Abstract
A highly acidic environment surrounds proximal tubular cells as a result of their reabsorption of HCO3−. It is unclear whether this luminal acidity affects proteinuria-induced progression of tubular cell damage. Here, we investigated the contribution of luminal acidity to superoxide (O2·−) production induced by oleic acid–bound albumin (OA-Alb) in proximal tubular cells. Acidic media significantly enhanced OA-Alb–induced O2·− production in the HK-2 proximal tubular cell line. Simultaneous treatment with both OA-Alb and acidic media led to phosphorylation of the intracellular pH sensor Pyk2. Highly phosphorylated Pyk2 associated with activation of Rac1, an essential subcomponent of NAD(P)H oxidase. Furthermore, knockdown of Pyk2 with siRNA attenuated the O2·− production induced by cotreatment with OA-Alb and acid. To assess whether luminal alkalinization abrogates proteinuria-induced tubular damage, we studied a mouse model of protein-overload nephropathy. NaHCO3 feeding selectively alkalinized the urine and dramatically attenuated the accumulation of O2·−-induced DNA damage and proximal tubular injury. Overall, these observations suggest that luminal acidity aggravates proteinuria-induced tubular damage and that modulation of this acidic environment may hold potential as a therapeutic target for proteinuric kidney disease.
Tubulointerstitial injury and the subsequent progressive loss of renal function is a common pathologic pathway in many forms of chronic proteinuric kidney disease.1–3 Reactive oxygen species (ROS) are among the most toxic cellular factors that directly induce tubulointerstitial injury.4–6 ROS are abundantly generated in the proximal tubular cells through the reabsorption of urinary albumin.4,5 Urinary albumin is reabsorbed by endocytosis that is mediated by the proximal tubular scavenger receptors megalin, cubilin, and CD36.4–9 Subsequently, these receptor-albumin complexes activate protein kinase C (PKC) signaling pathways, which lead to NAD(P)H oxidase-mediated (NOX-mediated) ROS generation.4–10
Physiologic but hostile intrinsic environmental factors can sometimes be an additional mechanism for the progression of chronic kidney disease (CKD).11,12 Of these factors, we assumed that the gradient of decreasing pH in proximal tubular fluid, which is associated with normal acid-base homeostasis, might play a prominent role in the progression of proteinuria-induced tubular damage. Recently, alkali supplementation has been reported to attenuate the deterioration of kidney function in CKD patients with metabolic acidosis.13,14 These data suggest a pathogenic role of an acidic environment for CKD progression, as well as the potential of therapeutic intervention targeting luminal acidity for CKD treatment. However, there has been only limited information as to how the physiologic low pH level may contribute to, or exacerbate, ROS accumulation in proteinuric kidney disease.
One of the most likely candidate molecules to mediate this ROS effect is proline-rich tyrosine kinase 2 (Pyk2), which may convert the signal generated by extracellular acidity into intracellular ROS generation. Pyk2 is a cytoplasmic nonreceptor tyrosine kinase, which is activated by autophosphorylation when cells are grown in an acidified medium.15,16 Phosphorylated Pyk2 directly activates c-Src,15,16 which subsequently activates Rac1, a functional cytosolic subunit of NOX.17,18 The NOX complex including Rac1 induces ROS production by transferring electrons from NAD(P)H to oxygen.17,18 Furthermore, Pyk2 is activated through a PKC pathway that is induced by several types of extracellular factors.19–21 Thus, the combined evidence led us to hypothesize that a proteinuric condition in combination with a physiologic acidic environment might cooperatively aggravate ROS production in renal proximal tubular cells through Pyk2-dependent pathways.
In the present study, we demonstrated that albumin treatment together with exposure to an acidic environment strongly activated the Pyk2 kinase signaling pathway and subsequently increased O2·− production in the proximal tubular cell line. In a mouse model of protein-overload nephropathy, luminal alkalinization by NaHCO3 feeding significantly attenuated oxidative DNA damage in tubular epithelial cells. Thus, luminal acidity can be a physiologic precipitating factor leading to more severe proteinuria-induced tubular damage.
RESULTS
Effect of Acidic Conditions on OA-Alb–Induced O2·− Production
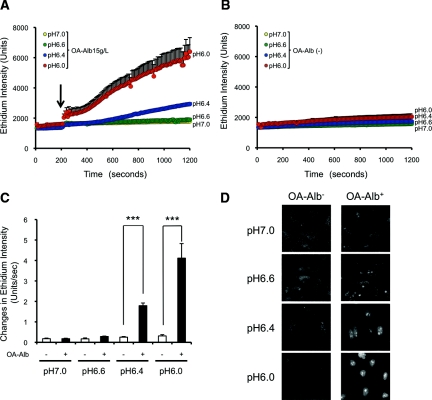
Oleic acid–bound albumin (OA-Alb) is a major component of free fatty acid–bound albumin (FFA-Alb) in the serum and urine of CKD patients.22 The oleic acid adduct has been reported to exacerbate albumin-induced ROS accumulation in proteinuric kidney disease.10,23 Therefore, we first examined the effect of acidification on OA-Alb–induced O2·− production in HK-2 cells. HK-2 cells were incubated at physiologically relevant pH levels (pH 7.0, 6.6, 6.4, or 6.0) with or without OA-Alb stimulation, and the cellular O2·− level was quantified using dihydroethidium (DHE), an O2·−-specific fluorescence probe. Flow cytometric analysis using SNARF, a fluorescence pH indicator, demonstrated that the intracellular pH level indeed decreased with increasing acidity of the culture media (Supplemental Figure 1). Acidification of the media alone did not induce O2·− production in the HK-2 cells (Figure 1, B through D). OA-Alb stimulation (15 g/L) induced little O2·− production under normo-pH (pH 6.6 to 7.0) conditions (Figure 1, A, C, and D). However, surprisingly, OA-Alb–induced O2·− production was dramatically increased under lower pH conditions (pH 6.0 or 6.4; Figure 1, A, C, and D). At pH 6.4, OA-Alb administration (15 g/L) significantly increased the rate of O2·− production, which was sevenfold higher than that in the unstimulated (OA-Alb−) control. Furthermore, at pH 6.0, the O2·− production rate increased up to 13-fold greater than the OA-Alb− control (Figure 1C). This increase in the O2·− production rate was dependent on the concentration of OA-Alb (5 to 30 g/L at pH 6.4; Figure 2, A and B).
Figure 1.
An acidic environment increases OA-Alb–induced superoxide (O2·−) production. HK-2 cells were incubated in HBSSH/l-arginine at a physiologically relevant pH (pH 7.0 to pH 6.0) with or without OA-Alb stimulation (15 g/L). O2·− production was assayed using the O2·−-specific fluorescence probe, DHE. (A) OA-Alb, administered at 200 seconds after DHE (10 μM) loading (arrow) led to increased O2·− production at pH 6.0 (red) and pH 6.4 (blue). (B) Acidification without OA-Alb stimulation did not induce O2·− production in HK-2 cells (P = 0.074 among all pH conditions). (C) The O2·− production rate was significantly increased up to 7-fold at pH 6.4, and up to 13-fold at pH 6.0, compared with the OA-Alb− control (***P < 0.001). (D) Representative photomicrographs of HK-2 cells, with or without OA-Alb stimulation at the indicated pH, 1000 seconds after DHE loading, are shown. The white signals indicate O2·− production. All the experiments were repeated at least six times, and data are expressed as the mean ± SEM.
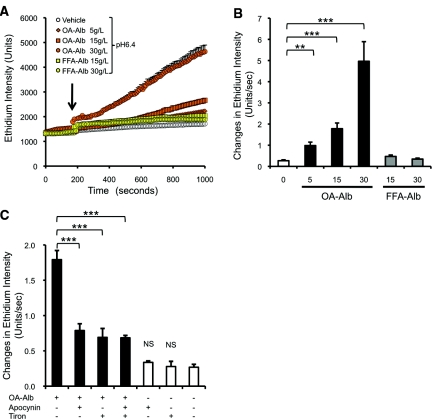
Figure 2.
OA-Alb administration induces superoxide (O2·−) production mediated by NAD(P)H oxidase in HK-2 cells. (A) OA-Alb administration (5 to 30 g/L) dose dependently induced O2·− production (orange symbols) in HK-2 cells. FFA-Alb–induced O2·− production in HK-2 cells was only slightly higher than that induced by the vehicle (yellow symbols). OA- or FFA-Alb was administered at 200 seconds after DHE (10 μM) loading (arrow) at pH 6.4. (B) The O2·− production rate was significantly increased by OA-Alb administration in a dose-dependent manner. (C) OA-Alb–induced O2·− production was attenuated by pretreatment with either the NAD(P)H oxidase inhibitor, apocynin (100 μM), or by the ROS scavenger, tiron (10 mM). Simultaneous preincubation with apocynin and tiron did not show additive attenuation (P = 0.66 versus apocynin treatment). Pretreatment with either apocynin or tiron alone did not increase O2·− production (P = 0.27 versus no treatment). All the experiments were repeated at least four times, and data are expressed as the mean ± SEM. The statistical significance of the differences are indicated (**P < 0.01 and ***P < 0.001).
We next attempted to examine whether oleic acid adducts have a greater effect on albumin-induced ROS generation than other types of fatty acid adducts. To this end, we administered the same dose of FFA-Alb, to which various types of fatty acids were randomly conjugated. Although the FFA-Alb administration has been reported to induce ROS accumulation in HK-2 cells over longer incubations (approximately 2 hours),23 the shorter exposure of FFA-Alb (approximately 1000 seconds) induced a relatively low level of O2·− production compared with that induced by OA-Alb at pH 6.4 (Figure 2, A and B). Overall, these results clearly demonstrate that an acidified medium enhances OA-Alb–induced O2·− production, and moreover that the oleic acid adduct evokes more significant cellular damage than the random FFA conjugates.
Contribution of NAD(P)H Oxidase to the OA-Alb–Induced O2·− Production
We next examined the contribution of NOX to O2·− production in OA-Alb–stimulated HK-2 cells under acidic conditions. Apocynin, a specific inhibitor of NOX, suppressed O2·− production by 56% (Figure 2C), suggesting that NOX activity contributes to O2·− production in OA-Alb–stimulated HK-2 cells. Addition of tiron, another type of ROS scavenger, also reduced O2·− production by 61%, although simultaneous treatment with both tiron and apocynin did not lead to any additive attenuation (Figure 2C).
To determine whether the increased O2·− production under acidic conditions depends on the accumulated level of OA-Alb that was absorbed by the cells, we quantified the intracellular OA-Alb level 15 minutes after exposure to OA-Alb under each pH condition. The level of intracellular OA-Alb accumulation was almost equivalent for each pH condition (Supplemental Figure 2). Therefore, this result indicated that the efficiency of OA-Alb absorption was not affected by the pH of the OA-Alb solution.
Intracellular Signaling Pathways of OA-Alb–Induced Superoxide Production
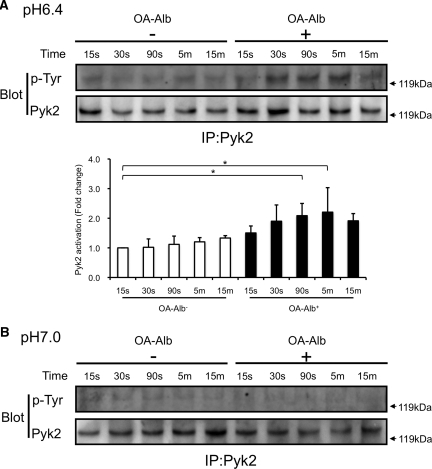
We next tried to delineate the underlying mechanism leading to the pH-dependent enhancement of O2·− production in OA-Alb–stimulated HK-2 cells. Initially, we assumed that the lowered intracellular pH level, and scavenger receptor-mediated PKC activation, cooperatively induced the Pyk2 signaling pathway, thereby resulting in synergistic ROS accumulation. To prove this hypothesis, we examined the phosphorylation status of Pyk2 in response to OA-Alb exposure at pH 6.4 and pH 7.0. Under normo-pH conditions (pH 7.0), Pyk2 phosphorylation was not augmented when the cells were stimulated with OA-Alb (Figure 3B). However, upon OA-Alb exposure at pH 6.4, strong phosphorylation of Pyk2 was observed from 30 seconds to 5 minutes after OA-Alb exposure (Figure 3A). This result is consistent with the significant increase in O2·− production observed in OA-Alb–stimulated HK-2 cells under acidic conditions (Figure 1A). Moreover, we observed that phosphorylated c-Src, which is known to be activated by Pyk2, was coimmunoprecipitated with Pyk2 upon OA-Alb exposure at pH 6.4, but not at pH 7.0, suggesting that Pyk2 phosphorylation is associated with subsequent c-Src phosphorylation (Figure 4, A and B).
Figure 3.
Pyk2 is strongly phosphorylated after OA-Alb administration under acidic conditions. HK-2 cells were incubated at pH 6.4 (A) or pH 7.0 (B), with or without OA-Alb stimulation (15 g/L) for the indicated times. Total cell lysates (diluted to 1 mg/ml) were immunoprecipitated with an anti-Pyk2 antibody and were immunoblotted with anti-Pyk2 and anti-phosphotyrosine (p-Tyr) antibodies. (A) At pH 6.4, Pyk2 was marginally phosphorylated. After OA-Alb stimulation marked Pyk2 phosphorylation was observed at pH 6.4 from 90 seconds onward. The lower panel indicates quantification of the phosphorylated Pyk2 level, which was normalized to the level of total Pyk2. The data are expressed as the mean ± SEM of at least four independent experiments. The statistical significance of the differences are indicated (*P < 0.05). (B) At pH 7.0 Pyk2 was hardly phosphorylated. OA-Alb administration did not induce phosphorylation. All gel data are representative of at least four independent experiments.
Figure 4.
c-Src phosphorylation is involved in the signaling pathway of OA-Alb–induced superoxide production. HK-2 cells were incubated at pH 6.4 (A) or at pH 7.0 (B), with or without OA-Alb stimulation (15 g/L) for the indicated times. Total cell lysates (diluted to 1 mg/ml) were immunoprecipitated with an anti-Pyk2 antibody and were then immunoblotted with anti-Pyk2, anti–c-Src, or p-Tyr antibodies. (A) Phosphorylated c-Src was coimmunoprecipitated with Pyk2 upon OA-Alb stimulation at pH 6.4. The lower panel indicates quantification of phosphorylated c-Src, which was normalized to the level of total Pyk2 protein. The data are expressed as the mean ± SEM of four independent experiments. The statistical significance of the differences are indicated (*P < 0.05). (B) At pH 7.0, c-Src was not coimmunoprecipitated with Pyk2. All gel data are representative of four independent experiments. p-Tyr, anti-phosphotyrosine.
Rac1, a member of the Rho family of small GTPases, is a subcomponent of NOX.24 c-Src is known to activate Rac1 through phosphorylation of the EGF receptor (EGFR) in a ligand-independent manner (Figure 11).18,25 On the basis of these data, we next sought to determine if Rac1 was activated upon stimulation with OA-Alb at pH 6.4. Expectedly, GTP-bound activated Rac1 was observed from 90 seconds after OA-Alb exposure (Figure 5, A and B). These observations strongly suggest that cellular OA-Alb exposure under acidic conditions accelerates the phosphorylation of Pyk2 and subsequent O2·− production via the activation of c-Src-, Rac1-, and NOX-dependent pathways.
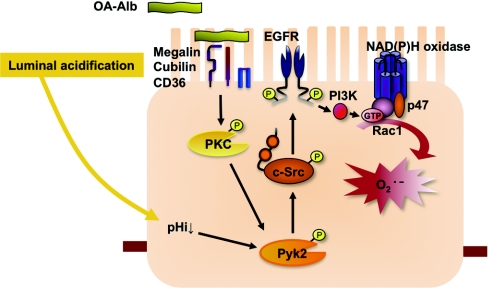
Figure 11.
Model of the intracellular signaling mechanisms underlying OA-Alb induced superoxide production in renal proximal tubular cells. Proteinuria associated with physiologic luminal acidification cooperatively induces robust Pyk2 activation in proximal tubular cells. The activated Pyk2 induces extensive ROS accumulation through activation of c-Src and NAD(P)H oxidase.
Figure 5.
Rac1, a subcomponent of NOX, is activated upon OA-Alb exposure at pH 6.4. (A) HK-2 cells were stimulated with OA-Alb (15 g/L) at pH 6.4 for the indicated times. The amount of GTP-bound active Rac1 was assessed using a glutathione-S-transferase (GST) pull-down assay with p21 activated kinase 1(PAK)-p21 binding domain (PBD) GST-fusion beads followed by immunoblotting with an anti-Rac1 antibody. GTP-bound active Rac1 increased in a time-dependent manner. (B) Quantification of GTP-bound active Rac1 normalized to total Rac1 levels. The total level of Rac1 did not change over time. The data are expressed as the mean ± SEM of three independent experiments. The statistical significance of the difference is indicated (*P < 0.05).
Role of Pyk2 in OA-Alb–Induced Apoptosis under Acidic Conditions
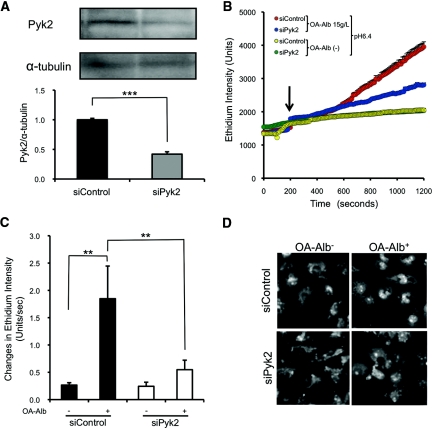
To further elucidate the functional significance of Pyk2 in O2·− production, we knocked down Pyk2 expression using specific small-interference RNA (siRNA). Transient introduction of an anti-Pyk2 siRNA suppressed Pyk2 protein expression by 58% (Figure 6A). Upon OA-Alb (15 g/L) administration at pH 6.4, O2·− production was significantly suppressed by transient Pyk2 knockdown (siPyk2) in HK-2 cells, in comparison with that in the negative control siRNA transfectants (siControl) (Figure 6, B through D).
Figure 6.
Pyk2 knockdown attenuates OA-Alb–induced O2·− production. (A) Pyk2-specific siRNA (siPyk2) reduced the protein level of Pyk2 by 58% as assessed by Western blotting. α-Tubulin was assayed as a loading control. (B) Pyk2 knockdown attenuated OA-Alb–induced O2·− production at pH 6.4. OA-Alb was administered 200 seconds after DHE (10 μM) loading (arrow). (C) OA-Alb–induced O2·− production was significantly attenuated by 70% by Pyk2 knockdown. (D) Representative photomicrographs of HK-2 cells with and without OA-Alb stimulation at 1000 seconds after DHE loading are shown. All the experiments were repeated at least four times, and the data in (A) and (C) are expressed as the mean ± SEM. The statistical significance of the differences in (A) and (C) are indicated (**P < 0.01 and ***P < 0.001).
To determine the consequences of Pyk2 knockdown for cellular viability upon treatment with OA-Alb under acidic conditions, we examined the apoptotic status of HK-2 cells by flow cytometric analysis using propidium iodide (PI)/Annexin-V costaining (Figure 7B; see Concise Methods).26,27 As expected, OA-Alb exposure at pH 6.4 induced a higher level of apoptosis either in siPyk2 or siControl cells (Figure 7, A and C). However, notably, Pyk2 knockdown significantly reduced the percentage of OA-Alb–induced apoptotic cells compared with the siControl cells (Figure 7, A and C). The combined observations clearly demonstrate a crucial role for Pyk2 in O2·− production and subsequent apoptotic cell death upon OA-Alb stimulation under acidic conditions.
Figure 7.
Protein overload with acidic exposure reduces cellular viability through Pyk2-dependent pathways. (A and B) Cellular viability was assessed using flow cytometry and Annexin V/PI costaining. Early apoptotic cells resided in the Annexin-V–single positive fraction (lower right), viable cells were negative for both Annexin-V and PI (lower left), and late apoptotic and necrotic cells stained positively for both stains (upper right). The percentage of cells in each quadrant is indicated. (C) The number of Annexin-V+/PI− early apoptotic cells (red rectangles in A) was increased upon exposure to OA-Alb (15 g/L) at pH 6.4. siRNA knockdown of Pyk2 suppressed the OA-Alb–induced apoptotic cell death. All the experiments were repeated at least four times. The data in (C) are expressed as the mean ± SD. The statistical significance of the differences are indicated (**P < 0.01 and ***P < 0.001).
Therapeutic Effect of Luminal Alkalinization on ROS Generation and Oxidative DNA Damage in a Murine Protein-Overload Nephropathy Model
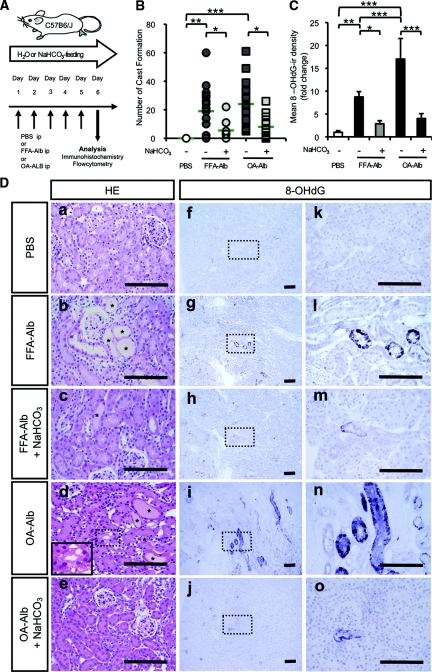
To further elucidate the role of proximal luminal pH on the progression of proteinuric nephropathy in vivo, we utilized the murine model of protein-overload nephropathy induced by either FFA-Alb or OA-Alb administration (Figure 8A).28,29 Histologic examination of either group of the protein-overloaded mice consistently demonstrated marked tubular dilation, cast formation, and interstitial edema in the medullary ray and at the outer stripe of the outer medulla, whereas no gross abnormality was observed in PBS-injected control animals (Figure 8, D-a, D-b, and D-d). Quantification of cast formation showed a statistically significant increase in both groups of protein-overloaded mice compared with the control group (Figure 8B). Notably, OA-Alb–administered mice showed more severe histologic changes than FFA-Alb treatment, consistent with the more profound toxicity of OA-Alb to HK-2 cells. Indeed, the number of cast formations was slightly higher in the OA-Alb group than in the FFA-Alb group (Figure 8B), and the tubular vacuolization was more predominantly observed in the OA-Alb group (inlet in Figure 8D-d and asterisks in Figure 9I). We next induced luminal alkalinization by feeding protein-overloaded mice with NaHCO3 in their drinking water. NaHCO3 feeding significantly alkalinized the urine pH, whereas the systemic acid-base balance was well maintained (Table 1). Interestingly, the NaHCO3 feeding dramatically attenuated the tubular injury in both groups of the protein-overloaded mice (Figure 8, D-c and D-e, and Figure 8B).
Figure 8.
Luminal alkalinization attenuates oxidative DNA damage in a mouse model of protein-overload nephropathy. (A) C57B6/J mice were treated with daily intraperitoneal (ip) FFA- or OA-Alb injections for 5 consecutive days. The mice were analyzed on day 6. PBS was injected into control animals (ip). Luminal alkalinization was induced by feeding 200 mM NaHCO3 in the drinking water. (D) Staining of kidney sections with H&E (D, left column) revealed that protein overload induced tubular dilation, flattening of tubular cells, cast formation (*), and interstitial edema (D-b and -d). Note OA-Alb injection induced vacuolization of tubular cells (inlet in D-d). Luminal alkalinization by feeding NaHCO3 attenuated the proteinuria-induced tubular damage (D-c and -e). Upon FFA- or OA-Alb injections, the cytoplasmic, that is, mitochondrial, and nuclear 8-OHdG-ir were detected in renal tubular cells located mainly in the outer stripe of outer medulla, extending to the cortex (D-g, -l, -i, and -n). Luminal alkalinization markedly attenuated the accumulation of 8-OHdG-ir (D-h, -m, -j, and -o). There were no abnormalities in the control sections (D-a, -f, and -k). Scale bars: 100 μm. (B) The number of casts formed in the randomly selected cortical area was markedly increased with protein overload, and luminal alkalinization reduced this histologic change (n > 8 in each group). (C) The mean 8-OHdG-ir density was significantly increased with protein overload and dramatically reduced by luminal alkalinization (n ≥ 4 in each group). The statistical significance of the differences are indicated (*P < 0.05, **P < 0.01, and ***P < 0.001).
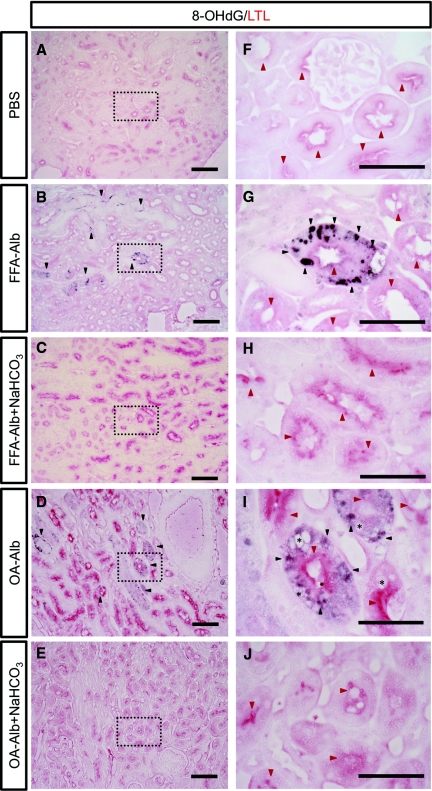
Figure 9.
Oxidative DNA damage accumulates in the proximal tubular cells. Kidney sections from FFA-Alb–injected (B, G) or OA-Alb–injected (D, I) or control PBS-injected (A, F) mice were analyzed by immunhistochemistry for colocalization of 8-OHdG-ir (black arrow heads) with LTL (red arrow heads), a marker of proximal tubule apical membranes [(F) through (J) are higher magnifications of the dotted square in (A) thorugh (E), respectively]. 8-OHdG-ir colocalized with LTL after FFA- or OA-Alb injections. Note that 8-OHdG-ir was observed in both dilated and nondilated tubules (B). Tubular vacuolization was more predominantly observed in the OA-Alb–injected group (asterisk, I). 8-OHdG-ir was not observed in the PBS-injected control section. Scale bars: 100 μm (A through E) and 50 μm (F through J). LTL, Lotus tetragonobulus lectin.
Table 1.
Urine and blood data of mice subjected to protein overload (n = 6)
| PBS | FFA-Alb | FFA-Alb + NaHCO3 | OA-Alb | OA-Alb + NaHCO3 | |
|---|---|---|---|---|---|
| Urine | |||||
| total protein/creatinine (mg/mg) | 15.96 ± 5.25 | 132.5 ± 52.8a | 137.2 ± 97.3a | 106.1 ± 28.2a | 135.6 ± 84.3a |
| pH | 6.6 ± 0.18 | 7.6 ± 0.91a | 9.2 ± 0.56a,b | 6.2 ± 0.06 | 6.7 ± 0.12c |
| Blood | |||||
| pH | 7.36 ± 0.034 | 7.39 ± 0.088 | 7.33 ± 0.063 | 7.43 ± 0.055 | 7.45 ± 0.041 |
| total CO2 (mmol/L) | 20.3 ± 2.3 | 23.9 ± 3.4 | 22.8 ± 3.4 | 24.6 ± 2.0 | 25.8 ± 2.6a |
All data are expressed as the mean ± SD.
aP < 0.05 compared with PBS-administered control.
bP < 0.05 compared with FFA-Alb–administered control.
cP < 0.05 compared with OA-Alb–administered control.
To evaluate the genotoxic effects of proteinuria-induced oxidative stress, paraffin sections of kidneys were stained with an anti–8-OHdG antibody as a marker of oxidative DNA damage.30 Interestingly, 8-OHdG-immunoreactivity (-ir) was markedly accumulated around the medullary ray and the outer stripe of the outer medulla in the protein-overloaded kidney, whereas little or no 8-OHdG-ir was observed in the control sections (Figure 8, D-f, D-k, D-g, D-l, D-i, and D-n, and Figure 8C). Expectedly, OA-Alb administration induced more abundant 8-OHdG-ir than FFA-Alb treatment, confirming the more potent toxicity of OA-Alb (Figure 8C). Interestingly, NaHCO3 feeding significantly lowered the numbers of 8-OHdG-ir cells in both groups of the protein-overloaded mice, in agreement with the histologic improvement (Figure 8, D-h, D-m, D-j, and D-o, and Figure 8C). To more precisely clarify the histologic distribution of proteinuria-induced tubular damage, we performed costaining with lotus tetragonobulus lectin (LTL), a proximal tubule-specific marker.31,32 LTL reactivity was observed along the brush border of the proximal tubule (Figure 9, A and F).31,32 Notably, 8-OHdG-ir colocalized with the LTL-positive proximal tubular cells, suggesting that O2·−-induced DNA damage occurred selectively in the proximal tubular cells (Figure 9, B, G, D, and I).
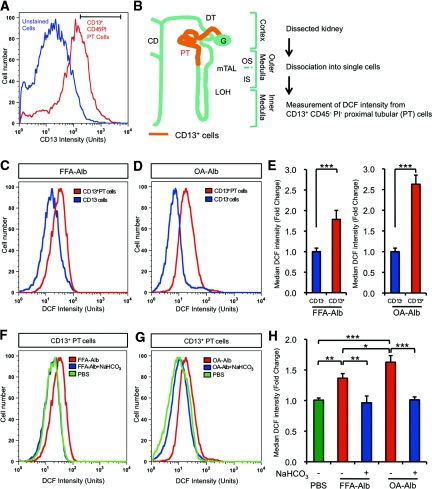
For a more quantitative ROS analysis focusing on the proximal tubular cells, we performed a series of flow cytometric analyses. For this purpose, we utilized PE-conjugated anti-CD13 antibody to isolate proximal tubular cells (Figure 10, A through H).33,34 Hematopoietic or dead cells were eliminated by gating out CD45+ and/or PI+ cells. CD13-positive proximal tubular (PT) cells (CD13+/CD45−/PI−) were subjected to subsequent analysis by 2′-7′-dichlorofluorescein diacetate (DCFDA), a fluorescence ROS indicator (horizontal bar in Figure 10A). CD13-positive PT cells either from OA-Alb– or FFA-Alb–overloaded mice exhibited a significantly higher intensity of DCFDA fluorescence than those from the CD13-negative cells (Figure 10, C through E). Moreover, OA-Alb–treated CD13-positive PT cells showed a more marked increase of DCFDA intensity than FFA-Alb–treated CD13-positive PT cells, in good agreement with the more abundant 8-OHdG-ir in OA-Alb–treated kidney (Figure 8, C and H). These observations clearly indicate that OA-Alb overload induces more significant ROS accumulation in the proximal tubular cells than FFA-Alb overload. Expectedly, luminal alkalinization dramatically decreased intracellular ROS to a level that was almost as low as that of the PBS-injected control (Figure 10, F through H). Therefore, luminal alkalinization by NaHCO3 supplementation exerts a favorable therapeutic effect on tubulointerstitial injury caused by protein-overload nephropathy.
Figure 10.
Luminal alkalinization attenuates ROS production in a mouse model of protein-overload nephropathy. ROS level of CD13+ renal PT cells from C57BL6/J mice treated with FFA-Alb or OA-Alb were analyzed by FACS using DCFDA. (A) A representative histogram of unstained kidney cells (blue line) and CD13+ PT cells (red line). The CD13+ PT cell fraction (indicated by the horizontal bar) was subjected to the DCFDA analysis. CD45+ hematopoietic cells and PI+ dead cells were gated out throughout the FACS experiments. (B) A diagram showing the tissue distribution of CD13+ PT cells in the nephron. (C) and (D) DCFDA FACS analysis. The ROS level in the CD13+ PT cell fraction (red line) was strongly increased upon FFA- or OA-Alb administration compared with the CD13− cell fraction (blue line). (E) The median intensity of the DCFDA fluorescence in (C) and (D). OA-Alb treatment induces more marked ROS accumulation in CD13+ PT cells than FFA-Alb treatment does. (F) and (G) FFA- or OA-Alb–induced ROS accumulation (red line) was attenuated by the luminal alkalinization induced by NaHCO3 feeding (blue line). The green line indicates the ROS level in the PBS-injected mouse. (H) The median intensity of the DCFDA fluorescence in (F) and (G). All data are expressed as the mean ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001; n = 6 in each group). LOH, loop of Henle; mTAL, medullary thick ascending limb; DT, distal tubule; CD, collecting duct; OS, outer stripe; IS, inner stripe.
DISCUSSION
In the present study, we have demonstrated three major findings. First, OA-Alb administration more prominently induced O2·− production in HK-2 cells than FFA-Alb, which was enhanced by acidic exposure. Second, Pyk2-dependent intracellular signaling pathways were activated during this accelerated O2·− production process. Finally, luminal alkalinization attenuated the oxidative DNA damage observed in a murine protein-overload nephropathy model. These findings represent a step forward in our understanding of how the acidity of urine affects oxidative stress production in diseased kidneys.
The pH-Dependent Oxidant Production
Assuming that the decreasing pH of proximal tubular fluid could exaggerate proteinuria-induced ROS accumulation, we first examined the effect of an acidified OA-Alb solution on O2·− production in HK-2 cells. Previous micropuncture studies using rat kidneys demonstrated that the luminal pH of the proximal portion of the proximal tubules was 7.06 ± 0.15, and that of the distal portion was 6.70 ± 0.50 or could reach pH 6.41 ± 0.068 with maximal reabsorption of HCO3−.35,36 Furthermore, another report showed that the pH of the distal portion of proximal tubule was even lower, i.e., pH 6.2, in acidotic rats.37 However, the pH level that was directly determined in those studies was measured only at accessible superficial sites (S1 and S2). The deeper and more distal portions of the proximal tubular fluid (S3) could not be directly examined in vivo.38 Meanwhile, an in vitro microperfusion study demonstrated that the S3 proximal tubule is still capable of reabsorbing bicarbonate,39 suggesting that the luminal pH in those deeper proximal tubules could be more acidic than that in the measurable superficial part, and could potentially even be as low as the pH of the final urine (pH approximately 6.0).38 On the basis of these studies, a reasonable estimate of the normal physiologic acidity in the renal proximal tubule is that it may reach pH 6.4 or lower.
Intracellular Signaling Mechanism
Pyk2 is a nonreceptor tyrosine kinase that is autophosphorylated upon direct sensing of the intracellular pH level in proximal tubular cells.15,16 Pyk2 phosphorylation is induced by phorbol 12,13-dibutyrate, a direct agonist of protein kinase C (PKC).19–21 Given that PKC is activated downstream of scavenger receptor-mediated albumin endocytosis,4–10 we surmise that simultaneous stimulation of both OA-Alb and intracellular pH acidification cooperatively amplifies Pyk2 phosphorylation. Indeed, Pyk2 was highly phosphorylated after OA-Alb administration under acidic conditions. In agreement with this observation, Pyk2 knockdown experiments demonstrated that Pyk2 plays a crucial role in the augmentation of O2·− production and the resultant apoptosis (Figure 11). To test in vivo significance of this intracellular pathway, it would be intriguing to examine whether Pyk2-deficient mice are resistant to proteinuria-induced tubular injury.
Effect of Luminal Alkalinization on Protein-Overload Nephropathy
The murine protein-overload nephropathy is one of the most popular animal models of tubulointerstitial injury caused by albuminuria.28,29,40–43 In this model, we observed dense nuclear 8-OHdG-ir, which indicates nuclear DNA damage in renal tubular cells.30 The luminal pH is highly acidic in the medullary ray and at the outer stripe of the outer medulla, where the straight portion of the proximal tubule (S3) is located.35–39 Importantly, a detailed histologic analysis revealed that 8-OHdG-ir cells were concomitantly distributed around the medullary ray and at the outer stripe of the outer medulla. Consistent with this observation, the histochemical analysis using LTL as well as the flow cytometric analysis using an anti-CD13 antibody demonstrated that albuminuria-induced oxidative stress was prominent in proximal tubular cells. This observation indicates that a physiologic lower luminal pH indeed enhances albuminuria-induced DNA damage in proximal tubular cells.
The favorable effects of administration of exogenous alkali have been reported in a murine polycystic kidney disease model and in a 5/6-nephrectomized rat model.44–47 More recently, daily bicarbonate supplementation was reported to halt the progression of proteinuric human CKD without metabolic acidosis.48 The underlying mechanism of these therapeutic effects could be explained by our present observation that luminal alkalinization suppresses intracellular ROS level in tubular cells. To firmly establish the therapeutic potential of luminal alkalinization, it would be necessary to examine whether other types of proteinuric tubular injury (e.g., diabetic nephropathy and chronic glomerulonephritis) are also attenuated by NaHCO3 feeding.
In conclusion, our results suggest that the regulation of luminal pH is a therapeutic target for chronic proteinuric nephropathy. It should be considered that this treatment could effectively buffer the progression to end-stage kidney disease.
CONCISE METHODS
Cell Culture
The human proximal tubular cell line, HK-2 (purchased from ATCC), was cultured at 37°C, 95% ambient air, and 5% CO2, in renal epithelial basal medium supplemented with REGM Singlequot Bulletkit (Lonza, CC-3190). HK-2 cells retain the functional characteristics of the original proximal tubular epithelial cells.49,50
Preparation of Oleic Acid–Bound Albumin
Oleic acid–bound albumin (OA-Alb) was prepared as described previously.23 Briefly, free fatty acid–bound albumin (FFA-Alb; Sigma, A-9430) was dissolved in phosphate-buffered saline. Oleic acid (Sigma, O-1008) was added to the FFA-Alb solution at a molar ratio of 6:1 (OA:Alb), incubated at 37°C for 2.5 hours, and then sterilized through a 0.22-μm filter. The OA-Alb solution was diluted with Hank's balanced salt solution (Invitrogen, 14025) with 20 mM Hepes (Sigma, H3375)/100 μM l-arginine (Sigma, A-8094) (HBSSH). The addition of OA-Alb did not alter the pH of the HBSSH solution (data not shown). We used a range of protein concentrations (5, 15, and 30 g/L) that were comparable to those used in other previous studies.4,5,7,23,51,52 Such concentrations are higher than those measured in the proximal tubular fluid of rats with experimental proteinuria using micropuncture studies in which the reported maximum albumin concentration was 7 g/L.53 However, since the duration of protein exposure is much longer in in vivo settings, the total amount of proteins processed by proximal tubular cells in vivo far exceeds that of the cultured HK-2 cells. Therefore, 15 g/L of OA-Alb used for most of our experiments is appropriate for in vitro protein-overload model.23,54
Measurement of O2·− Production in HK2 Cells (In Vitro Protein-Overload Model)
DHE (Molecular Probes, D11347), an O2·−-specific and pH-resistant fluorescent dye, was used to detect intracellular O2·− production as described previously.55–57 Menadione sodium bisulfite (2.5 mM; Sigma, M2518) was added to serve as a positive control stimulus for mitochondrial O2·− production and was a test of appropriate dye loading and cell viability. Semiconfluent cells, grown on collagen (KOKEN, IPC30) coated 15-mm coverslips (Matsunami, C015001), were first washed with vehicle solution (HBSSH/l-arginine with pH adjusted to 6.0, 6.4, 6.6, or 7.0). These cells were preloaded with 10 mM DHE for 200 seconds. OA-Alb (5, 15, or 30 g/L) dissolved in the pH-adjusted vehicle solution was then added. The cells were exposed to 480-nm light to excite the ethidium and the emitted fluorescence was recorded using a 505-nm dichroic mirror with a 605/55 nm band pass filter (Olympus) and the 605-nm filter of the Lambda 10-2 optical filter changer (Sutter Instruments). Circular regions of interest (ROI) were determined inside the HK-2 cells and the mean intensities within ROI were recorded every 10 seconds. The increasing rate of ethidium fluorescence intensity (units per second) after OA-Alb stimulation (increments from 400 to 800 seconds after OA-Alb exposure), representing a change in O2·− production, was compared between each experimental condition. Each experiment was repeated at least six times. For NAD(P)H oxidase inhibitor and ROS-scavenger studies, semiconfluent HK-2 cells were pretreated with apocynin (100 μM; Sigma, W508454) and/or Tiron (10 mM; DOJINDO, 347-02741) for 4 hours before the analysis of intracellular ROS formation. Each experiment was repeated at least four times.
The imaging system consisted of an IX71 inverted microscope (Olympus), an Ixon digital CCD camera (Andor Co.), a Lambda 10-2 optical filter changer, and a Sutter DG-4 175-W xenon arc lamp (Sutter Instruments). Images were displayed and analyzed using Metaflor image-analysis software (Universal Imaging Co.). Cells were placed in the series-20 temperature-controlled imaging chamber system at 37°C (Warner Instruments).
Intracellular pH Measurement
Intracellular pH was measured as previously reported with some modification.58 Briefly, HK-2 cells were grown to semiconfluence and then harvested. Cells (1 × 106) were incubated in serum-free medium containing 10 μM carboxy-SNARF-1 (Invitrogen, C1271) for 30 minutes. Single-cell suspensions were then exposed to Hank's balanced salt solution (pH 6.0, 6.4, 6.6, or 7.0) or to a standard solution (130 mM KCl, 10 mM NaCl, 1 mM MgSO4, and 10 mM Na-MOPS with 10 μg/ml nigericin) for 15 minutes. The pH-dependent spectral shift was measured as the ratio of fluorescence emission (640 nm/580 nm).
Measurement of Albumin Uptake
Albumin uptake was quantified as described previously with some modification.23 Briefly, HK-2 cells were grown to confluence and were serum-starved for 24 hours. After the cells were exposed to 15 g/L OA-Alb for 15 minutes, a total cell lysate was prepared using RIPA buffer (Santa Cruz, sc-24948). Protein (20 μg) was subjected to Western blotting using an anti-bovine serum albumin antibody (1:200; Nordic Laboratories, RAB/ALB/7S). Band intensity was quantified using National Institute of Health imaging and normalized to β-actin, which was probed by blotting the same sample with an anti–β-actin antibody (1:20,000; Sigma, A5441). Each experiment was repeated six times.
Immunoprecipitation
HK-2 cells were grown to confluence and serum-starved for 48 hours. The cells were lysed with radio-immuno-precipitation assay (RIPA) buffer (Santa Cruz, sc-2498) after treatment with vehicle or OA-Alb solution for the times indicated in Figures 4 and 5. All subsequent manipulations were performed on ice. The cells were harvested using a scraper and the supernatant was obtained as recommended by the manufacturer. The supernatant was diluted to 1 mg protein/ml and then incubated overnight at 4°C with 5 μg/ml of a goat anti-Pyk2 antibody (Santa Cruz, sc-1514), with rotation. Protein G-Plus agarose (30-μl bead volume; Calbiochem) was then added and the mixture was incubated overnight at 4°C and then centrifuged. The pelleted beads were washed five times with ice-cold PBS and then suspended in 60 μl of 2× SDS loading buffer and boiled for 5 minutes. After centrifugation, the supernatant was divided into two aliquots. Each aliquot was subjected to electrophoresis on a 7.5% SDS-PAGE gel and transferred to polyvinylidene fluoride membranes. After blocking the membranes with blocking buffer (1% BSA, 0.05% tris-buffered saline tween-20 [TBST]), one aliquot was subjected to immunoblotting with a precipitating antibody and the other aliquot was subjected to immunoblotting with a nonprecipitating antibody.
Rac1 Activation Pull-down Assay
A Rac1 activation assay was performed according to the manufacturer's instructions (Cytoskeleton, BK035). Briefly, the cells were cultured to 40% confluence and then serum-starved for 48 hours. The subconfluent cells (80 to 90%) were subsequently lysed with lysis buffer after OA-Alb solution exposure (15 g/L, pH 6.4) for the times indicated in Figure 5. Samples were aliquoted for determination of the total amounts of Rac1 and GTP-bound Rac1. The aliquot for determination of GTP-bound Rac1 was diluted to 0.5 mg/ml and incubated with 20 μg of p21 activated kinase 1(PAK)-p21 binding domain (PBD) glutathione affinity beads at 4°C for 1 hours. The pelleted beads were then suspended in 10 μl of 2× SDS loading buffer and boiled for 5 minutes. The GTP-bound Rac1 and total content of Rac1 in these samples were determined by SDS-PAGE and immunoblotting with an anti-Rac1 antibody (1:500). Each experiment was repeated three times. Bands were visualized using ECL (GE Healthcare) and quantified using densitometry.
Western Blotting
Samples were subjected to SDS-PAGE and were then transferred to PVDF membranes (Immobilon-P; Millipore). Blots were blocked with 1% BSA in TBST at 4°C overnight. The blots were then probed with anti-Pyk2 (1:500; Upstate Biology, 06-559), anti-phosphotyrosine (1:50; Santa Cruz, sc-7020), anti–c-Src (1:200; Santa Cruz, sc-18), and anti–α-tubulin (1:2000; Sigma, 096K1777) antibodies overnight at 4°C. The blots were then incubated with the appropriate secondary antibodies. Bands were visualized using ECL (GE Healthcare).
Pyk2 Knockdown Assay
For Pyk2 knockdown, HK-2 cells were transfected with Pyk2-specific and random control Stealth RNAi (Invitrogen, VHS50360 and 12935-400) using the Lipofectamine 2000 reagent (Invitrogen, 11678) as recommended by the manufacturer. Briefly, 30 to 50% confluent cells were treated with 50 nM dsRNA using lipofectamine 2000 (1:400). The cells were incubated for an additional 48 hours and then subjected to analysis. The efficacy of Pyk2 knockdown was confirmed by Western blotting.
Detection of Apoptosis
Apoptotic cell analysis was performed using flow cytometry and Annexin-V as described previously.26,27 After stimulation of HK-2 cells with OA-Alb in acidic media (pH 6.4) for 120 minutes, the cells were then stained with FITC-conjugated Annexin-V (Pharmingen) and propidium iodide (PI) for 15 minutes. Early apoptotic cells stain solely with Annexin-V, whereas late apoptotic and necrotic cells stain positively for both Annexin-V and PI. Viable cells were negative for both Annexin-V and PI. Analysis was performed using FACScalibur, and the data were analyzed using CellQuest software (BD Bioscience). Each experiment was repeated at least four times.
Murine Protein-Overload Nephropathy Model
A murine protein-overload nephropathy model was performed as described previously with some modifications (Figure 8A).28,29 Briefly, male and female C57B6/J mice (age, 5 to 8 weeks; body weight, 20 g) were divided into the following five groups (n > 8 each): (1) Free fatty acid-bound albumin–injected (FFA-Alb–injected), (2) oleic acid-bound albumin–injected (OA-Alb–injected), (3) FFA-Alb injected with luminal alkalinization, (4) OA-Alb injected with luminal alkalinization, and (5) PBS-injected groups. FFA-Alb (Sigma, A-9430) was dissolved in 0.45-μm filtered PBS (5 g of FFA-Alb in 15 ml of PBS; FFA-Alb solution). To circumvent the fatal toxicity of OA-Alb, we used a lower molar ratio of oleic acid to albumin (3:1) for animal studies as previously reported.43 Oleic acid (Sigma, O-1008) was added to the FFA-Alb solution and incubated at 37°C for 2.5 hours.23 Mice were administered daily intraperitoneal bolus injections of 0.3 g/20 g body wt of FFA-Alb or OA-Alb for 5 consecutive days. All control animals received intraperitoneal injections of an equal volume of PBS on an identical schedule. All groups of mice were sacrificed on day 6. The luminal alkalinization group was fed with 200 mM sodium bicarbonate solution ad libitum throughout the experiment.44,45 Development of proteinuria was confirmed by the Albustix protein measurement assay (Siemens) on days 4 and 5.
The mice were maintained in a facility free of specific pathogens and all protocols performed were approved by the Tohoku University animal care committee.
Urine and Blood Tests
Twelve-hour urine was collected using a metabolic cage after 5 consecutive days of protein overload. The concentration of creatinine and total protein was determined using enzymatic detection (Pure Auto S CRE-L, Sekisui Medical) and a pyrogallol red-molybdate method (Micro TP-AR, Wako). Blood samples were collected directly before euthanization. The total CO2 concentration was measured using i-Stat according to the manufacturer's protocol (Abott).
Histologic Analysis and 8-Dihydro-2′-deoxyguanosine (8-OHdG) Immunohistochemistry
Pentobarbital-anesthetized mice were intracardially perfused with ice-cold PBS followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The dissected kidneys were fixed overnight and then processed for paraffin embedding. Tissue sections were stained with hematoxylin and eosin to evaluate morphologic abnormalities. Staining with 8-OHdG was performed as described previously with some modification.30 Tissue sections (3-μm thick) were antigen unmasked by boiling in 10 mM sodium citrate (pH 6.0) and blocked with mouse-on-mouse anti-mouse IgG blocking reagent (Vector Labs, PK-2200) for 1 hour at room temperature. Sections were incubated with a 1:200 diluted monoclonal anti–8-OHdG antibody (Japan Institute for the Control of Aging, MOG-20P) overnight at 4°C. Sections were then incubated with biotinylated anti-mouse IgG (Vector Labs) for 10 minutes at room temperature followed by ABC reagent (Vector Labs) for 5 minutes at room temperature. The signal was visualized by staining with DAB solution containing nickel chloride hexahydrate (DAB Ni, Wako).
For the coimmunostaining assay, biotinylated lotus tetragonobulus lectin (Vector Labs, B-1325; 1:50) was incubated with the tissue section for 45 minutes at room temperature,31,32 and then with Vector ALP reagent (Vector Labs, D0396) for 30 minutes at room temperature. The signal was visualized by staining with an AP substrate (Vector Labs, SK-5100). Antigen retrieval was then performed. Immunoreactivity of 8-OHdG was assessed using the above-mentioned anti–8-OHdG antibody and the Envision polymer reagent (K4000, DAKO). The signal was visualized by staining with DAB Ni.
Quantitative Evaluation of Tubulointerstitial Injury
Tubulointerstitial injury was evaluated by counting the number of cast formations and by quantifying the 8-OHdG-immunoreactive (-ir) area. Quantification of the cast number was performed manually in four randomly selected 1.5-mm2 cortical areas from a transverse kidney section in the maximum ordinate level. The 8-OHdG-ir area was morphometrically quantified as a ratio to the selected 1.5-mm2 cortical area using Image-J software (NIH Imaging). All assessments were performed by blinded observers.
Fluorescence Activated Cell Sorting (FACS) Analysis of Intracellular ROS Levels
The kidneys were dissected out after intracardiac perfusion with ice-cold PBS. The kidneys were decapsulated and cut into pieces of approximately 1 mm3. The minced kidney tissues were digested in collagenase solution (1 mg/ml; Sigma, C9407) with DNase I (15 U/ml; Roche Diagnostics). The suspension was incubated for 1 hour at 37°C and was then sieved through a 35-μm nylon mesh. The resulting single-cell suspension was loaded with 10 μM DCFDA (Molecular Probe) for 2 hours at 37°C and then incubated with a PE-conjugated (phycoerythrin fluorescence-conjugated) antibody against CD13 (aminopeptidase N, a type II transmembrane glycoprotein; BD Bioscience, 558745) for 30 minutes at 4°C.33,34 Dead cells were excluded by the addition of 1 μg/ml PI. Because CD13 is also expressed in myeloid lineage cells, we excluded blood cells using an anti-CD45 antibody (BD Biosciences). FACS analysis was performed using FACSaria (BD Bioscience), and the data were analyzed using FACSDiva software (BD Bioscience).
Immunocytochemistry
HK-2 cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 15 minutes at room temperature and were blocked with 1% BSA in Tris-buffered saline for 30 minutes at room temperature. Cells were then incubated with a 1:500 diluted polyclonal anti-aquaporin 1 antibody (Abcam, ab65837) overnight at 4°C, and then with Alexa Fluor 488-conjugated anti-rabbit IgG antibody (Invitrogen) for 1 hour at room temperature. Cells were stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:1000) for nuclear staining. The fluorescence signal was observed with a LSM510 confocal imaging system (Carl Zeiss).
Data and Statistical Analysis
Data are expressed as means ± SEM. Comparisons between groups were made using one-way ANOVA or an unpaired t test as appropriate. For multiple comparisons, the Tukey-Kramer test was used. Data were considered statistically significant when the P value was <0.05.
DISCLOSURES
None.
Acknowledgments
This work was supported by Grants-in-Aid for Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 18790156), the 21st Century COE Program Special Research Grant from the Ministry of Education, Sports, and Culture. The authors thank Prof. Allen W. Cowley, Jr., Medical College of Wisconsin, for his invaluable advice and for critically reviewing our manuscript, and Dr. Shiwaku, Ms. Suzuki, Ms. Kisu, and Ms. Nakamura for their technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Risdon RA, Sloper JC, De Wardener HE: Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 [DOI] [PubMed] [Google Scholar]
- 2. Benigni A, Zoja C, Remuzzi G: The renal toxicity of sustained glomerular protein traffic. Lab Invest 73: 461–468, 1995 [PubMed] [Google Scholar]
- 3. D'Amico G: Tubulointerstitium as predictor of progression of glomerular diseases. Nephron 83: 289–295, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Tang S, Leung JC, Abe K, Chan KW, Chan LY, Chan TM, Lai KN: Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 111: 515–527, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, Ghilardi M, Remuzzi G: Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol 13: 1179–1189, 2002 [PubMed] [Google Scholar]
- 6. Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Iwao Y, Nakajou K, Nagai R, Kitamura K, Anraku M, Maruyama T, Otagiri M: CD36 is one of important receptors promoting renal tubular injury by advanced oxidation protein products. Am J Physiol 295: F1871–F1880, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Li M, Balamuthusamy S, Simon EE, Batuman V: Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am J Physiol Renal Physiol 295: F82–F90, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Gekle M: Renal tubule albumin transport. Annu Rev Physiol 67: 573–594, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Arici M, Brown J, Williams M, Harris KP, Walls J, Brunskill NJ: Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: A role for protein kinase C. Nephrol Dial Transplant 17: 1751–1757, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Neuhofer W, Beck FX: Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005 [DOI] [PubMed] [Google Scholar]
- 13. de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Li S, Sato S, Yang X, Preisig PA, Alpern RJ: Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Preisig PA: The acid-activated signaling pathway: Starting with Pyk2 and ending with increased NHE3 activity. Kidney Int 72: 1324–1329, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK: Angiotensin II stimulation of NAD(P)H oxidase activity: Upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW: Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21: 489–495, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J: Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature 376: 737–745, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Sabri A, Govindarajan G, Griffin TM, Byron KL, Samarel AM, Lucchesi PA: Calcium- and protein kinase C-dependent activation of the tyrosine kinase PYK2 by angiotensin II in vascular smooth muscle. Circ Res 83: 841–851, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley ED, Owada KM, Marumo F, Hirata Y: Involvement of PYK2 in angiotensin II signaling of vascular smooth muscle cells. Hypertension 33: 201–206, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Ghiggeri GM, Ginevri F, Candiano G, Oleggini R, Perfumo F, Queirolo C, Gusmano R: Characterization of cationic albumin in minimal change nephropathy. Kidney Int 32: 547–553, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Ishola DA, Jr., Post JA, van Timmeren MM, Bakker SJ, Goldschmeding R, Koomans HA, Braam B, Joles JA: Albumin-bound fatty acids induce mitochondrial oxidant stress and impair antioxidant responses in proximal tubular cells. Kidney Int 70: 724–731, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ushio-Fukai M: Localizing NADPH oxidase-derived ROS. Sci STKE 22: re8, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Hunyady L, Catt KJ: Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H: Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun 372: 51–56, 2008 [DOI] [PubMed] [Google Scholar]
- 27. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP: Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31: 1–9, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Suzuki Y, Lopez-Franco O, Gomez-Garre D, Tejera N, Gomez-Guerrero C, Sugaya T, Bernal R, Blanco J, Ortega L, Egido J: Renal tubulointerstitial damage caused by persistent proteinuria is attenuated in AT1-deficient mice: Role of endothelin-1. Am J Pathol 159: 1895–1904, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamijo Y, Hora K, Kono K, Takahashi K, Higuchi M, Ehara T, Kiyosawa K, Shigematsu H, Gonzalez FJ, Aoyama T: PPARalpha protects proximal tubular cells from acute fatty acid toxicity. J Am Soc Nephrol 18: 3089–3100, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Sun Z, Zhang X, Ito K, Li Y, Montgomery RA, Tachibana S, Williams GM: Amelioration of oxidative mitochondrial DNA damage and deletion after renal ischemic injury by the KATP channel opener diazoxide. Am J Physiol 294: F491–F498, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Laitinen L, Virtanen I, Saxén L: Changes in the glycosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochem Cytochem 35: 55–65, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Kiley SC, Thornhill BA, Belyea BC, Neale K, Forbes MS, Luetteke NC, Lee DC, Chevalier RL: Epidermal growth factor potentiates renal cell death in hydronephrotic neonatal mice, but cell survival in rats. Kidney Int 68: 504–514, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Helbert MJ, Dauwe SE, Van der Biest I, Nouwen EJ, De Broe ME: Immunodissection of the human proximal nephron: Flow sorting of S1S2S3, S1S2 and S3 proximal tubular cells. Kidney Int 52: 414–428, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Kurita N, Honda S, Usui K, Shimizu Y, Miyamoto A, Tahara-Hanaoka S, Shibuya K, Shibuya A: Identification of the Fcα/μR isoform specifically expressed in the kidney tubules. Mol Immunol 46: 749–753, 2009 [DOI] [PubMed] [Google Scholar]
- 35. DuBose TD, Jr., Pucacco LR, Lucci MS, Carter NW: Micropuncture determination of pH, PCO2, and total CO2 concentration in accessible structures of the rat renal cortex. J Clin Invest 64: 476–482, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malnic G, de Mello-Aires M: Kinetic study of bicarbonate reabsorption in proximal tubule of the rat. Am J Physiol 220: 1759–1767, 1971 [DOI] [PubMed] [Google Scholar]
- 37. Wilcox CS, Granges F, Kirk G, Gordon D, Giebisch G: Effects of saline infusion on titratable acid generation and ammonia secretion. Am J Physiol 247: F506–F519, 1984 [DOI] [PubMed] [Google Scholar]
- 38. Gottschalk CW, Lassiter WE, Mylle M: Localization of urine acidification in the mammalian kidney. Am J Physiol 198: 581–585, 1960 [DOI] [PubMed] [Google Scholar]
- 39. Kurtz I, Star R, Balaban RS, Garvin JL, Knepper MA: Spontaneous luminal disequilibrium pH in S3 proximal tubules. Role in ammonia and bicarbonate transport. J Clin Invest 78: 989–996, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eddy AA: Interstitial nephritis induced by protein-overload proteinuria. Am J Pathol 135: 719–733, 1989 [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ: Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol 283: F640–F647, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, Hirata Y, Goto A, Fujita T, Omata M: Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int 62: 1628–1637, 2002 [DOI] [PubMed] [Google Scholar]
- 43. van Timmeren MM, Bakker SJ, Stegeman CA, Gans RO, van Goor H: Addition of oleic acid to delipidated bovine serum albumin aggravates renal damage in experimental protein-overload nephrosis. Nephrol Dial Transplant 20: 2349–2357, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Torres VE, Mujwid DK, Wilson DM, Holley KH: Renal cystic disease and ammoniagenesis in Han:SPRD rats. J Am Soc Nephrol 5: 1193–1200, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Torres VE, Cowley BD, Jr., Branden MG, Yoshida I, Gattone VH: Long-term ammonium chloride or sodium bicarbonate treatment in two models of polycystic kidney disease. Exp Nephrol 9: 171–180, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Tanner GA: Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 9: 1242–1248, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B: HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Whitin JC, Bhamre S, Tham DM, Cohen HJ: Extracellular glutathione peroxidase is secreted basolaterally by human renal proximal tubule cells. Am J Physiol 283: F20–F28, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Reich H, Tritchler D, Herzenberg AM, Kassiri Z, Zhou X, Gao W, Scholey JW: Albumin activates ERK via EGF receptor in human renal epithelial cells. J Am Soc Nephrol 16: 1266–1278, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Whaley-Connell AT, Morris EM, Rehmer N, Yaghoubian JC, Wei Y, Hayden MR, Habibi J, Stump CS, Sowers JR: Albumin activation of NAD(P)H oxidase activity is mediated via Rac1 in proximal tubule cells. Am J Nephrol 27: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Lewy JE, Pesce A: Micropuncture study of albumin transfer in aminonucleoside nephrosis in the rat. Pediatr Res 7: 553–559, 1973 [DOI] [PubMed] [Google Scholar]
- 54. Abbate M, Remuzzi G, Zoja C: Role of Proteinuria in Progression. In: Seldin and Giebisch's The Kidney, 4th Ed., edited by Alpern RJ, Hebert SC. London, Academic, 2008, pp 2563–2576 [Google Scholar]
- 55. Cutaia M, Kroczynski J, Tollefson K: pH-dependent oxidant production following inhibition of the mitochondrial electron transport chain in pulmonary endothelial cells. Endothelium 9: 109–121, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB: Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol 29: 2571–2583, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW, Jr: Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol 291: F350–F357, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Bond J, Varley J: Use of flow cytometry and SNARF to calibrate and measure intracellular pH in NS0 cells. Cytometry A 64: 43–50, 2005 [DOI] [PubMed] [Google Scholar]