Abstract
Hyperplasia of the PTG underlies the secondary hyperparathyroidism (SHPT) observed in CKD, but the mechanism underlying this hyperplasia is incompletely understood. Because aberrant cyclooxygenase 2 (COX2) expression promotes epithelial cell proliferation, we examined the effects of COX2 on the parathyroid gland in uremia. In patients with ESRD who underwent parathyroidectomy, clusters of cells within the parathyroid glands had increased COX2 expression. Some COX2-positive cells exhibited two nuclei, consistent with proliferation. Furthermore, nearly 78% of COX2-positive cells expressed proliferating cell nuclear antigen (PCNA). In the 5/6-nephrectomy rat model, rats fed a high-phosphate diet had significantly higher serum PTH levels and larger parathyroid glands than sham-operated rats. Compared with controls, the parathyroid glands of uremic rats exhibited more PCNA-positive cells and greater COX2 expression in the chief cells. Treatment with COX2 inhibitor celecoxib significantly reduced PCNA expression, attenuated serum PTH levels, and reduced the size of the glands. In conclusion, COX2 promotes the pathogenesis of hyperparathyroidism in ESRD, suggesting that inhibiting the COX2 pathway could be a potential therapeutic target.
Secondary hyperparathyroidism (SHPT) is one of the most common abnormalities of mineral metabolism in patients with chronic kidney disease (CKD), characterized by hyperplasia of the parathyroid gland (PTG).1 It is well documented that increased parathyroid hormone (PTH) contributes to bone disorders and cardiovascular complications of ESRD patients and is associated with the morbidity and mortality of this population.2,3 Strong evidence indicates that PTG hyperplasia is positively linked to the magnitude of circulating levels of PTH, and the size of the PTG is associated with the controllability of parathyroid function in CKD patients.4,5 The mechanism underlying PTG hyperplasia is incompletely defined.
Under normal conditions, the turnover rate of the PTG is very low, and mitoses are seldom observed.1,6 However, under certain pathologic conditions such as CKD, hyperphosphatemia, and vitamin D deficiency, increased proliferation of the PTG can be detected.7,8 In CKD patients, the PTG shows diffuse and polyclonal proliferation at early stages of SHPT. With progression of the disease, the PTG can transform with monoclonal and aggressive proliferation.9–11 It is well accepted that PTG hyperplasia is associated with reduced 1,25-vitamin D synthesis from the kidney and downregulation of the vitamin D receptor (VDR)12 and calcium-sensing receptor in the PTG.13,14 In the hyperparathyroidism tissue, the expression of the cyclin-dependent kinase inhibitors p21 and p27 is depressed in a VDR-dependent manner.15,16 Recently, EGF receptor (EGFR) activation by TGF-α has also been suggested to play an important role in the growth of the PTG and the downregulation of VDR, which may be associated with vitamin D resistance in advanced SHPT in ESRD.17 EGFR activation may lead to activation of cyclin D1, an important contributor to parathyroid hyperplasia in humans.18
Cyclooxygenase (COX) catalyzes the rate-limiting step in the synthesis of prostaglandins (PGs) from arachidonic acid (AA). Two isoforms have been identified, COX1 and COX2. COX1 is constitutively expressed, whereas COX2 is induced by various mitogenic and inflammatory stimuli.19–21 Multiple lines of evidence suggest that COX2 plays a significant role in carcinogenesis by promoting cell proliferation, inhibiting apoptosis, and stimulating angiogenesis.20,22 Nonselective COX inhibitors and selective COX2 inhibitors have been shown to have chemopreventive and therapeutic effects on many types of tumors.23
COX-derived PGs were reported in bovine PTGs.24 COX1 and COX2 expression has been observed via immunohistochemistry in the oxyphil cells of the PTG from patients.25 PGE2, a bioactive product of COX, was reported to increase PTH production.26,27 These studies are consistent with a potential role of COX-derived PGs in the pathogenesis of SHPT and led us to propose that COX2 may be involved in the pathogenesis of parathyroid hyperplasia.
RESULTS
COX2 is Expressed in the Hyperplastic PTG from Hemodialysis Patients and Is Associated with Cell Proliferation
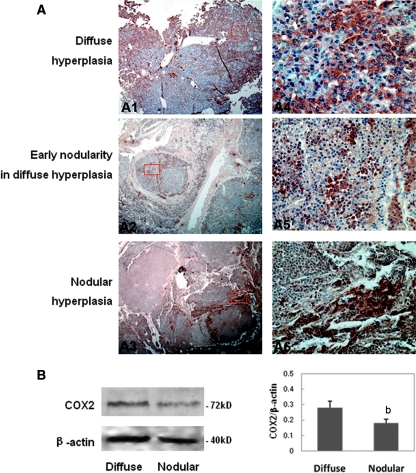
Among 39 PTGs obtained from hemodialysis patients, 12 glands showed diffuse hyperplasia and 27 glands exhibited nodular hyperplasia. Chief and oxyphil cells were present in the hyperplastic regions. Using an immunohistochemistry method, COX2 immunoprotein was detected in clusters of cells within the hyperplasia PTGs (Figure 1A), whereas COX1 expression was barely detectable (data not shown). Interestingly, the distribution of COX2 positivity seems to be influenced by the degree of tissue hyperplasia. In the diffuse hyperplastic parathyroid, the distribution of COX2-positive cells was diffuse and the intensity was relatively uniform (Figure 1, A1 and A4), whereas in the nodular hyperplastic parathyroid, COX2-positive cells were mainly distributed in the periphery of the nodule and the cells within the nodule showed weak COX2 immunoreactivity (Figure 1, A2, A3, A5, and A6). Immunoblot analysis further indicated that the levels of COX2 expression in the diffuse hyperplasic parathyroid tissues were higher than those in nodular hyperplasic tissues (Figure 1B).
Figure 1.
COX2 is expressed in the hyperplastic PTGs from hemodialysis patients. (A) COX2 immunoreactivity in PTGs with diffuse hyperplasia (A1, A4), early nodular hyperplasia (A2, A5), and nodular hyperplasia (A3, A6). Magnifications: ×5 in A1 through A3; ×400 in A4 through A6. (B) Immunoblot of diffuse hyperplasia and nodular hyperplasia PTGs using antibodies for COX2 and β-actin as a loading control. Quantitative analysis result was expressed as COX2/β-actin protein ratio. bP < 0.01 versus diffuse hyperplasia tissue.
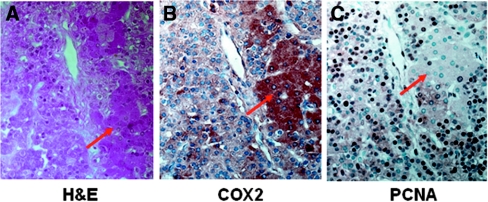
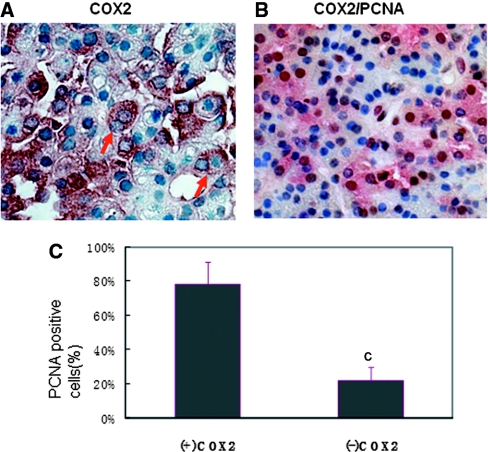
To examine whether COX2 expression is associated with cell proliferation and to determine the cell type that expresses COX2, we performed immunohistochemistry and hemotoxylin and eosin (H&E) staining in serial sections of PTGs. Cell proliferation was marked by PCNA expression as previously reported.7 COX2 and PCNA expression were observed in eosinophilic (oxyphil) cells and chief cells of human hyperplastic PTGs (Figure 2). Nearly 78% of COX2-positive cells also expressed PCNA (Figure 3, B and C), supporting that COX2 overexpression was associated with cell proliferation in the uremic parathyroids. Expression of COX2 in proliferating cells was further supported by the immunohistochemistry showing COX2 expression in cells with two nuclei (Figure 3A).
Figure 2.
COX2 and PCNA immunoproteins are observed in both chief cells and oxyphil cells of hyperplastic PTGs from hemodialysis patients. Immunohistochemistry and H&E staining in consecutive sections of the PTG show (A) H&E staining to differentiate chief cells and oxyphil cells (eosinophilic cells, red arrow), (B) cytoplasmic positivity for COX2 in oxyphil cells and chief cells, and (C) nuclear positivity for PCNA in oxyphil cells and chief cells. Magnifications: ×400.
Figure 3.
Most COX2-positive PTG cells are proliferative cells. (A) The immunohistochemistry detected some COX2-positive cells in the hyperplasia parathyroids with two nuclei (red arrow). (B) COX2 (red) and PCNA (brown) co-staining in the paraffin-embedded sections of hyperplastic PTGs from hemodialysis patients. Magnifications: ×400. (C) Percentage of PCNA-positive cells in COX2-positive or COX2-negative PTG cells per microscope field. cP < 0.001 versus COX2-positive cells.
COX2 Inhibitor Suppresses the Hyperplasia of Parathyroid in the 5/6-Nephrectomized Rats Fed with a High-Phosphate Diet
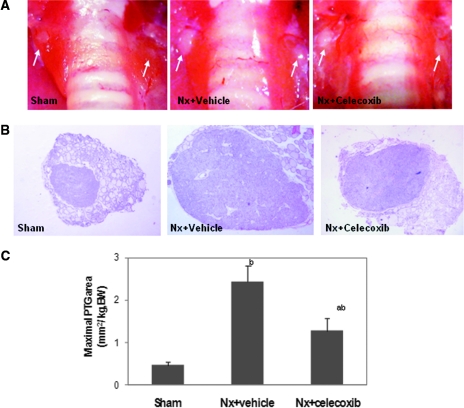
A 5/6-nephrectomy (Nx) rat fed with high dietary phosphate is a widely used animal model of human SHPT and is characterized by diffuse proliferation of chief cells in the PTG. To examine the role of COX2 in hyperplasia of the parathyroid, we treated the rats with the COX2 inhibitor celecoxib 1 month after Nx for subsequent 2 months. Table 1 illustrates the body weights and serum chemistries in Nx and sham-operated rats fed with or without celecoxib. As expected, body weight gain was retarded in uremic rats compared with the sham-operated rats. There was no significant difference in serum calcium levels among three groups of animals. Serum creatinine, blood urea nitrogen (BUN), and serum phosphorus increased significantly in the uremic animals fed with the high-phosphate diet. Serum PTH levels and the size of the PTG were also significantly increased in the Nx rats fed with a high-phosphate diet (Figure 5).
Table 1.
Baseline data of sham-operated and uremic rats
| Sham-Operated Rats | Uremic Rats |
||
|---|---|---|---|
| Nx-Vehicle | Nx-Celecoxib | ||
| Diet | Phosphate 0.8% | Phosphate 1.2% | Phosphate 1.2% |
| Number of rats | 20 | 17 | 18 |
| Body weight (gram) | 645 ± 30.20 | 486 ± 19.60a | 540 ± 20.70a |
| Serum creatinine (mg/dl) | 0.50 ± 0.04 | 1.27 ± 0.16a | 1.08 ± 0.14a |
| BUN (mg/dl) | 27.13 ± 0.81 | 77.28 ± 10.08a | 66.33 ± 10.50a |
| Serum calcium (mg/dl) | 9.50 ± 0.32 | 10.14 ± 0.24 | 9.94 ± 0.28 |
| Serum phosphorus (mg/dl) | 7.37 ± 0.50 | 8.73 ± 0.81a | 7.80 ± 0.77a |
| Intact PTH (pg/ml) | 22.65 ± 2.63 | 124.39 ± 8.38b | 88.20 ± 4.21d,b |
Data are mean ± SEM.
aP < 0.05 versus sham group;
dP < 0.05 versus Nx + vehicle group;
bP < 0.01 versus sham group.
Figure 5.
COX2 inhibition reduces the size of PTGs in uremic rats. (A) Gross observation of PTGs as indicated by white arrows during surgical procedures. (B) Representative histologic observation of maximal PTGs in each group. H&E stain. Magnifications: ×400. (C) Quantification of maximal PTG area. Values are mean ± SEM. bP < 0.01 versus sham group; aP < 0.05 versus Nx + vehicle group.
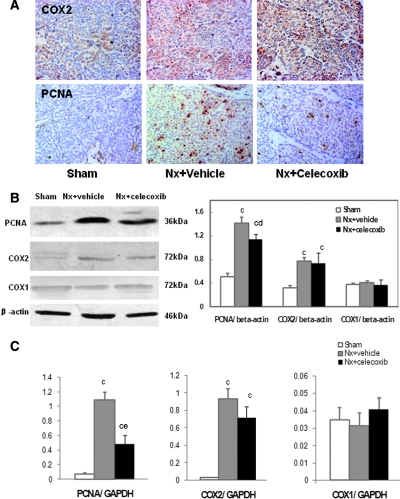
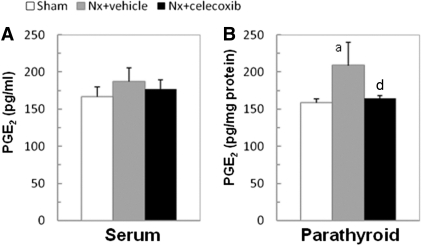
Consistent with the studies in uremic patients, the uremic rats on a high-phosphate diet demonstrated increased COX2 expression (Figure 6A) and PGE2 biosynthesis in the PTG (Nx: 208.76 ± 31.28 versus sham: 159.01 ± 5.20 pg/mg protein, P < 0.05) but not in serum (Figure 4). The COX2 protein and mRNA levels in the uremic parathyroid were 2.5-fold and 39-fold compared with normal rats, respectively (P < 0.001) (Figure 6, B and C). The increased PGE2 production was abolished by the COX2 inhibitor celecoxib (164.82 ± 3.10 pg/mg protein) (Figure 4B). Treatment with celecoxib for 2 months also significantly attenuated serum PTH levels (Table 1) and reduced the maximal PTG area to 50% of those without celecoxib (Figure 5). Celecoxib treatment had no significant effect on serum creatinine, BUN, and phosphorus levels.
Figure 6.
COX2 inhibition suppresses the enhanced expression of PCNA in PTGs of uremic rats. (A) Immunohistochemistry with antibody for COX2 and PCNA in PTGs, showing enhanced staining of COX2 and PCNA in the chief cells of PTGs from uremic rats. Magnifications: ×400. (B) Immunoblot of PTGs using antibodies for PCNA/COX2/COX1 and β-actin as a loading control. (C) Real-time PCR analysis for PCNA/COX2/COX1 mRNA of RNA extracted from microdissected PTGs. The expression of COX2 and PCNA in PTGs was markedly increased in uremic rats fed with a high-phosphate diet compared with the sham group. COX2 inhibitor celecoxib significantly reduced PCNA protein and mRNA expression. Values are mean ± SEM. cP < 0.001 versus sham group; dP < 0.05 versus Nx + vehicle group; eP < 0.001 versus Nx + vehicle group.
Figure 4.
COX2 derived PGE2 biosynthesis is increased in PTGs of uremic rats. PGE2 levels of rats were determined by an ELISA kit. (A) No difference of serum PGE2 levels was observed among three groups (P > 0.05). (B) Local production of PGE2 significantly increased in the parathyroids from 5/6-Nx rats compared with control rats. Celecoxib reduced the PGE2 production by the PTGs of 5/6-Nx rats to control levels. Values are mean ± SEM. aP < 0.05 versus sham group; dP < 0.05 versus Nx + vehicle group.
Immunohistochemistry analysis demonstrated a few PCNA-positive cells in the PTGs of sham-operated rats. A high-phosphate diet led to a marked increase in PCNA-positive cells and COX2-positive cells in uremic PTGs compared with those in controls. The COX2 inhibitor celecoxib not only reduced PGE2 production but also significantly reduced PCNA-positive staining in PTGs (Figure 6A). Immunoblot and real-time PCR analysis (Figure 6, B and C) further supported the effect of celecoxib on PCNA expression. In uremic rats fed with a high-phosphate diet, the protein and mRNA levels of PCNA in parathyroid tissues markedly increased by 2.8-fold and 25-fold compared with those of control rats (P < 0.001). Administration of COX2 inhibitor significantly decreased the excessive protein and gene expression of PCNA by 22% (P < 0.05) and 56% (P < 0.001) but had no effects on COX2 expression. In contrast, the COX1 expression in the PTG was very little and there was no significant difference between uremic and normal rats. Therefore, these results strongly suggest that COX2- but not COX1-derived PG plays an important role in the pathogenesis of uremic proliferation of PTGs.
DISCUSSION
The mechanism by which hyperparathyroidism is developed in uremic patients is incompletely defined. Phosphate retention, impaired vitamin D metabolism, and the downregulation of the calcium-sensing receptor have been demonstrated to be involved in the pathogenesis of SHPT in CKD patients.7,13,14 The study presented here suggests that COX2 also plays an important role in parathyroid hyperplasia in patients with ESRD and participates in the pathogenesis of hyperparathyroidism in these patients. The data presented here show that COX2 expression was markedly increased in the PTGs of uremic patients and rats, COX2 expression was associated with cell proliferation, and COX2 inhibitor significantly attenuated parathyroid proliferation and reduced PTH release in the rat model of uremic hyperparathyroidism.
In early 1980s, the cells from PTGs were reported to produce various PGs that were associated with cAMP accumulation and PTH release.24,26 Later studies detected COX2 expression in human PTGs, primarily in the oxyphil cells.25 The study presented here demonstrates that the PTGs of the patients with severe SHPT expressed large amount of COX2. The increased COX2 was localized to oxyphil and chief cells by immunohistochemistry. Elevated COX2 expression was also observed in chief cells of the PTGs in 5/6-nephrectomized rats fed with a high-phosphate diet for 3 months. We only detected a few COX1-positive cells in PTGs, and no change in COX1 protein expression was observed in hyperplastic PTGs. These results suggest that COX2 is selectively induced in the uremic condition and plays a major role in catalyzing the biosynthesis of PGs in uremic parathyroids.
Hyperplasia is one of the characteristics of SHPT, which not only results in 10- to 40-fold increases in parathyroid mass28 but is also a major contributor for enhanced PTH production. The study presented here provides strong evidence that COX2 plays an important role in cell proliferation in the uremic PTG. Increased PCNA-positive cells were observed in the parathyroid tissue of SHPT from humans and the rat model, consistent with published studies.7,29,30 Most of the PCNA-positive cells simultaneously expressed COX2. In the nodular hyperplasia PTG, COX2 was detected primarily in the peripheral region of the nodule, where high proliferative activity has been reported.30 It is well documented that aberrant COX2 expression plays a key role in cell proliferation in a wide range of premalignant and malignant epithelial tumors.22,31 Our studies are consistent with a potential role of COX2 in the proliferation of PTGs from uremic individuals.
The association of AA and hyperparathyroids was noticed in the early 1990s by Bourdeau et al., who showed that AA inhibited PTH production.32 The inhibitory effect of AA on PTH synthesis has been demonstrated through its lipoxygenase (LO) pathway products 12- and 15-hydroxyeicosatetraenoic acid, and high phosphorus has been shown to reduce AA synthesis.33–35 A recent study found that VDR gene expression in the PTG was also regulated by AA through activation of the extracellular signal-regulated kinase 1/2-mitogen-activated protein kinase.36 AA is a common substrate for the COX, LO, and P450 monoxygenase pathways. The studies presented here show that the COX2 pathway of the AA metabolite (prostanoid) was increased in uremic SHPT and could stimulate PTH production and parathyroid cell growth. Because increased COX activity in hyperparathyroidism may also shunt AA products from the LO pathway to the COX pathway, whether reduced LO products may also contribute to increased PTH production remains to be determined.
To further examine the role of COX2 in the proliferation of parathyroid cells in SHPT, we generated an animal model by 5/6-Nx plus a high-phosphate diet.7,37 Immunohistochemical and immunoblot studies revealed that COX2 was markedly induced in the PTGs of the rats with decreased renal mass that were fed a high-phosphate diet. To determine whether increased COX2 expression contributes to increased proliferation of the PTG and increased PTH release, we treated those rats with the COX2 inhibitor celecoxib at the dosage of 100 mg/kg per day, which has been shown to efficiently inhibit COX2 activity and reduce cell proliferation in animal models.38–42 Two-month celecoxib treatment dramatically suppressed the increase in maximal PTG area and PCNA expression in the parathyroid tissues, accompanied by reduced serum PTH levels. Celecoxib did not alter renal function or calcium-phosphate levels in uremic rats. Previous studies reported that chronic administration of a selective COX2 inhibitor significantly decreased proteinuria and attenuated development of glomerular sclerosis in rats with reduced renal mass.43–45 However, in our study, celecoxib, administered 1 month after Nx, did not significantly improve renal function in the uremic rats with SHPT. Delayed celecoxib treatment after Nx may contribute to the less-protective effect on renal function. It appears that the suppressive effects of the COX2 inhibitor on the parathyroid hyperplasia in uremia is not due to its effect on improving renal function but rather comes from its direct effect on the hyperplastic PTG. Our immunoblot showed that the levels of COX2 expression in the diffuse hyperplasic parathyroid tissues were higher compared with nodular hyperplasic tissues. This may, at least in part, reflect relatively less COX2-positive cells in nodular tissues as shown in our immunohistochemistry studies. Whether COX2 expression levels differ at the cellular level between nodular and diffuse tissues is not clear. Nevertheless, colocalization of COX2 and PCNA in nodular and diffuse hyperplasic parathyroid tissues supports an association of COX2 expression and cell proliferation. Whether COX2-derived prostanoids play a more important role in polyclonal than monoclonal parathyroid hyperplasia remains to be investigated.
COX converts AA to PGH2, which is subsequently metabolized to multiple bioactive PGs, including PGE2, PGI2, PGD2, PGA2, and thromboxane A2.46 PGE2 has been demonstrated to stimulate many cellular processes such as proliferation, angiogenesis, and invasion while inhibiting the immune response and apoptosis. The antitumor effects of nonsteroidal anti-inflammatory drugs or COX2 inhibitors are associated with marked reductions in tumor PGE2 levels.20,47 PGE2 exerts its cellular effects by binding to four distinct E-prostanoid receptors (EP1 to EP4) that belong to the family of seven transmembrane G-protein-coupled receptors.48 Previous data show that parathyroid tissue and parathyroid cells per se produce various PGs.24 PGE2 stimulates cAMP accumulation and PTH release.26,27,49 However, the downstream mechanism by which COX2 mediates parathyroid hyperplasia remains to be determined.
The mechanism by which COX2 is induced in hyperparathyroidism is also unclear. Hypocalcemia, hyperphosphatemia, and vitamin D deficiency have been documented to be critical contributors to the hyperplastic parathyroid growth in CKD.7,8,50 The study presented here showed that high-phosphate diet induced an overexpression of COX2 in hyperplastic PTGs and increased the release of PGE2 in uremic rats. Recent works have demonstrated a crosstalk between the COX2 and the EGFR,20 TGF,51 endothelin-1,52 and other cytokines in cancer research. Coincidentally, these potent growth promoters were also found to be markedly enhanced in the parathyroid and were identified as the main cause of parathyroid hyperplasia in experimental CKD.50,53 In any event, further in vitro and in vivo studies will be required before the significance of the COX2 function in the control of the parathyroid response to traditional and novel risk factors of hyperplasia can be established.
In conclusion, we have demonstrated a new direct relationship between COX2 overexpression and parathyroid hyperplasia from hemodialysis patients and high-phosphate induced Nx-SHPT rats. COX2 inhibition ameliorated SHPT and the parathyroid cell growth in experimental rats, independent of renal function. These results suggest that the COX2 pathway may be a potential therapeutic target for SHPT in CKD patients.
CONCISE METHODS
Human Parathyroid Tissue Preparations
Parathyroid tissues were obtained at the time of parathyroidectomy surgery from 12 maintenance hemodialysis patients (four men and eight women, mean age 49.7 ± 8.0 years, mean duration of hemodialysis therapy 8.8 ± 1.6 years) in our hospital between the years 2005 and 2008 with approval of the Ethics Committee on Human Research at Huashan Hospital, Fudan University, Shanghai, People's Republic of China. All of the patients had advanced SHPT that was resistant to >6 months of administration of calcitriol pulse therapy. Presence of one or more PTGs >1 cm3 was confirmed by ultrasonography. Immediately after resection, a piece of hyperplastic parathyroid tissue from each gland was fixed with 4% paraformaldehyde and embedded in paraffin for histologic studies. The rest of the tissues were frozen and stored at −70°C for protein analysis.
In Vivo Experiments in Rats
The experimental protocol was approved by the Animal Study Committee at Huashan Hospital, Fudan University. 5/6-Nx was performed on 200- to 250-g male Sprague–Dawley rats in a two-step procedure as previously reported.54 A high-phosphate (1.2%) rodent chow was given to 5/6-nephrectomized rats for 1 month.55,56 These SHPT rats were then divided into two groups: (1) those treated with the selective COX2 inhibitor celecoxib (100 mg/kg per day; n = 18), and (2) those treated with vehicle (n = 17). Celecoxib in 1 ml of sterile water or vehicle was administered daily via gavage to the SHPT rats. Twenty sham-operated rats fed a normal-phosphate diet (0.8%) were regarded as the control group. Rodent chows (normal and high phosphorus) were purchased from Slac Laboratory Animal, Chinese Academy of Science. These chows contain identical calcium (1.2%), vitamin D (750 IU/kg), carbohydrate, lipid, and protein contents.
Two months after the above treatment, the rats were sacrificed and blood samples were obtained for creatinine, BUN, calcium, phosphorus, intact PTH, and PGE2 measurements. The PTGs were immediately removed using microdissection equipment and prepared for histologic, protein, and mRNA analysis following the protocols described above. For ELISA analysis of PGE2 concentration, part of the parathyroid tissues were homogenized (in ice) in the buffer, which contained 0.1 M phosphate and 10 μM indomethacin at a pH of 7.4. The resultant homogenate was centrifuged for 30 minutes at 4000 rpm at 4°C. The supernatant was aspirated into polypropylene test tubes and stored at −70°C.
Laboratory Measurements
Serum creatinine, BUN, calcium, and phosphorus were determined by an autoanalyzer (Hitachi 7170, Japan). Serum intact PTH was measured by an ELISA kit special for rats (R&D Systems). The amount of PGE2 in serum and parathyroid tissue homogenate was detected using an ELISA kit from R&D Systems.
Histologic Studies
Paraffin-embedded PTG tissue from each hemodialysis patients was cut in multiple consecutive sections for H&E staining and immunohistochemistry. Immunohistochemistry was also performed on paraffin-embedded parathyroid tissue from rats. Sections (4 μm thick) were cut and blocked with 10% horse serum/PBS for 30 minutes at room temperature. The sections were then incubated with the following primary antibodies: (1) anti-COX2 polyclonal antibody (1:100 dilution; Cayman Chemical), (2) anti-COX1 polyclonal antibody (1:100 dilution; Cayman Chemical), and (3) anti-PCNA rabbit polyclonal antibody (1:100 dilution; Santa Cruz Biotechnology, Inc.). After 60 minutes of incubation, the sections were washed and incubated with biotinylated anti-IgG secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) for 60 minutes. Signals were visualized with a VECTA STAIN Elite ABC standard kit (Vector Laboratories) and a diaminobenzidine substrate kit (Vector Laboratories). Human hyperplastic PTG specimens were also double-stained with anti-COX2 antibody and anti-PCNA antibody. COX2 was detected with Texas-red-conjugated streptavidin (AK Substrate Kit; Vector). COX2-positive cells, PCNA-positive cells, and co-positive cells were counted per microscope field with the PTG sections completely filling the microscope field. For each patient, five microscope fields were counted and the mean was used for analysis.
Assessment of the Size of the Rat PTG
Serial sections of the paraffin-embedded rat PTGs were cut (4 μm) and stained with H&E. The maximal two-dimensional area of the right or left PTG was determined from at least ten serial sections according to the method described by Nagano et al.57 The sections were examined under a light microscope (Olympus CX40) connected to a computer. A digitizer tablet carefully traced the outside edge of the PTG, and the area inside of the circle was calculated by an image analysis system (Image J). The area of each section was the average of nine measurements by three different investigators (three measurements by each investigator).
Immunoblot
Freshly removed human PTG tissue and microdissected rat PTG tissue free of thyroid tissue were homogenized and extracted in lysis buffer containing 150 mM NaCl, 100 mM Tris-buffered saline (pH 8.0), 1% Tween 20, 50 mM diethyldithiocarbamate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, and 10 μg/ml pepstatin. Protein concentration was determined using a bicinchoninic acid protein assay (Sigma). Twenty micrograms of protein extract were separated on a 10% SDS-PAGE gel. Protein was transferred to a polyvinylidene difluoride membrane at 22 V overnight at 4°C. The membrane was washed three times with Tris-buffered saline–Tween (50mM Tris, 150 mM NaCl, and 0.05% Tween 20) and then incubated in blocking buffer (150 mM NaCl, 50 mM Tris, 0.05% Tween 20, and 5% Carnation nonfat dry milk [pH 7.5]) for 1 hour at room temperature. The membrane then was incubated with antibodies (1:200) raised against PCNA, COX2, and COX1 (Cayman Chemical) overnight at 4°C. After three washes, the membrane was incubated with a horseradish-peroxidase-conjugated secondary antibody (1:10,000; Jackson Immunoresearch Laboratories) for 1 hour at room temperature followed by three 15-minute washings. Antibody labeling was visualized by electrochemiluminescence (Amersham Biosciences, England, United Kingdom).
Isolation of Total RNA and Real-Time PCR
Total RNA was extracted from microdissected rat parathyroid tissue using Trizol reagent (Invitrogen, Carlsbad, CA) and then treated with DNase (Takara Bio Inc, Otsu, Shiga, Japan) according to the manufacturer's protocol. Reverse transcription was carried out using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA). PCR reactions were carried out using 50 ng of total parathyroid RNA under the following conditions: 95°C for 2 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 30 seconds. A real-time PCR reaction mixture was 25 μl (12.5 μl TaqMan Mix, 0.5 μl cDNA, 0.6 μl forward primer, 0.6 μl reverse primer, 0.3 μl probe, and 10.5 μl water). Primers and TaqMan probes for each target are listed in Table 2. Values were normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA levels in each sample as determined by a TaqMan rodent glyceraldehyde 3-phosphate dehydrogenase control reagent kit (Applied Biosystems). The results of real-time PCR were analyzed with BIORAD iCycler software.
Table 2.
Primers and TaqMan probes used for RT-PCR
| Target | Sequence |
|---|---|
| PCNA | |
| forward | TAGCCATATTGGAGATGCTGTGG |
| reverse | TCAGAGCAAAAGTTAGCTGAACTGG |
| probe | CTCCTGTGCAAAGGACGGGGTGAAG |
| COX2 | |
| forward | CACGGACTTGCTCACTTTGTTGA |
| reverse | GTGTAAGGTTTCAGGGAGAAGCG |
| probe | CAGACAGATTGCTGGCCGGGTTGCT |
| COX1 | |
| forward | GGGGTAGGAACTTTGACTACCATG |
| reverse | ACCATATAGCTCCTCCAACTCAGC |
| probe | TGCATGTGGCCGAGGATGTCATCAA |
Statistical Analysis
Values were reported as mean ± SEM. Statistical significance was assessed by the nonparametric ANOVA test with SPSS version 16.0 software. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by the China Natural Science Foundation (30971373; to Jing Chen), a New Century Grant for Talented People by the National Education Committee of China (to Jing Chen), a research grant from the Public Health Bureau of Shanghai Municipality (2007123; to Yanwen Lu), and the scientific research foundation of Shanghai Medical College at Fudan University (126; to Haiming Li). We thank Dr. Hongying Wang and Dr. Qiang Zou (Department of General Surgery, Huashan Hospital, Fudan University, Shanghai, China) for their assistance in the preparation of patient PTGs. Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology; November 14 through 19, 2006; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Parfitt AM: The hyperparathyroidism of chronic renal failure: A disorder of growth. Kidney Int 52: 3–9, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Gonzalez EA, Martin KJ: Renal osteodystrophy: Pathogenesis and management. Nephrol Dial Transplant 10[Suppl 3]: 13–21, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Fukagawa M, Kitaoka M, Yi H, Fukuda N, Matsumoto T, Ogata E, Kurokawa K: Serial evaluation of parathyroid size by ultrasonography is another useful marker for the long-term prognosis of calcitriol pulse therapy in chronic dialysis patients. Nephron 68: 221–228, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Katoh N, Nakayama M, Shigematsu T, Yamamoto H, Sano K, Saito I, Nakano H, Kasai K, Kubo H, Sakai S, Kawaguchi Y, Hosoya T: Presence of sonographically detectable parathyroid glands can predict resistance to oral pulsed-dose calcitriol treatment of secondary hyperparathyroidism. Am J Kidney Dis 35: 465–468, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Wang Q, Palnitkar S, Parfitt AM: The basal rate of cell proliferation in normal human parathyroid tissue: Implications for the pathogenesis of hyperparathyroidism. Clin Endocrinol (Oxf) 46: 343–349, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Naveh-Many T, Rahamimov R, Livni N, Silver J: Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest 96: 1786–1793, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silver J, Kilav R, Naveh-Many T: Mechanisms of secondary hyperparathyroidism. Am J Physiol Renal Physiol 283: F367–F376, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Arnold A, Brown MF, Urena P, Gaz RD, Sarfati E, Drueke TB: Monoclonality of parathyroid tumors in chronic renal failure and in primary parathyroid hyperplasia. J Clin Invest 95: 2047–2053, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tominaga Y, Kohara S, Namii Y, Nagasaka T, Haba T, Uchida K, Numano M, Tanaka Y, Takagi H: Clonal analysis of nodular parathyroid hyperplasia in renal hyperparathyroidism. World J Surg 20: 744–750; discussion 750–752, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Tominaga Y, Tsuzuki T, Uchida K, Haba T, Otsuka S, Ichimori T, Yamada K, Numano M, Tanaka Y, Takagi H: Expression of PRAD1/cyclin D1, retinoblastoma gene products, and Ki67 in parathyroid hyperplasia caused by chronic renal failure versus primary adenoma. Kidney Int 55: 1375–1383, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y: Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest 92: 1436–1443, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kifor O, Moore FD, Jr, Wang P, Goldstein M, Vassilev P, Kifor I, Hebert SC, Brown EM: Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab 81: 1598–1606, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Goodman WG, Quarles LD: Development and progression of secondary hyperparathyroidism in chronic kidney disease: Lessons from molecular genetics. Kidney Int 74: 276–288, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Tokumoto M, Tsuruya K, Fukuda K, Kanai H, Kuroki S, Hirakata H: Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int 62: 1196–1207, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Cozzolino M, Lu Y, Finch J, Slatopolsky E, Dusso AS: p21WAF1 and TGF-alpha mediate parathyroid growth arrest by vitamin D and high calcium. Kidney Int 60: 2109–2117, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Arcidiacono MV, Sato T, Alvarez-Hernandez D, Yang J, Tokumoto M, Gonzalez-Suarez I, Lu Y, Tominaga Y, Cannata-Andia J, Slatopolsky E, Dusso AS: EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J Am Soc Nephrol 19: 310–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dusso A, Cozzolino M, Lu Y, Sato T, Slatopolsky E: 1,25-Dihydroxyvitamin D downregulation of TGFalpha/EGFR expression and growth signaling: A mechanism for the antiproliferative actions of the sterol in parathyroid hyperplasia of renal failure. J Steroid Biochem Mol Biol 89-90: 507–511, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Smith WL, Garavito RM, DeWitt DL: Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 271: 33157–33160, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Mann JR, Backlund MG, DuBois RN: Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol 2: 202–210, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR: TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem 266: 12866–12872, 1991 [PubMed] [Google Scholar]
- 22. Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K: Cyclo-oxygenase 2: A pharmacological target for the prevention of cancer. Lancet Oncol 2: 544–551, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Harris RE: Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 17: 55–67, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Brown EM, Swartz SL: Production of prostaglandins by dispersed cells and fragments from bovine parathyroid glands. Prostaglandins 29: 35–46, 1985 [DOI] [PubMed] [Google Scholar]
- 25. Bell CD, Vidal S, Kovacs K, Anderson J, Rotondo F: Cox-2 expression in the oxyphilic cells of the normal, hyperplastic, and adenomatous parathyroid gland. Endocr Pathol 15: 29–38, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Gardner DG, Brown EM, Attie MF, Aurbach GD: Prostaglandin-mediated stimulation of adenosine 3′,5′-monophosphate accumulation and parathyroid hormone release in dispersed human parathyroid cells. J Clin Endocrinol Metab 51: 20–25, 1980 [DOI] [PubMed] [Google Scholar]
- 27. Gardner DG, Brown EM, Windeck R, Aurbach GD: Prostaglandin E2 stimulation of adenosine 3′,5′-monophosphate accumulation and parathyroid hormone release in dispersed bovine parathyroid cells. Endocrinology 103: 577–582, 1978 [DOI] [PubMed] [Google Scholar]
- 28. Krause MW, Hedinger CE: Pathologic study of parathyroid glands in tertiary hyperparathyroidism. Hum Pathol 16: 772–784, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Yamaguchi S, Yachiku S, Morikawa M: Analysis of proliferative activity of the parathyroid glands using proliferating cell nuclear antigen in patients with hyperparathyroidism. J Clin Endocrinol Metab 82: 2681–2688, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Ohta K, Manabe T, Katagiri M, Harada T: Expression of proliferating cell nuclear antigens in parathyroid glands of renal hyperparathyroidism. World J Surg 18: 625–628; discussion 628–629, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Muller-Decker K, Furstenberger G: The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog 46: 705–710, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Bourdeau A, Souberbielle JC, Bonnet P, Herviaux P, Sachs C, Lieberherr M: Phospholipase-A2 action and arachidonic acid metabolism in calcium-mediated parathyroid hormone secretion. Endocrinology 130: 1339–1344, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Bourdeau A, Moutahir M, Souberbielle JC, Bonnet P, Herviaux P, Sachs C, Lieberherr M: Effects of lipoxygenase products of arachidonate metabolism on parathyroid hormone secretion. Endocrinology 135: 1109–1112, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Almaden Y, Canalejo A, Ballesteros E, Anon G, Rodriguez M: Effect of high extracellular phosphate concentration on arachidonic acid production by parathyroid tissue in vitro. J Am Soc Nephrol 11: 1712–1718, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Canalejo A, Canadillas S, Ballesteros E, Rodriguez M, Almaden Y: Importance of arachidonic acid as a mediator of parathyroid gland response. Kidney Int Suppl June: S10–S13, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Canadillas S, Canalejo R, Rodriguez-Ortiz ME, Martinez-Moreno JM, Estepa JC, Zafra R, Perez J, Munoz-Castaneda JR, Canalejo A, Rodriguez M, Almaden Y: Upregulation of parathyroid VDR expression by extracellular calcium is mediated by ERK1/2-MAPK signaling pathway. Am J Physiol Renal Physiol 298: F1197–F1204, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Denda M, Finch J, Slatopolsky E: Phosphorus accelerates the development of parathyroid hyperplasia and secondary hyperparathyroidism in rats with renal failure. Am J Kidney Dis 28: 596–602, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, Wong MC: Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: Inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys 67: 888–896, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Chang CL, Ma B, Pang X, Wu TC, Hung CF: Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther 17: 1365–1372, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G: COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J 15: 2742–2744, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Dovedi SJ, Kirby JA, Davies BR, Leung H, Kelly JD: Celecoxib has potent antitumour effects as a single agent and in combination with BCG immunotherapy in a model of urothelial cell carcinoma. Eur Urol 54: 621–630, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, Ohta T, Koichi M: A COX-2 inhibitor prevents the esophageal inflammation-metaplasia-adenocarcinoma sequence in rats. Carcinogenesis 26: 565–570, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wang JL, Cheng HF, Shappell S, Harris RC: A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int 57: 2334–2342, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Fujihara CK, Antunes GR, Mattar AL, Andreoli N, Malheiros DM, Noronha IL, Zatz R: Cyclooxygenase-2 (COX-2) inhibition limits abnormal COX-2 expression and progressive injury in the remnant kidney. Kidney Int 64: 2172–2181, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Sanchez PL, Salgado LM, Ferreri NR, Escalante B: Effect of cyclooxygenase-2 inhibition on renal function after renal ablation. Hypertension 34: 848–853, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Hao CM, Breyer MD: Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70: 357–377, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Turini ME, DuBois RN: Cyclooxygenase-2: A therapeutic target. Annu Rev Med 53: 35–57, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Breyer RM, Bagdassarian CK, Myers SA, Breyer MD: Prostanoid receptors: Subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Gardner DG, Brown EM, Windeck R, Aurbach GD: Prostaglandin F2alpha inhibits 3′,3′-adenosine monophosphate accumulation and parathyroid hormone release from dispersed bovine parathyroid cells. Endocrinology 104: 1–7, 1979 [DOI] [PubMed] [Google Scholar]
- 50. Dusso AS, Sato T, Arcidiacono MV, Alvarez-Hernandez D, Yang J, Gonzalez-Suarez I, Tominaga Y, Slatopolsky E: Pathogenic mechanisms for parathyroid hyperplasia. Kidney Int Suppl July: S8–S11, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Tian M, Schiemann WP: PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-beta signaling during mammary tumorigenesis. FASEB J 24: 1105–1116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A: Inhibition of cyclooxygenase-1 and -2 expression by targeting the endothelin a receptor in human ovarian carcinoma cells. Clin Cancer Res 10: 4670–4679, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Fujii Y, Moreira JE, Orlando C, Maggi M, Aurbach GD, Brandi ML, Sakaguchi K: Endothelin as an autocrine factor in the regulation of parathyroid cells. Proc Natl Acad Sci U S A 88: 4235–4239, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shvil Y, Naveh-Many T, Barach P, Silver J: Regulation of parathyroid cell gene expression in experimental uremia. J Am Soc Nephrol 1: 99–104, 1990 [PubMed] [Google Scholar]
- 55. Mizobuchi M, Hatamura I, Ogata H, Saji F, Uda S, Shiizaki K, Sakaguchi T, Negi S, Kinugasa E, Koshikawa S, Akizawa T: Calcimimetic compound upregulates decreased calcium-sensing receptor expression level in parathyroid glands of rats with chronic renal insufficiency. J Am Soc Nephrol 15: 2579–2587, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Takahashi F, Denda M, Finch JL, Brown AJ, Slatopolsky E: Hyperplasia of the parathyroid gland without secondary hyperparathyroidism. Kidney Int 61: 1332–1338, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Nagano N, Miyata S, Obana S, Ozai M, Kobayashi N, Fukushima N, Burke SK, Wada M: Sevelamer hydrochloride (Renagel), a non-calcaemic phosphate binder, arrests parathyroid gland hyperplasia in rats with progressive chronic renal insufficiency. Nephrol Dial Transplant 16: 1870–1878, 2001 [DOI] [PubMed] [Google Scholar]