Abstract
Mesangial cells in diabetic mice and human kidneys with diabetic nephropathy exhibit increased type VIII collagen, a nonfibrillar protein that exists as a heterodimer composed of α1(VIII) and α2(VIII), encoded by Col8a1 and Col8a2, respectively. Because TGF-β1 promotes the development of diabetic glomerulosclerosis, we studied whether type VIII collagen modulates the effects of TGF-β1 in mesangial cells. We obtained primary cultures of mesangial cells from wild-type, doubly heterozygous (Col8a1+/−/Col8a2+/−), and double-knockout (Col8a1−/−/Col8a2−/−) mice. TGF-β1 bound normally to double-knockout mesangial cells. In wild-type mesangial cells, TGF-β1 inhibited proliferation, but in double-knockout cells, it stimulated proliferation, promoted cell cycle progression, and reduced apoptosis; we could reverse this effect by reconstituting α1(VIII). Furthermore, in wild-type cells, TGF-β1 mainly stimulated the Smad pathways, whereas in double-knockout cells, it activated the MAPK and PI3K/Akt pathways and induced expression of fibroblast growth factor 21 (FGF21). Inhibiting FGF21 expression by either interfering with activation of the MAPK and PI3K/Akt pathways or by FGF21 siRNA attenuated the TGF-β1-induced proliferation of double-knockout mesangial cells. In vivo, diabetic double-knockout mice had significantly higher expression of renal FGF21 mRNA and protein compared with diabetic wild-type mice. Immunohistochemistry revealed strong expression of FGF21 in both glomerular (mesangial) and tubular cells of diabetic mice. Taken together, these data suggest that type VIII collagen significantly modulates the effect of TGF-β1 on mesangial cells and may therefore play a role in the pathogenesis of diabetic nephropathy.
Diabetic nephropathy is clinically characterized by proteinuria and progressive renal insufficiency and is the leading cause of end-stage renal failure in developed countries.1 Podocyte injury, glomerular basement membrane thickening, mesangial hypertrophy, and expansion with subsequent development of glomerulosclerosis and tubulointerstitial fibrosis have long been recognized as pathologic hallmarks.2,3 Several hormones, cytokines, and growth factors are known to play an important role in the genesis and progression of diabetic nephropathy. In particular, the multifunctional cytokine TGF-β1 has been identified as stimulating the synthesis and inhibiting the degradation of extracellular matrix molecules, and promoting mesangial cell hypertrophy, one of the cardinal characteristics of diabetic glomerulopathy.4,5 It has been found that TGF-β1 mRNA and protein expressions are elevated in the glomeruli and renal cortex of humans with diabetic nephropathy and in various experimental models of diabetic nephropathy.6,7 On the other hand, neutralization of TGF-β1 by an anti-TGF-β antibody prevented glomerular hypertrophy and attenuated enhanced extracellular matrix gene expression in experimental diabetic nephropathy.8,9 TGF-β1 initially stimulates mesangial cell proliferation but subsequently inhibits cell cycle progression by induction of cell cycle inhibitors such as p27Kip1, resulting in mesangial hypertrophy.10–12 Moreover, TGF-β1 simulates the expression of key extracellular matrix molecules including different types of collagen, fibronectin, and laminin, and suppresses, in parallel, the degradation of these molecules.5,13 This leads to the complex accumulation of matrix, resulting in diffuse glomerular sclerosis and progressive tubulointerstitial fibrosis.14
In addition to the classical collagen types I, III, IV, and VI found in diabetic nephropathy, a strong specific induction of type VIII collagen has been found in mesangial cells in diabetic mice and in kidney biopsies from patients with human diabetic nephropathy compared with normal kidneys.15,16 Type VIII collagen, a nonfibrillar collagen protein, exists as a heterodimer composed of two distinct polypeptide chains designated as α1(VIII) and α2(VIII) (genes: Col8a1 and Col8a2).17,18 Although increasing evidence indicates that type VIII collagen, expressed by a limited number of cell types, may play an important role in mesangial cell function and the pathogenesis of diabetic glomerulosclerosis, the detailed physiologic function of this matrix protein remains unclear. This study investigates the potential role of type VIII collagen in modulating the biologic effects of TGF-β1 on mesangial cells.
RESULTS
TGF-β1 Induces the Expression of Col8a1 in Mouse Mesangial Cells
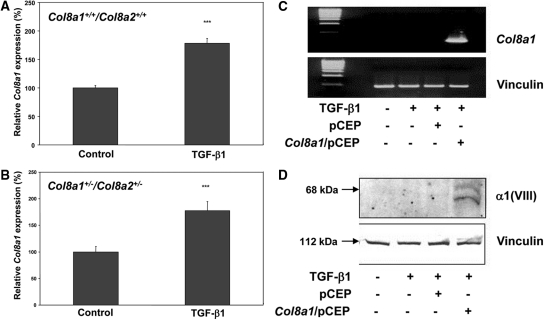
Previous studies have shown that high ambient glucose induces expression of Col8a1 in mouse mesangial cells.15 Therefore, the effect of recombinant TGF-β1 was tested. Figure 1 shows that stimulation with 2.5 ng/ml TGF-β1 for 24 hours significantly enhanced the expression of Col8a1 mRNA in wild-type (by 78.2 ± 7.8%; Figure 1A) as well as in Col8a1+/−/Col8a2+/− heterozygous (HZ) (by 77.5 ± 16.3%; Figure 1B) mesangial cells. No specific Col8a1 mRNA or α1(VIII) protein was detected in the absence or presence of TGF-β1 in Col8a1−/−/Col8a2−/− knockout (KO) mesangial cells (Figure 1, C and D). However, α1(VII) expression could be reconstituted in KO mesangial cells using the mammalian expression vector pCEP in which the Col8a gene transcription is driven by a cytomegalovirus promoter (Figure 1, C and D).15
Figure 1.
Induction of Col8a1 mRNA expression in mesangial cells from wildtype (A) and Col8a1+/−/Col8a2+/− heterozygous mice (B). Values were normalized to 18S rRNA expression, and the control was assigned as an arbitrary value of 100% (n = 9). ***P < 0.001 versus controls). (C) No Col8a1 mRNA expression was found after RT-PCR in the absence or presence of TGF-β1 in Col8a1−/−/Col8a2−/− KO mesangial cells. However, Col8a1 mRNA expression can be reconstituted by the Col8a1/pCEP expression vector, whereas the empty pCEP vector was without effect. (D) Western blot for α1(VIII) collagen. No expression was found in Col8a1−/−/Col8a2−/− KO mesangial cells, but α1(VIII) collagen expression was reconstituted with the Col8a1/pCEP expression vector. The data are representative of two separate experiments.
Mesangial Cell Proliferation Is Induced by TGF-β1 in Col8a1−/−/Col8a2−/− KO Mesangial Cells
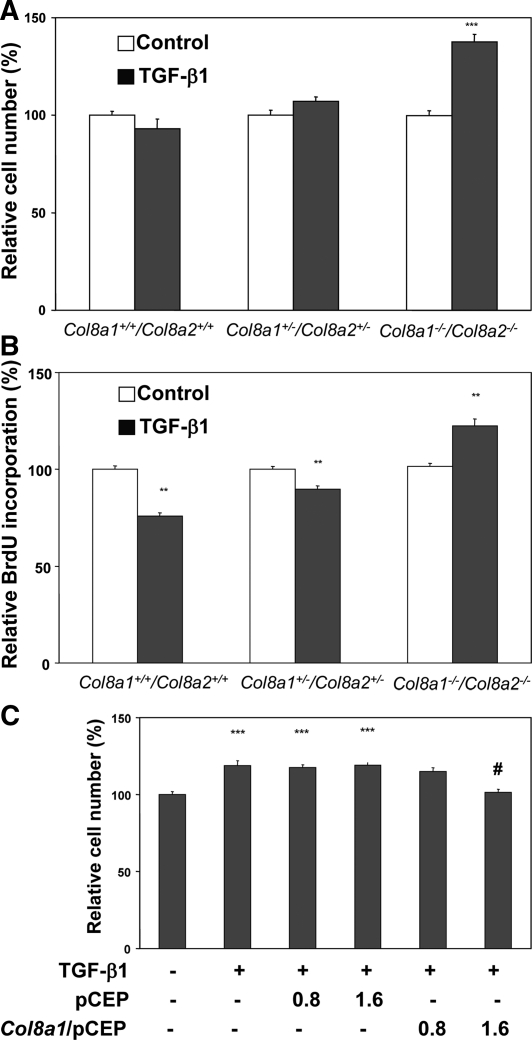
We have previously shown that TGF-β1 inhibits proliferation of mesangial cells and induces cell hypertrophy.10,11 Therefore, we further investigated whether endogenous type VIII collagen expression may modulate the influence of TGF-β1 on the proliferation. Wild-type, Col8a1+/−/Col8a2+/− HZ, and Col8a1−/−/Col8a2−/− KO mesangial cells were stimulated with 2.5 ng/ml TGF-β1 for 24 hours. Cell proliferation was assessed by direct cell counting as well as DNA synthesis rate. Under these conditions, TGF-β1 significantly increased the total cell number of Col8a1−/−/Col8a2−/− KO (by 37.5 ± 7.3%), whereas no increase was seen in Col8a1+/−/Col8a2+/− HZ and wild-type mesangial cells (Figure 2A). To confirm these findings with an alternative approach, we investigated the DNA synthesis rate under similar conditions. Cells were labeled with bromodeoxyuridine (BrdU), and the incorporation into DNA was assessed. Induction of DNA synthesis by TGF-β1 was apparent in Col8a1−/−/Col8a2−/− KO, whereas the cytokine inhibited DNA synthesis in Col8a1+/−/Col8a2+/− HZ as well as wild-type mesangial cells (Figure 2B). Forced reconstitution of Col8a1 expression using a cytomegalovirus-promoter plasmid, but not the empty control vector, in Col8a1−/−/Col8a2−/− KO cells abrogated the TGF-β1-induced proliferation (Figure 2C).
Figure 2.
TGF-β1-mediated effects on proliferation are modified by the presence of α1(VIII) collagen. (A) Total cell number is significantly stimulated by 2.5 ng/ml TGF-β1 for 24 hours only in Col8a1−/−/Col8a2−/− KO mesangial cells but not in wild-type or Col8a1+/−/Col8a2+/− HZ cells (n = 10 independent experiments with triplicates, ***P < 0.001 versus controls). (B) DNA synthesis determined as bromodeoxyuridine incorporation. Whereas 2.5 ng/ml TGF-β1 induced a significant decrease in DNA synthesis in wild-type and Col8a1+/−/Col8a2+/− HZ cells, it clearly stimulated DNA synthesis in Col8a1−/−/Col8a2−/− KO mesangial cells (n = 10 independent experiments done four times each, **P < 0.01 versus controls of the same genotype). (C) Effect of a Col8a1 reconstitution on cell number. Col8a1−/−/Col8a2−/− KO mesangial cells were transfected either with control plasmid pCEP or Col8a1/pCEP (each 0.8 and 1.6 μg/ml) for 12 hours and stimulated with 2.5 ng/ml TGF-β1 for a further 24 hours. Reconstitution of Col8a1 expression reduced the TGF-β1 induced proliferation to basal levels, whereas the empty control vector had no effect (n = 9, ***P < 0.001 versus controls without TGF-β1, #P < 0.001 versus cells transfected with control vector [1.6 μg/ml] treated with TGF-β1).
Col8a1 Expression Influences the Effect of TGF-β1 on Cell Cycle and Apoptosis
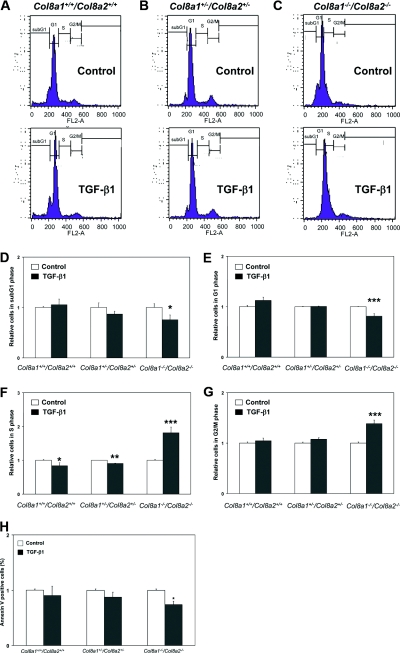
It is well known that in many cell types, TGF-β1 can affect the cell cycle and induce apoptosis.11,19 To address this, we measured cell cycle and apoptosis by fluorescence-activated cell sorter (FACS) analyses in mesangial cells exposed to 2.5 ng/ml TGF-β1 (Figure 3, A through G). Cell cycle analysis revealed that TGF-β1 increased the number of Col8a1−/−/Col8a2−/− KO cells in the S phase (by 81.4 ± 17.0%), whereas in wild-type as well as in Col8a1+/−/Col8a2+/− HZ mesangial cells, the DNA synthesis was significantly inhibited by TGF-β1 (Figure 3, A through C and F). Moreover, TGF-β1 raised the number of Col8a1−/−/Col8a2−/− KO cells in the G2/M phase (by 38.5 ± 7.2%) compared with wild-type cells (Figure 3, A through C and G), indicating completion of the cell cycle. In contrast, TGF-β1-mediated cell cycle arrest in wild-type cells was attenuated in Col8a1−/−/Col8a2−/− KO mesangial cells as demonstrated by a reduction of the number of cells in the G1 phase (by 18.9 ± 5.0%) as well as in the sub-G1 phase (by 24.6 ± 9.8%) by treatment of TGF-β1 (Figure 3, A through E). Because cells in the sub-G1 phase may be apoptotic, we assessed annexin V-fluorescein binding and propidium-iodide staining with FACS analysis (Figure 3H). TGF-β1 significantly reduced the rate of apoptotic (annexin-positive but propidium iodide-negative) Col8a1−/−/Col8a2−/− KO mesangial cells rate (by 26 ± 6%) in contrast to wild-type mesangial cells (Figure 3H).
Figure 3.
The effect of TGF-b1 on cell cycle and apoptosis depends on the presence of type VIII collagen. (A through C) Representative FL-2A histograms of Col8a1+/+/Col8a2+/+ wild-type, Col8a1+/−/Col8a2+/− HZ, and Col8a1−/−/Col8a2−/− KO mesangial cells treated with 2.5 ng/ml TGF-β1 or control medium. (D) Sub-G1 phase. TGF-β1 significantly reduced the number of Col8a1−/−/Col8a2−/− KO mesangial cells (n = 12, *P < 0.05 versus controls). (E) G1 phase of cell cycle. Treatment of Col8a1−/−/Col8a2−/− KO mesangial cells with TGF-β1 significantly reduced the number of cells in the G1 phase (n = 12, **P < 0.01 versus controls). (F) S phase. Whereas TGF-β1 significantly lowered the number of wild-type and Col8a1+/−/Col8a2+/− HZ mesangial cells in the S1 phase, it increased the number of Col8a1−/−/Col8a2−/− KO in the S phase (n = 12, *P < 0.05 versus controls, **P < 0.01 versus controls, ***P < 0.001 versus controls of the same genotype). (G) TGF-β1 for 24 hours significantly increased only the number of Col8a1−/−/Col8a2−/− KO mesangial cells in the G2/M phase of the cell cycle (n = 12, ***P < 0.001 versus controls). (H) Apoptosis as determined by annexin V-FITC and propidium-iodide staining followed by FACS analysis. TGF-β1 significantly reduces apoptosis of Col8a1−/−/Col8a2−/− KO mesangial cells (n = 12, *P < 0.01 versus controls).
The Receptor Affinity for TGF-β1 Is Not Different
To exclude the possibility that the different effects of TGF-β1 may be caused by changes in the expression of receptors for TGF-β1, saturation binding studies with 125I-TGF-β1 were performed. Binding occurred in a concentration-dependent manner over the range of 0.5 to 25 pM and was saturable (data not shown). Scatchard analysis of binding data revealed a single class of high affinity receptors for TGF-β1 with similar dissociation constants (Kd) (Kd values of wild-type cells: 15.5 ± 1.1 pM; Kd of Col8a1−/−/Col8a2−/− KO 14.2 ± 0.9 pM, n = 6, NS). Furthermore, maximal binding capacity for TGF-β1 was equal in both cell types (data not shown).
TGF-β1 Signaling Pathways Differ Depending on Type VIII Collagen Expression
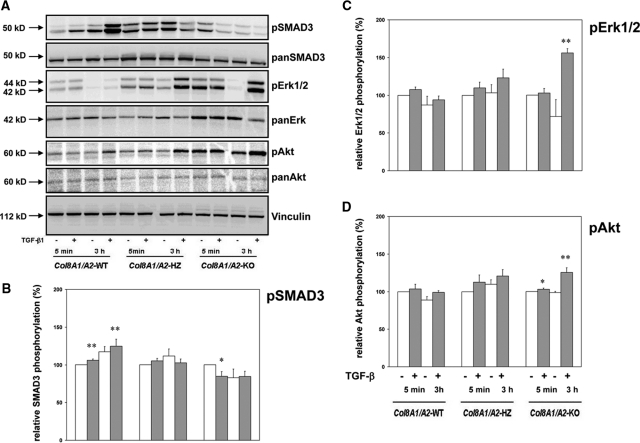
Because TGF-β1 can activate diverse signaling pathways including Smad and mitogen-activated protein (MAP) kinase pathways,20,21 we tested whether these second messengers are differentially regulated. Wild-type, Col8a1+/−/Col8a2+/− HZ, and Col8a1−/−/Col8a2−/− KO mesangial cells were exposed to 2.5 ng/ml TGF-β1 for different time periods, and cell extracts were analyzed by Western blotting with antibodies against Smad3, Erk 1,2, and Akt (Figure 4). In contrast to wild-type cells, TGF-β1 activated in Col8a1−/−/Col8a2−/− KO cells activated MAP kinase and Akt pathways rather than Smad3 (Figure 4). Furthermore, phosphorylated and total Erk 1,2 and PI3K/Akt was increased in Col8a1−/−/Col8a2−/− KO mesangial cells compared with the wild-type even in the absence of TGF-β1.
Figure 4.
TGF-b1 induced signal transduction is different in wildtype and Col8a1−/−/Col8a2−/− KO mesangial cells. (A) Signal transduction pathways. Wild-type, Col8a1+/−/Col8a2+/− HZ cells, and Col8a1−/−/Col8a2−/− KO mesangial cells were stimulated for 5 minutes or 3 hours with 2.5 ng/ml with (+) or without (−) TGF-β1, and phosphorylation of Smad3, Erk1,2, and Akt was analyzed by Western blots using phospho-specific antibodies. (B through D) Statistical analysis after densitometry of the blots. Whereas TGF-β1 leads to phosphorylation of Smad3 and only relatively little phosphorylation of Erk1,2 and Akt in wild-type cells, MAP kinase activation and Akt phosphorylation was significantly stronger in Col8a1−/−/Col8a2−/− KO mesangial cells after challenge with TGF-β1 (C and D, *P <0.05 versus no TGF-β1, **P <0.01 versus no TGF-β1, n = 3 independent experiments). In contrast Smad3 phosphorylation was attenuated. Col8a1+/−/Col8a2+/− cells compared with the wild-type (B, *P <0.05 versus no TGF-β1, **P <0.01 versus no TGF-β1, n = 3 independent experiments) HZ mesangial cells revealed an intermediate response. The blots are representative for three independent experiments with qualitatively similar results.
Difference in TGF-β1-induced Gene Expression in Wild-type and Col8a1−/−/Col8a2−/− KO Mesangial Cells
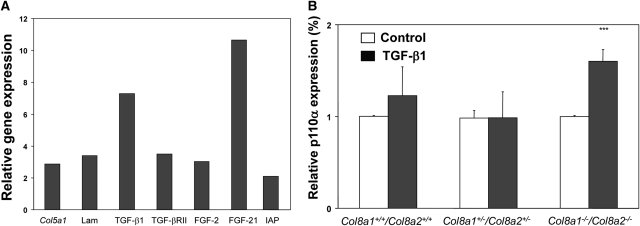
We next assessed potential differences in the TGF-β1-induced transcriptome in wild-type and Col8a1−/−/Col8a2−/− KO mesangial cells using microarray analysis. Out of the more than 39,000 transcripts on the mouse Affymetrix array, 945 showed a reliable signal. The number of genes induced or repressed at least two-fold was 110. Gene array analysis was performed according the MIAME Guidelines,22,23 and data have been submitted the microarry informatic database of the European Bioinformatics Institute (accession number E-MEXP-2658). Further information how the analysis of gene arrays was performed and selected results are shown in the supplemental materials. Thirty-three known genes were upregulated, and 77 were down-regulated (data not shown). We found that genes involved in, for example, extracellular matrix formation, cell cycle, apoptosis, survival, MAP kinase signaling, and DNA replication showed altered expression levels. Several genes (collagen type V [2.9-fold], laminin [3.4-fold], TGF-β1 [7.3-fold], TGF-β receptor type II [3.5-fold], fibroblast growth factor 2 [3.0-fold], fibroblast growth factor (FGF21) [10.7-fold], and inhibitor of apoptosis protein [2.1-fold]) were highly induced by TGF-β1 in Col8a1−/−/Col8a2−/− KO compared with TGF-β1-challenged wild-type mesangial cells (Figure 5A). Real-time PCR analyses of NF-κB and PI3K, both well known as important regulators of cell survival,24 confirmed the finding that TGF-β1 is able to induce components of cell survival pathways in the absence of type VIII collagen expression (increase of p110α expression by 40.9 ± 12.7%) (Figure 5B).
Figure 5.
Differential expression of target genes in wildtype and Col8a1−/−/Col8a2−/− KO mesangial cells after stimulation with TGF-β1. (A) Affymetrix analysis of eight selected genes from 33 induced by 2.5 ng/ml TGF-β1 for 24 hours in Col8a1−/−/Col8a2−/− KO compared with wild-type mesangial cells (n = 3). Col5a1, α1(V) collagen; Lama, laminin; TGFBRII, TGF-beta receptor type II; FGF2, fibroblast growth factor 2. (B) Quantitative RT-real-time PCR for p110α, a catalytic subunit of PI3K. TGF-β1 significantly stimulated p110α mRNA expression only in Col8a1−/−/Col8a2−/− KO mesangial cells (n = 12, ***P < 0.001 versus controls of the same genotype).
TGF-β1-induced FGF21 Expression Is Increased in the Absence of Type VIII Collagen
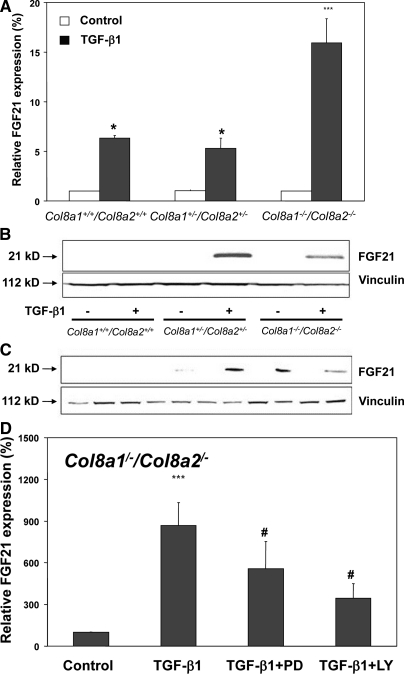
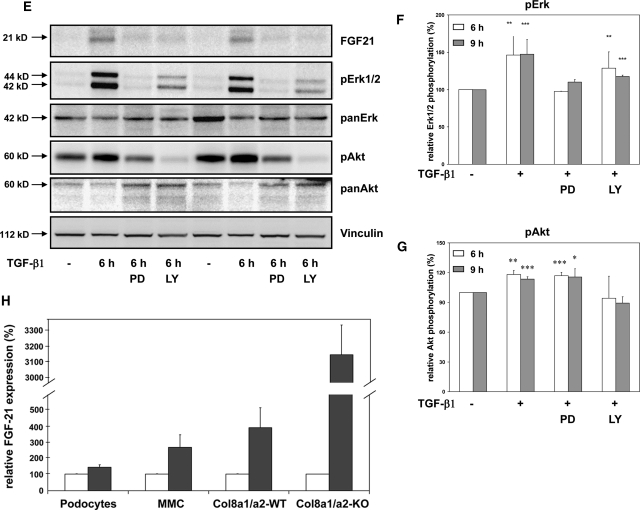
Affymetrix analysis indicated that the TGF-β1-mediated FGF21 gene expression is much higher in Col8a1−/−/Col8a2−/− KO compared with TGF-β1-treated wild-type mesangial cells. Because it has been recently reported that FGF21 is a novel metabolic factor regulating glucose uptake,25 we further focused on this cytokine. As shown in Figure 6A, FGF21 mRNA expression was inducible by TGF-β1 in all three mesangial cells, but induction of FGF21 mRNA was significantly higher (approximately three-fold) in Col8a1−/−/Col8a2−/− KO mesangial cells than in wild-type cells. This finding was confirmed by Western blot analysis (Figure 6B). Time-course analysis of FGF21 expression in Col8a1−/−/Col8a2−/− KO mesangial cells showed that TGF-β1 stimulated expression of FGF21 protein in a time-dependent manner (Figure 6C). Next, we investigated whether the TGF-β1-induced FGF21 expression is dependent on Erk 1,2 and PI3K/Akt pathways. Interference with the Erk 1,2 (with PD98059) as well as PI3K/Akt (with LY294002) pathways attenuated the TGF-β1-mediated upregulation of FGF21 mRNA Col8a1−/−/Col8a2−/− KO mesangial cells (Figure 6D). Moreover, TGF-β1-induced FGF21 protein was reversed to basal levels by PD 098059 or LY294002 (Figure 6E). This Western blot also clearly demonstrates that the inhibitors prevented phosphorylation of Erk 1,2 and Akt (Figure 6E). To test the cell specificity of the TGF-β1-induced FGF21 mRNA expression, podocytes as well as a mouse mesangial cell line (MMC) were studied.26 As shown in Figure 6F, TGF-β1 significantly stimulated FGF21 mRNA expression in mesangial cells with the highest induction in Col8a1−/−/Col8a2−/− KO mesangial cells but not in podocytes.
Figure 6.
(A through E) FGF21 expression induced by TGF-beta is strongest in Col8a1−/−/Col8a2−/− KO mesangial cells. (A) Although TGF-β1 for 24 hours increased FGF21 mRNA expression in all three cell types, the induction was much stronger in Col8a1−/−/Col8a2−/− KO mesangial cells. Quantitative RT-real-time PCR normalized to 18 S rRNA (n = 12, *P < 0.01 versus controls, **P < 0.001 versus controls of the same phenotype). (B) Western blot analysis for FGF21 protein expression. Stimulation of wild-type mesangial cells for 24 hours with TGF-β1 resulted in no detectable band, whereas Col8a1+/−/Col8a2+/− HZ and Col8a1−/−/Col8a2−/− KO mesangial cells clearly expressed FGF21 protein after TGF-β1 treatment. This blot is representative of three independent experiments with qualitatively similar results. (C) Time-course analysis of FGF21 protein expression in Col8a1−/−/Col8a2−/− KO mesangial cells. The cells were stimulated with 2.5 ng/ml TGF-β1 (+) or control medium without TGF-β1 (−) for the indicated length of time, and FGF21 protein expression was analyzed by Western blot. TGF-β1-mediated FGF21 protein expression occurred after 3 hours, peaked at 6 to 9 hours, and diminished after 24 hours. This blot is representative of two separate experiments. (D) Effect of MAP kinase (PD98059, PD) or PI3K inhibitors (LY294002, LY) on TGF-β1-induced FGF21 mRNA expression Col8a1−/−/Col8a2−/− KO mesangial cells. The cells were preincubated with 20 μM PD 98059 and 10 μM LY294002 for 30 minutes and then stimulated with TGF-β1 for further 24 hours. Both inhibitors significantly reduced the TGF-β1-mediated increase in FGF21 mRNA (n = 8, *P < 0.001 versus controls, #P < 0.01 versus TGF-β1 only). (E) Representative blots of FGF21 protein expression in the presence or absence of MAP kinase (PD98059) or PI3K inhibitors (LY294002) in Col8a1−/−/Col8a2−/− KO mesangial cells. The cells were treated with TGF-β1 (2.5 ng/ml) for the indicated times. Either 20 μM PD98059 or 10 μM LY294002 were given for 30 minutes before TGF-β1 treatment. PD98059 inhibits TGF-β1 phosphorylation of Erk 1,2 and LY294002 Akt phosphorylation, respectively. TGF-β1 induced FGF21 protein expression was abolished by PD98059 as well as LY294002. This blot is representative of three independent experiments. (F) Quantification of ERK phosphorylation in Col8a1−/−/Col8a2−/− KO mesangial cells (**P < 0.01 versus time-matched controls without TGF-b1, ***P < 0.001 versus time-matched controls without TGF-b1, n=3). (G) Quantification of AKT phosphorylation in Col8a1−/−/Col8a2−/− KO mesangial cells (**P < 0.01 versus time-matched controls without TGF-b1, ***P < 0.001 versus time-matched controls without TGF-b1, n = 3). (H) FGF21 mRNA expression is induced by TGF-b1 in mesangial cells. Primary mesangial cells (wildtype and Col8a1−/−/Col8a2−/− KO), a mouse mesangial cell line (MMC), and mouse podocytes were stimulated with TGF-b1 for 24 hours and the FGF21 mRNA expression was determined with RT-real-time PCR analysis. Data of relative mRNA expression are normalized to 18S rRNA. TGF-b1 induced only in mesangial cells FGF21 mRNA with the strongest increase in Col8a1−/−/Col8a2−/− KO cells (n = 12, **P < 0.01 versus controls, ***P < 0.001 versus controls of the same genotype, #P <0.01 versus Col8a1+/+/Col8a2+/+ wildtype mesangial cells treated with TGF-b1).
Inhibition of FGF21 Expression Attenuates the TGF-β1-mediated Proliferation in Col8a1−/−/Col8a2−/− KO Mesangial Cells
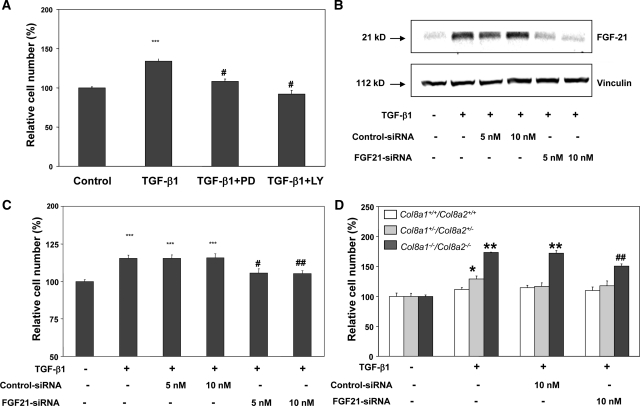
Inhibition of Erk 1,2 or PI3K/Akt pathways attenuated the TGF-β1-stimulated proliferation of Col8a1−/−/Col8a2−/− KO mesangial cells (Figure 7A). To further investigate the role of FGF21 in the TGF-β1-induced proliferation of Col8a1−/−/Col8a2−/− KO mesangial cells, FGF21 expression was inhibited by siRNA. 5 and 10 nM FGF21 siRNA, but not control siRNA, inhibited TGF-β1-mediated FGF21 protein expression in Col8a1−/−/Col8a2−/− KO mesangial cells (Figure 7B). Inhibition of FGF21 protein expression using specific siRNA inhibited the TGF-β1-stimulated proliferation of Col8a1−/−/Col8a2−/− KO mesangial cells (Figure 7C).
Figure 7.
TGF-β1-induced proliferation depends on FGF21 expression. (A) Inhibition of MAP kinases with PD98059 or PI3K with LY294002 completely attenuates TGF-β1-inuduced proliferation of Col8a1−/−/Col8a2−/− KO mesangial cells (n = 24, ***P < 0.001 versus control, #P < 0.001 versus TGF-β1 only). (B) FGF21 siRNA inhibits TGF-β1-induced FGF21 protein expression in Col8a1−/−/Col8a2−/− KO mesangial cells. A control siRNA was without effect. The blot is representative of two independent experiments. (C) Transfection of Col8a1−/−/Col8a2−/− KO mesangial cells with FGF21 siRNA, but not with control siRNA, inhibits TGF-β1-induced proliferation (n = 12, ***P < 0.001 versus controls; #P < 0.01 versus TGF-β1 only, ##P < 0.001 versus TGF-β1 only). (D) TGF-β1-induced a modest proliferative effect in heterozygous Col8a1+/−/Col8a2+/− (*P < 0.05 versus no TGF-β1, n = 12) and a stronger increase in cell number in Col8a1−/−/Col8a2−/− KO mesangial cells (**P < 0.01 versus no TGF-β1, n = 12). These effects were antagonized by FGF21 siRNA (##P < 0.01 versus control siRNA, n = 12). Neither the control siRNA nor the FGF21 siRNA exhibited any influence on cell number of Col8a1+/+/Col8a2+/+ wild-type mesangial cells in the presence or absence of TGF-β1 (n = 12).
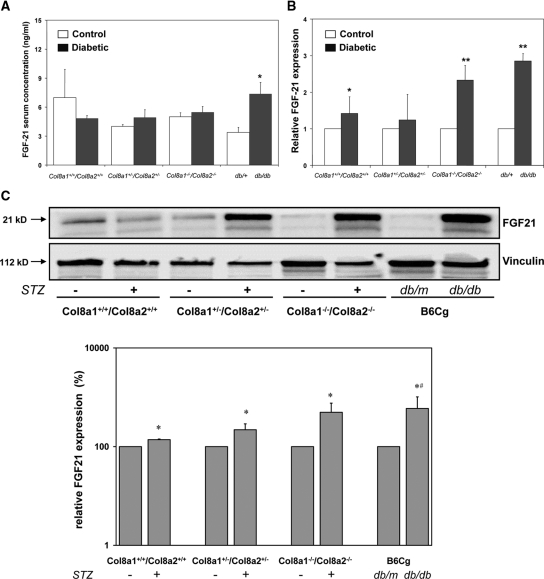
Enhanced Renal FGF21 Expression in Diabetic Col8a1−/−/Col8a2−/− KO Mice
To address the in vivo situation, we investigated the levels of FGF21 in serum and kidney homogenate in the streptozotocin-induced diabetic wild-type, Col8a1+/−/Col8a2+/− HZ, and Col8a1−/−/Col8a2−/− KO mice. Because it has been recently demonstrated that serum FGF21 levels and FGF21 mRNA expression in different tissues are increased in obese and diabetic mice,27 we used db/db diabetic mice as a control. Serum FGF21 concentrations significantly increased only in db/db mice, a model of type2 diabetes, compared with nondiabetic db/m littermates, whereas no significant difference was observed in streptozotocin-induced diabetic mice compared with nondiabetic controls (Figure 8A). However, renal FGF21 mRNA (Figure 8B) and protein (Figure 8C) expression was significantly higher in diabetic Col8a1−/−/Col8a2−/− KO mice compared with diabetic wild-type animals.
Figure 8.
FGF21 expression in diabetic mice is significantly higher in diabetic mice. (A) Serum FGF21 concentrations were only significantly enhanced in diabetic db/db mice compared with nondiabetic db/+ animals. STZ-induced diabetes in wild-type, Col8a1+/−/Col8a2+/− HZ, and Col8a1−/−/Col8a2−/− KO mice did not significantly change serum FGF21 concentrations (n = 5 animals in each group, *P < 0.01 versus db/+). (B) Renal FGF21 mRNA expression. FGF21 transcripts were highest in STZ-induced diabetic Col8a1−/−/Col8a2−/− KO and db/db mice (n = 5 animals per group, *P < 0.05 versus nondiabetic controls of the same genotype, **P < 0.01 versus nondiabetic controls of the same genotype). (C) Western blot analysis of FGF21 protein expression in whole renal homogenates from diabetic mice. In general expression of FGF21 protein was significantly higher in diabetic mice (STZ or db/db) compared with nondiabetic controls (*P < 0.05 versus nondiabetic controls, n = 2). In addition, bands for FGF21 protein are significantly stronger in homogenates from diabetic Col8a1−/−/Col8a2−/− KO mice compared with diabetic wild-type mice (#P < 0.05 versus diabetic wild-type, n = 2).
DISCUSSION
Mesangial cells play an important role in the development of diabetic glomerulosclerosis. In vitro and in vivo studies have demonstrated that mesangial cells exposed to high glucose or the diabetic environment initially undergo a very limited proliferation and become subsequently arrested in the G1 phase of the cell cycle.10,26 This G1 phase arrest is associated with the development of cellular hypertrophy and production of extracellular matrix proteins leading to mesangial expansion and sclerosis.28 TGF-β1 is a key factor induced by high glucose, mediating G1 phase arrest by induction of cell cycle inhibitors such as p27Kip1.11,12
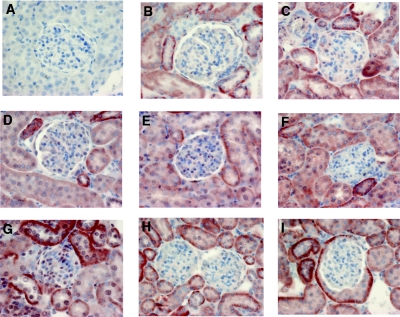
We have recently found in biopsies from patients with diabetic nephropathy that type VIII collagen mRNA and protein expression (Fig. 9) is upregulated compared with healthy control kidneys.16 Furthermore, type VIII collagen expression in human diabetic nephropathy was not correlated with renal function and proteinuria, suggesting that the diabetic environment itself may be primarily responsible for the induction of expression.16 In fact, high glucose-containing medium induced Col8a1 mRNA expression independent of its osmolarity in a MMC, but not in murine tubular and glomerular endothelial cells.15 Col8a2 was not induced by high glucose in mesangial cells. Further studies in STZ-induced diabetic Col8a1−/−/Col8a2−/− KO mice revealed less mesangial matrix deposition and albuminuria compared with diabetic wild-type animals. Because it has been previously shown that type VIII collagen may stimulate migration of smooth vascular muscle cells29 but may inhibit proliferation of endothelial cells,30 this study addresses whether type VIII collagen modulates the influence of TGF-β1, one of the major cytokines in diabetic nephropathy, on cell cycle regulation in mesangial cells. We used mesangial cells isolated from genetically modified mice deficient in both Col8 genes to test whether there is a relationship between the biologic effects of exogenous TGF-β1 and the expression of type VIII collagen.31 We provide evidence that TGF-β1 induced proliferation of mesangial cells in the absence of endogenous type VIII collagen expression. TGF-β1-induced signal transduction changed from utilization of the Smad system to activation of Erk 1,2 and PI3K/Akt pathways in the absence of type VIII collagen, but TGF-β1-receptor affinity and expression was not modulated. Limited GeneChip analysis revealed that TGF-β1-induced FGF21 expression is markedly increased in mesangial cells deficient of type VIII collagen. A greater renal expression of FGF21 was also seen in diabetic Col8a1−/−/Col8a2−/− KO mice compared with diabetic wild-type animals. FGF21 is a recently discovered novel endocrine and paracrine metabolic regulator.32 When given to diabetic animals, FGF21 stimulates glucose uptake into cells and lowers plasma glucose as well as triglycerides.33 On the other hand, FGF21 mRNA expression itself is induced in the liver by glucose.34 Stimulation of the PI3K/Akt signaling pathway induces FGF21.35 However, Wente et al. have previously demonstrated that FGF21 stimulates insulin gene transcription and insulin biosynthesis via activation of Erk 1,2 signaling in the pancreatic β-cell, promotes β-cell survival, and inhibits β-cell apoptosis via activation of PI3K/Akt signaling, which resulted in a strong reduction in circulating glucose levels and an increased number of islets and β-cells in diabetic mice after long-term administration of FGF21.36 Renal effects of FGF21 have not been previously described. FGF21 signals through cell-surface receptors composed of the classic FGF receptors complexed with β-Klotho but can also act through all four FGR isotypes.37–39 Klotho is highly expressed in the kidney and mesangial cells, and these cells have been found to also express FGF receptors.40,41 Thus, our findings that FGF21 mediates the TGF-β1 induced proliferation in Col8a1−/−/Col8a2−/− KO mesangial cells are in accordance with these earlier findings. Because it has been previously shown that FGF21 expression is induced by the PI3K/Akt pathway and there are multiple interactions between the PI3K/Akt and Erk 1,2 pathways,35,42,43 the shift from TGF-β1-induced Smad activation to the noncanonical Erk 1,2 and PI3K/Akt in the absence of type VIII collagen is the cause of mesangial proliferation. However, the increase in whole kidney FGF21mRNA expression as well the enhanced serum concentration of FGF21 in diabetic animal is likely due to stimulated tubular expression as detected in the immunohistochemistry. Nevertheless, the discrete increase in mesangial cell staining for FGF21 in diabetic Col8a1−/−/Col8a2−/− KO mice but not in the diabetic wild-type is presumably sufficient to stimulate local mesangial proliferation.
Figure 9.
Increase in FGF21 mesangial expression only in diabetic Col8a1−/−/Col8a2−/− KO mice. (A through H) Immunohistology for FGF21-protein expression. (A) Negative control with normal rabbit serum. (B) Nondiabetic Col8a1+/+/Col8a2+/+ wild-type mice. (C) Diabetic Col8a1+/+/Col8a2+/+ wild-type animals. (D) Nondiabetic Col8a1+/−/Col8a2+/− heterozygous mice. (E) Diabetic Col8a1+/−/Col8a2+/− heterozygous mice. (F) Nondiabetic Col8a1+/−/Col8a2+/− KO mice. (G) Diabetic Col8a1+/−/Col8a2+/− KO mice. (H) Nondiabetic db/+ mice. (I) diabetic db/db mice (at 4 weeks). Although all diabetic animals revealed an increase in tubular staining for FGF21, a weak increase in mesangial FGF21 expression was only found in diabetic Col8a1+/−/Col8a2+/− KO mice (G). Interestingly, diabetic db/db mice showed an increase in FGF21 expression in visceral cells of Bowman's capsule. No specific staining was found with nonimmune serum (A). This staining is representative of five sections obtained from each kidney from five individual animals with qualitatively similar results (magnification, ×400).
How exactly type VIII collagen modulates TGF-β1-associated signal transduction pathways is currently unclear, but a few suggestions can be made. Older studies have shown that collagen type V is required for the growth and inhibitory actions of TGF-β1 nontransformed epithelial cells.44 The NC1 domain of the α1 chain of type VIII collagen inhibits the proliferation of bovine aortic endothelial cells, but the mechanisms behind these anti-proliferative effects were not further investigated.30 Several studies have also demonstrated that various collagens can exert anti-proliferative effects on mesangial cells.45,46 For example, collagen type I suppresses Erk 1,2 activation and is antiproliferative for mesangial cells.46 Discoidin domain receptors (DDR1 and 2) are receptor tyrosine kinases that function as receptors for various collagens.47 DDR1 is expressed on mesangial cells, and type VIII collagen binds to and activates this receptor.48,49 Interestingly, Curat and Vogel report that DDR1-knockout mesangial cells exhibited an increased proliferation and activation of Erk 1,2.50 Thus, the lack of type VIII collagen synthesis after TGF-β1 stimulation in Col8a1−/−/Col8a2−/− KO mesangial cells may lead to a reduced DDR1 activation with concomitant stimulation of the Erk 1,2 pathway. This, together with the induced FGF21 synthesis and activation of other pathways (e.g. NF-κB), ultimately leads to TGF-β1-induced proliferation and not hypertrophy in Col8a1−/−/Col8a2−/− KO mesangial cells. Further studies that are beyond the scope of this report are necessary to define the exact role of DDR1 in TGF-β1-induced proliferation of Col8a1−/−/Col8a2−/− KO mesangial cells.
Is TGF-β1-mediated mesangial proliferation in the absence of type VIII collagen better than hypertrophy? Regression of diabetic mesangial matrix expansion and glomerulosclerosis have occurred in humans and in experimental models of diabetic nephropathy.51,52 It is reasonable to assume that this process requires some limited proliferation of mesangial cells to restore an intact environment besides proteolytic clearance of the deposited extracellular matrix proteins. Diabetic p27Kip1 knockout mice have a reduced glomerular matrix expansion and less albuminuria despite an increase in the mesangial cell number compared with diabetic wild-type animals.53 Bone marrow-derived cells can differentiate into mesangial cells after renal injury.54 Bone-marrow infusion can ameliorate progressive glomerulosclerosis in different experimental models.55,56 Although de novo formation of endothelial cells is important in this process, there is experimental evidence that the repair also involves mesangial cells.57 Glomerular mesangial cells provide structural support, and a repair process after diabetic nephropathy likely requires a well-balanced recruitment of glomerular endothelial and mesangial cells. Alternatively, one may see the increase in type VIII collagen expression during diabetic nephropathy as an intrinsic renal attempt to limit progression of disease.
In summary, we found that TGF-β1 induces a proliferation of mesangial cells in the absence of concomitant type VIII collagen expression. This effect is in strong contrast to wild-type mesangial cells in which TGF-β1 is anti-proliferative and induces cell cycle arrest. The proliferative effect of TGF-β1 in Col8a1−/−/Col8a2−/− KO mesangial cells is associated with a shift from Smad to noncanonical TGF-β1-pathways and depends on the induction of FGF21.
CONCISE METHODS
Animal Experiments
All of the animal experiments were approved by the Thüringer Landesamt für Lebensmittelsicherheit und Verbraucherschutz and done in accordance with the German Animal Protection Law. The generation and phenotype of Col8a1−/−/Col8a2−/− KO mice has been previously described in detail.31 Col8a1−/−/Col8a2−/− KO and Col8a1+/−/Col8a2+/− HZ mice were crossed back for at least 20 generations into the C57Bl/6 background,31 and wild-type mice were maintained in a pathogen-free facility. All of the animals had free access to water and were on the standard rodent chow. Ten- to twelve-week-old mice were randomly divided into groups treated with streptozotocin (STZ; Sigma, St. Louis, MO) or left untreated. STZ was dissolved in sterile 10 mM sodium citrate (pH 5.5) and injected intraperitoneally (50 mg/kg body wt) for five consecutive days. To render the animals hyperglycemic without becoming ketoacidotic, a subcutaneous insulin implant (LinShin, Toronto, Canada) was administered.15 Venous blood glucose concentrations were measured with Free Style Lite (Abbott Diabetes Care, Wiesbaden, Germany). The mice were sacrificed after 40 days. In addition, diabetic db/db mice and control db/m mice (Jackson Laboratory, Bar Harbor, ME) were used at the age of 4 months. At the end of the experiment, venous blood was collected in tubes on ice containing aprotinin (0.6 TIU/ml of blood; Phoenix Pharmaceuticals, Burlingame, CA) to inhibit the activity of proteinases. FGF21 protein concentrations were determined in serum samples using a mouse FGF21 RIA kit (Phoenix Pharmaceuticals, Burlingame, CA). In addition, the kidneys were harvested and mechanically homogenized with the homogenizer SpeedMill P12 (Analytik Jena Bio Solutions, Jena, Germany), and the total RNA and protein were isolated as described below.
Isolation and Culture of Mesangial Cells
Mesangial cells were obtained from the isolated glomeruli of Col8a1+/+/Col8a2+/+ wild-type, Col8a1+/−/Col8a2+/− HZ, and Col8a1−/−/Col8a2−/− KO mice kidneys according to previously described protocols of sieving and centrifugation for the preparation of primary mesangial cell cultures.15,25 Our studies used subcultures of the fifteenth to twentieth passages of mesangial cells grown in RPMI 1640 medium (Promo Cell, Heidelberg, Germany), containing 5 mM glucose and 10% heat-inactivated FCS at 37°C in 5% CO2. Routine identification of the mesangial cells was performed by indirect immunofluorescence microscopy using antibodies against α-smooth-muscle actin (Progen Biotechnik, Heidelberg, Germany), synaptopodin (Sigma), E-cadherin (Abcam, Cambridge, UK), and PECAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA). Used cells stained positive for α-smooth-muscle actin but were negative for synaptopodin, E-cadherin, and PECAM-1. The cells were seeded in appropriate tissue culture plates and cultured for 24 hours. The cells were serum-starved (0.1% FCS) overnight and exposed as described for the indicated lengths of time.
Proliferation Assays
A colorimetric immunoassay kit for BrdU incorporation (Roche Diagnostics, Mannheim, Germany) was used for quantification of DNA synthesis.58 1 × 103 cells were seeded in 96-well plates; after incubation in RPMI supplemented with 0.1% FCS for 24 hours, the cells were treated with 2.5 ng/ml human recombinant TGF-β1 (Peprotech, Hamburg, Germany) for 24 hours. Mesangial cells were then labeled with BrdU for 4 hours at 37°C, and the incorporation was determined with the assay kit following the manufacturer's instructions. The absorbance of the samples was measured at 450 nm using a microplate reader (Tecan, Crailsheim, Germany).
For automated cell counting, 5 × 104 cells were seeded in 12-well culture plates and cultured for 24 hours. The cells were serum-starved overnight and exposed to agonists for a further 24 hours. The cells were detached from the culture dishes by trypsinization and counted in a CASY Cell Counter (Schärfe System, Reutlingen, Germany) using the Analyzer System Model DT routine according to the manufacturer's instructions.
For reconstitution experiments, full-length Col8a1 cDNA15 cloned into pCEP-Pu vector or pCEP-Pu control vector were transfected into mesangial cells (80 to 90% confluence) using LipofectaminTM 2000 reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's protocol.
Cell Cycle Analysis and Apoptosis
Cell cycle analysis and apoptosis assay were performed using flow cytometry. After 24 hours of treatment with 2.5 ng/ml TGF-β1, the cells were harvested by trypsinization and washed twice with PBS. The cells were suspended in 200 μl of PBS with 2% FCS and incubated in staining solution (10 mM 1,4-piperazinediethanesulfonic acid, 2 mM MgCl2, 0.1 M NaCl, 0.1% Triton X-100, 25 μg/ml RNase [Roche Diagnostics], 10 μg/ml propidium iodide [Sigma]) at 4°C for 30 minutes in the dark. Then each sample was analyzed using a FACScalibur flow cytometer (Becton Dickinson, Franklin Lake, NJ), and the percentages of cells within the sub-G1, G1, S, and G2/M phases of the cell cycle were determined. The total number of cells analyzed for each sample was 10,000, and raw data were processed using CellQuestPro and WinMDI software.
Annexin V binding was determined with an apoptosis assay kit (Roche Diagnostics) following the manufacturer's instructions. Fluorescence was measured on the FACScalibur flow cytometer.
TGF-β1 Receptor Binding Assay
5 × 104 Col8a1+/+/Col8a2+/+ wild-type and Col8a1−/−/Col8a2−/− KO mesangial cells were plated per well in a 12-well plate and grown for 24 hours. After serum deprivation for 12 hours, the cells were washed once with ice-cold PBS and preincubated with binding buffer (serum-free RPMI with 5 mM MgCl2, 10 mM 6-aminocaproic acid, 1 mM phenylmethylsulfonyl fluoride, 0.05% aprotinin, and 1 mg/ml bovine serum albumin) on a shaking platform at 4°C for 30 minutes. For saturation binding assays, increasing amounts (0.5 to 25 pM) of human recombinant 125I-TGF-β1 (specific activity, 3287 Ci/mmol) (PerkinElmer, Waltham, MA) were added in binding buffer. Nonspecific binding was determined in the presence of 0.5 to 25 nM human recombinant TGF-β1 (Peprotech, Hamburg, Germany). After incubation on a shaking platform at 4°C for 1 hour, the cells were gently washed three times with ice-cold binding buffer and lysed with 1 ml of 5 M NaOH with 0.5% Triton X-100. The amount of radioactivity was counted in a gamma scintillation counter. Control wells without radioactivity were trypsinized, and the cell number was determined with a CASY Cell Counter (Schärfe System, Reutlingen, Germany). Nonspecific binding was subtracted, and the data were analyzed with GraphPad Prism (GraphPad Software, La Jolla, CA). The results are presented as Scatchard plots of saturation binding data, and each point represents the mean of three individual experiments.
RNA Extraction, cDNA Synthesis, and Reverse Transcription (RT) Real-time PCR
For RT real-time PCR analysis, total RNA was isolated from treated cells or kidney homogenates using the RNeasy kit (Qiagen, Hilden, Germany), and 1 μg was reverse-transcribed using the Reverse Transcription System (Promega, Madison, WI) following the manufacturer's instructions. The expression levels of genes were determined by quantitative PCR using LightCycler-FastStart DNA Master SYBR Green I (Roche Diagnostics) on a Real-Plex Mastercycler (Eppendorf, Hamburg, Germany). PCRs were carried out in a total volume of 20 μl containing 2 μl of cDNA and sense and antisense primers in a concentration of 0.25 μM each. To normalize for differences in the amount of cDNA in each sample, we performed amplification of 18 S ribosomal RNA as an endogenous control. The amplification program included an initial denaturation step (10 minutes, 95°C), 40 cycles of amplification (denaturation for 10 seconds at 95°C), primer annealing (temperature see Table 1) for 15 seconds, extension for 10 seconds at 72°C, an additional heating step to melt potential primer dimers for 5 seconds at 95°C), and a melting curve program (denaturation for 10 seconds at 95°C, cooling and holding for 15 seconds at 60°C, and then heating at a speed of 0.1°C/s to 95°C). Finally, a cooling step of 2 minutes at 40°C was performed. Table 1 shows the sequences of the primer pairs. The transcript levels were normalized to the mean value of samples from the unstimulated controls.
Table 1.
List of primer pairs and annealing temperature
| Gene | Sense Primers | Antisense Primers | Tann. |
|---|---|---|---|
| 18 S rRNA | 5′-CACGGCCGGTACAGTGAAAC-3′ | 5′-GATCTGATAAATGCACGC-3′ | 58°C |
| Col8a1 | 5′-CGGGAGTAGGAAAACCAGGAGTG-3′ | 5′-CTCGGCCCAAGAACCCCAGGAA-3′ | 62°C |
| FGF21 | 5′-ATGGAATGGATGAGATCTAGAGTTGG-3′ | 5′-TCTTGGTGGTCATCTGTGTAGAGG-3′ | 62°C |
| NFκB | 5′-AGTACCTGCCAGATACAGACGAT-3′ | 5′-GATGGTGCTCAGGGATGACGTA-3′ | 58°C |
| p110α | 5′-GAACCAGTAGGCAACCGTGAA-3′ | 5′-GCATCCTCCCCAGCATTAT-3′ | 56°C |
| Vinculin | 5′-GCCAAGCAGTGCACAGATAA-3′ | 5′-TTCCTTTCTGGTGTGTGAAGC-3′ | 62°C |
Microarray Experiments
After stimulation of Col8a1+/+/Col8a2+/+ wild-type and Col8a1−/−/Col8a2−/− KO mesangial cells with 2.5 ng/ml TGF-β1 for 24 hours, total RNA was extracted as described above. The GeneChip Mouse Genome 430 2.0 Array containing probes of over 39000 transcripts (Affymetrix, Santa Clara, CA) was used for analysis. Array hybridization was performed according to the supplier's instructions using the GeneChip Expression 3′Amplifikation One-Cycle Target Labeling and Control reagents from Affymetrix. The scanning of the microarray was done with the GeneChip Scanner 3000 (Affymetrix) at 1.56 μ resolution. Data analysis was performed with the MAS 5.0 (Microarray Suite statistical algorithm; Affymetrix) probe level analysis using GeneChip Operating Software (GCOS 1.4), and the final data extraction was done with the DataMining Tool 3.1 (Affymetrix). Each experiment (stimulation of cells, isolation of RNA, chip hybridization, and analysis) was independently performed three times, and only differentially expressed transcripts in all sets of experiments were further analyzed. For the details of data analysis according to the MIAME Guidelines,22,23 see the online supplemental materials.
FGF21 siRNA
To inhibit the FGF21 mRNA expression, mesangial cells were transfected with siRNA targeting FGF21 (5′-TTGGAATAATAAAGAGTCTGA-3′; NM_020013) or control siRNA (Qiagen) using HiPerfect Transfection Reagent (Qiagen). After incubation with the transfection complexes under normal growth conditions overnight, the medium was changed, and the cells were stimulated with 2.5 ng/ml TGF-β1 for 24 hours.
Western Blot Analysis
Cultured mesangial cells and kidney homogenate were lysed using Complete LysisM (Roche Diagnostics) and supplemented with the phosphatase inhibitor sodium orthovanadate (100 μM). Lysates were cleared by centrifugation, and 50 μg of protein/lane was separated by SDS-PAGE. Western blotting was performed as described previously.59 The primary antibodies used were as follows: anti-mouse collagen α1(VIII) (Abcam, Cambridge, UK), anti-phospho-Erk 1,2, anti-phospho-Akt, anti-phospho-Smad3 (Cell Signaling Technology, Danvers, MA), and anti-mouse FGF21 (R&D Systems, Minneapolis, MN). The blots were subsequently reprobed with anti-α-vinculin (Sigma), anti-Erk 2, anti-Akt (Santa Cruz Biotechnology), and anti-Smad3 (Cell Signaling Technology). Peroxidase-labeled secondary antibodies to rabbit IgG and mouse IgG (1:10,000) were purchased from KPL (Gaithersburg, MD). Western Lightning Chemiluminescence Reagent Plus (ECL; PerkinElmer LAS, Boston, MA) was used for detection of signals, and the images were captured using a Fuji LAS-3000 imaging system (Fujifilm Life Science, Düsseldorf, Germany) as described previously.58,59
Immunohistochemistry for FGF21 Expression
Paraffin-embedded kidneys were sectioned at 4 μm. For the detection of FGF21, a rabbit polyclonal to FGF21 (Abcam, Cambridge, UK) with a concentration of 0.30 mg/ml was used in a 1.100 dilution (this antibody reacts with human and murine FGF21). Detection was performed with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) using an anti-rabbit secondary antibody as described previously.59
Statistical Analyses
The values given in this article are presented as the means ± SEM. The results were analyzed with the Kruskal-Wallis test for multigroup comparison followed by the Mann-Whitney U-test. A P value of <0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
We would like to acknowledge Simone Schönfelder and Sybille Franke for excellent support, Martin Förster for help with FACS analysis, Claus Liebmann for helpful discussions of the receptor binding studies, and Wolfgang Schmidt-Heck for the help with the data analysis of microarray studies. This work was supported by a grant from the Deutsche Forschungsgemeinschaft to G.W.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Wolf G: New insights into the pathophysiology of diabetic nephropathy: From haemodynamics to molecular pathology. Eur J Clin Invest 34: 785–796, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Raptis AE, Viberti G: Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes 109: 424–437, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Wolf G, Ziyadeh FN: Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol 106: 26–31, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Ziyadeh FN: Different roles for TGF-β and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract 82(Suppl. 1):S38–S41, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Ziyadeh FN, Sharma K, Ericksen M, Wolf G: Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest 93: 536–542, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA: Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U.S.A. 90: 1814–1818, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hong SW, Isono M, Chen S, Iglesias-de la Cruz MC, Han DC, Ziyadeh FN: Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol 158: 1653–1663, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma K, Jin Y, Guo J, Ziyadeh FN: Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45: 522–530, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Ziyadeh FN, Hoffman BB, Han DC, Iglesias-de la Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K: Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U.S.A. 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN: High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney Int 42: 647–656, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Monkawa T, Hiromura K, Wolf G, Shankland SJ: The hypertrophic effect of transforming growth factor-beta is reduced in the absence of cyclin-dependent kinase-inhibitors p21 and p27. J Am Soc Nephrol 13: 1172–1178, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Wolf G, Schroeder R, Zahner G, Stahl RA, Shankland SJ: High glucose-induced hypertrophy of mesangial cells requires p27Kip1, an inhibitor of cyclin-dependent kinases. Am J Pathol 158: 1091–1100, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krag S, Nyengaard JR, Wogensen L: Combined effects of moderately elevated blood glucose and locally produced TGF-β1 on glomerular morphology and renal collagen production. Nephrol Dial Transplant 22: 2485–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Sternberg M, Cohen-Forterre L, Peyroux J: Connective tissue in diabetes mellitus: Biochemical alterations of the intercellular matrix with special reference to proteoglycans, collagens and basement membranes. Diabete Metab 11: 27–50, 1985 [PubMed] [Google Scholar]
- 15. Hopfer U, Hopfer H, Meyer-Schwesinger C, Loeffler I, Fukai N, Olsen BR, Stahl RAK, Wolf G: Lack of type VIII collagen in mice ameliorates diabetic nephropathy. Diabetes 58: 1672–1681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerth J, Cohen CD, Hopfer U, Lindenmeyer MT, Sommer M, Gröne HJ, Wolf G: Collagen type VIII expression in human diabetic nephropathy. Eur J Clin Invest 37: 767–773, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Sage H, Trüeb B, Bornstein P: Biosynthetic and structural properties of endothelial cell type VIII collagen. J Biol Chem 258: 13391–13401, 1983 [PubMed] [Google Scholar]
- 18. Plenz GAM, Deng MC, Robenek H, Völker W: Vascular collagens: Spotlight on the role of type VIII collagen in atherogenesis. Atherosclerosis 166: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Khera T, Martin J, Riley S, Steadman R, Phillips AO: Glucose enhances mesangial cell apoptosis. Lab Invest 86: 566–577, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Böttinger E, Bitzer M: TGF-β signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G: TGF-β and fibrosis in different organs: molecular pathway imprints. Biochim Biophys Acta 1792: 746–756, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Hosltege FCP, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M: Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet 29: 365–371, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Rayner TF, Rocca-Serra P, Spellman PT, Causton HC, Farne A., Holloway E, Irizarry RA, Liu J, Maier DS, Miller M, Petersen K, Quackenbush J, Sherklock G, Stoeckert CJ, Jr., White J, Whetzel PL, Wymore F, Parkinson H, Sarkans U, Ball CA, Brazma A: A sinmple spreadsheet-based, MIAME-supportive format for microarray data: NAGE-TAB. Biomed Central 2010, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kliewer SA, Mangelsdorf DJ: Fibroblast growth factor 21: From pharmacology to physiology. Am J Clin Nutr 91[Suppl]: 254S–257S, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolf G, Haberstroh U, Neilson EG: Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol 140: 95–107, 1992 [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X, Yeung DCY, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RLC, Chow WS, Tso AWK, Lam KSL, Xu A: Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57: 1246–1253, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Young BA, Johnson RJ, Alpers CE, Eng E, Gordon K, Floege J, Couser WG: Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int 47: 935–944, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Wolf G, Ziyadeh FN: Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 56: 393–405, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Hou G, Mulholland D, Gronska MA, Bendeck MP: Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinases synthesis after arterial injury. Am J Pathol 156: 467–476, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu R, Yao ZY, Xin L, Zhang Q, Li TP, Gan RB: NC1 domain of human type VIII collagen (alpha 1) inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochem Biophys Res Commun 289: 264–268, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Hopfer U, Fukai N, Hopfer H, Wolf G, Joyce N, Li E, Olsen BR: Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J 19: 1232–1244, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Nishimura T, Nakatake Y, Konishi M, Nobuyuki I: Identification of a novel FGF, FGF21, preferentially expressed in the liver. Biochim Biophys Acta 1492: 203–206, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB: FGF21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iizuka K, Takeda J, Horikawa Y: Glucose induces FGF21 mRNA expression trough ChREBP activation in rat hepatocytes. FEBS Lett 583: 2882–2886, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitoenkov A, Wals K: FGF21 is an Akt-regulated myokine. FEBS Lett 582: 3805–3810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J: Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55: 2470–2478, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM: Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281: 15694–15700, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beenken A, Mohammadi M: The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8: 235–253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T: bKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptors (FGFR) 1c and FGFR3c. Mol Endocrinol 22: 1006–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB: Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int 68: 2621–2628, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Chambard JC, Lefloch R, Pouysségur J, Lenormand P: ERK implication in cell cycle regulation. Biochim Biophys Acta 1773: 1299–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Kato M, Yuan H, Xu ZG, lanting L, Li SL, Wang M, Hu MCT, Reddy MA, Natarajan R: Role of the Akt/FoxO3a pathway in TGF-β1-mediated mesangial cell dysfunction: A novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 17: 3325–3335, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Sarubbi DJ, Narayanan R, Telang NT, Newmann MJ: Evaluation of the role of extracellular matrix proteins, polyunsaturated fatty acids and c-myc expression in the inhibition of the serum-free growth of epithelial cells by TGF-β1. In Vitro Cell Dev Biol 26: 1195–1201, 1990 [DOI] [PubMed] [Google Scholar]
- 45. He CJ, Striker LJ, Tsokos M, Yang CW, Peten EP, Striker GE: Relationships between mesangial cell proliferation and types I and IV collagen mRNA levels in vitro. Am J Physiol 269: C554–C562, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Miralem T, Templeton DM: Inactivation of kinase cascades in mesangial cells grown on collagen type I. Am J Physiol 275: F585–F594, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Leitinger B, Hohenester E: Mammalian collagen receptors. Matrix Biology 26: 146–155, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Lee R, Eidman KE, Kren SM, Hostetter TH, Segal Y: Localization of discoidin domain receptors in rat kidney. Nephron Exp Nephrol 97: e62–e70, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Hou G, Vogel W, Bendeck MP: The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest 107: 727–735, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curat CA, Vogel WF: Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J Am Soc Nephrol 13: 2648–2656, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Fioretto P, Steffes MW, Sutherland DER, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Teles F, Machado FG, Ventura BH, Malheiros DMA, Fujihara CK, Silva LFF, Zatz R: Regression of glomerular injury by losartan in experimental diabetic nephropathy. Kidney Int 75: 72–79, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Wolf G, Schanze A, Stahl RAK, Shankland SJ, Amann K: p27Kip1 knockout mice are protected from diabetic nephropathy: Evidence for p27Kip1 haplotype insufficiency. Kidney Int 68: 1583–1589, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Roufosse C, Cook HT: Stem cells and renal regeneration. Nephron Exp Nephrol 109: e39–e45, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Guo JK, Schedl A, Krause DS: Bone marrow transplantation can attenuate the progression of mesangial sclerosis. Stem Cells 24: 406–415, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Li B, Morioka T, Uchiyama M, Oite T: Bone marrow cell infusion ameliorates progressive glomerulosclerosis in an experimental rat model. Kidney Int 69: 323–330, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Imasawa T, Utsunomiya Y, Kawamura T, Zhong Y, Nagasawa R, Okabe M, Maruyama N, Hosoya T, Ohno T: The potential of bone marrow-derived cells to differentiate to glomerular mesangial cells. J Am Soc Nephrol 12: 1401–1409, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Bondeva T, Wolf G: Advanced glycation end products suppress neuropilin-1 expression in podocytes by a reduction in SP1-dependent transcriptional activity. Am J Nephrol 30: 336–345, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Hammerschmidt I, Loeffler I, Wolf G: Morg1 heterozygous mice are protected from acute renal ischemia-reperfuision injury. Am J Physiol Renal Physiol 287: F1273–F1287, 2009 [DOI] [PubMed] [Google Scholar]