | √ | [150, 151] | |
| Chlamydia Trachomatis | √ | [152, 153] | ||||
| Lyme | [154, 155] | Lyme | √ | [156] | ||
| Klebsiealla | √ | [157] | ||||
| West Nile Virus | [28] | |||||
| Cancer | ||||||
| Renal | [158] | Renal | √ | √ | [86] | |
| Colon | [159] | Colon | √ | [160] | ||
| Melanoma | [161] | Melanoma | √ | √ | [161] | |
| Lymphoma | [162–164] | Lymphoma | √ | [165] | ||
| Breast | [166] | Breast | √ | √ | [166, 167] |
Induction of CXCR3 on T cells
Naïve T cells differentiate into Th1 cells under the direction of the cytokine IL12 and the transcription factor Tbet (Tbx21, T-box expressed in T cells). Th1 cell-mediated inflammation is characterized by the recruitment of IFNγ producing CD4 T cells that normally mediate protection against intracellular pathogens, but are also dysregulated in autoimmunity. T-bet is the master transcription factor of Th1 and CTL commitment. T-bet directly activates transcription of a set of genes important for Th1 and CTL cell function, including those encoding IFNγ [19–21]. The resistance of T-bet deficient mice to inflammatory diseases is characterized by a lack of T cell infiltration at the pathological site [22, 23]. T-bet imprints a migratory program upon developing effector T cells to ensure appropriate homing to inflammatory sites. Defects in migration of T-bet deficient (−/−) T cells are primarily due to loss of CXCR3 expression, as T-bet directly transactivates CXCR3, and retroviral-mediated CXCR3 expression in T-bet −/− CD8 T cells reconstitutes their ability to infiltrate inflamed tissues [20, 24, 25]. Indeed, the deficiency of Cxcr3 or one of its ligands has been shown to limit the infiltrations of Th1 and CTL cells in a multitude of Th1-driven disease models (Table 1).
CXCR3 permits entry into inflammatory sites
CXCR3 expression on effector T cells grants them entry into sites otherwise restricted. This seems particularly true during infections in the brain. CXCR3 is required on CD8+ cells for infiltration into the brain during P. berghei ANKA infection for the development of cerebral malaria symptoms [26, 27]. Cxcr3−/− mice are protected from cerebral malaria due to reduced CD8+ CTL sequestration in the brain. This protection is mediated by both CXCL9 and CXCL10, as mice deficient in either one of these ligands showed partial disease protection [26]. In other models of brain inflammation, CXCL10 appeared to have a primary role in the recruitment of effector T cells into the brain. In West Nile virus infection, CXCL10 is expressed by neurons, and directs the migration of CD8+ T cells into the brain [28]. T cell infiltration of mucosal tissues is also highly dependent on CXCR3 expression. This is true during Herpes Simplex Virus-2 (HSV-2) infection of the vaginal mucosa [29–31] and during colitis. In the IL10 null Inflammatory Bowel Disease (IBD) model, CXCL10 and CXCR3 are highly expressed at sites of colitis due to local production of the ligands leading to the recruitment of CXCR3 positive T cells. In this model, CXCL10 neutralization could attenuate the severity of colitis [32]. In the adoptive transfer model of colitis CD4+CD25- T cells require expression of CXCR3 to cause disease in Rag1−/−mice. Interestingly, the transfer of Tregs for disease protection in this model does not require CXCR3, indicating that these cells access the site of suppression via a different mechanism [33]. Accumulation of effector T cells at sites of autoimmune inflammation is strongly correlated with CXCR3 expression. In addition to autoimmune rheumatoid arthritis synovium where CXCR3 expressing cells were first characterized in a human disease [7] and subsequently shown to regulate T cell recruitment in murine models [34, 35], deficiency in CXCR3 also reduces autoimmune insulitis diabetes and infiltration of T cells into the kidney in systemic lupus erythematosus (SLE) [36–38].
CXCR3 chemokine ligands
Despite the demonstration of their importance in multiple disease models, surprisingly few studies have investigated in detail the specific cellular sources of CXCR3 ligand production during inflammation. Much of the work in this area has been done using quantitative PCR on whole tissues. While this method confirms timing and tissue expression, it offers little information about the types of cells that elicit effector T cell recruitment, and the molecules that induce this expression. However, in the cerebral malaria model mentioned above, immunohistochemistry of P. berghei infected mice revealed that CXCL9 was predominantly expressed by endothelial cells and CXCL10 was predominantly expressed by neurons perhaps explaining the non overlapping roles of these two CXCR3 ligands in the pathogenesis of cerebral malaria [26]. Likewise, in a murine model of granulomatous liver disease induced by Propionibacterium acnes, hepatic LN DCs produced CXCL10, while hepatic granuloma cells in the liver parenchyma produced CXCL9, perhaps explaining why neutralization of CXCL9 and CXCL10 gave different results [39].
As their original names suggest, IFNγ (TypẹỊIinterferon) mediates the induction of all three CXCR3 ligands [40–42]. However, the CXCR3 ligands are also differentially regulated by other stimuli, such as the Type̤IinterferonṣIFNα/β̤and NF-κB. CXCL10 induction is more sensitive to innate stimuli, such as the Type I interferons and NF-κB induction, leading to the preferential induction of CXCL10 by Toll like receptors that activate IRF3 and the release of Type I interferon, while CXCL9 is more dependent and more strongly induced by IFNγ. CXCL10 is also preferentially induced by hypoxia-reperfusion injury via NF-κB activation [43], and has been shown to play an early role in the hypoxia-induced inflammation associated with solid organ transplantation, such as the heart and lung [43, 44].
Possible role for CXCR3 on other T helper subsets
While the expression of CXCR3 is tightly linked to Th1 CD4+ and CD8+ effector cells, it has also been observed on populations of IL4-secreting Th2 cells [5, 14, 15] and IL17-secreting Th17 cells [38, 45] in inflamed tissues. Cxcr3- and Cxcl9-deficiency protect MRL/lpr mice from autoimmune lupus-like inflammation [37]. Recently it was shown that deficiency of Cxcr3 in this model is associated with a block in the infiltration of not only Th1 cells, but also IL17 secreting cells into the kidney [38]. It is likely that the high levels of IFNγ or IFNα/β present in the kidney result in production of CXCL9, CXCL10 and CXCL11. While this study did not investigate the presence of double positive IFNγ and IL17 expressing cells, one third of IL17 secreting cells also expressed CXCR3, suggesting these cells may express transcription factors for both Th1 and Th17 effector cells. Further studies of this kind will evaluate a role for CXCR3 in the recruitment of CD4+ helper subsets other than Th1, or effector populations that do not fit into the paradigm of CD4+ cell polarization, such as cells dually expressing IFNγ and IL17 [45].
CXCR3-dependent amplification of immune responses
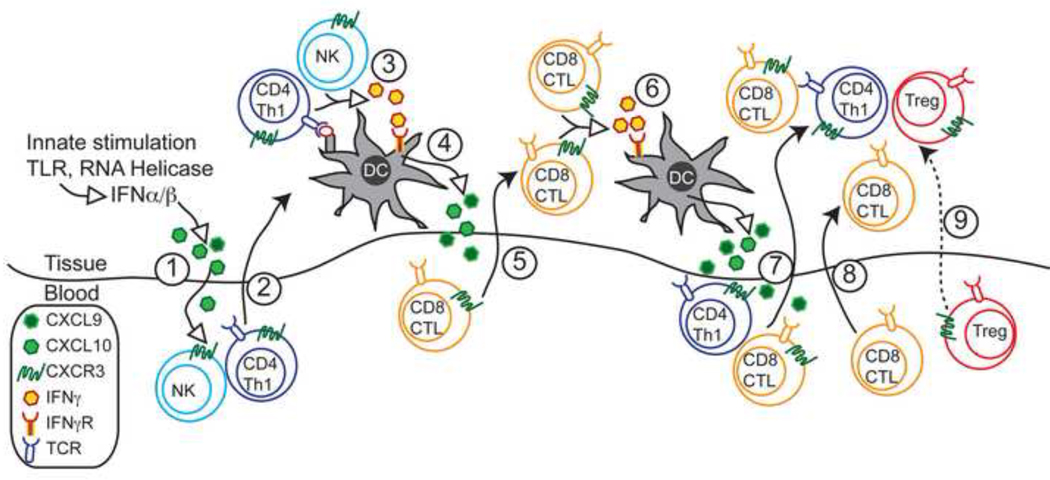
Multiple studies have demonstrated that recruitment of effector T cells and inflammation drives further recruitment and inflammation. This has been observed in the context of CXCR3-dependent inflammation whereby CXCR3-dependent T cell recruitment permits the entry of CXCR3 negative and Cxcr3−/− T cells into immune privileged sites [26, 38]. However, in addition to promoting the effector cell inflammation via CXCR3-independent means, an inflammatory CXCR3-chemokine dependent amplification loop also exits (Figure 1). For example, during malarial infection, the entry of CXCR3+CD8+ cells into the brain leads to increases in the levels of CXCL9 and CXCL10 [26]. Although not assessed in this work, presumably this is due to the local secretion of IFNγ by T cells in inflamed tissues. The increased secretion of CXCR3 ligands promotes additional recruitment of CXCR3+ effector cells. In turn, these effectors secrete IFNγ locally, which further amplifies infiltration of effector cells. This inflammatory loop allows CXCR3 and its ligands to coordinate T cell responses in the inflamed periphery, and suggests why expression of CXCR3 is tightly linked to autoimmunity. This CXCR3-dependant coordination of inflammation has also been elegantly demonstrated in the vaginal mucosa during HSV-2 infection [29]. In this model, CXCR3 permits the entry of CTL CD8+ cells into the infected tissues. This recruitment is dependent on the invasion of mucosa by CD4+ Th1 helper cells, which enter prior to CTLs. Critically, IFNγ specifically from the infiltrating CD4+ T cells is required in this process to increase CXCL9 and CXCL10 production by cells in the vaginal epithelium (Figure 1). IFNγ from CD4+ cells is likely due to the presentation of antigen by DCs in the infected tissue [46]. Presumably, IFNγ activates the transcription factor Stat in tissue resident cells to induce the production of CXCL9, CXCL10 and CXCL11 in inflammatory tissues [47]. The upregulation of CXCR3 ligands then mediates the mobilization of effector CTLs to the peripheral site of infection, as Cxcr3−/− CTLs failed to migrate into virally infected vaginal tissues [29]. This work is supported by previous results showing Cxcl9−/− and Cxcl10−/− are more susceptible to HSV-2 due to delayed recruitment of CTL CD8+ cells into the vaginal tissue [31]. NK cells recruitment into the mucosa was also reduced in Cxcr3 ligand−/− mice. How these cells contribute to the amplification of inflammation in this setting was not tested in this model. However, in a murine model of CMV infection, NK cells were an important source of early IFNγ, which induces the expression of CXCR3 ligands in the liver resulting on the recruitment of CXCR3+ T cells [48]. IFNγ from NK cells has also been shown drive CD4+ Th1 cell differentiation in the lymph node, which may also apply Th1 responses in inflamed tissues [49]. Interestingly, effector CD4+ T cell entry into the vaginal tissue also required IFNγ secretion and responsiveness to IFNγ by the CD4+ themselves. The role NK cells play in this mechanism, or dependence on CXCR3 for the entry of Th1 CD4+ cells into the inflamed vaginal tissue is likely, but was not assessed [29]. It remains to be investigated if CXCR3 is required for CTL entry into all virus-infected tissues (e.g., brain). The CXCR3-inflammatory loop potentially may not only increase recruitment of CTLs into peripheral tissues, but also may enhance the generation of CTLs [50] and promote increased effector responses through STAT1 signaling [51].
Figure 1. Model for Interferon-CXCR3 chemokine ligand-dependent inflammatory amplification in the periphery.
- Initial innate challenge, such as bacterial or viral infection, activates TLRs and RNA helicases leading to the release of IFNα/β and subsequent secretion of chemokines, predominantly CXCL10.
- CXCL10 recruits CD4+ Th1 cells and possibly NK cells into the target tissue.
- DCs in the tissue present antigen to CD4+ Th1 cells, which in turn secrete IFNγ.
- Tissue resident DCs and other cells take up IFNγ leading to secretion of CXCL9 and CXCL10.
- CXCL9 and CXCL10 recruit CD8+ CTLs into the tissue
- Migrated CD8+ CTLs secrete IFNγ, further stimulating tissue resident cells to produce more CXCL9 and CXCL10.
- Increased chemokine release amplifies inflammation, leading to further recruitment of CXCR3-expressing Th1 T cells and CTLs.
- Increased inflammation can also attract in CTLs in a CXCR3-independent manner.
- CXCR3-expressing CD4+ Treg cells may also enter the tissue, to counterbalance the CTL and Th1 cell response and lead to resolution of Th1 inflammation.
Although concept of an IFNγ-CXCR3-chemokine-dependent inflammatory loop is firmly established, interesting questions about the cellular sources of the ligands in different types of inflammation still remain unanswered. Whether the cells upregulating CXCR3 ligands are peripheral dendritic cell subsets and/or non-immune structural cells in the site of inflammation remains to be determined. In addition, it is not clear if the T cells involved in this amplification loop directly contact cells that upregulate CXCL9 and CXCL10, or if soluble chemokine gradients dispersed throughout the inflamed tissue recruit more effector cells.
Breaking the Inflammatory Loop – a role for CXCR3+ T regulatory cells
Although strong Th1 and CTL responses are beneficial during infection, these responses must be counterbalanced to prevent unwanted tissue destruction and immunopathology. Indeed, as detailed above, the identification of CXCR3+ Th1 cells was originally characterized in the affected tissues of patients with autoimmune arthritis and many autoimmune diseases are thought to result from deregulated Th1 responses. The induction of (Tregs) expressing the transcription factor FOXP3 is important for maintaining immune tolerance and preventing autoimmunity [52, 53]. The presence Tregs during diabetes induction inhibited expression of CXCR3 on effector CD4+ T cells, reducing pancreatic islet infiltration [54].
Tregs exert their suppressive activity on CD4+ Th1 or CTLs at the site of peripheral antigen presentation [46, 55]. Transfecting CD4+CD25+Foxp3+ cells with Cxcr3 increases their accumulation in target organs, indicating these cells can be recruited to sites of inflammation in a manner similar to the effector cells they suppress [56]. Recently, a subset of Th1-specific Tregs has been identified that express CXCR3 [57]. Presumably, although not yet formally demonstrated, it is likely the same cytokine (IFNγ)-chemokine (Cxcl9/10) inflammatory loop that amplifies effector responses, may also “invite” Tregs into peripheral tissues as Th1-specific Tregs are activated in a similar cytokine milieu as CD4 helper T cells. Although IL12 signaling does not appear necessary, IFNγ signaling through Ifnγr1 and Stat1 is required for the induction of CXCR3+ Tregs [57]. In this study, the source of IFNγ was not directly examined. It will be interesting to learn if Tregs are induced in these conditions, when effector cells are deficient in IFNγ, or if accessory cells, such as NK cells, are responsible for Treg induction. IFNγ derived from NKT cells have been shown to promote the migration of CXCR3+ Tregs into the inflamed liver [58]. IFNγ signaling induces the Th1 transcription factor T-bet, which in turn promotes the expression of CXCR3 expression, which may allow Th1-specific Tregs to gain to sites of inflammation, such as the lung, draining lymph node and spleen following aerosol infection with Mycobacterium tuberculosis [57].
T-bet-expressing Foxp3+ Tregs specifically suppress Th1 inflammation, and not Th2- or Th17-driven inflammation, which have separately been shown to be suppressed by IRF4-expressing and Stat3-expressing Tregs, respectively [59, 60]. These observations may explain the subtle phenotypes seen in Cxcr3 knockout mice in models that rely on multiple effector populations, such as Experimental Autoimmune Encephalomyelitis (EAE), the mouse model for Multiple Sclerosis, which is dependent on both Th1 and Th17 cells. In this model, Cxcr3−/− mice display no difference in incidence and severity of disease, but show reduced disease contraction accompanied by a reduction in the recruitment of Tregs into the spinal cord [61]. In addition, Cxcr3−/− Tregs that did enter the spinal cord, failed to cluster in CD3+Foxp3- T cell areas, as was seen for wildtype Tregs. These results indicate that CXCR3 expression is required for Treg entry into peripheral tissues, and also their function and interaction with effector T cells in these sites.
However, not all Th1 inflammation appears to induce CXCR3-expressing Tregs. Acute MHC-mismatch allograft rejection is characterized by infiltrating CXCR3+ effector T cells. Combined blocking of CXCR3 and CCR5 in this model, induces the recruitment of Treg cells, indicating unique chemokine requirements for these cells [62]. Similarly, in the ApoE−/− mouse model of atherogenesis, deficiency in Cxcl10 reduced the number of CD4+ T effector cells, but enhanced the accumulation of Tregs [63].
It is clear that CXCR3+ Tregs are induced and recruited by pro-inflammatory cytokines in order to circumvent the consequences of chronic inflammation. However, the presence of Tregs in sites of persistent inflammation argues that these suppressor cells may not be fully functional [64–66]. Interestingly, Tregs found in synovial fluid from patients with rheumatoid arthritis (RA) showed increased suppressive activity [65]. It is likely that these Tregs are either out numbered or their suppressive ability diminished by other cells, such that these Tregs are unable to counteract the over-exuberant effector T cells that drive persistent inflammation [65, 66].
While much work has been done on CD4+ Tregs, much less is known about CD8+ regulatory T cells. Recently, CXCR3 was shown to be a marker of CD8+ IL10 producing cells with suppressive activity in both mice and humans [67]. Further work is required to determine how these cells respond during in vivo immune responses, as their cell markers overlap with the CD8+ central memory population, and thus it is not clear if these cells will remain suppressive or if they will enter the effector pool.
CXCR3 in the induction and recall of immune responses
It is well established that CXCR3 expression by effector and regulatory T cells allows these cells entry into inflamed peripheral tissues. By contrast, the requirements for CXCR3 and its ligands during primary and secondary responses in the LN remain incompletely studied. Indeed, the use of in vitro activated effector cell transfer models in order to specifically investigate events in peripheral tissue has avoided scrutiny of the potential importance of CXCR3 during CD4+ and CD8+ T cell activation. The immediate up regulation of CXCR3 on CD4+ and CD8+ following DC activation, makes this an interesting proposal, as activated T cells remain sequestered in draining LN for up to 4 days prior to being recruited to peripheral tissues. Interestingly, T cell upregulation of CXCR3 coincides with the expression of Cxcl10 and Cxcl9 in the lymphoid organs at times relevant to T cell polarization and restimulation [31, 39]. Although not directly focused on, multiple studies have hinted an important role to be likely.
Potential role for CXCR3 during T cell priming
The original paper describing the Cxcl10−/− mouse showed decreased T cell activation, proliferation and IFNγ secretion following Th1-skewed in vivo immunization and mouse hepatitis virus (MHV) infection [68]. However, these outcomes were not correlated with differences in T cell-DC interactions in the LN. In vitro CXCL10 increases tethering of T cells to antigen presenting cells and synergy has been shown between CXCR3 and CD3ε signaling, due to spatial association of these receptors and subsequent tyrosine phosphorylation of the same signaling molecules [69–71]. DEC-205+ DCs appear to be a primary source of CXCL10 in the draining LN. These DCs rapidly up regulate Cxcl10 following Propionibacterium acnes infection [39]. CXCL10-expressing DCs actively interact with T cell clusters, indicating a role for CXCL10 in the DC-CD4+ interactions in draining LNs. This hypothesis is further supported, as blocking CXCL10 resulted in a decrease in clusters between proliferating CD4+ T cells and DEC-205+ DCs [39]. Although the effect of this blocking antibody treatment was assessed in the periphery, the impact of treatment on LN T cell activation and cytokine secretion, and Th1 polarization was not addressed. In contrast, it was recently demonstrated in a model of allograft rejection that CXCL9 produced by allograft DCs promotes priming towards CTL CD8+ and Th1 CD4+ IFNγ-producing T cells [72]. Surprisingly, CXCL10 appeared to have the opposite effect on T cell priming, where a deficiency of Cxcl10 in the allograft resulted in increased IFNγ-producing CD8+ T cells [72]. Cxcr3−/− T cells exhibited impaired CD8+ cytotoxicity, and reduced expression of T-bet, IFNγ, perforin and granzyme B following HSV-2 infection [30]. While these phenotypes were seen in the LN, this study correlated these findings with reduced plasmacytoid DC recruitment and activation, and did not investigate the relevance of CXCR3 loss exclusively on T cells. Future studies are needed to determine a role for CXCR3 and it’s ligands during initial T cell activation.
Potential role for CXCR3 during induction of T cell memory
In addition to a potential role during primary responses, CXCR3 expression by T cells in LNs may be important for the induction of T cell memory. CXCR3+ cells make up between 60–90% of CD8+ memory T cells [73, 74] and 40% of CD4+ memory T cells [75, 76]. CXCR3 expression has been proposed as a reliable marker for T memory responses, as viral recall responses for both CD8+ and CD4+ T cells are largely restricted to CXCR3+ cells [74, 76]. The expression of CXCR3 indicates the ability for recall responses, although the requirement for CXCR3 interactions in this process has not formally been tested using Cxcr3−/− T cells. The CXCR3 ligands expressed at times of antigen recall, either expressed by LN stroma or antigen presenting cells have also yet to be investigated.
Once effector T cells have left the LN for peripheral tissues, T effector memory cells (TEM) can return to reactive LNs to either halt or promote future immune responses. CXCL9 is displayed on LN high endothelial venules (HEV) rapidly and transiently following induction of a reactive LN. This window allows CD8+ T effector memory (TEM) cells to gain access to the LN to interact with antigen presenting DCs [73]. CXCR3+ CD8+ memory cells engage and kill DCs as an efficient mechanism to regulate and reduce future T cell responses. In contrast, CD4+ TEM cells gain access to reactive LNs via CXCR3-independent means to interact with DCs and increase future T cell priming through CD40 upregulation [49]. Once TEM cells enter the LN, it is unknown if CD8+ or CD4+ interactions with DCs are also dependent on CXCR3 and its ligands, although it has been shown that CD40L stimulated DCs increase expression of CXCL10 [77]. Future studies into the role of CXCR3-dependent interactions in the development of primary, secondary and contraction of adaptive immune responses will therefore yield interesting results with applications for vaccinology.
Concluding comments and future directions
In the past few years much has been learned of the involvement of the CXCR3 chemokine system during inflammation. These studies have highlighted the role for CXCR3-dependent interactions in the coordination of inflammatory events in the periphery, both to increase recruitment of CD4+ and CD8+ effector T lymphocytes to drive inflammation, but also perhaps for the induction and recruitment of Th1-specific T regulatory cells to dampen over exuberant responses. How CXCR3 balances the choice between these critical responses remains to be determined. In addition, the factors influencing the described CXCR3-dependent amplification loop between IFNγ-expressing T cells and the cells expressing the IFNγ inducible ligands needs to be further characterized. Finally, although not studied in great detail yet, it is now becoming increasingly clear that the CXCR3 chemokine system plays important roles in the migratory behavior and cellular interactions of T cells in the lymphoid compartment and within peripheral tissue that likely have consequences for the generation of effector, regulatory and memory T cells. Future studies aimed at studying the role of the CXCR3 and its ligands in these processes in detail will undoubtedly shed new light on this important chemokine system in the control of T cell function.
Acknowledgements
JRG was supported by Australian Government National Health and Medical Research Counsel and Overseas Biomedical Based Fellowship and ADL was supported by NIH grant CA69212.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 2.Campanella GS, Grimm J, Manice LA, Colvin RA, Medoff BD, Wojtkiewicz GR, Weissleder R, Luster AD. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177:6991–6998. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- 3.Campanella GS, Medoff BD, Manice LA, Colvin RA, Luster AD. Development of a novel chemokine-mediated in vivo T cell recruitment assay. J Immunol Methods. 2008;331:127–139. doi: 10.1016/j.jim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Lu B, Humbles A, Bota D, Gerard C, Moser B, Soler D, Luster AD, Gerard NP. Structure and function of the murine chemokine receptor CXCR3. Eur J Immunol. 1999;29:3804–3812. doi: 10.1002/(SICI)1521-4141(199911)29:11<3804::AID-IMMU3804>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas SY, Hou R, Boyson JE, Means TK, Hess C, Olson DP, Strominger JL, Brenner MB, Gumperz JE, Wilson SB, Luster AD. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 9.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 10.Nanki T, Takada K, Komano Y, Morio T, Kanegane H, Nakajima A, Lipsky PE, Miyasaka N. Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2009;11:R149. doi: 10.1186/ar2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 12.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–6243. [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, Rosa R, Di Salvo J, Mudgett J, Peterson LB, Wicker LS, DeMartino JA. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73:771–780. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, Miyawaki T. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J Leukoc Biol. 2000;68:568–574. [PubMed] [Google Scholar]
- 17.Langenkamp A, Nagata K, Murphy K, Wu L, Lanzavecchia A, Sallusto F. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003;33:474–482. doi: 10.1002/immu.200310023. [DOI] [PubMed] [Google Scholar]
- 18.Khan IA, MacLean JA, Lee FS, Casciotti L, DeHaan E, Schwartzman JD, Luster AD. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 19.Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem. 2006;281:11992–12000. doi: 10.1074/jbc.M513613200. [DOI] [PubMed] [Google Scholar]
- 20.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 21.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juedes AE, Rodrigo E, Togher L, Glimcher LH, von Herrath MG. T-bet controls autoaggressive CD8 lymphocyte responses in type 1 diabetes. J Exp Med. 2004;199:1153–1162. doi: 10.1084/jem.20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taqueti VR, Grabie N, Colvin R, Pang H, Jarolim P, Luster AD, Glimcher LH, Lichtman AH. T-bet controls pathogenicity of CTLs in the heart by separable effects on migration and effector activity. J Immunol. 2006;177:5890–5901. doi: 10.4049/jimmunol.177.9.5890. [DOI] [PubMed] [Google Scholar]
- 26.Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A. 2008;105:4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miu J, Mitchell AJ, Muller M, Carter SL, Manders PM, McQuillan JA, Saunders BM, Ball HJ, Lu B, Campbell IL, Hunt NH. Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol. 2008;180:1217–1230. doi: 10.4049/jimmunol.180.2.1217. [DOI] [PubMed] [Google Scholar]
- 28.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virusinfected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapa M, Carr DJ. CXCR3 deficiency increases susceptibility to genital herpes simplex virus type 2 infection: Uncoupling of CD8+ T-cell effector function but not migration. J Virol. 2009;83:9486–9501. doi: 10.1128/JVI.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thapa M, Welner RS, Pelayo R, Carr DJ. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. 2008;180:1098–1106. doi: 10.4049/jimmunol.180.2.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW., Jr CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(−/−) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res. 2008;28:31–43. doi: 10.1089/jir.2007.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kristensen NN, Gad M, Thomsen AR, Lu B, Gerard C, Claesson MH. CXC chemokine receptor 3 expression increases the disease-inducing potential of CD4+ CD25- T cells in adoptive transfer colitis. Inflamm Bowel Dis. 2006;12:374–381. doi: 10.1097/01.MIB.0000217337.15442.e1. [DOI] [PubMed] [Google Scholar]
- 34.Mohan K, Issekutz TB. Blockade of chemokine receptor CXCR3 inhibits T cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol. 2007;179:8463–8469. doi: 10.4049/jimmunol.179.12.8463. [DOI] [PubMed] [Google Scholar]
- 35.Salomon I, Netzer N, Wildbaum G, Schif-Zuck S, Maor G, Karin N. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J Immunol. 2002;169:2685–2693. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- 36.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 37.Menke J, Zeller GC, Kikawada E, Means TK, Huang XR, Lan HY, Lu B, Farber J, Luster AD, Kelley VR. CXCL9, but not CXCL10, promotes CXCR3-dependent immune-mediated kidney disease. J Am Soc Nephrol. 2008;19:1177–1189. doi: 10.1681/ASN.2007111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, Velden J, Hopfer H, Fehr S, Krieger T, Meyer-Schwesinger C, Meyer TN, Helmchen U, Mittrucker HW, Stahl RA, Panzer U. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183:4693–4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, Ichida T, Asakura H, Matsushima K. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J Exp Med. 2002;195:1257–1266. doi: 10.1084/jem.20011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 41.Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole KE, A SC, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, Farber JM, Luster AD. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol. 2006;176:7087–7095. doi: 10.4049/jimmunol.176.11.7087. [DOI] [PubMed] [Google Scholar]
- 44.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 46.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikhak Z, Fleming CM, Medoff BD, Thomas SY, Tager AM, Campanella GS, Luster AD. STAT1 in peripheral tissue differentially regulates homing of antigen-specific Th1 and Th2 cells. J Immunol. 2006;176:4959–4967. doi: 10.4049/jimmunol.176.8.4959. [DOI] [PubMed] [Google Scholar]
- 48.Hansen DS, Bernard NJ, Nie CQ, Schofield L. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol. 2007;178:5779–5788. doi: 10.4049/jimmunol.178.9.5779. [DOI] [PubMed] [Google Scholar]
- 49.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 50.Yoon SH, Yun SO, Park JY, Won HY, Kim EK, Sohn HJ, Cho HI, Kim TG. Selective addition of CXCR3(+) CCR4(−) CD4(+) Th1 cells enhances generation of cytotoxic T cells by dendritic cells in vitro. Exp Mol Med. 2009;41:161–170. doi: 10.3858/emm.2009.41.3.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, Woodland DL. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009;183:4378–4384. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 53.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 54.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 55.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasegawa H, Inoue A, Kohno M, Lei J, Miyazaki T, Yoshie O, Nose M, Yasukawa M. Therapeutic effect of CXCR3-expressing regulatory T cells on liver, lung and intestinal damages in a murine acute GVHD model. Gene Ther. 2008;15:171–182. doi: 10.1038/sj.gt.3303051. [DOI] [PubMed] [Google Scholar]
- 57.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49:1267–1276. doi: 10.1002/hep.22761. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, Campbell IL. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- 62.Schnickel GT, Bastani S, Hsieh GR, Shefizadeh A, Bhatia R, Fishbein MC, Belperio J, Ardehali A. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180:4714–4721. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 63.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 64.Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, Shetty S, Harki J, Shaw JC, Eksteen B, Hubscher SG, Walker LS, Adams DH. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 65.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 66.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–742. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 67.Shi Z, Okuno Y, Rifa'i M, Endharti AT, Akane K, Isobe K, Suzuki H. Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ Treg. Eur J Immunol. 2009;39:2106–2119. doi: 10.1002/eji.200939314. [DOI] [PubMed] [Google Scholar]
- 68.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma- inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 69.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 70.Dar WA, Knechtle SJ. CXCR3-mediated T-cell chemotaxis involves ZAP-70 and is regulated by signalling through the T-cell receptor. Immunology. 2007;120:467–485. doi: 10.1111/j.1365-2567.2006.02534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton P, O'Boyle G, Jenkins Y, Ali S, Kirby JA. T cell extravasation: demonstration of synergy between activation of CXCR3 and the T cell receptor. Mol Immunol. 2009;47:485–492. doi: 10.1016/j.molimm.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenblum JM, Shimoda N, Schenk AD, Zhang H, Kish DD, Keslar K, Farber JM, Fairchild RL. CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules during the priming of alloreactive T cell effectors. J Immunol. 2010;184:3450–3460. doi: 10.4049/jimmunol.0903831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743–752. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 74.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim CH, Nagata K, Butcher EC. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J Immunol. 2003;171:152–158. doi: 10.4049/jimmunol.171.1.152. [DOI] [PubMed] [Google Scholar]
- 76.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Y, Dai J, Lu P, Huang Y, Zhu Y, Zhang X. Distinct effect of CD40 and TNF- signaling on the chemokine/chemokine receptor expression and function of the human monocyte-derived dendritic cells. Cell Mol Immunol. 2008;5:121–131. doi: 10.1038/cmi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gottlieb AB, Luster AD, Posnett DN, Carter DM. Detection of a gamma interferon- induced protein IP-10 in psoriatic plaques. Journal of Experimental Medicine. 1988;168:941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 80.Goebeler M, Toksoy A, Spandau U, Engelhardt E, Brocker EB, Gillitzer R. The C-X-C chemokine Mig is highly expressed in the papillae of psoriatic lesions. J Pathol. 1998;184:89–95. doi: 10.1002/(SICI)1096-9896(199801)184:1<89::AID-PATH975>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 81.Agostini C, Cassatella M, Sancetta R, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Newman W, Luster AD, Semenzato G. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J. Immunol. 1998;161:6413–6420. [PubMed] [Google Scholar]
- 82.Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, Sone S. CXCL9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clin Exp Immunol. 2007;149:317–326. doi: 10.1111/j.1365-2249.2007.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, Huang C, Zisman DA, Fishbein M, Lynch JP, 3rd, Strieter RM, Elashoff RM, Belperio JA. CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis. Eur Respir J. 2009;34:676–686. doi: 10.1183/09031936.00157508. [DOI] [PubMed] [Google Scholar]
- 84.Tsubaki T, Takegawa S, Hanamoto H, Arita N, Kamogawa J, Yamamoto H, Takubo N, Nakata S, Yamada K, Yamamoto S, Yoshie O, Nose M. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin Exp Immunol. 2005;141:363–371. doi: 10.1111/j.1365-2249.2005.02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martini G, Zulian F, Calabrese F, Bortoli M, Facco M, Cabrelle A, Valente M, Zacchello F, Agostini C. CXCR3/CXCL10 expression in the synovium of children with juvenile idiopathic arthritis. Arthritis Res Ther. 2005;7:R241–R249. doi: 10.1186/ar1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, Dubinett SM, Strieter RM. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol. 2006;176:1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 87.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol. 2007;179:1901–1912. doi: 10.4049/jimmunol.179.3.1901. [DOI] [PubMed] [Google Scholar]
- 88.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, Andrew DP, Wu L, Butcher EC, Wardlaw AJ. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 89.Bochner BS, Hudson SA, Xiao HQ, Liu MC. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol. 2003;112:930–934. doi: 10.1016/j.jaci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, Dufour JH, Luster AD. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002;168:5278–5286. doi: 10.4049/jimmunol.168.10.5278. [DOI] [PubMed] [Google Scholar]
- 91.Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y, Nakajima Y, Kimura H. A small- molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31:783–789. doi: 10.1183/09031936.00111507. [DOI] [PubMed] [Google Scholar]
- 92.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. The interferon-gamma inducible CXC chemokines IP-10, Mig, and I-TAC are differentially expressed by human atheroma-associated cells: Implications for lymphocyte recruitment in atherogenesis. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, Charo IF, Mach F. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 94.Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, Lassmann H, Olsen DB, Strieter RM, Ransohoff RM, Sellebjerg F. Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 95.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Balashov K, Rottman J, Weiner H, Hancock W. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1 and IP-10 are expressed in demyelinating brain lesions. PNAS. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sporici R, Issekutz TB. CXCR3 blockade inhibits T-cell migration into the CNS during EAE and prevents development of adoptively transferred, but not actively induced, disease. Eur J Immunol. 2010;40:2751–2761. doi: 10.1002/eji.200939975. [DOI] [PubMed] [Google Scholar]
- 98.Wildbaum G, Netzer N, Karin N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2002;168:5885–5892. doi: 10.4049/jimmunol.168.11.5885. [DOI] [PubMed] [Google Scholar]
- 99.Kohler RE, Comerford I, Townley S, Haylock-Jacobs S, Clark-Lewis I, McColl SR. Antagonism of the chemokine receptors CXCR3 and CXCR4 reduces the pathology of experimental autoimmune encephalomyelitis. Brain Pathol. 2008;18:504–516. doi: 10.1111/j.1750-3639.2008.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu L, Huang D, Matsui M, He TT, Hu T, Demartino J, Lu B, Gerard C, Ransohoff RM. Severe disease, unaltered leukocyte migration, and reduced IFN-gamma production in CXCR3−/− mice with experimental autoimmune encephalomyelitis. J Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 101.Klein RS, Izikson L, Means T, Gibson HD, Lin E, Sobel RA, Weiner HL, Luster AD. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J Immunol. 2004;172:550–559. doi: 10.4049/jimmunol.172.1.550. [DOI] [PubMed] [Google Scholar]
- 102.Schroepf S, Kappler R, Brand S, Prell C, Lohse P, Glas J, Hoster E, Helmbrecht J, Ballauff A, Berger M, von Schweinitz D, Koletzko S, Lacher M. Strong overexpression of CXCR3 axis components in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1882–1890. doi: 10.1002/ibd.21312. [DOI] [PubMed] [Google Scholar]
- 103.Hyun JG, Lee G, Brown JB, Grimm GR, Tang Y, Mittal N, Dirisina R, Zhang Z, Fryer JP, Weinstock JV, Luster AD, Barrett TA. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11:799–805. doi: 10.1097/01.mib.0000178263.34099.89. [DOI] [PubMed] [Google Scholar]
- 104.Pignatti P, Brunetti G, Moretto D, Yacoub MR, Fiori M, Balbi B, Balestrino A, Cervio G, Nava S, Moscato G. Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis. Am J Respir Crit Care Med. 2006;173:310–317. doi: 10.1164/rccm.200502-244OC. [DOI] [PubMed] [Google Scholar]
- 105.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31:395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 106.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, Li Y, Tager AM, Goetinck PF, Luster AD, Noble PW. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uno S, Imagawa A, Saisho K, Okita K, Iwahashi H, Hanafusa T, Shimomura I. Expression of chemokines, CXC chemokine ligand 10 (CXCL10) and CXCR3 in the inflamed islets of patients with recent-onset autoimmune type 1 diabetes. Endocr J. 2010 doi: 10.1507/endocrj.k10e-076. [DOI] [PubMed] [Google Scholar]
- 109.Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159:338–343. doi: 10.1111/j.1365-2249.2009.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol. 2003;171:6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 111.Enghard P, Humrich JY, Rudolph B, Rosenberger S, Biesen R, Kuhn A, Manz R, Hiepe F, Radbruch A, Burmester GR, Riemekasten G. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 2009;60:199–206. doi: 10.1002/art.24136. [DOI] [PubMed] [Google Scholar]
- 112.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, Panoskaltsis- Mortari A, Gregersen PK, Behrens TW, Baechler EC. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong KO, Tan AW, Thong BY, Lian TY, Cheng YK, Teh CL, Koh ET, Chng HH, Law WG, Lau TC, Leong KP, Leung BP, Howe HS. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin Exp Immunol. 2009;156:134–140. doi: 10.1111/j.1365-2249.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, Sinigaglia F, Fabbri LM. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 115.Brozyna S, Ahern J, Hodge G, Nairn J, Holmes M, Reynolds PN, Hodge S. Chemotactic mediators of Th1 T-cell trafficking in smokers and COPD patients. COPD. 2009;6:4–16. doi: 10.1080/15412550902724164. [DOI] [PubMed] [Google Scholar]
- 116.Nie L, Xiang R, Zhou W, Lu B, Cheng D, Gao J. Attenuation of acute lung inflammation induced by cigarette smoke in CXCR3 knockout mice. Respir Res. 2008;9:82. doi: 10.1186/1465-9921-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nie L, Xiang RL, Liu Y, Zhou WX, Jiang L, Lu B, Pang BS, Cheng DY, Gao JM. Acute pulmonary inflammation is inhibited in CXCR3 knockout mice after short-term cigarette smoke exposure. Acta Pharmacol Sin. 2008;29:1432–1439. doi: 10.1111/j.1745-7254.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 118.Yuan J, Liu Z, Lim T, Zhang H, He J, Walker E, Shier C, Wang Y, Su Y, Sall A, McManus B, Yang D. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ Res. 2009;104:628–638. doi: 10.1161/CIRCRESAHA.108.192179. [DOI] [PubMed] [Google Scholar]
- 119.Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, Hancock WW, Briscoe D, M Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 120.Fahmy NM, Yamani MH, Starling RC, Ratliff NB, Young JB, McCarthy PM, Feng J, Novick AC, Fairchild RL. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75:72–78. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 121.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rosenblum JM, Zhang QW, Siu G, Collins TL, Sullivan T, Dairaghi DJ, Medina JC, Fairchild RL. CXCR3 antagonism impairs the development of donor-reactive, IFN-gamma-producing effectors and prolongs allograft survival. Transplantation. 2009;87:360–369. doi: 10.1097/TP.0b013e31819574e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Uppaluri R, Sheehan KC, Wang L, Bui JD, Brotman JJ, Lu B, Gerard C, Hancock WW, Schreiber RD. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86:137–147. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zerwes HG, Li J, Kovarik J, Streiff M, Hofmann M, Roth L, Luyten M, Pally C, Loewe RP, Wieczorek G, Banteli R, Thoma G, Luckow B. The chemokine receptor Cxcr3 is not essential for acute cardiac allograft rejection in mice and rats. Am J Transplant. 2008;8:1604–1613. doi: 10.1111/j.1600-6143.2008.02309.x. [DOI] [PubMed] [Google Scholar]
- 125.Kwun J, Hazinedaroglu SM, Schadde E, Kayaoglu HA, Fechner J, Hu HZ, Roenneburg D, Torrealba J, Shiao L, Hong X, Peng R, Szewczyk JW, Sullivan KA, DeMartino J, Knechtle SJ. Unaltered graft survival and intragraft lymphocytes infiltration in the cardiac allograft of Cxcr3−/− mouse recipients. Am J Transplant. 2008;8:1593–1603. doi: 10.1111/j.1600-6143.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 126.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. CXCR3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 128.Piper KP, Horlock C, Curnow SJ, Arrazi J, Nicholls S, Mahendra P, Craddock C, Moss PA. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 129.Mapara MY, Leng C, Kim YM, Bronson R, Lokshin A, Luster A, Sykes M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12:623–634. doi: 10.1016/j.bbmt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Z, Kaptanoglu L, Tang Y, Ivancic D, Rao SM, Luster A, Barrett TA, Fryer J. IP-10-induced recruitment of CXCR3 host T cells is required for small bowel allograft rejection. Gastroenterology. 2004;126:809–818. doi: 10.1053/j.gastro.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 131.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a gamma interferon- induced protein (IP-10) in delayed immune responses in human skin. Journal of Experimental Medicine. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T Cell-Specific CXC Chemokines IP-10, Mig, and I-TAC Are Expressed by Activated Human Bronchial Epithelial Cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- 133.Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One. 2008;3:e3901. doi: 10.1371/journal.pone.0003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fadel SA, Bromley SK, Medoff BD, Luster AD. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur J Immunol. 2008;38:3376–3387. doi: 10.1002/eji.200838628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 136.Norose K, Kikumura A, Luster AD, Hunter CA, Harris TH. CXCL10 is required to maintain T cell populations and control parasite replication during chronic ocular toxoplasmosis. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, Adjei AA, Gyasi RK, Tettey Y, Wiredu EK, Tongren JE, Udhayakumar V, Stilesv JK. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Becerra A, Warke RV, Martin K, Xhaja K, de Bosch N, Rothman AL, Bosch I. Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo. J Med Virol. 2009;81:1403–1411. doi: 10.1002/jmv.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu-Hsieh BA, Gerard C, Luster A, Liao F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol. 2006;177:1855–1863. doi: 10.4049/jimmunol.177.3.1855. [DOI] [PubMed] [Google Scholar]
- 140.Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Apolinario A, Majano PL, Alvarez-Perez E, Saez A, Lozano C, Vargas J, Garcia- Monzon C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861–2870. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- 142.Askarieh G, Alsio A, Pugnale P, Negro F, Ferrari C, Neumann AU, Pawlotsky JM, Schalm SW, Zeuzem S, Norkrans G, Westin J, Soderholm J, Hellstrand K, Lagging M. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010;51:1523–1530. doi: 10.1002/hep.23509. [DOI] [PubMed] [Google Scholar]
- 143.Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, Lane TE. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 144.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carr DJ, Chodosh J, Ash J, Lane TE. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brainard DM, Tager AM, Misdraji J, Frahm N, Lichterfeld M, Draenert R, Brander C, Walker BD, Luster AD. Decreased CXCR3+ CD8 T cells in advanced human immunodeficiency virus infection suggest that a homing defect contributes to cytotoxic T- lymphocyte dysfunction. J Virol. 2007;81:8439–8450. doi: 10.1128/JVI.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Foley JF, Yu CR, Solow R, Yacobucci M, Peden KW, Farber JM. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte- derived macrophages, dendritic cells, and lymph nodes. J Immunol. 2005;174:4892–4900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 148.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 149.Hailu A, van der Poll T, Berhe N, Kager PA. Elevated plasma levels of interferon (IFN)-gamma, IFN-gamma inducing cytokines, and IFN-gamma inducible CXC chemokines in visceral leishmaniasis. Am J Trop Med Hyg. 2004;71:561–567. [PubMed] [Google Scholar]
- 150.Rosas LE, Barbi J, Lu B, Fujiwara Y, Gerard C, Sanders VM, Satoskar AR. CXCR3−/− mice mount an efficient Th1 response but fail to control Leishmania major infection. Eur J Immunol. 2005;35:515–523. doi: 10.1002/eji.200425422. [DOI] [PubMed] [Google Scholar]
- 151.Gupta G, Bhattacharjee S, Bhattacharyya S, Bhattacharya P, Adhikari A, Mukherjee A, Bhattacharyya Majumdar S, Majumdar S. CXC chemokine-mediated protection against visceral leishmaniasis: involvement of the proinflammatory response. J Infect Dis. 2009;200:1300–1310. doi: 10.1086/605895. [DOI] [PubMed] [Google Scholar]
- 152.Olive AJ, Gondek DC, Starnbach MN. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2010 doi: 10.1038/mi.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Belay T, Eko FO, Ananaba GA, Bowers S, Moore T, Lyn D, Igietseme JU. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect Immun. 2002;70:844–850. doi: 10.1128/IAI.70.2.844-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mullegger RR, Means TK, Shin JJ, Lee M, Jones KL, Glickstein LJ, Luster AD, Steere AC. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75:4621–4628. doi: 10.1128/IAI.00263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 156.Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zeng X, Moore TA, Newstead MW, Deng JC, Kunkel SL, Luster AD, Standiford TJ. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect Immun. 2005;73:8226–8236. doi: 10.1128/IAI.73.12.8226-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reckamp KL, Figlin RA, Moldawer N, Pantuck AJ, Belldegrun AS, Burdick MD, Strieter RM. Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL-2 therapy. J Immunother. 2007;30:417–424. doi: 10.1097/CJI.0b013e31802e089a. [DOI] [PubMed] [Google Scholar]
- 159.Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, Witz IP, Ben-Baruch A. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]
- 160.Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, Melero I, Prieto J. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol. 2000;164:3112–3122. doi: 10.4049/jimmunol.164.6.3112. [DOI] [PubMed] [Google Scholar]
- 161.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–632. [PubMed] [Google Scholar]
- 163.Jones D, O'Hara C, Kraus MD, Perez-Atayde AR, Shahsafaei A, Wu L, Dorfman DM. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood. 2000;96:685–690. [PubMed] [Google Scholar]
- 164.Trentin L, Agostini C, Facco M, Piazza F, Perin A, Siviero M, Gurrieri C, Galvan S, Adami F, Zambello R, Semenzato G. The chemokine receptor CXCR3 is expressed on malignant B cells and mediates chemotaxis. J Clin Invest. 1999;104:115–121. doi: 10.1172/JCI7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, Vanguri P, Burd PR, Sheiki N, Gupta G, Teruya-Feldstein J, Tosato G. Interferon- inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc. Natl. Acad. Sci. USA. 1996;93:13791–13796. doi: 10.1073/pnas.93.24.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol Cancer Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 167.Luster AD, Leder P. IP-10, A -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. Journal of Experimental Medicine. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]