Abstract
Patients with CKD are at higher risk for major events after percutaneous coronary intervention (PCI) compared with subjects with normal renal function. The aims of this study were to evaluate responsiveness to clopidogrel in patients with CKD and to examine the effect of antiplatelet drug response on post-PCI outcome. We retrospectively evaluated a consecutive cohort of 1567 patients with symptomatic coronary artery disease undergoing PCI, 648 (41%) of whom had stage 3 to 5 CKD. We assessed responsiveness to clopidogrel by ADP-induced platelet aggregation after oral administration of a 600-mg clopidogrel loading dose and 100 mg of aspirin. In a multivariate survival analysis that included 1335 (85%) of the cohort, stage 3 to 5 CKD and low response to clopidogrel were independent predictors of the primary end point (composite of myocardial infarction, ischemic stroke, and death within 1 year). In summary, a low response to clopidogrel might be an additional risk factor for the poorer outcomes in patients with stage 3 to 5 CKD compared with patients with better renal function.
Variability of platelet response to antiplatelet drugs is a multifactorial phenomenon, and the underlying mechanisms are of a nongenetic and genetic nature. Recently, we found a number of distinct clinical variables to be associated with persistent high platelet reactivity despite clopidogrel treatment,1 resulting in an increased rate of artherothrombotic complications.2
Patients with CKD are at high risk to develop cardiovascular events, and renal insufficiency is associated with higher comorbidity and mortality after percutaneous coronary interventions (PCI). Impaired renal function has been characterized as a major predictor for artherothrombotic complications including thrombotic stent occlusions3–6 as well as bleeding events.3 Management of coronary artery disease in patients with CKD can represent a challenge as many of the drugs used in the treatment and prevention of coronary artery disease are metabolized or excreted by the kidney. The role of renal function for responsiveness to antiplatelet therapy has been addressed in only a small number of studies.7,8 Recently, a small randomized study suggested that impairment of renal function is associated with poorer response to clopidogrel.9 The aim of this study was to evaluate the role of renal function on platelet reactivity in patients receiving dual antiplatelet therapy with aspirin and clopidogrel and the prognostic effect of antiplatelet drug responsiveness on the outcome in patients with chronic kidney disease.
RESULTS
1567 patients were enrolled in the platelet aggregation study. The cohort consisted of consecutive, unselected patients who underwent coronary stenting for symptomatic coronary artery disease. Baseline characteristics for patients stratified to quartiles of residual platelet aggregation (RPA) are shown in Table 1. There were major differences between the groups. Thus, there was a clear trend in the associations of older age and cardiovascular risk factors like diabetes with higher levels of RPA. Additionally, we observed relevant differences of risk factor distribution between patients with CKD and normal renal function (not shown). This was mainly due to the higher frequency of comorbidities associated with CKD. Thus, CKD patients were more likely to have diabetes (P < 0.001), arterial hypertension (P < 0.01), and poor left ventricular function (P < 0.001) compared with patients without CKD. Additionally, patients with CKD were older, more often female, and more likely to be treated with angiotensin-receptor blockers, calcium-channel blockers, and diuretics.
Table 1.
Characteristics of the study population
| Variable | Total | First Quartile of PA | Second Quartile of PA | Third Quartile of PA | Fourth Quartile of PA | P value for Fourth Quartile versus First, Second, and Third Quartiles |
|---|---|---|---|---|---|---|
| Gender, men/women (%) | 1170 (74.7)/397 (25.3) | 310 (74.5)/106 (25.5) | 330 (77.8)/94 (22.2) | 313 (75.8)/100 (24.2) | 290 (72.5)/110 (27.5) | 0.12 |
| Age (years)a | 67.4 ± 0.27 | 65.1 ± 12.4 | 67.5 ± 10.4 | 68.5 ± 9.8 | 69.2 ± 9.6 | 0.001 |
| Acute coronary syndrome (%) | 855 (54.9) | 207 (50.5) | 219 (51.8) | 211 (51.3) | 228 (57.3) | 0.05 |
| Left ventricular function (%) | ||||||
| Ejection fraction | ||||||
| 45 to 55% | 359 (23.1) | 99 (24.2)) | 84 (19.9) | 86 (20.8) | 102 (25.8) | <0.001 |
| 35 to 45% | 247 (15.9) | 50 (12.2) | 62 (14.7) | 54 (13.1) | 87 (22.0) | |
| <35% | 167 (15.9) | 33 (8.1) | 39 (9.2) | 47 (11.4) | 53 (13.4) | |
| Hypertension (%) | 1259 (80.3) | 314 (76.6) | 348 (82.1) | 346 (84.4) | 316 (80.2) | 0.67 |
| Hyperlipidemia (%) | 943 (60.2) | 251 (61.2) | 261 (61.6) | 266 (64.9) | 227 (57.6) | 0.1 |
| Diabetes (%) | 499 (32.4) | 96 (23.9) | 118 (28.2) | 143 (35.5) | 155 (39.7) | <0.001 |
| Smoking history (%) | 521 (33.2) | 176 (26.7) | 172 (40.6) | 156 (38.0) | 156 (39.6) | 0.54 |
| Glomerular filtration rate (MDRD; ml/min per 1.73 m2)a | 64.4 (± 22.7) | 67.5 (± 22.2) | 65.2 (± 22.9) | 65.5 (± 22.6) | 59.6 (± 23.5) | <0.001 |
| Contrast agent (ml)a | 252 ± 101.5 | 242 ± 96.6 | 263.8 ± 107 | 268.7 ± 102.5 | 254.7 ± 95.9 | 0.5 |
| Medication | ||||||
| ACE inhibitors (%) | 1241 (80.3) | 324 (79.0) | 335 (80.0) | 340 (83.1) | 315 (80.4) | 0.95 |
| Angiotensin receptor blockers (%) | 202 (13.1) | 54 (13.2) | 40 (9.5) | 54 (13.2) | 63 (16.1) | 0.08 |
| Beta-Blockers (%) | 1430 (92.7) | 376 (91.7) | 392 (93.8) | 379 (93.1) | 358 (91.3) | 0.26 |
| Calcium-channel blockers (%) | 228 (14.8) | 61 (25.1) | 51 (21.0) | 74 (30.5) | 57 (23.5) | 0.96 |
| Diuretics (%) | 739 (48.4) | 186 (46.4) | 203 (48.4) | 191 (47.5) | 200 (51.5) | 0.13 |
| Statins (%) | 1369 (88.4) | 362 (87.9) | 378 (89.6) | 365 (89.5) | 339 (86.5) | 0.28 |
| Aspirin (100 mg/day) | 698 (44.6) | 162 (41.4) | 180 (46.8) | 184 (45.9) | 172 (44.4) | 0.86 |
| Clopidogrel (75 mg/day) | 70 (4.5) | 27 (6.9) | 17 (4.4) | 17 (4.2) | 9 (2.3%) | 0.022 |
| GPIIb-IIIa inhibitor (abciximab) | 191 (11.7) | 47 (11.4) | 32 (7.6) | 57 (14.0) | 55 (13.9) | 0.13 |
| Number of stentsa | 1.41 (± 0.72) | 1.34 (± 0.66) | 1.41 (± 0.74) | 1.44 (± 0.77) | 1.42 (± 0.69) | 0.72 |
| Type of stent: bare metal/drug eluting/both | 1143 (74.1)/297 (19.2)/103 (6.7) | 284 (73.7)/78 (20.3)/23 (6.0) | 286 (71.7)/79 (19.8)/34 (8.5) | 282 (73.6)/74 (19.4)/27 (7.0) | 291 (77.3)/66 (17.6)/19 (5.1) | 0.11 |
ACE, angiotensin-converting enzyme.
aMean ± standard deviation.
All of the patients were prescribed clopidogrel (75 mg/d) and aspirin (100 mg/d) treatment for at least 3 months after the index PCI. There was no significant difference concerning the prevalence of clopidogrel dosing regimens and antiplatelet pretreatment depending on subgroups of patients with different stages of CKD. Residual platelet aggregation was divided into quartiles of equal-sized groups. Thus, the border of the upper quartile (75% percentile) was defined by a value of >47.5%. There was a significant trend of low responders (as defined by final RPA >75th percentile) among groups classified according to the Kidney Disease Outcomes Quality Initiative (K/DOQI) index (Figure 1).
Figure 1.
Percentage of low responders to clopidogrel increases according to deterioration of renal function.
There were 31 patients with K/DOQI V participating in a chronic dialysis program, of which two patients received postprocedural hemodialysis on the day of coronary intervention. Platelet function testing was performed between PCI and hemodialysis.
Incidence of Major Events
A consecutive cohort of 1335 (85.2%) patients were followed up regarding the occurrence of death, myocardial infarction, and ischemic stroke for a mean follow-up of 266 days. Within this time a total of 122 of these events (5.9%) occurred in the study population. Cumulative event rate was significantly higher in patients with poor renal function (K/DOQI IV+V) (Figure 2). There was higher percentage of combined events and mortality in low responders to clopidogrel, especially in those with poor baseline renal function (Table 2).
Figure 2.
In adjusted cumulative event analysis including myocardial infarction and death, patients with severely impaired renal function (K/DOQI IV+V) show the highest risk for combined events.
Table 2.
Incidence of major events according to clopidogrel response status and renal function
| Major Events within Follow-up (n) (%) | Low-Responder Group 1 (n = 165) | Responder Group 1 (n = 396) | P | Low-Responder Group 2 (n = 161) | Responder Group 2 (n = 613) | P |
|---|---|---|---|---|---|---|
| First combined major event within follow-up | 30 (18.2) | 46 (11.6) | 0.038 | 11 (6.8) | 35 (5.7) | 0.59 |
| Myocardial infarctiona | 17 (10.2) | 20 (5.1) | 0.024 | 9 (5.7) | 21 (3.4) | 0.20 |
| Ischemic strokea | 2 (1.2) | 16 (4.1) | 0.08 | 1 (0.6) | 6 (1.0) | 0.67 |
| Deatha | 17 (10.3) | 22 (5.6) | 0.04 | 3 (1.9) | 10 (1.6) | 0.85 |
aThe numbers do not sum up to a total combined event rate because of multiple occurrences of major events within follow-up in some patients.
Multivariate and Survival Analysis
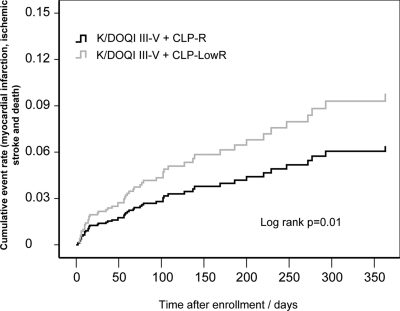
After inclusion of relevant variables in Cox proportional hazard survival regression (enter method), clopidogrel low response and renal function impairment could be identified as independent predictors of the combined end point in addition to left ventricular dysfunction, acute coronary syndrome on admission, and diabetes mellitus. Concomitant use of β-blockers and pretreatment with clopidogrel was associated with an apparent beneficial effect on the outcome in the multivariate analysis (Table 3). In a Kaplan-Meier analysis, patients belonging to group I (K/DOQI III–V) and a final aggregation >75th percentile showed a significantly higher cumulative event rate compared with those with normal to low platelet aggregation (log rank 0.01; Figure 3). Additionally, we demonstrated an interaction effect between clopidogrel response and CKD stage by means of a Cox-regression analysis adjusted for the previously included covariates as the hazard significantly increased in low responders with poor renal function (Table 3).
Table 3.
Identification of independent predictors of the combined end point selected by Cox regression analysis
| Unadjusted Hazard |
Sig. | Adjusted Hazard |
p | |||||
|---|---|---|---|---|---|---|---|---|
| Ratio | 95% Confidence Interval |
Ratio | 95% Confidence Interval |
|||||
| Lower | Upper | Lower | Upper | |||||
| eGFR (MDRD formula) | 0.97 | 0.63 | 0.98 | <0.001 | 0.99 | 0.98 | 1.0 | 0.004 |
| Clopidogrel low response (RPA >75% percentile) | 2.08 | 1.42 | 3.03 | <0.001 | 1.64 | 1.06 | 2.54 | 0.026 |
| Diabetes mellitus | 2.17 | 1.49 | 3.16 | <0.001 | 1.68 | 1.1 | 2.57 | 0.02 |
| ACS | 2.2 | 1.49 | 3.26 | <0.001 | 1.78 | 1.14 | 2.77 | 0.01 |
| Impaired left ventricular function | 2.42 | 1.64 | 3.59 | <0.001 | 1.66 | 1.04 | 2.66 | 0.04 |
| ACE inhibitors | 0.75 | 0.48 | 1.17 | 0.203 | 0.68 | 0.38 | 1.23 | 0.21 |
| Pretreatment with aspirin | 0.9 | 0.62 | 1.29 | 0.56 | 0.98 | 0.63 | 1.52 | 0.91 |
| Pretreatment with clopidogrel | 0.63 | 0.31 | 1.3 | 0.21 | 0.34 | 0.15 | 0.77 | 0.01 |
| GPIIb-IIIa inhibitors (abciximab) | 0.81 | 0.45 | 1.44 | 0.46 | 0.8 | 0.42 | 1.52 | 0.49 |
| AT1 blockers | 1.07 | 0.61 | 1.87 | 0.819 | 0.84 | 0.39 | 1.79 | 0.84 |
| Beta blockers | 0.31 | 0.19 | 0.50 | <0.001 | 0.53 | 0.29 | 0.94 | 0.03 |
| Calcium-channel blockers | 0.99 | 0.59 | 1.67 | 0.98 | 0.75 | 0.4 | 1.39 | 0.36 |
| Diuretics | 1.22 | 0.84 | 1.78 | 0.29 | 1.02 | 0.67 | 1.54 | 0.94 |
| Statins | 0.45 | 0.28 | 0.7 | <0.001 | 0.67 | 0.39 | 1.16 | 0.15 |
| Age | 1.04 | 1.02 | 1.06 | <0.001 | 1.00 | 0.98 | 1.03 | 0.7 |
| Tobacco use | 0.65 | 0.44 | 0.98 | 0.039 | 0.95 | 0.59 | 1.53 | 0.82 |
| Arterial hypertension | 0.66 | 0.44 | 1.01 | 0.058 | 0.62 | 0.39 | 1.00 | 0.052 |
| Gender | 1.13 | 0.76 | 1.7 | 0.54 | 0.84 | 0.52 | 1.37 | 0.49 |
| Interaction effect CKD group 1, clopidogrel low responsea | 2.08 | 1.61 | 2.67 | <0.001 | 1.37 | 1.13 | 1.66 | 0.001 |
ACE, angiotensin-converting enzyme; Sig., significance; ACS, acute coronary syndrome.
aWhen adjusted for diabetes mellitus, acute coronary syndromes, impaired left ventricular, gender arterial hypertension, tobacco use, age, cardiovascular comedication, antiplatelet pretreatment, clopidogrel low response, and age.
Figure 3.
In adjusted cumulative event analysis including myocardial infarction and death, CLP-LowR significantly increases risk for combined events in patients with poor renal function.
DISCUSSION
The major finding of our study is that antiplatelet drug efficacy as measured by post-treatment platelet function represents a factor that contributes to an increased risk of patients with chronic kidney disease regarding short- and long-term adverse clinical outcomes after PCI. To our knowledge, this is the largest systematic analysis of the association between post-treatment platelet function and renal function as measured by the estimated GFR. We found that a low response to clopidogrel defined by a high post-treatment platelet aggregation >75th percentile significantly added to the risk for combined major adverse events and mortality after PCI in patients with chronic kidney disease. CKD is in any case associated with poorer clinical outcome after coronary interventions.10–14 Additionally, assessment of renal function by GFR rather than absolute creatinine values better predicts morbidity and mortality, especially in younger patients undergoing PCI.15
Recently, there have been some suggestions that clopidogrel responsiveness is reduced in patients with CKD.8 A subanalysis of the Clopidogrel for the Reduction of Events during Observation trial suggested that clopidogrel may have decreased efficacy in patients with mild to moderate CKD. That finding was not related to an increased bleeding rate.16 In a retrospective analysis of patients in the CHARISMA trial, a higher mortality was observed in asymptomatic patients with diabetic nephropathy who were randomized to clopidogrel, suggesting that clopidogrel may be harmful in that particular group.17 Recently, we established a multivariate risk model showing that renal failure as defined by a serum creatinine >1.5 mg/dl was an independent risk factor for high post-treatment platelet aggregation in PCI patients receiving clopidogrel.1
The mechanisms for decreased antiplatelet drug effects in patients with chronic kidney disease are poorly understood and could be explained by a number of disturbances found in chronic kidney disease and possibly leading to altered platelet function and reduced sensitivity to antiplatelet drugs. This includes an increase in the platelet turnover rate18 by means of increased levels of thrombospondin19,20; poor bioavailability of the active clopidogrel metabolite caused by impaired absorption or drug metabolization21,22; procoagulant factors, such as thrombin-antithrombin III complex, D-dimer, fibrinogen, fibrinopeptide A, and von Willebrand factor23,24; altered metabolism of prostaglandin25; changes in thromboxane A2-dependent platelet activation and altered expression of platelet surface receptors26,27; and extrinsic factors such as uremic toxin, anemia, and abnormality of nitric oxide synthesis.28,29
We are aware of several limitations associated with this study. First, the creatinine levels used to calculate the estimated GFR were measured only at a single time point on admission, limiting our ability to discriminate chronic kidney disease and acute worsening of pre-existing kidney injury that may occur as a result of hemodynamic compromise in the setting of acute coronary events. Platelet function was only assessed by a single measurement and a single method. Although we used the “gold standard” of platelet function testing (light transmittance aggregometry), we did not attempt to reproduce the results using different available assays. This study uses observational data, and conclusions are not based on randomized study results. Thus, outcome may be related to unidentified risk factors especially those evolving over time. Thus, we cannot exclude the effect of procedural and plaque characteristics to have an additional influence on clinical outcome in these patients, and increased coronary calcification has been described in patients with chronic kidney disease,30,31 representing a moderate prognostic factor in this risk group.32,33 Finally, we were unable to determine bleeding events in a systematic way. Because bleeding is a major risk factor for post-PCI mortality and renal failure is associated with increased bleeding risk,34 we cannot exclude a higher rate of major bleeding in patients with CKD being responsible for the higher mortality rate observed. We are fully aware that this study is merely hypothesis generating. However, because of the sparse data in this field, there is further need to characterize antiplatelet drug efficacy and to re-evaluate first observations deriving from smaller studies and case reports. To our knowledge, this is the largest cohort study that systematically investigates the association of kidney function and antiplatelet drug response.
In conclusion, these data suggest that chronic kidney disease is associated with poor responsiveness to thienopyridine (clopidogrel) treatment and that high post-treatment platelet aggregation significantly worsens the prognosis of patients with CKD undergoing PCI. Therefore, platelet function should be monitored in individuals with renal impairment to adjust antiplatelet therapies after careful consideration of bleeding risk.
Concise Methods
This was a single center observational study conducted at the University Hospital (Tübingen, Germany). Patients admitted for percutaneous coronary intervention for symptomatic coronary artery disease (stable coronary artery disease and acute coronary syndromes) were consecutively investigated by platelet function analysis after receiving a 600-mg clopidogrel loading dose and registered in a database. The study design was approved by the local ethics committee, and signed informed consent was obtained from all subjects. Serum samples for creatinine measurement were collected before catheterization, and renal function (GFR) was determined according to modification of diet in renal disease (MDRD) formula: GFR (ml/min per 1.73 m2) = 186 × (Pcr)−1.154 × (age)−0.203 (× 0.742 if female). The patients enrolled were all Caucasian. Individuals were followed up for the incidence of major cardiovascular events within a planned period of 365 days by telephone interview or review of patients' charts on admission. The predefined primary end point was the first occurrence of death, myocardial infarction, or ischemic stroke.
Definition of Renal Failure
Chronic kidney disease was defined according to the guidelines of the K/DOQI.35,36 Patients with moderate to severe chronic kidney disease were defined with a K/DOQI index of >III, and individuals were stratified to either group 1 (K/DOQI index III–V) or group 2 (K/DOQI index I–II).
Blood Sampling and Platelet Aggregation
Blood samples for platelet function analysis were obtained at an earliest time point of 6 hours after administration of 600 mg of clopidogrel, when maximum platelet inhibition was anticipated as described previously.37,38 A minority of patients already on chronic clopidogrel treatment received a loading dose of 300 mg and were measured approximately 24 hours after drug administration. Platelet function testing was performed according to previously reported protocols.39,40 In patients with CKD K/DOQI V who received chronic dialysis, platelet function testing was performed before a planned postprocedural hemodialysis session.
Definition of Clopidogrel Low Responders
Patients with a final platelet aggregation beyond the 75th percentile of all values in the study collective were classified as low responders to clopidogrel treatment. This definition and cut-off value have been used in previous reports.41,42
Statistical Analyses
The primary objective was to evaluate the association of renal function and post-treatment platelet reactivity and the first combined major event (myocardial infarction, death, or ischemic stroke) within 1 year. Taken from recent registry data investigating the effect of renal function on the 1-year rate of events after PCI,3 we assumed a difference of the combined end point of at least 5% between patients with K/DOQI III–V (group 1) and KDOQI I–II (group 2). With an estimated prevalence of 35%, a sample size of 1090 was calculated to detect this difference of event rate with a statistical power of 90% at a two-sided α level of 5%. The continuous data are expressed as the means ± SD; not normally distributed data are presented as medians and interquartile ranges. Dichotomous variables are shown as number (%), and the equality of distribution between patients with platelet aggregation (PA) >75th percentile versus those with platelet aggregation (PA) ≤75th percentile was analyzed by chi-squared test (two-sided Pearson χ2). Cox proportional hazards survival regression was used to identify predictors of the primary end point. Prognostic factors and factors significantly different between groups, in detail left ventricular function, age, gender, acute coronary syndromes, diabetes mellitus, recent tobacco use, arterial hypertension, cardiovascular comedication, antiplatelet pretreatment, eGFR, and clopidogrel low response were entered in the final model. Survival curves were estimated by the Kaplan-Meier method and adjusted for above covariates. Survival across different groups were plotted for K/DOQI classes I, II, III, and IV/V and for patients with poor renal function (classes III–V) stratified according to clopidogrel response. Subgroups were compared by using the Mantel (log-rank) test. The proportional hazard assumption was assessed by a plot of log(−log(survival function)) versus time for categorical variables. Interaction between clopidogrel response and CKD stages was tested by categorizing patients according to clopidogrel low response and CKD group. Statistical analysis was performed with SPPS, version 15, for Windows (SPSS, Inc., Chicago, IL).
DISCLOSURES
None.
Acknowledgments
We greatly appreciate the excellent technical help of Florian Reichenauer Elena Ninci, and Christine Flaum. The study was supported in part by the Deutsche Forschungsgemeinschaft, the Sonderforschungsbereich Transregio TR-19, the Karl & Lore-Klein-Stiftung, and a personal grant from the European Society of Cardiology (TG).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Geisler T, Grass D, Bigalke B, Stellos K, Drosch T, Dietz K, Herdeg C, Gawaz M: The PREDICT (Residual Platelet Aggregation after Deployment of Intracoronary Stent)-Score. J Thromb Haemost 6: 54–61, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Geisler T, Zürn C, Simonenko R, Rapin M, Kraibooj H, Kilias A, Bigalke B, Stellos K, Schwab M, May AE, Herdeg C, Gawaz M: Early but not late stent thrombosis is influenced by residual platelet aggregation in patients undergoing coronary interventions. Eur Heart J 31: 59–66, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Latif F, Kleiman NS, Cohen DJ, Pencina MJ, Yen CH, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ, Saucedo JF, EVENT Investigators: In-hospital and 1-year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug-eluting stents: a report from the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) registry. JACC Cardiovasc Interv 2: 37–45, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, Kim SW, Bui A, Gevorkian N, Xue Z, Smith K, Fourn-adjieva J, Suddath WO, Satler LF, Pichard AD, Kent KM, Waksman R: Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation 113: 1108–1113, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Jacques M, Nicolas D, Jean ML, Jean MF, Jean LB, Stephanie M, Alison F, Jean LQ, Hélène E, Gérald V: Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients. J Am Coll Cardiol 50: 501–508, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A: Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293: 2126–2130, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Deray G, Bagnis C, Brouard R, Necciari J, Leenhardt AF, Raymond F, Baumelou A: Clopidogrel activities in patients with renal function impairment. Clin Drug Investig 16: 319–328, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, Ernst A, Sawhney NS, Schatz RA, Teirstein PS: Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 29: 992–1000, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Park SH, Kim W, Park CS, Kang WY, Hwang SH, Kim W: A comparison of clopidogrel responsiveness in patients with versus without chronic renal failure. Am J Cardiol 104: 1292–1295, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Naidu SS, Selzer F, Jacobs A, Faxon D, Marks DS, Johnston J, Detre K, Wilensky RL: Renal insufficiency is an independent predictor of mortality after percutaneous coronary intervention. Am J Cardiol 92: 1160–1164, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB: The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol 39: 1113–1119, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Blackman DJ, Pinto R, Ross JR, Seidelin PH, Ing D, Jackevicius C, Mackie K, Chan C, Dzavik V: Impact of renal insufficiency on outcome after contemporary percutaneous coronary intervention. Am Heart J 151: 146–152, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Sadeghi HM, Stone GW, Grines CL, Mehran R, Dixon SR, Lansky AJ, Fahy M, Cox DA, Garcia E, Tcheng JE: Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation 108: 2769–2775, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Cardarelli F, Bellasi A, Ou FS, Shaw LJ, Veledar E, Roe MT, Morris DC, Peterson ED, Klein LW, Raggi P: Combined impact of age and estimated glomerular filtration rate on in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction (from the American College of Cardiology National Cardiovascular Data Registry). Am J Cardiol 103: 766–771, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, Califf RM, Topol EJ; CREDO Investigators: The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: Results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J 155: 687–693, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Dasgupta A, Steinhubl SR, Bhatt DL, Berger PB, Shao M, Mak KH, Fox KA, Montalescot G, Weber MA, Haffner SM, Dimas AP, Steg PG, Topol EJ; CHARISMA Investigators: Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial). Am J Cardiol 103: 1359–1363, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Himmelfarb J, Holbrook D, McMonagle E, Ault K: Increased reticulated platelets in dialysis patients. Kidney Int 51: 834–839, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Linthorst GE, Folman CC, van Olden RW, von dem Borne AE: Plasma thrombopoietin levels in patients with chronic renal failure. Hematol J 3: 38–42, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Gawaz MP, Ward RA. Effects of hemodialysis on platelet-derived thrombospondin: Kidney Int 40: 257–265, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Touchette MA, Slaughter RL: The effect of renal failure on hepatic drug clearance. DICP 25: 1214–1224, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V: Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol 12: 326–332, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Sagripanti A, Cupisti A, Baicchi U, Ferdeghini M, Morelli E, Barsotti G: Plasma parameters of the prothrombotic state in chronic uremia. Nephron 63: 273–278, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Irish A: Cardiovascular disease, fibrinogen and the acute phase response: Associations with lipids and blood pressure in patients with chronic renal disease. Atherosclerosis 137: 133–139, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Remuzzi G, Cavernaghi AE, Mecca G, Donati MB, de Gaetano G: Prostacyclin like activity and bleeding in renal failure. Lancet 2: 1195–1197, 1977 [DOI] [PubMed] [Google Scholar]
- 26. Gawaz MP, Dobos G, Späth M, Schollmeyer P, Gurland HJ, Mujais SK: Impaired function of platelet membrane glycoprotein IIb-IIIa in end-stage renal disease. J Am Soc Nephrol 5: 36–46, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Ballow A, Gader AM, Huraib S, Al-Husaini K, Mutwalli A, Al-Wakeel J: Platelet surface receptor activation in patients with chronic renal failure on hemodialysis, peritoneal dialysis and those with successful kidney transplantation. Platelets 16: 19–24, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Churchill DN, Muirhead N, Goldstein M, Posen G, Fay W, Beecroft ML, Gorman J, Taylor DW: Probability of thrombosis of vascular access among hemodialysis patients treated with recombinant human erythropoietin. J Am Soc Nephrol 4: 1809–1813, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Noris M, Benigni A, Boccardo P: Enhanced nitric oxide synthesis in uremia: Implications for platelet dysfunction and dialysis hypotension. Kidney Int 44: 445–450, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Dellegrottaglie S, Saran R, Gillespie B, Zhang X, Chung S, Finkelstein F, Kiser M, Sanz J, Eisele G, Hinderliter AL, Kuhlmann M, Levin NW, Rajagopalan S: Prevalence and predictors of cardiovascular calcium in chronic kidney disease (from the Prospective Longitudinal RRI-CKD Study). Am J Cardiol 98: 571–576, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Arad Y, Goodman K, Roth M, Newstein D, Guerci AD: Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: The St. Francis Heart Study J Am Coll Cardiol 46: 158–165, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358: 1336–1345, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Attallah N, Yassine L, Fisher K, Yee J: Risk of bleeding and restenosis among chronic kidney disease patients undergoing percutaneous coronary intervention. Clin Nephrol 64: 412–418, 2005 [DOI] [PubMed] [Google Scholar]
- 35. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 36. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G: National Kidney Foundation: National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Müller I, Seyfarth M, Rudiger S, Wolf B, Pogatsa-Murray G, Schömig A, Gawaz M: Effect of a high loading dose of clopidogrel on platelet function in patients undergoing coronary stent placement. Heart 85: 92–93, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, Bestehorn HP, Büttner HJ, Neumann FJ: Time Dependence of Platelet Inhibition after a 600-mg Loading Dose of Clopidogrel in a Large, Unselected Cohort of Candidates for Percutaneous Coronary Intervention Circulation 111: 2560–2564, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Gawaz M, Ruf A, Neumann FJ, Pogátsa-Murray G, Dickfeld T, Zohlnhöfer D, Schömig A: Effect of glycoprotein IIb-IIIa receptor antagonism on platelet membrane glycoproteins after coronary stent placement. Thromb Haemost 80: 994–1001, 1998 [PubMed] [Google Scholar]
- 40. Neumann FJ, Hochholzer W, Pogatsa-Murray G, Schomig A, Gawaz M: Antiplatelet effects of abciximab, tirofiban and eptifibatide in patients undergoing coronary stenting. J Am Coll Cardiol 37: 1323–1328, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Hochholzer W, Trenk D, Bestehorn HP, Fischer B, Valina CM, Ferenc M, Gick M, Caputo A, Büttner HJ, Neumann FJ: Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol 48: 1742–1750, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H: Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 109: 3171–3175, 2004 [DOI] [PubMed] [Google Scholar]