Abstract
In men, the penile urethra is a primary infection site for sexually transmitted pathogens. Research on the immunology of this mucosal site has been limited in part due to sampling challenges, but available evidence indicates that the urethra contains a rich contingent of immunological mediators that can mount vigorous innate and adaptive immune responses against infectious organisms. Further research is needed to define approaches to stimulate immunity at this mucosal site to prevent the transmission of HIV-1 and other sexually transmitted pathogens.
Keywords: Penis, urethra, sexually transmitted infection, mucosal immunity, innate immunity, vaccine
Introduction
Although sexually transmitted pathogens infect both male and female genital tissues, much more is known about the pathogenesis of infections and immune defense of the female genital tract. This is due in part to the relative accessibility and abundance of female genital secretions and tissues for research. In women the cervical transformation zone, where the stratified squamous vaginal epithelium transitions into the glandular columnar epithelium of the endocervix, is a major site of infection and immune defense. In men the comparable site is the opening of the penile urethra, where the relatively impermeable stratified squamous epithelium of the penile skin and fossa navicularis transitions into the glandular columnar epithelium of the penile urethra. Sampling urethral secretions by swabs or lavage can be painful, and urethral tissues are not normally removed during routine surgical procedures. Most of the descriptive studies on immune cells in the human penile urethra have been conducted on autopsy tissue. Recently, researchers have begun to use alternative methods for sampling urethral secretions for immunological studies, including collection of preejaculatory fluid and first catch urine, and use of epithelial cell lines to characterize innate and adaptive immune defense mechanisms in the penile urethra. In this report we review published research findings and unpublished data from our laboratory providing further evidence that the human penile urethra is an immunologically dynamic site.
Anatomy of the human penile urethra
During embryonic development the urethra develops from the primordial urethral plate. Urethral folds are formed which enclose the urethral sinus. This urethral sinus then fuses ventrally in a basal to distal manner and lengthens to produce the penile urethra. Anatomically, the urethra is a direct duct from the bladder, approximately 20cm in length. It provides an exit for urine as well as semen, and is comprised of four distinct segments. Starting at the bladder, the first urethral segment is called the preprostatic urethra and is approximately 1 cm long. The second segment, called the prostatic segment, penetrates the prostate gland and is joined by the ejaculatory ducts that carry sperm from the testis and epididymis, as well as numerous ducts from the prostate and the prostatic utricle. These openings into the prostatic urethra are collectively called the verumontanum. The next segment, the short membranous urethra (1–2 cm in length), passes through the skeletal muscle of the urogenital diaphragm. The final segment, the penile or spongy urethra, runs the entire length of the penis and exits through the fossa navicularis, a distinct region located just inside the urethral orifice, or meatus (Fig. 1a).
Figure 1.
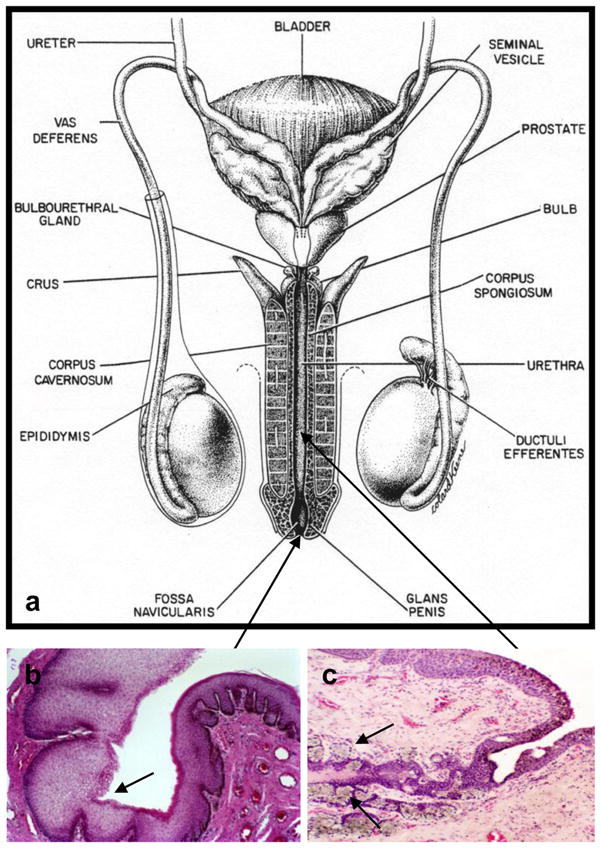
Fig. 1. a) Diagram of human male genital tract (From: Gray H. Anatomy of the Human Body. Philadelphia: Lea and Febiger, 1918), b) Transition from the fossa navicularis on the right (stratified squamous epithelium) to the penile urethra (pseudostratified columnar epithelium) on the left, c) glands of Littre (arrows)
Histologically, these different regions of the urethral mucosa comprise distinct epithelial types, each with its own immunological characteristics and microenvironment. At the urethral orifice, which is approximately 0.6–1.2 cm in diameter, the keratinized stratified squamous epithelium of the penile skin transforms abruptly into a non-keratinizing stratified squamous epithelium that lines the fossa navicularis; this mucosa then transitions as it enters the shaft of the penis into the pseudostratified columnar epithelium that lines the length of the penile urethra (Fig. 1b). This epithelium develops many, often deep, invaginations that form the periurethral glands of Littre (Fig. 1c). A specialized viscous mucus secreted from the glands of Littre during sexual excitement, called preejaculate, is thought to neutralize acidity of residual urine present in the lumen of the urethra and provide a lubricant during sexual intercourse. These glands also produce a number of immunologically relevant molecules that play an important role in innate and acquired immunity.
Innate immunity
Mucus
Mucosal epithelial surfaces are coated with a mucus layer that plays an important role in first line immune defense by trapping and eliminating microbes before they reach the epithelial surface. Mucus obtains its structural characteristics from mucins, a family of large hydrophilic glycoproteins that contain tandem repeats of serine- and threonine-rich domains that are sites of extensive O-glycan attachment. To date, at least 18 mucin genes have been cloned, and based on sequencing data, two classes of mucins have been identified: membrane-associated mucins (MUC1, MUC3A, MUC3B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, and MUC20), and secreted mucins, which include large gel-forming mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC19) and small soluble mucins (MUC7 and MUC9) (Dekker et al., 2002).
Membrane-associated mucins are expressed at the apical surface of epithelial cells throughout the male and female genital tracts (Gipson et al., 1997, Russo et al., 2006). These mucins have hydrophobic domains near the carboxy terminus of the protein that allow them to span the cell membrane. The extracellular domain of membrane-associated mucins may extend up to 500 nm from the epithelial cell surface and form a dense glycocalyx along the apical surface of the epithelia. Negatively charged carbohydrate residues on the mucin protein backbone confer disadhesive properties. The extracellular domain is shed from apical surfaces and thus these mucins may contribute to protection/lubrication of the epithelia in both soluble and membrane-tethered forms. Several membrane-associated mucins (i.e. MUC1, MUC4) are multifunctional molecules, providing not only barrier and lubrication functions but also signal transduction through their juxtamembrane regions (Theodoropoulos and Carraway, 2007).
Secreted mucins are classified into two groups: large gel-forming mucins and smaller soluble mucins. The gel-forming mucins, formed by linking multiple mucin monomers, can be extremely long, with lengths up to several microns and molecular weights ranging from 0.5 to 200 Mda. Secreted mucins are produced in abundance by epithelial goblet cells and submucosal glands.
Functional studies have recently been conducted with cervicovaginal mucus, which is hormonally regulated and comprised primarily of MUC4 and MUC5b (Gipson, 2005). The pores in the mesh structure of the mucus layer range in size from 50–1800 nm, significantly larger than the diameter of sexually transmitted viruses, but viruses diffuse very slowly through this mucus (Lai et al., 2010). Virus penetration of cervicovaginal mucus is impeded by surface charge (Lai et al., 2009), and by antibodies, especially polyvalent IgA and IgM, which can agglutinate pathogens into clusters that are too large to diffuse through mucus. Immunoglobulins also bind to mucin fibers through low-affinity molecular bonds with the Fc and SC components of the molecules to promote trapping of pathogens within the mucus gel (Harada et al., 1997, Kobayashi et al., 1991, Olmsted et al., 2001). Soluble antimicrobial factors produced by the cells within the epithelium also may accumulate in the mucus layer (see below).
The amount, molecular size and morphology of mucin glycoproteins can be altered in disease states. Studies on mucin production in the lung and gastrointestinal tract have shown that proinflammatory cytokines (eg. IL-4, IL-13, TNFα), bacterial lipopolysaccharides (LPS), and certain host proteases can upregulate mucin gene expression, whereas other glycosidases, proteinases and glycosulfatases produced by pathogenic organisms can degrade mucins (Lievin-Le Moal and Servin, 2006, Owen, 2005).
Mucin gene expression in the human penile mucosa was recently characterized (Russo et al., 2006). Several membrane–associated mucins (MUC1, MUC3, MUC4, MUC13, MUC15, MUC17 and MUC20) were confirmed to be expressed by the urethral epithelium, and one gel-forming mucin, MUC5AC, was detected in urethral glands. Further characterization of the structure and function of urethral mucins in health and disease states will be important for a complete understanding of immune defense at this site.
Pattern Recognition Receptors
The ability to discriminate self from nonself is a central feature of both acquired and innate immune defense. Innate immune recognition is principally mediated by cellular receptors known as pattern-recognition receptors (PRRs). These molecules detect virulent microorganisms through recognition of invariant pathogen-associated molecular patterns (PAMPs). PRRs are distributed throughout extracellular, cell membrane and cytoplamic compartments to formulate a fairly complete line of defense against extracellular and intracellular pathogens of viral, bacterial, fungal and protozoan origin.
Prominent members of the PRR class of molecules are the Toll-like receptors (TLRs), a family of membrane (either plasma or endosomal)-associated proteins that recognize a variety of microbe-derived molecules and activate both innate and acquired immune responses. A comprehensive survey of the expression of TLRs along the length of the human male genital tract has recently been reported (Pudney and Anderson, 2010). Unlike the deeper tissues of the male genital tract where TLR expression is relatively rare, a large number of cells in the penile urethra expressed diverse TLRs. The mucosal epithelium of the penile urethra was one of few genital tissues that expressed TLR9, a receptor that detects viral nucleic acids and plays an important role in antiviral immune defense (Hemmi et al., 2000) (Fig 2a). Urethral samples from the majority of cases examined contained TLR9+ epithelial cells, and the staining pattern occurred as positive granules located in the cytoplasm which is consistent with its endosomal location. Immune cells in the urethral mucosal epithelium also expressed a variety of TLRs. TLR1+ cells with a morphologic appearance of macrophages were commonly observed along the length of the urethral mucosa in most patient samples. If only few TLR1+ cells were present, they were restricted to the lamina propria, whereas if there were numerous TLR1+ cells they could be found distributed between the lamina propria and the epithelium. In a previous study we reported that granulocytes were extremely abundant in urethral samples from men who had recently experienced Foley catheters (Pudney and Anderson, 1995a). These neutrophil elastase positive cells were located in the lamina propria and epithelium of the penile urethra and fossa navicularis (Fig. 2b), and expressed TLR1 (Fig. 2c). Phagocytic cells such as granulocytes play a role in innate immunity by ingesting and eliminating pathogens limiting the possibility that these organisms may spread to infect other tissue sites.
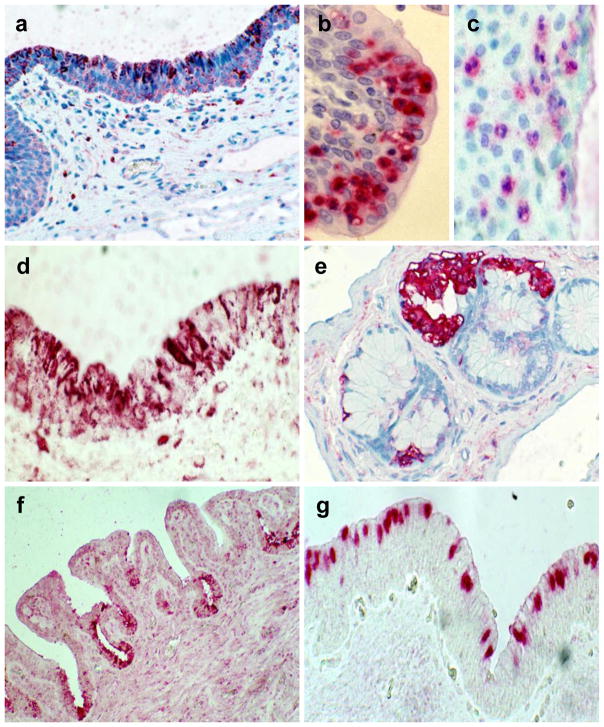
Figure 2.
Mediators of innate immunity in the penile urethral mucosa. a) Expression of TLR9 by urethral epithelium, b) Granulocytes present in urethral epithelium, c) expression of TLR1 by urethral granulocytes, d) Expression of interferon-α in urethral epithelium, e) lysozyme detected in the glands of Littre, f) Lactoferrin detected in urethral epithelium, g) SLPI in urethral epithelium.
Samples of penile urethra contained very few cells expressing TLR2, TLR3 or TLR4, important receptors for the detection of bacterial pathogens and double stranded viral RNA. When present these cells had a lymphocytic morphology and were primarily restricted to the lamina propria. TLR5, a receptor that recognizes flagellin, was consistently observed on lymphocytes in the lamina propria of the urethral mucosa, and occasionally on cells in the epithelium of the fossa navicularis and penile urethra. Cells expressing TLR6 or TLR8 were not detected, whereas TLR7, a receptor that detects RNA viruses, and TLR9 that detects bacterial and viral DNA were detected on cells of macrophage morphology. The TLR7+ and TLR9+ immune cells varied in numbers along the length of the urethra from few to abundant and were mostly located within the epithelium.
Functional studies have not been performed to delineate molecular mechanisms underlying the role of TLRs in innate immunity of the human penile urethra. Furthermore, a number of other PRRs including the NOD-like receptors (NOD1, NOD2; NALP), mannose receptors, and RNA helicases (RIG-I, MDA5) have been detected at other mucosal sites but have not been studied in urethral tissues or cell lines. Further research is needed to determine whether any of these play a role in innate immunity of the penile urethral mucosa.
Antimicrobial peptides (AMPs)
AMPs, an important feature of innate immunity, are widely expressed in mucosal epithelia throughout the body (Lai and Gallo, 2009). They are constituitively expressed by numerous cell types in mucosal epithelia including dendritic cells, macrophages, neutrophils, natural killer and epithelial cells, and their expression can be upregulated during initial stages of infection. AMPs that have been studied in mucosal secretions include small inorganic molecules (i.e., zinc, hydrogen peroxide, nitric oxide), small antimicrobial proteins (i.e., defensins and cathelicidins) and large proteins (i.e., lysozyme, azurocidin, cathepsin G, phospholipase A2, serine leukocyte protease inhibitor (SLPI) and lactoferrin) (Cole, 2006, Doss et al., 2010). The expression of AMPs differs depending on the cell and tissue type and presence of microbial organisms, but in most cases AMPs are co-expressed as groups that act together. Furthermore, many AMPs must be proteolytically cleaved to assume an active form; this is often achieved by enzymes released by granulocytes that quickly infiltrate sites of infection (Wilson et al., 1999). The abundance and redundancy of antimicrobial peptides in mucosal secretions suggest that they work in aggregate to limit the expansion of invading pathogens and provide time for more effective host innate and adaptive immunity to be generated.
Defensins, an important family of AMPs with activity against a broad range of pathogens, have been detected in female and male genital secretions (Porter et al., 2005, Quayle et al., 1998). Defensins are often divided into categories based on the cell type that produces them: human β defensins (HBD) −1, −2 and −3, and human α-defensins (HD) −5 and −6 are primarily expressed by epithelial cells, whereas human neutrophil α-defensins 1–4 (HNPs 1–4) are predominantly expressed by granulocytes. Defensin profiles were recently studied in urethral secretions from men with and without acute STIs. Whereas little HBD-1 and HBD-2 were detected, HD-5 was secreted as a propeptide and its expression was upregulated by Chlamydia trachomatis and Neisseria gonorrhea infections. HD-5 was activated when levels of HNP 1–3 were elevated, suggesting that neutrophils contribute key proteases to convert proHD-5 to its bioactive form in the urethra during infection (Porter et al., 2005).
Activation of the innate immune defense system can also entail the secretion of type 1 interferons, which exert potent antiviral effects, and other chemokines and cytokines that attract and activate effector cells at the site of microbial invasion. We surveyed urethral tissue sections for cells expressing type 1 interferons (IFNα and IFNβ), cytokines that play an important role in antiviral immune defense. Highly localized interferon expression was detected in the epithelium in a subset of cases that also expressed high levels of TLR9 (Fig. 2d). The basal layer of epithelial cells in the fossa navicularis was consistently positive for IFNβ. Apart from its anti-viral properties, IFNβ also has pleiotropic biological activities and its expression by the basal epithelial cells may be related to regulation of cell division and/or differentiation that occur in this layer.
IFNα and IFNβ-positive immune cells were commonly observed in the lamina propria and were especially abundant in samples from 2 patients with urethral catheters that had numerous granulocytes. Occasionally IFNα- and IFNβ-positive immune cells were also observed within the epithelial layer. We examined urethral tissues for the presence of several other classical AMPs. Lysozyme was often detected in the glands of Littre (Fig 2e) and in intraepithelial cells resembling macrophages. Lactoferrin was consistently expressed by columnar epithelial cells of the urethra. The production of lactoferrin was most prevalent in crypt-like infoldings that were present along the length of the urethral epithelium (Fig 2f.). Lactoferrin positive cells ranging in numbers from few to many were also detected in the lamina propria. Serine leukocyte protease inhibitor (SLPI), a component of innate immunity that plays a role in reducing inflammation as well as inhibiting infection by bacteria, viruses and fungi, was abundantly expressed by both columnar epithelial cells and urethral glands in the penile mucosa (Fig 2g.).
We recently measured concentrations of SLPI and lactoferrin in urethral lavages of men with and without acute gonnorhea infections (Anderson, personal communication). SLPI was detected in both groups, and was not elevated in secretions from the GC group (999 ± 675 pg/ml vs 596 ± 343 pg/ml, p>0.05). Lactoferrin was also detected in both groups and was significantly elevated in urethral secretions from the GC group (130 ± 54 pg/ml vs. 1,696 ± 373 pg/ml, p < 0.01). These data provide evidence that AMPs play an important role in limiting urethral infections.
Natural Killer Cells
Natural killer (NK) cells play an important role in innate immunity by destroying virally infected cells. The presence of NK cells along the length of the penile urethra has been reported (Pudney and Anderson, 1995b). Furthermore, CD1d, a molecule that bridges innate and adaptive immunity by presenting glycolipid microbial antigens to invariant NKT cells, was detected on an immortalized penile urethral cell line (Kawana et al., 2008).
Adaptive Immunity
Cellular immunity
The descriptive data presented in this section is summarized from a comprehensive study of T cell subsets and antigen presenting cells in the human penile urethra mucosa (Pudney and Anderson, 1995a), and from recent reports on HIV target cells in the penile urethra (Fischetti et al., 2009, McCoombe and Short, 2006). CD1a+ dendritic cells are present in the mucosal epithelia of the meatus and fossa navicularis; they are more abundant in the refection of skin covering the meatus than in the non-keratinizing stratified squamous epithelium of the fossa navicularis (Fig 3a). In the pseudostratified columnar epithelium of the urethra proper, CD1a + dendritic cells are absent and macrophages comprise the major population of antigen presenting cells. Macrophages were detected mostly in the epithelium with few located in the lamina propria (Fig. 3b). Abundant T-lymphocytes are present in the urethral mucosa. CD8+ lymphocytes are the predominant sub-population occurring in both the epithelium and lamina propria (Fig. 3c). By contrast CD4+ lymphocytes are primarily restricted to the lamina propria (Fig. 3d). Intraepithelial lymphocytes represent a distinct and specialized population of cells having specific functions in immunological defense and tolerance at mucosal surfaces (Parker et al., 1992). Small numbers of naive T lymphocytes expressing CD45RA were detected in the urethra, mostly in the lamina propria. In contrast, large concentrations of CD45RO (memory) T lymphocytes were present in both the epithelium and lamina propria of the urethra. Many of these T lymphocytes also expressed CD103, the αEβ7 integrin that mediates adhesion of mucosal lymphocytes to E-cadherin on epithelial cells (Cerf-Bensussan et al., 1987) (Fig. 3e). Urethral T lymphocytes present in the epithelium and lamina propria also expressed TIA-1, a cytotoxic granule-associated protein found in lymphocytes with cytotoxic functions (Russell et al., 1993) (Fig 3f). These data indicate that the human penile urethra has classical mucosal T cell and antigen-presenting cell populations potentially capable of mounting local immune defense.
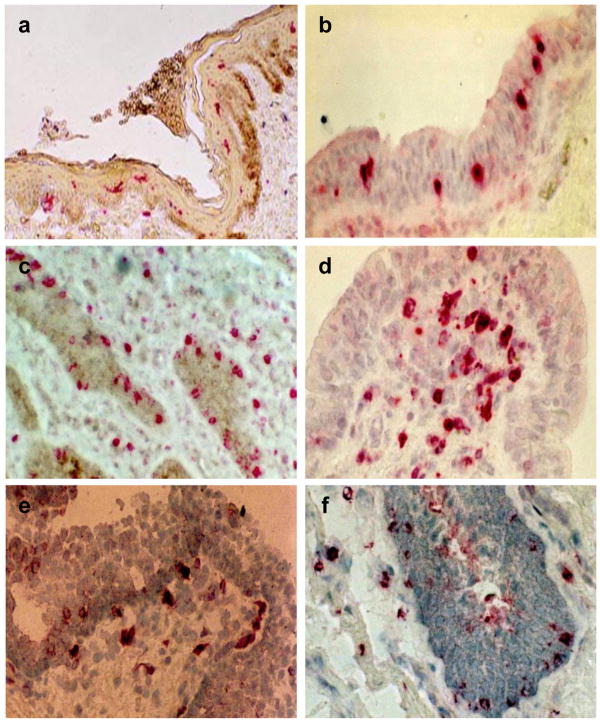
Figure 3.
Mediators of adaptive immunity in the penile urethral mucosa. a) CD1a+ dendritic cells in epithelium of the meatus, b) macrophages present in the epithelium of the penile urethra, c) CD8+ lymphocytes in the urethral epithelium, d) CD4+ lymphocytes in the lamina propria of the urethral mucosa, e) CD103+ lymphocytes in the urethral mucosa, f) TIA+ lymphocytes in the urethral epithelium and lamina propria.
Whereas a number of animal studies have begun to characterize cellular immune responses to infections in the penile urethra (Lehner et al., 1994, Pal et al., 2009, Wang et al., 2010), few studies have been conducted on urethral samples from men with sexually transmitted infections. One study analyzed T lymphocytes, presumably of urethral origin, in first-catch urine samples. The numbers of T cells were low, but significantly more were recovered from men with gonorrhoea (67 × 104) or chlamydia infections (35 × 104) than from men with nongonococcal urethritis (15 × 104) (Shahmanesh et al., 1996). Another study from our group assessed levels of immunologically relevant cytokines in urethral lavages, performed with a 10-cc syringe filled with saline and fitted with a pediatric catheter. Men with gonorrhoea infections had significantly higher concentrations of interleukin (IL)-1β (1,803 ± 795 pg/ml) and IFNγ (22 ± 7.9 pg/ml) in urethral lavages than men without infections (18 ± 11 pg/ml and 1.2 ± 1.1 pg ml respectively, p<0.05) (Anderson et al. personal communication). It should be possible to use these collection techniques to more fully describe cellular immune responses occurring at this site.
Humoral Immunity
Our study on cellular mediators of immune defense in the human penile urethra also described Ig-producing plasma cells and polymeric Ig receptor expression in this tissue (Pudney and Anderson, 1995a). Numerous IgA and IgM producing plasma cells were present in the lamina propria (Fig 4a and b). Furthermore most of these plasma cells were positive for the presence of the J chain indicating secretion of polymeric IgA or IgM (Fig 4c). Fewer IgG + plasma cells compared to IgA+ plasma cells were detected in the urethra. Polymeric IgA and IgM are transported across epithelia by means of the polymeric Ig receptor (pIgR) which is mediated via the secretory component (SC). The epithelium of the urethra highly expressed the pIgR as evidenced by the intense positive staining for SC (Fig 4d). Also the glands of Littre and their contents were often positive for the presence of IgA, J chain and SC. Furthermore, the mucus layer covering the surface of the urethral epithelium was also strongly positive for IgA and SC. This suggests that the mucosal secretion produced by the glands of Littre contains abundant secretory IgA and coats the epithelial surface to form an immunological barrier against invading pathogens. The stratified squamous epithelium lining the meatus and fossa navicularis did not express pIgR. Whereas few plasma cells of any phenotype were present in the meatus, numerous IgA+ and J chain+ plasma cells were observed in the mucosa of the fossa navicularis.
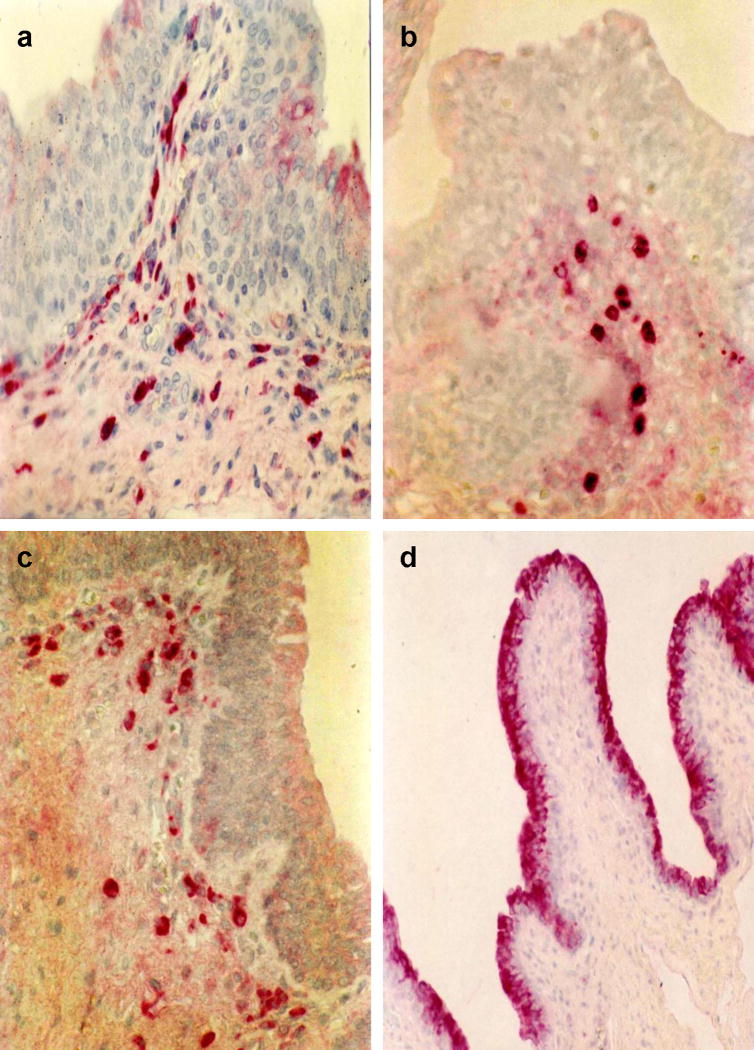
Figure 4.
Mediators of humoral immunity in the penile urethral mucosa. a) IgA+ plasma cells, b) IgM+ plasma cells, c) Plasma cells expressing J-chain, d) Urethral epithelium expressing the polymeric Ig Receptor.
Surprisingly little data are available concerning immunoglobulin levels and isotypes in human penile urethral secretions. Based on the immunohistologic evidence of abundant IgA+ plasma cells and polymeric Ig receptor in urethral tissues, it is likely that secretory IgA predominates in urethral secretions. Evidence that this may be the case has been provided from two recent clinical studies. C. trachomatis-specific IgA (and to a lesser extent IgG) was detected in urethral secretions from C. trachomatis infected men (Pate et al., 2001). HIV-1 specific IgA was detected in urethral swabs from HIV-highly exposed uninfected men (Lo Caputo et al., 2003).
Conclusions
Pathogens that commonly infect the male genital tract include N. gonorrhea, C. trachomatis, Trichomonas vaginalis, Ureaplasma urealyticum, gram-negative bacteria (particularly Escherichia coli) and viruses such cytomegalovirus, human papilloma virus, herpes simplex virus-2 and the human immunodeficiency viruses 1 and 2. The penile urethra is an initial site of exposure to and infection by these pathogens. If untreated these infections could ascend the genital tract resulting in infertility and/or systemic dissemination.
Limited studies on the immunology of the human penile urethra indicate that this mucosal site is immunologically competent and capable of mounting both innate as well as cellular and humoral adaptive immunological responses. The urethral mucosa can recognize pathogens through the expression of TLRs on epithelial cells and intraepithelial immune cells, and is capable of secreting a wide array of antimicrobial factors which may be retained by a viscous mucosal layer secreted by glands of Littre. Little is known about adaptive immune responses at this site, but the urethral mucosal appears to have an entire contingent of humoral and cellular immunological mediators that could participate in a local immune response. It may also be possible to target the penile urethra in vaccine strategies to prevent the sexual transmission of HIV-1 and other sexually transmitted pathogens.
Acknowledgments
Supported by NIH grants PO1 AI46518, R56AI071909, R33AI076966 (DJA)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- CERF-BENSUSSAN N, JARRY A, BROUSSE N, LISOWSKA-GROSPIERRE B, GRISCELLI C, GUY-GRAND D. Monoclonal antibodies specific for human and rat intestinal lymphocytes. Adv Exp Med Biol. 1987;216A:483–91. doi: 10.1007/978-1-4684-5344-7_56. [DOI] [PubMed] [Google Scholar]
- COLE AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- DEKKER J, ROSSEN JW, BULLER HA, EINERHAND AW. The MUC family: an obituary. Trends Biochem Sci. 2002;27:126–31. doi: 10.1016/s0968-0004(01)02052-7. [DOI] [PubMed] [Google Scholar]
- DOSS M, WHITE MR, TECLE T, HARTSHORN KL. Human defensins and LL-37 in mucosal immunity. J Leukoc Biol. 2010;87:79–92. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHETTI L, BARRY SM, HOPE TJ, SHATTOCK RJ. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. AIDS. 2009;23:319–28. doi: 10.1097/QAD.0b013e328321b778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIPSON IK. Human endocervical mucins. Ernst Schering Res Found Workshop. 2005:219–44. doi: 10.1007/3-540-27147-3_10. [DOI] [PubMed] [Google Scholar]
- GIPSON IK, HO SB, SPURR-MICHAUD SJ, TISDALE AS, ZHAN Q, TORLAKOVIC E, PUDNEY J, ANDERSON DJ, TORIBARA NW, HILL JA., 3RD Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997;56:999–1011. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- HARADA N, II, JIMA S, KOBAYASHI K, YOSHIDA T, BROWN WR, HIBI T, OSHIMA A, MORIKAWA M. Human IgGFc binding protein (FcgammaBP) in colonic epithelial cells exhibits mucin-like structure. J Biol Chem. 1997;272:15232–41. doi: 10.1074/jbc.272.24.15232. [DOI] [PubMed] [Google Scholar]
- HEMMI H, TAKEUCHI O, KAWAI T, KAISHO T, SATO S, SANJO H, MATSUMOTO M, HOSHINO K, WAGNER H, TAKEDA K, AKIRA S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- KAWANA K, MATSUMOTO J, MIURA S, SHEN L, KAWANA Y, NAGAMATSU T, YASUGI T, FUJII T, YANG H, QUAYLE AJ, TAKETANI Y, SCHUST DJ. Expression of CD1d and ligand-induced cytokine production are tissue specific in mucosal epithelia of the human lower reproductive tract. Infect Immun. 2008;76:3011–8. doi: 10.1128/IAI.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI K, HAMADA Y, BLASER MJ, BROWN WR. The molecular configuration and ultrastructural locations of an IgG Fc binding site in human colonic epithelium. J Immunol. 1991;146:68–74. [PubMed] [Google Scholar]
- LAI SK, HIDA K, SHUKAIR S, WANG YY, FIGUEIREDO A, CONE R, HOPE TJ, HANES J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI SK, WANG YY, HIDA K, CONE R, HANES J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI Y, GALLO RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–41. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHNER T, TAO L, PANAGIOTIDI C, KLAVINSKIS LS, BROOKES R, HUSSAIN L, MEYERS N, ADAMS SE, GEARING AJ, BERGMEIER LA. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J Virol. 1994;68:1624–32. doi: 10.1128/jvi.68.3.1624-1632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEVIN-LE MOAL V, SERVIN AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–37. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LO CAPUTO S, TRABATTONI D, VICHI F, PICONI S, LOPALCO L, VILLA ML, MAZZOTTA F, CLERICI M. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS. 2003;17:531–9. doi: 10.1097/00002030-200303070-00008. [DOI] [PubMed] [Google Scholar]
- MCCOOMBE SG, SHORT RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–5. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- OLMSTED SS, PADGETT JL, YUDIN AI, WHALEY KJ, MOENCH TR, CONE RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–7. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN CA. Proteinases and oxidants as targets in the treatment of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:373–85. doi: 10.1513/pats.200504-029SR. discussion 394–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAL S, SARCON AK, DE LA MAZA LM. C3H male mice with severe combined immunodeficiency cannot clear a urethral infection with a human serovar of Chlamydia trachomatis. Infect Immun. 2009;77:5602–7. doi: 10.1128/IAI.00766-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER CM, CEPEK KL, RUSSELL GJ, SHAW SK, POSNETT DN, SCHWARTING R, BRENNER MB. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1924–8. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATE MS, HEDGES SR, SIBLEY DA, RUSSELL MW, HOOK EW, 3RD, MESTECKY J. Urethral cytokine and immune responses in Chlamydia trachomatis-infected males. Infect Immun. 2001;69:7178–81. doi: 10.1128/IAI.69.11.7178-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER E, YANG H, YAVAGAL S, PREZA GC, MURILLO O, LIMA H, GREENE S, MAHOOZI L, KLEIN-PATEL M, DIAMOND G, GULATI S, GANZ T, RICE PA, QUAYLE AJ. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun. 2005;73:4823–33. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUDNEY J, ANDERSON D. Effects of fixation and paraffin embedding on the immunohistological detection of cell-associated HIV-1 by different monoclonal antibodies. J Histochem Cytochem. 1995a;43:857–62. doi: 10.1177/43.9.7543912. [DOI] [PubMed] [Google Scholar]
- PUDNEY J, ANDERSON DJ. Immunobiology of the human penile urethra. Am J Pathol. 1995b;147:155–65. [PMC free article] [PubMed] [Google Scholar]
- PUDNEY J, ANDERSON DJ. Expression of Toll-like Receptors in Genital Tract Tissues from Normal and HIV-infected Men. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE AJ, PORTER EM, NUSSBAUM AA, WANG YM, BRABEC C, YIP KP, MOK SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–58. [PMC free article] [PubMed] [Google Scholar]
- RUSSELL GJ, NAGLER-ANDERSON C, ANDERSON P, BHAN AK. Cytotoxic potential of intraepithelial lymphocytes (IELs). Presence of TIA-1, the cytolytic granule-associated protein, in human IELs in normal and diseased intestine. Am J Pathol. 1993;143:350–4. [PMC free article] [PubMed] [Google Scholar]
- RUSSO CL, SPURR-MICHAUD S, TISDALE A, PUDNEY J, ANDERSON D, GIPSON IK. Mucin gene expression in human male urogenital tract epithelia. Hum Reprod. 2006;21:2783–93. doi: 10.1093/humrep/del164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAHMANESH M, PANDIT PG, ROUND R. Urethral lymphocyte isolation in non-gonococcal urethritis. Genitourin Med. 1996;72:362–4. doi: 10.1136/sti.72.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODOROPOULOS G, CARRAWAY KL. Molecular signaling in the regulation of mucins. J Cell Biochem. 2007;102:1103–16. doi: 10.1002/jcb.21539. [DOI] [PubMed] [Google Scholar]
- WANG Y, NAGARAJAN U, HENNINGS L, BOWLIN AK, RANK RG. Local host response to chlamydial urethral infection in male guinea pigs. Infect Immun. 2010;78:1670–81. doi: 10.1128/IAI.01339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON CL, OUELLETTE AJ, SATCHELL DP, AYABE T, LOPEZ- BOADO YS, STRATMAN JL, HULTGREN SJ, MATRISIAN LM, PARKS WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]