Abstract
The current study tested the hypotheses that chronic loss of inhibitory GABAergic tone in the bed nucleus of the stria terminalis (BNST), a region implicated in anxiety behavior, results in generalized anxiety disorder-like behaviors without panic-like responses (i.e., tachycardia, hypertension and tachypnea) following panicogenic stimuli (e.g., sodium lactate infusions). To test this hypothesis, the GABA synthesis inhibitor L-allylglycine (L-AG) or its inactive isomer D-AG was chronically infused into the BNST of male rats via osmotic minipumps. L-AG, but not D-AG, treated rats had increased anxiety-like behavior as measured by social interaction (SI) and elevated plus maze paradigms. Restoring GABAergic tone, with 100pmoles/100nl of muscimol (a GABAA receptor agonist), in the BNST of L-AG treated rats attenuated L-AG-induced anxiety-like behavior in the SI test. To assess panic-like states, L-AG treated rats were intravenously infused with 0.5M sodium lactate, a panicogenic agent, prior to assessing SI and cardiorespiratory responses. L-AG decreased SI duration again; however, sodium lactate did not induce panic-like cardiorespiratory responses. These findings demonstrate that GABA inhibition in the BNST elicits anxiety-like behavior without increasing sensitivity to lactate, thus suggesting a behavioral profile similar to that of generalized anxiety-like behavior rather than that of panic.
Keywords: Amygdala, elevated-plus maze, fear, anxiety disorder, muscimol, panic, BNST, stria terminalis, social interaction
Introduction
The bed nucleus of the stria terminalis (BNST) is a heterogeneous nucleus defined as part of the extended amygdala. Anatomically, the extended amygdala arises from both the central and the medial nuclei, with the central nucleus giving rise to the lateral BNST and the medial nucleus projecting to the medial BNST (Cassell et al., 1999; McDonald, 2003). Furthermore, the BNST has extensive connections with the different nuclei of the amygdala, the hypothalamus and other limbic structures (Ter Horst and Luiten, 1986; Alheid et al., 1998; Cassell et al., 1999; Dong et al., 2001; Dong and Swanson, 2004; Spencer et al., 2005), suggesting a role for this structure in a variety of cognitive and emotional behaviors.
Several studies have shown that the BNST is involved in the generation of anxiety-like responses. Davis and colleagues found that administration of AMPA antagonists into the BNST results in a loss of light-enhanced startle, with no effect on fear-potentiated startle (Walker and Davis, 1997). Subsequently, one conclusion they proposed was that the BNST appears to play a critical role in unconditioned responses as compared to the central nucleus of the amygdala which is involved in conditioned fear behaviors (Davis et al., 1997; Walker and Davis, 1997; Davis, 1998; Davis and Shi, 1999; Walker, 2003). Hammack et al. (2004) found that the typical freezing and increase in escape latency associated with uncontrollable or inescapable shock were also blocked following BNST lesioning, while Sullivan et al. (2004) found that lesions of this nucleus did not disrupt specific-cue related fear responses, but a more general contextual cue-mediated anxiety. Unlike the amygdala, the BNST is not activated by the panicogenic drug doxapram (Choi et al., 2005). Based on such findings, an argument has been made for the role of the BNST in the regulation of innate anxiety behaviors and as a potential substrate for generalized anxiety-like as opposed to panic-like symptoms (Davis, 1998).
The function of the BNST is regulated by an extensive network of internal GABAergic neurons (Sun and Cassell, 1993; Egli and Winder, 2003; Bali et al., 2005). Microinjections of the GABAA receptor agonist muscimol directly into the BNST can inhibit freezing behavior in rats following exposure to predator odor (Fendt et al., 2003). Thus an intact GABAergic response within the BNST appears to be critical for normal regulation of anxiety behaviors and, based on the findings reviewed above, may not induce panic-like states.
In the basolateral amygdala (BLA) and dorsomedial hypothalamus (DMH), two other limbic structures bi-directionally connected to the BNST, GABAergic tone appears to be important for regulating central autonomic control in addition to anxiety-like behavior (Sanders and Shekhar, 1991, 1995; Shekhar and Katner, 1995; Samuels et al., 2004). Subthreshold disruption of GABAergic tone in the BLA and DMH results in the development of not only chronic anxiety-like responses but also panic-like sensitivity to intravenous sodium lactate infusions in rats (Shekhar et al., 1996; Sajdyk and Shekhar, 2000; Johnson and Shekhar, 2006), a response similar to that of individuals with panic disorder (Pitts, 1967; Reiman et al., 1984; Liebowitz et al., 1986; Coplan et al., 1998). Thus, subthreshold inhibition of GABAergic tone in the BLA and DMH produce conditions mimicking panic-like states rather than generalized anxiety. In order to test the hypothesis that chronic inhibition of GABAergic tone in the BNST leads to states resembling generalized anxiety rather than panic-like states, we chronically removed GABAergic tone in the BNST in a similar manner as is done in the DMH panic-model (Shekhar et al., 1996) and used two accepted tests for measuring anxiety [i.e., social interaction (SI) test and elevated plus maze (EPM)] to assess general anxiety-like behavior. Panic-like states were also tested in rats with inhibited GABAergic tone in the BNST by intravenously infusing 0.5M sodium lactate and monitoring heart rate, blood pressure and respiration rate, as well as conducting a SI test.
Methods and Materials
Animals and housing conditions
All experiments were conducted on adult male Sprague-Dawley rats (275–300 g), which were purchased from Harlan Laboratories and were housed individually in plastic cages under standard environmental conditions (22 °C; 12/12 light/dark cycle; lights on at 7:00 A.M.) for 7–10 days prior to the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80–23; revised 1996) and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
Surgical Procedure
Prior to surgery, rats were anesthetized in a closed plexiglas box which was connected to an isoflurane system (MGX Research Machine; Vetamic, Rossville IN). They were removed from the box and placed on a plexiglas platform face up and connected to a nose cone which delivered constant anesthetic throughout surgery. On day 1 (see Fig. 1) all rats were fitted with femoral arterial catheters for measurement of mean arterial blood pressure (MAP), heart rate (HR) and respiration rate (RR) and with venous catheters for intravenous (i.v.) infusions, as previously described (Sajdyk et al., 2003).
Figure 1.
An illustration representing a timeline of experimental conditions (listed above arrows) from day 1 (D1) to day 26 (D26) along the horizontal bar. See methods for detailed description. Gray shading of horizontal bar indicates the timeline of D-AG or LAG infusions.
Ten days later all rats were implanted with osmotic pumps fitted with guide cannula (Model 2004, 28 GA, 28 day pumps) unilaterally into the BNST (from bregma: 1.0 mm anterior, +2.5 mm lateral, +7.0 mm ventral and adjusted for approaching at a +10 degree [medial-lateral] with the stereotaxic incisor bar elevated 5 mm above the interaural line; see Fig. 1). The pumps were filled with the GABA synthesis inhibitor LAG or the inactive isomer D-AG (Orlowski et al., 1977) just prior to the start of surgery. The L-AG or D-AG was infused into the BNST at a rate of 7.0 nmoles/0.25 μl/hr. Implantation of Osmotic mini-pumps have been described previously (Shekhar et al., 1996). Upon completion of implantation the pump was sutured under the skin in the nape of the neck and the connector and cannula were cemented to the skull in the same manner as previously explained (Shekhar A, 1996).
Social Interaction
The modified social interaction test is a paradigm that we have used extensively in our laboratory to measure anxiety-like behaviors in rats (Sajdyk et al., 2004). The protocol involves placing the treated animal simultaneously into the SI box (91.44 cm. L x 91.44 cm. W x 30.48 cm H box with an open top) with an experimentally naïve partner rat. All partner rats are sex and weight matched; housed under identical conditions; and have no previous contact with the treated rat. The testing was carried out under low light (40 watt red lighting), familiar conditions. All testing was video taped via a camera mounted on the ceiling directly above the SI box and scored at a later time. The total amount of time the experimental rat spent initiating social interaction (i.e., sniffing, grooming and crawling) with the partner rat was scored from the video tape by two blinded raters with an inter-rater reliability of 83%. Scoring of SI times were determined as previously described (Shekhar and Katner, 1995; Sajdyk and Shekhar, 1997).
Elevated Plus-Maze
The elevated plus-maze comprised of two open arms (50 × 10 cm) and two closed arms (50 × 10 cm) elevated 50 cm above ground with an open top. The arms were situated such that the two open and the two closed arms were directly opposite to each other. Rats were placed in the center of the arms and their exploration of the maze was recorded from a video mounted on the ceiling for the total of the five min testing period. The total amount of time and the number of arm entries the rat spends in each arm was scored from the videotape by a treatment-blind rater.
Experimental Protocol
On experimental day 1 (see Fig. 1 for timeline), all rats underwent surgery for implantation of femoral venous and arterial catheterization. On day 5, each rat was placed in the SI test for baseline assessment of anxiety-like behavior and then 24 hours later (day 6) tested on the elevated plus-maze. On experimental day 7, each rat was randomly assigned to one of two treatments (saline or sodium lactate). Sodium lactate was utilized as a panicogenic agent for this study and a detailed procedure has been previously described (Shekhar et al., 1996). Briefly, rats were administered i.v. infusions of 0.9% saline and 0.5 M sodium lactate (10 ml/kg; 1 ml/min), similar to clinical lactate infusions (Liebowitz et al., 1986). They were then connected to a pressure transducer (Dynograph, Sensormedics R511) via their arterial catheter to obtain baseline measurements for heart rate (HR), blood pressure (BP), and indirect measurement of respiration rate (RR) from normal sinus arrhythmia]. After a steady state baseline for all parameters was maintained for at least 20 min, the rat was infused with their assigned treatment via the venous catheter. Immediately following the infusion the experimental animal was assessed for anxiety-like behavior in the SI test. The procedure was repeated again on day 9; however, each rat received the treatment not originally given on day 7.
Twenty-four hours later (on day 10) the rats were again anesthetized with isoflurane. They were then placed on the stereotaxic instrument and surgically implanted with osmotic pumps into the BNST. In a randomized manner, half of the animals received L-AG and the other half D-AG. The rats were allowed to recover for four days (only those rats fully recovered were utilized for the study) and then on day 14 all animals were reassessed in the SI test and compared to their scores on day 5 (pre-pump vs post-pump) to determine if the blockade of GABA synthesis in the BNST had an effect on anxiety-like behavior.
On days 16, 18 and 20, rats with L-AG pumps received 100nl injections of saline or muscimol (20 or 100 pmoles, obtained from RBI) directly into the BNST in a counterbalanced design. The pump was briefly disconnected and the assigned treatment was delivered via the cannula that was connected to the pump. Fifteen min after BNST injections, rats were placed in the SI test for behavioral assessment of anxiety. Rats with the D-AG pumps were not tested with muscimol in this study because previous work in our lab has shown that these same doses of muscimol into the BNST have no effect on SI behavior (unpublished data).
On days 22 and 24, L-AG treated rats were connected to the physiograph and a steady baseline for heart rate, blood pressure and respiration rate was obtained. Upon obtaining a steady baseline recording of at least 20 min, rats were infused with sodium lactate (10 ml/kg) and then administered either saline or muscimol (100 pmoles) with half the rats receiving a given treatment on each day. Recordings for cardiovascular parameters were taken throughout the infusion. Forty-eight hours later (day 26), all rats were reassessed on the elevated plus-maze test, then immediately following the study, rats were killed and their brains removed for histological verification of pump placement.
Statistical Analysis
All experiments were conducted utilizing a within animal design. The combined L-AG and D-AG data for SI duration and EPM was analyzed with a repeated measures one-way ANOVA with allylglycine infusion as the independent variable and time as the repeated measure. Social interaction data from multiple doses of muscimol was analyzed using a one-way ANOVA. Tukey’s (Honestly Significant Difference) HSD post hoc test was used for multiple comparisons. Due to the directional hypothesis with L-AG and muscimol treatments, one tailed paired t-tests were used when comparing only two treatment groups. All p values were set at ≤ 0.05 and graphical presentation of data is done with bars representing the mean and error bars representing the standard error of the mean (SEM).
Results
Elevated Plus-Maze
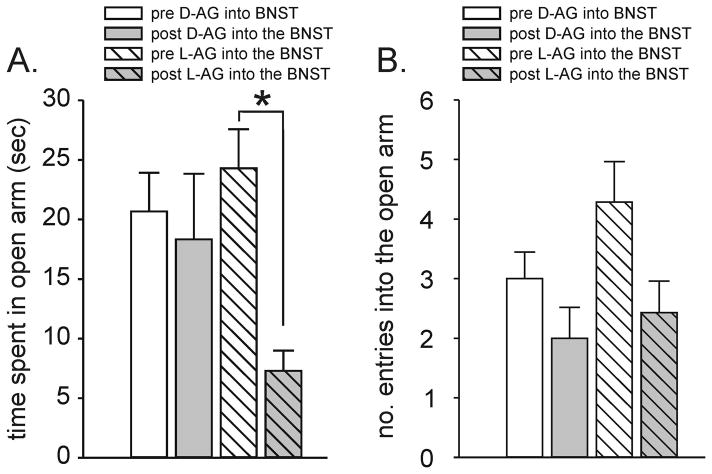
Two groups of rats were assessed for anxiety-like behavior in the elevated plus-maze. The first group received Osmotic mini-pumps implanted into the BNST and filled with L-AG (n=7), the active form of the GABA synthesis inhibitor, while the second group was given D-AG (n=6), the inactive isomer. A repeated measures ANOVA plus tukey’s posthoc test revealed that rats had a significant decrease in time spent in open arm and the number of open arm entries post L-AG, but not D-AG, infusions [L-AG x time interaction, F(1,11) = 6.2, p < 0.04, Fig. 2A]. No difference was observed in the number of open arm entries [L-AG x time interaction F(1,11) = 0.1, p = 0.345, Fig. 2B] or the total number of combined open arm or closed arm entries [L-AG x time interaction F(1,11) = 2.9, p = 0.116, data not shown] between pre or post L-AG and D-AG infusions.
Figure 2.
EPM test data from pre (day 6) and post (day 26) D-AG and L-AG infusions. A) The amount of time (sec) spent in the open arm of the elevated plus-maze or B) the number of entries into the open arm during a 5 min test of rats with previous infusions of the GABA synthesis inhibitor L-AG or its inactive isomer D-AG into the BNST. Bars represent the mean and error bars represent the SEM. *, p<0.05.
Social Interaction
Rats implanted with Osmotic mini-pumps containing either L-AG or D-AG were assessed in the SI test prior to pump placement (one week prior) and then again after surgery (day 4 post-surgery). Rats that received L-AG into the BNST showed a significant decrease in SI time by day 4 (n=7) compared to pre L-AG SI baseline, whereas the SI duration in the control rats with the D-AG inactive isomer did not differ from the pre D-AG SI baseline (n=6) [L-AG x time interaction F(1,11) = 12.1, p < 0.01, Fig. 2B]; Fig. 3].
Figure 3.
The amount of time (sec) that the experimental rats spent initiating interaction with the partner rats during a 5 min SI test, both before (day 5) and after (day 14) pump surgery. The top graph depicts the average times for animals implanted with L-AG pumps into the BNST, while the bottom graph shows times for those with D-AG pumps into the BNST. Bars represent the mean and error bars represent the SEM. *, p<0.05.
The osmotic mini-pump cannula also contained a microinjection cannula as part of the apparatus. This design allowed for a brief insertion of an injection cannula so that muscimol, a GABAA receptor agonist, be given through the cannulae. Two different doses of muscimol (20 or 100 pmoles) were administered to determine if restoring local GABAergic tone in the BNST could reverse the effects of L-AG on SI behavior. The results for this study (Fig. 4) show that infusing L-AG into the BNST decreased SI duration (paired t-test, T=5.7, df=3, p<0.01). This L-AG induced reduction in SI duration was reversed in a dose-dependent manner when rats received prior injections of muscimol, but not saline, into the BNST [F(2,9) = 13.6; p<0.003, (n=4 for all groups)]. In the subsequent experiment, the stylet for delivering the muscimol and saline injections became blocked preventing completion of the crossover design in 3 of the 7 L-AG treated rats reducing the n to 4 in this experiment and in the following sodium lactate experiment.
Figure 4.
The amount of time (secs) that the experimental rats spent initiating interaction with the partner rats during a 5 min SI test, under four conditions. The first bar represents pre L-AG infusion baseline. Four days after L-AG infusion an SI test was done following an injection of saline (bar 2), 20 pmoles of muscimol (bar 3), or 100 pmoles of muscimol into the BNST. Treatments 2 – 4 were administered in a counter balanced design. Bars represent the mean and error bars represent the SEM. * Significantly different from pre-LAG baseline (paired t-test) and both doses of muscimol (Tukey’s posthoc test following ANOVA), # significantly different from 100 pmoles of muscimol (Tukey’s posthoc test following ANOVA). *,#, p<0.05.
Sodium Lactate Infusions
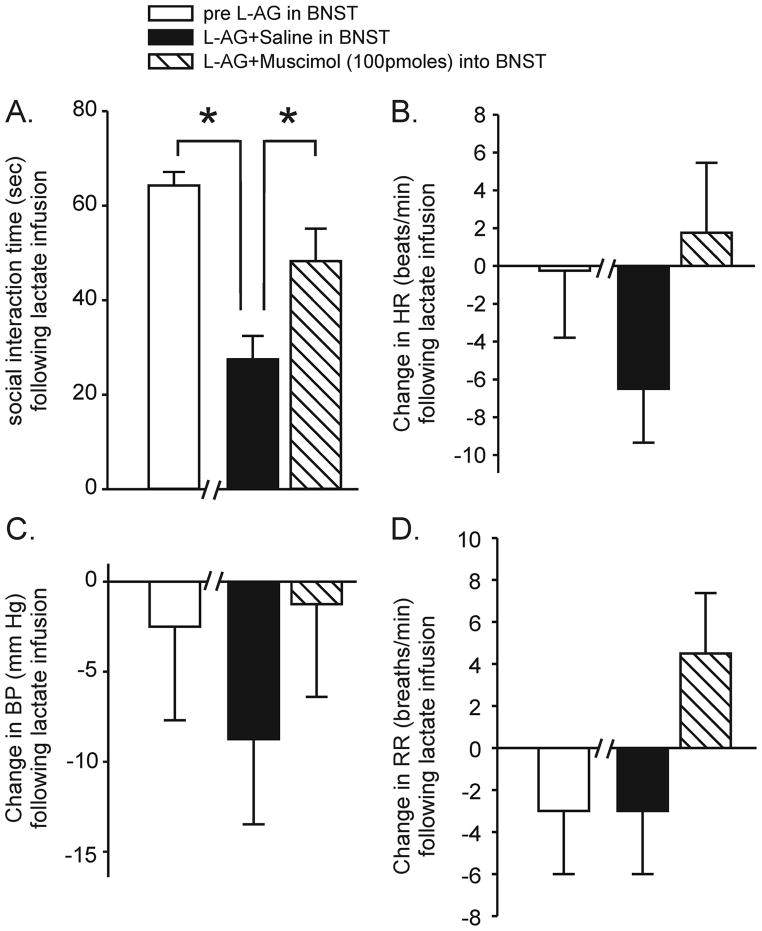
Similar L-AG infusions in other anxiety-like response eliciting structures (i.e., the DMH) not only produces similar results on SI behavior, but also makes rats susceptible to displaying panic-like responses (i.e., tachycardia, tachypnea and increases in blood pressure) in response to mild interoceptive stressors such as i.v. infusions of 0.5M sodium lactate (Shekhar et al., 1996; Johnson and Shekhar, 2006; Shekhar et al., 2006). In light of this, SI tests and peripheral physiological measures (i.e., heart rate, blood pressure and respiration rate) were recorded in a separate group of rats at baseline as well as after they had L-AG pumps following i.v. sodium lactate infusion. Infusing L-AG into the BNST+ i.v. lactate decreased SI duration (T=14.2, df=3, p<0.001, Fig. 5A), but had no effect on HR (T=1.5, df=3, p=0.120, Fig. 5B), BP (T=2.1, df=3, p=0.066, Fig. 5C) or RR (T=0.0, df=3, p=0.5, Fig. 5D) when compared to baseline (i.e. pre L-AG into BNST+ i.v. lactate). Comparing the rats treated with L-AG+saline into the BNST to rats treated with L-AG+100 pmoles of muscimol into the BNST prior to lactate challenge revealed that the 100 pmoles of muscimol increased the SI duration (T=3.0, df=3, p<0.03, Fig. 5A) near baseline levels without altering HR (T=0.8, df=3, 0.238, Fig. 5B), BP (T=1.4, df=3, p=0.127, Fig. 5C) or RR (T=1.5, df=3, p=0.120, Fig. 5D).
Figure 5.
Changes in A) social interaction time, B) heart rate, C) blood pressure and D) respiration rate in rats infused with i.v. sodium lactate prior to L-AG pump placement in the BNST, and on day 20 or 22 after beginning infusions with the GABA synthesis inhibitor L-AG and pre-injections of either saline or muscimol 100 pmoles into the BNST. There were no significant peripheral responses to lactate infusions in these animals either at baseline or after GABA synthesis inhibition in the BNST which resulted in increased baseline anxiety. In these animals, there was no difference in lactate responses after saline or muscimol pre-injections into the BNST, suggesting no differences in lactate sensitivity with or without baseline anxiety increases. * significant against pre L-AG in BNST (*, paired t-test, p<0.002) and L-AG in BNST + 100 pmoles muscimol (*, paired t-tests, p≤0.050). Bars represent the mean and error bars represent the SEM.
Histological verification of pump implantation sites
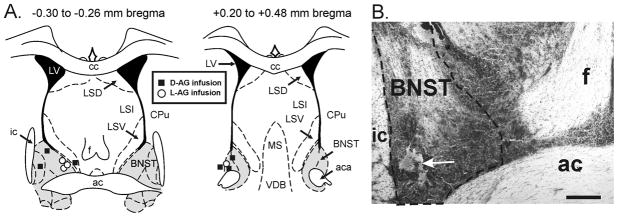
The post-mortem histological examination was utilized to confirm that the data were analyzed from only those animals with distribution of L-AG (GABA synthesis inhibitor) or D-AG (control isomer) filled osmotic minipumps in the BNST region. Cresyl violet staining was done on 60 μm coronal brain sections from 4 out of 7 L-AG reated rats and all 6 D-AG treated rats (Fig. 6A and 6B). Confirmation of 3 L-AG minipump placements in the BNST region were visually done on coronal brain sections cut at 300μm and micropunched for future chemical assays.
Figure 6.
Distribution of L-allylglycine (L-AG: GABA synthesis inhibitor) or D-allylglycine (D-AG: control isomer) filled osmotic minipumps in the bed nucleus of the stria terminalus (BNST) region of all 6 D-AG treated rats and 4 of 7 L-AG treated rats. Placements were confirmed visually on 300 μm coronal brain section for the other 3 L-AG treated rats and then microdissected. A) Diagrammatic illustration of two coronal rat brain sections (−0.30 to −0.26 mm bregma and +0.20 to +0.48mm bregma) with minipump placement and treatment indicated by symbols (D-AG infusion site = black square and LAG infusion site = white circle) and boundaries of the BNST indicated by gray shading. B) Low magnification photograph of cresyl violet staining in the BNST region; the arrow denotes the minipump placement. Scale bar: 400 μm. Abbreviations: ac, anterior commissure; aca, anterior part of anterior commissure; cc, corpus collosum; CPu, caudate putamen; f, fornix; ic, internal capsule; LSD, dorsal part of lateral septum; LSI, intermediate part of lateral septum; LSV, ventral part of lateral septum; LV, lateral ventricle; MS, medial septum; VDB, nucleus of the vertical limb of the diagonal band.
Discussion
The present study demonstrates that a decrease in GABA inhibition due to chronic infusion of L-AG but not D-AG, the inactive isomer, into the BNST increases anxiety-like behaviors as measured by decreases in the amount of time spent in the open arms in the elevated plus-maze, as well as reductions in the duration of social interactions. These results are consistent with the idea that increased activation of the BNST is associated with the expression of anxiety-like responses. In the current study we assessed animals in the elevated plus-maze before and following treatment. Previously, File and colleagues demonstrated a change in benzodiazepine response on the 2nd trial of the maze (File and Zangrossi, 1993; File et al., 1993), suggesting that on the 2nd trial the response is confounded by 1 trial learning and thus different than that in the 1st test. However, the response to benzodiazepines is re-established after 2 weeks of no further exposure to EPM. As a result, we assess our animals in the 2nd trial at least 2 weeks from the first session.
Another important finding is that L-AG infusion into the BNST of the rats increased anxiety-like behavior without eliciting panic-like symptoms (i.e., tachycardia, tachypnea and increased blood pressure) in response to sodium lactate. This lack of responsivity is in contrast to that seen in our other animal models of anxiety. For example, similar L-AG infusions into the DMH produces anxiety-like behaviors in SI, but also makes the animals susceptible to displaying panic-like characteristics (i.e., tachycardia, tachypnea and increases in blood pressure) in response to i.v. infusions of 0.5M sodium lactate (Shekhar et al., 1996; Johnson and Shekhar, 2006; Shekhar et al., 2006). Overall, this data supports the hypothesis that BNST plays an important role in regulating general anxiety. More importantly, these findings show that removing GABAergic tone in the BNST may be a useful tool in studying general anxiety-related circuits.
The data presented here are also consistent with results from other studies. Singewald et al. (2003) found that systemic administration of four different anxiogenic compounds consistently increased cellular responses (i.e., c-Fos expression) in the BNST, while Lee and Davis (1997) have shown that directly activating the BSNT with the stress associated neuropeptide corticotrophin releasing factor (CRF) will enhance acoustic startle responses (For review see Bale and Vale, 2004). Interestingly, activation of the BNST with CRF will also induce reinstatement of cocaine use similar to footshock stress, while the CRF receptor antagonist D-Phe CRF will block reinstatement following the stress (Erb and Stewart, 1999). The current findings together with the other behavioral studies strongly indicate that activation of the BNST is associated with increases in anxiety-like behaviors.
In addition to regulating anxiety-associated behavior, the BNST appears to be important in regulating social behavior. Studies conducted by Insel and colleagues have focused on social behavior in rats and prairie voles and found that oxytocin is a key neurotransmitter in inducing prosocial behaviors and have proposed that oxytocin may elicit prosocial behaviors through interactions with oxytocin receptors in the BNST (Insel, 1992). In addition, recent studies indicate that the administration of the CRF receptor antagonist D-Phe CRF into the BNST blocks the submissive-defensive behaviors of hamsters normally associated with social defeat (Jasnow et al., 2004). These results suggest that the BNST is also an important limbic structure for the expression of appropriate social behaviors across several species.
Animals in the L-AG treatment group demonstrated a decrease in social interaction time and open arm time, thus it could be suggested that L-AG infusions into the BNST may be reducing overall locomotor activity. However, there was not a significant difference between the L-AG and the D-AG treatment groups in the total number of entries (data not shown), therefore it would suggest that locomotion was not a confounding factor.
In order to confirm that L-AG infusions were inducing anxiety states by removing local GABAergic tone in the BNST and not other possible non-specific effects, local GABAergic tone was restored with acute injections of muscimol (a GABAA receptor agonist) into the BNST. Injecting the highest dose of muscimol into the BNST of L-AG treated rats attenuated L-AG-induced anxiety responses in two separate experiments. Another possible confound would be diffusion of the L-AG into other nuclei. Earlier studies characterizing site specificity of L-AG infusions with exactly the same type of pumps in another brain area (the DMH) showed no significant effect in the adjacent areas beyond about one mm of radius when studies by either immunocytochemical or biochemical methods (Johnson and Shekhar, 2006; Shekhar et al., 2006), suggesting a limited area of diffusion from the pumps. Overall, these data are consistent with the hypotheses that L-AG infusions in the BNST elicits anxiety by removing local GABAergic tone and also suggests that the anxiolytic effects of GABA in the BNST are mediated via the GABAA receptor. Furthermore, the chronic anxiogenic-like effects of GABA inhibition (i.e., L-AG treated) in the BNST suggests that GABA is tonically inhibiting the BNST. Intracellular recordings within the dorsal oval nucleus of the BNST, the area targeted for the pumps, reveals that some of the local GABAergic neurons are tonically active and that administration of bicuculline methiodide (BMI, a GABAA receptor antagonist) attenuates this tonic inhibition (Rainnie, 1999; bEgli and Winder, 2003). Interestingly, rats undergoing ethanol withdrawal display marked anxiety-like behavior, which coincides with robust increases in CRF levels in the BNST (Olive et al., 2002). If alcohol is given to these animals CRF levels in the BNST return to normal. However, if they are only given a regular diet, the levels will continue to increase 100% above baseline. This phenomenon may explain the anxiety associated with withdrawal as well as the increase in socialization following alcohol consumption.
Previous work in our laboratory has shown that an acute decrease in GABA functioning in the BLA or the DMH of rats will also elicit anxiety-like responses (Shekhar et al., 1990; Sanders and Shekhar, 1991; Shekhar, 1993; Shekhar et al., 1993; Sanders and Shekhar, 1995; Shekhar and Katner, 1995; Shekhar et al., 1996; Sajdyk and Shekhar, 1997, 2000). When we induced chronic loss of GABAergic inhibition in these regions of rats, either via L-AG infusions in the DMH or repeated subthreshold doses of BMI into the BLA, the animals display increased anxiety-related behavior and develop a sensitivity to i.v. sodium lactate similar to that of individuals with panic disorder [i.e., tachycardia, hypertension and tachypnea (Shekhar et al., 1996; Shekhar and Keim, 1997; Sajdyk and Shekhar, 2000; Shekhar and Keim, 2000)].
Intravenous sodium lactate infusions have been best studied in subjects with panic disorder and consistently elicit panic attacks in the majority of panic disorder patients, often similar to naturally occurring episodes (Gorman et al., 1983; Carr et al., 1986; Gaffney et al., 1988; Den Boer et al., 1989; Hollander et al., 1989; Yeragani et al., 1989). Although panic-like response to lactate infusions is most consistent in panic disorder, some sensitivity to lactate infusions have been reported in post-traumatic stress disorder, where it primarily induces flash-backs (Rainey et al., 1987; Jensen et al., 1997), and a subset of patients with premenstrual syndrome (Facchinetti et al., 1992; Sandberg et al., 1993). A disorder that also shares a biological linkage with panic disorder (Facchinetti et al., 1998). In contrast, lactate infusions are ineffective in patients with obsessive compulsive disorder (Gorman et al., 1985), social anxiety disorder (Liebowitz et al., 1985) and major depression without history of panic attacks (Cowley et al., 1987). Thus, lactate induced panic has been proposed as a reasonable biological probe to separate panic versus other anxiety disorders (Cowley and Arana, 1990). The lack of responsiveness to sodium lactate in the present study suggests that the anxiety-like behavior displayed by the rats with the L-AG pumps placed in the BNST is not like that of panic and may be more similar to that of generalized or social anxiety disorder.
The likelihood of BNST mechanisms being a possible substrate for generalized anxiety disorders is further supported by studies with the administration of buspirone, a 5-HT1A agonist, into the BNST. Buspirone blocks light-enhanced startle (Davis et al., 1997). It has also been shown that a natural stressor, such as chronic restraint, increases dendritic remodeling and arborization in the BNST, indicating a function in stressed-induced facilitation of anxiety (Vyas et al., 2003). Furthermore, electrophysiological studies in the BNST also support a GABAergic and serotonergic mechanisms for reducing activation (Rainnie, 1999; bEgli and Winder, 2003) and thus reduce anxiety.
Acknowledgments
Supported by NIMH grant R01 MH52619 and MH 65702
References
- Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L. The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience. 1998;84:967–996. doi: 10.1016/s0306-4522(97)00560-5. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci Lett. 2005;380:60–65. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- bEgli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sheehan DV, Surman OS, Coleman JH, Greenblatt DJ, Heninger GR, Jones KJ, Levine PH, Watkins WD. Neuroendocrine correlates of lactate-induced anxiety and their response to chronic alprazolam therapy. Am J Psychiatry. 1986;143:483–494. doi: 10.1176/ajp.143.4.483. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim SJ, Park SH, Moon BH, Do E, Chun BG, Lee MS, Shin KH. Doxapram increases corticotropin-releasing factor immunoreactivity and mRNA expression in the rat central nucleus of the amygdala. Peptides. 2005;26:2246–2251. doi: 10.1016/j.peptides.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Goetz R, Klein DF, Papp LA, Fyer AJ, Liebowitz MR, Davies SO, Gorman JM. Plasma cortisol concentrations preceding lactate-induced panic. Psychological, biochemical, and physiological correlates. Arch Gen Psychiatry. 1998;55:130–136. doi: 10.1001/archpsyc.55.2.130. [DOI] [PubMed] [Google Scholar]
- Cowley DS, Arana GW. The diagnostic utility of lactate sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47:277–284. doi: 10.1001/archpsyc.1990.01810150077012. [DOI] [PubMed] [Google Scholar]
- Cowley DS, Dager SR, Dunner DL. Lactate infusions in major depression without panic attacks. J Psychiatr Res. 1987;21:243–248. doi: 10.1016/0022-3956(87)90025-2. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Boer JA, Westenberg HG, Klompmakers AA, van Lint LE. Behavioral biochemical and neuroendocrine concomitants of lactate-induced panic anxiety. Biol Psychiatry. 1989;26:612–622. doi: 10.1016/0006-3223(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Egli RE, Winder DG. Dorsal and ventral distribution of excitable and synaptic properties of neurons of the bed nucleus of the stria terminalis. J Neurophysiol. 2003;90:405–414. doi: 10.1152/jn.00228.2003. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinetti F, Tarabusi M, Nappi G. Premenstrual syndrome and anxiety disorders: a psychobiological link. Psychother Psychosom. 1998;67:57–60. doi: 10.1159/000012260. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Romano G, Fava M, Genazzani AR. Lactate infusion induces panic attacks in patients with premenstrual syndrome. Psychosom Med. 1992;54:288–296. doi: 10.1097/00006842-199205000-00005. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Zangrossi H., Jr “One-trial tolerance” to the anxiolytic actions of benzodiazepines in the elevated plus-maze, or the development of a phobic state? Psychopharmacology (Berl) 1993;110:240–244. doi: 10.1007/BF02246980. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Viana M, Graeff FG. Trial 2 in the elevated plus-maze: a different form of fear? Psychopharmacology (Berl) 1993;111:491–494. doi: 10.1007/BF02253541. [DOI] [PubMed] [Google Scholar]
- Gaffney FA, Fenton BJ, Lane LD, Lake CR. Hemodynamic, ventilatory, and biochemical responses of panic patients and normal controls with sodium lactate infusion and spontaneous panic attacks. Arch Gen Psychiatry. 1988;45:53–60. doi: 10.1001/archpsyc.1988.01800250063008. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Liebowitz MR, Fyer AJ, Dillon D, Davies SO, Stein J, Klein DF. Lactate infusions in obsessive-compulsive disorder. Am J Psychiatry. 1985;142:864–866. doi: 10.1176/ajp.142.7.864. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Levy GF, Liebowitz MR, McGrath P, Appleby IL, Dillon DJ, Davies SO, Klein DF. Effect of acute beta-adrenergic blockade on lactate-induced panic. Arch Gen Psychiatry. 1983;40:1079–1082. doi: 10.1001/archpsyc.1983.01790090041006. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hollander E, Liebowitz MR, Gorman JM, Cohen B, Fyer A, Klein DF. Cortisol and sodium lactate-induced panic. Arch Gen Psychiatry. 1989;46:135–140. doi: 10.1001/archpsyc.1989.01810020037007. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Jensen CF, Keller TW, Peskind ER, McFall ME, Veith RC, Martin D, Wilkinson CW, Raskind MA. Behavioral and neuroendocrine responses to sodium lactate infusion in subjects with posttraumatic stress disorder. Am J Psychiatry. 1997;154:266–268. doi: 10.1176/ajp.154.2.266. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci. 2006;26:7093–7104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. Journal of Neuroscience. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman JM, Fyer A, Dillon D, Levitt M, Klein DF. Possible mechanisms for lactate’s induction of panic. American Journal of Psychiatry. 1986;143:495–502. doi: 10.1176/ajp.143.4.495. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Fyer AJ, Gorman JM, Dillon D, Davies S, Stein JM, Cohen BS, Klein DF. Specificity of lactate infusions in social phobia versus panic disorders. Am J Psychiatry. 1985;142:947–950. doi: 10.1176/ajp.142.8.947. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Ann N Y Acad Sci. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M, Reingold DF, Stanley ME. D-and L-stereoisomers of allylglycine: convulsive action and inhibition of brain L-glutamate decarboxylase. Journal of Neurochemistry. 1977;28:349–353. doi: 10.1111/j.1471-4159.1977.tb07754.x. [DOI] [PubMed] [Google Scholar]
- Pitts FN, JN lactate metabolism in anxiety neruosis. New England Journal of Medicine. 1967;227:1329–1336. doi: 10.1056/NEJM196712212772502. [DOI] [PubMed] [Google Scholar]
- Rainey JM, Jr, Aleem A, Ortiz A, Yeragani V, Pohl R, Berchou R. A laboratory procedure for the induction of flashbacks. Am J Psychiatry. 1987;144:1317–1319. doi: 10.1176/ajp.144.10.1317. [DOI] [PubMed] [Google Scholar]
- Rainnie DG. Neurons of the bed nucleus of the stria terminalis (BNST). Electrophysiological properties and their response to serotonin. Ann N Y Acad Sci. 1999;877:695–699. doi: 10.1111/j.1749-6632.1999.tb09304.x. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310:683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acidA receptor blockade in the basolateral amygdala of rats. Journal of Pharmacology & Experimental Therapeutics. 1997;283:969–977. [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. European Journal of Pharmacology. 2000;394:265–273. doi: 10.1016/s0014-2999(00)00128-x. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A, Gehlert DR. Interactions Between NPY and CRF in the Amygdala Regulate Emotionality. Neuropeptides. 2004;38:225–234. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Keim SR, Thielen SK, Fitz SD, Shekhar A. Measurements of anxiety-like behavior following intravenous infusion of sodium lactate in primed rats. Current Protocols in Neuroscience. 2003;2(Suppl 24) doi: 10.1002/0471142301.ns0917s24. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. American journal of physiology. 2004;287:R472–478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Sandberg D, Endicott J, Harrison W, Nee J, Gorman J. Sodium lactate infusion in late luteal phase dysphoric disorder. Psychiatry Res. 1993;46:79–88. doi: 10.1016/0165-1781(93)90010-e. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Research. 1991;567:101–110. doi: 10.1016/0006-8993(91)91441-3. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacology, Biochemistry & Behavior. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Shekhar A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. I. Behavioral measures. Brain Research. 1993;627:9–16. doi: 10.1016/0006-8993(93)90742-6. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacology, Biochemistry & Behavior. 1995;50:253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. Journal of Neuroscience. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. LY354740, a potent group II metabotropic glutamate receptor agonist prevents lactate-induced panic-like response in panic-prone rats. Neuropharmacology. 2000;39:1139–1146. doi: 10.1016/s0028-3908(99)00215-4. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Research. 1990;512:81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sims LS, Bowsher RR. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plus-maze test. II. Physiological measures. Brain Res. 1993;627:17–24. doi: 10.1016/0006-8993(93)90743-7. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacology, Biochemistry & Behavior. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, Gehlert DR, DiMicco JA. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar AKS, Simon JR, McBride WJ. Pharmacology Biochemistry & Behavior. Vol. 55. 1996. Dorsomedial Hypothalamic GABA Dysfunction Produces Physiological Arousal Following Sodium Lactate Infusions; pp. 249–256. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: Possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Research Bulletin. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Yeragani V, Balon R, Pohl R. Lactate infusions in panic disorder patients and normal controls: autonomic measures and subjective anxiety. Acta Psychiatr Scand. 1989;79:32–40. doi: 10.1111/j.1600-0447.1989.tb09231.x. [DOI] [PubMed] [Google Scholar]