Abstract
Stem cells may contribute to renal recovery following acute kidney injury, and this may occur through their secretion of cytokines, chemokines, and growth factors. Here, we developed an acellular, nanofiber-based preparation of self-assembled peptides to deliver the secretome of embryonic stem cells (ESCs). Using an integrated in vitro and in vivo approach, we found that nanofibers preconditioned with ESCs could reverse cell hyperpermeability and apoptosis in vitro and protect against lipopolysaccharide-induced acute kidney injury in vivo. The renoprotective effect of preconditioned nanofibers associated with an attenuation of Rho kinase activation. We also observed that the combined presence of follistatin, adiponectin, and secretory leukoprotease during preconditioning was essential to the renoprotective properties of the nanofibers. In summary, we developed a designer-peptide nanofiber that can serve as a delivery platform for the beneficial effects of stem cells without the problems of teratoma formation or limited cell engraftment and viability.
Stem cells hold great promise for the kidney, and multiple studies have suggested that stem cells and specifically mesenchymal stem cells may contribute to the recovery of kidneys after acute kidney injury (AKI).1–11 However, the rate of stem cell engraftment in many cases appears too low to explain the significant renal improvement after AKI. An alternative hypothesis has been recently advanced, suggesting that the beneficial effects of stem cells in the kidney may not depend on their capacity to reconstitute the denuded cells directly, but rather on their paracrine/endocrine ability to release cytokines, chemokines, and growth factors.12–15
In the current study, we describe the development of a novel self-assembled peptide nanofiber-based preparation, which effectively delivers paracrine/endocrine factors secreted from mouse embryonic stem cells (ESCs) both in vitro and in vivo. We hypothesized that the use of nanofibers preconditioned with ESCs might enhance kidney tissue repair by providing an acellular delivery platform for bioactive molecules released from ESCs. We argued that this novel approach could harness the beneficial effects of stem cells in the repair and remodeling of damaged organs, while circumventing many limitations associated with the use of ESCs in vivo, including issues with limited cell engraftment, cell viability, immune tolerance, and formation of teratomas.
Self-assembling nanofibers are oligopeptides that consist of alternating hydrophilic and hydrophobic amino acids that rapidly assemble into nanofibers.16,17 Although nanofibers can be prepared in a number of different ways, self-assembly is one of the most powerful methods because of the excellent control over chemical composition, size in all dimensions, and the dynamic nature of the assembled fiber. The self-assembly method uses carefully designed assembler molecules that interact with one another through multiple weak, noncovalent interactions. Substantial progress in the development of novel nanofibers for delivery and controlled release of bioactive molecules has been made in recent years.18–21 Several groups, including our own, have previously utilized peptide self-assembly to create biocompatible and bioactive nanofibers.22–25 Nanofibers have been most recently popularized as drug delivery agents21 and as scaffolds to support cell proliferation and differentiation.26,27 Nanofibers are also increasingly playing a major role in providing new types of therapy for cancer and heart disease.16,19,20 For instance, it has been recently shown that delivery of PDGF by self-assembling nanofibers decreases infarct size and improves cardiac function after myocardial infarction.17 Likewise, tethering of IGF-1 to self-assembling peptide nanofibers increases survival of neonatal rat cardiomyocytes after myocardial infarction.16
We have previously reported the development of a biodegradable peptide nanofiber platform that can undergo spontaneous self-assembly.22–25 Similar peptide nanofibers have been used to influence stem cell differentiation by providing a three-dimensional scaffold microenvironment.28 However, whether biodegradable nanofibers could also be employed as acellular delivery platforms for secretome released from stem cells remains unknown.
This report provides the first example of the effect of preconditioned nanofibers with ESCs on the recovery of organs after acute injury. To our knowledge, this is the first study to investigate the use of preconditioned peptide nanofibers with stem cells in an experimental model in vivo.
RESULTS
Synthesis of Self-assembled Nanofibers
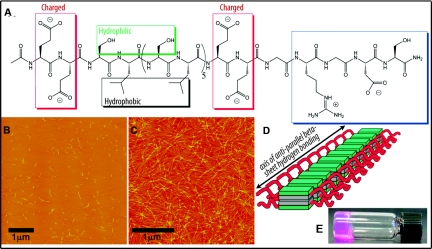
Nanofibers were self-assembled in a two-step process unique to the multidomain peptides (MDPs).22,23,25 The chemical structure of MDPs utilized in this study is shown in Figure 1A. Peptides were found to self-assemble into nanofibers with dimensions of approximately 2-nm high and 6-nm wide, and with lengths ranging from 50 to 250 nm (Figure 1B). Upon addition of MgCl2, average fiber length increased to several micrometers, whereas height and width remained relatively unchanged (Figure 1C). Cross-linking also dramatically increased as can be seen by atomic force microscopy (AFM) and macroscopically by gel formation (Figure 1, C through E).
Figure 1.
MDPs self-assemble into nanofibrous hydrogels. (A) Chemical structure of MDP nanofiber used in this study. (B) Short nanofibers after first step of self-assembly as visualized by AFM. (C) Cross-linked nanofibers after gelation with Mg2+. (D) Illustration of the assembled nanofibers. (E) A nanofibrous hydrogel of the MDP as formed in cell culture media.
Preconditioned Nanofibers Reverse LPS-Induced Endothelial Cell Hyperpermeability
The low-dose intraperitoneal (ip) injection of lipopolysaccharide (LPS) is an established model of endotoxemia as described in several recent studies.29–32 The underlying molecular mechanisms leading to LPS-induced kidney injury are complex and incompletely understood. However, it is clear that endothelial cell hyperpermeability and cell apoptosis are key mechanisms involved in the pathogenesis of the disease.32–35 Thus, we set out to assess the effect of preconditioned nanofibers on these key features of LPS-induced AKI both in vitro and in vivo.
We initially examined the effect of preconditioned nanofibers on LPS-induced endothelial cell hyperpermeability under in vitro conditions by determining the permeability of 125I-BSA across confluent endothelial cell monolayers.36 Our initial strategy was to employ two experimental preparations of nanofibers: (1) encapsulated nanofibers in which ESCs were embedded in designer peptide nanofibers for 1 hour (Figure 2A) or (2) preconditioned nanofibers that were prepared by exposing nanofibers to secretome from ESCs in a transwell coculture system for 24 hours (Figure 2B). To investigate the effect of preconditioned nanofibers on endothelial cell permeability, we employed a transwell system containing confluent kidney microvascular endothelial cells in the upper chamber separated from preconditioned nanofibers (100 μl) in the lower chamber by a cell-impermeable membrane (Figure 2C). Figure 2D depicts the effect of LPS on endothelial cell monolayer integrity. Upon addition of LPS (100 ng/ml) in the upper chamber, we detected a significant increase in endothelial cell permeability. In contrast, cocultures of endothelial cells with either ESCs alone, encapsulated nanofibers, or preconditioned nanofibers prevented LPS-induced endothelial cell hyperpermeability. However, nonconditioned nanofibers had no effect on LPS-induced cell permeability.
Figure 2.
Preconditioned nanofibers reverse endothelial cell hyperpermeability. (A) Scanning electron micrograph of mouse ESCs encapsulated in self-assembled peptide nanofibers. (B) Schematic representation of the two-compartment transwell coculture system for preparation of preconditioned nanofibers. (C) Schematic representation of the coculture system for determining the permeability of 125I-BSA across confluent endothelial cell monolayer in the presence of preconditioned nanofibers. (D) Quantification of kidney microvascular endothelial cell permeability exposed to LPS (100 ng/ml) for 4 hours. Bar graphs summarize results as mean ± SEM (n = 3 separate experiments in triplicate). (E) Quantification of kidney microvascular endothelial cell permeability exposed to VEGF (50 ng/ml) for 6 hours (n = 3 separate experiments in triplicate).
To test whether the modulatory effect of preconditioned nanofibers on cell permeability is restricted to LPS, we assessed the effect of preconditioned nanofibers on vascular endothelial growth factor–induced (VEGF-induced) cell permeability. As shown in Figure 2E, preconditioned nanofibers, encapsulated nanofibers, and ESCs alone also prevented VEGF-induced (50 ng/ml VEGF) endothelial cell hyperpermeability. Taken together, these results strongly suggest that preconditioning of nanofibers allows delivery of bioactive molecules from ESCs to targeted cells in vitro. Furthermore, the modulatory effect of preconditioned nanofibers on endothelial cell permeability is not restricted to LPS, and potentially involves a critical downstream target of cell permeability pathway.
Preconditioned Nanofibers Decrease LPS-Induced Apoptosis
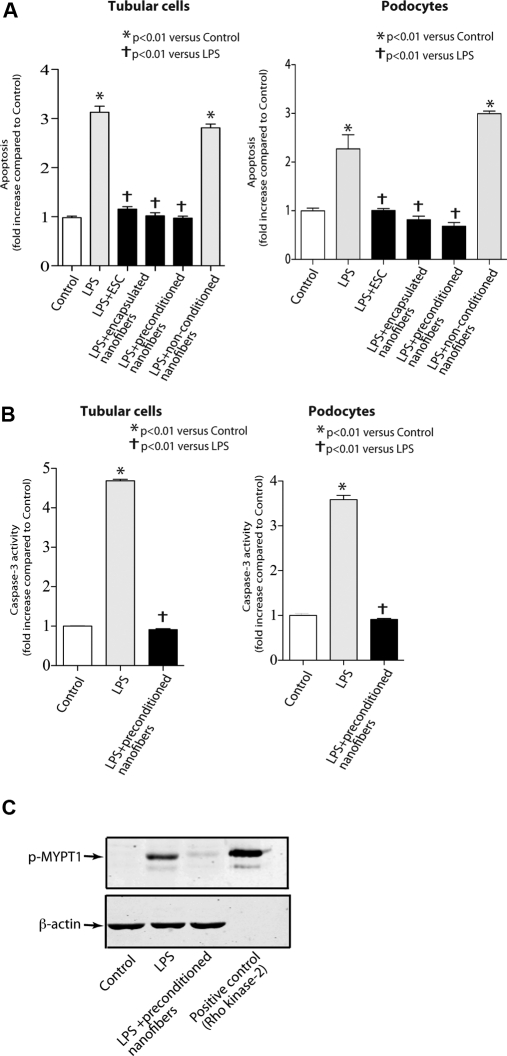
Having found that preconditioned nanofibers protect against LPS-induced cell permeability, we assessed their effect on LPS-induced cell apoptosis. We used the transwell coculture system in which preconditioned nanofibers were placed in the upper chamber, whereas confluent tubular or podocytes were seeded in the lower chamber. We found that LPS treatment (100 ng/ml) significantly increased cell apoptosis in tubular cells and podocytes (Figure 3A). In contrast, effect of LPS on cell apoptosis was prevented in the presence of ESCs alone, encapsulated nanofibers, or preconditioned nanofibers. Because caspase-3 was previously shown to play a critical role in LPS-induced apoptosis,37 we next asked whether the effect of preconditioned nanofibers on cell apoptosis involves inhibition of caspase-3 activation. LPS exposure resulted in a significant increase in caspase-3 activation in tubular cells and podocytes. The increase in caspase-3 activation, however, was prevented when cells were cocultured with preconditioned nanofibers (Figures 3B). These results suggest that preconditioned nanofibers prevent LPS-induced cell apoptosis in vitro.
Figure 3.
Preconditioned nanofibers mitigate cell apoptosis and inhibit Rho kinase activation. (A) Effect of preconditioned nanofibers on LPS-induced apoptosis in podocytes and tubular cells. Data are expressed as fold change in absorbance. (B) Effect of preconditioned nanofibers on LPS-induced caspase-3 activation in podocytes and tubular cells. Bar graphs summarize results of three separate experiments in triplicate. (C) Rho kinase activity in cells exposed to LPS for 4 hours. Rho kinase activation was evaluated by measuring the p-MYPT-1 at threonine 853 in endothelial cell lysates. The figure is representative of three separate experiments.
Effect of Preconditioned Nanofibers on Rho Kinase Activation
We initially used two separate strategies to deliver secretome from ESCs. However, after our initial success of employing preconditioned nanofibers in vitro, we continued the rest of the experiments by using only the preconditioned platform. We considered several possibilities to explain the effect of preconditioned nanofibers in vitro. Because we and others have previously reported that the RhoA/Rho kinase pathway plays a critical role in both cell permeability and apoptosis,38,39 we hypothesized that Rho kinase may also be involved in the cytoprotective effects of preconditioned nanofibers. Rho kinase is a serine/threonine protein kinase that has been identified as an important effector for activated RhoA.40,41 We assessed the effect of preconditioned nanofibers on LPS-induced Rho kinase activation. Rho kinase activity significantly increased with exposure to LPS (100 ng/ml) (Figure 3C). Preconditioned nanofibers, however, prevented LPS-induced Rho kinase activation in endothelial cells. These findings suggest that preconditioned nanofibers modulate Rho kinase activation in vitro.
Effect of Preconditioned Nanofibers on the LPS Model of AKI in Mice
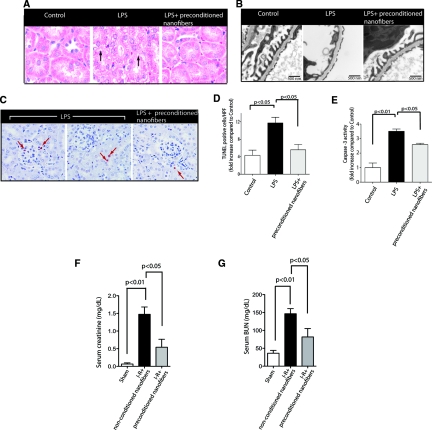
The experiments described above provided strong evidence for a protective effect of preconditioned nanofibers in vitro. However, it was unclear whether preconditioned nanofibers exhibited renoprotective effects in vivo. To address this question, we examined the effect of preconditioned nanofibers on the LPS model of AKI in mice.29 A single intraperitoneal (ip) injection of LPS (10 μg/g body wt) produced a significant increase in albuminuria and caused an abrupt rise in serum BUN and creatinine (Figure 4, A through C). In contrast, treatment with preconditioned nanofibers (200 μl) ip 1 hour after LPS injection markedly prevented LPS-induced proteinuria (Figure 4, A and B). Likewise, serum BUN and creatinine levels were significantly reduced in mice allocated to preconditioned nanofibers (Figure 4C). Interestingly, preconditioned nanofibers significantly reduced the concentrations of the liver enzyme AST, suggesting a systemic protective effect of preconditioned nanofibers (Figure 4D).
Figure 4.
Mice treated with preconditioned nanofibers are protected from LPS-induced kidney injury. (A) Quantitative analysis of albumin/creatinine ratio (n = 6 to 11 per group). (B) Representative SDS-PAGE analysis of the urine. (C) Quantitative analysis of serum creatinine and BUN (n = 6 to 11 per group). (D) Liver enzyme AST was measured in the serum 24 hours after intraperitoneal administration of LPS. (E) Quantitative analysis of albumin/creatinine ratio in mice allocated to nanofibers preconditioned with mouse embryonic fibroblasts (n = 3 mice per group).
To specifically address the role of ESCs in the preconditioned nanofibers, we also prepared nanofibers preconditioned with mouse embryonic fibroblasts (MEF) as an additional control. The MEF-preconditioned nanofibers were then administered in LPS-treated mice intraperitoneally. Consistent with our previous results, LPS significantly increased albuminuria (Figure 4E). However, the administration of preconditioned nanofibers with MEF did not prevent LPS-induced albuminuria, suggesting that peptide nanofibers require secretome from ESCs for their renoprotective effects.
Histologic analyses of kidneys obtained from animals allocated to preconditioned nanofibers with ESCs also showed a remarkable improvement (Figure 5A). Tubular epithelial cells were swollen and vacuolated 24 hours after LPS administration, but significantly improved in mice that received treatment with preconditioned nanofibers. We also assessed the effect of preconditioned nanofibers on podocyte injury based on two recent studies.29,30 As depicted in Figure 5B, preconditioned nanofibers ameliorated LPS-induced podocyte effacement observed in some glomerular areas. Similarly, although administration of LPS caused a significant increase in cell apoptosis in peritubular, vascular, tubular, and podocytes, the use of preconditioned nanofibers markedly mitigated the number of apoptotic cells in the kidneys (Figure 5, C and D). Furthermore, we also performed caspase-3 activation assays. LPS caused a significant increase in caspase-3 activation (Figure 5E). This increase was prevented in mice allocated to preconditioned nanofibers. Together, these results suggest that the preconditioned nanofibers protect against LPS-induced apoptosis in the kidney in vivo.
Figure 5.
Preconditioned nanofibers are renoprotective in experimental models of AKI. (A) Renal histology was assessed by H&E staining 24 hours after LPS administration. Tubular injury is indicated by vacuolization of tubular epithelial cells (arrows) (original magnification: ×400). (B) Representative transmission electron microscopy micrographs of the glomerular capillary wall. LPS-treated mice exhibit effacement of foot processes, whereas mice treated with preconditioned nanofibers show a significant improvement in podocytes (original magnification: ×15,000). (C) Apoptosis was quantified by TUNEL staining of formalin-fixed kidney tissue. After LPS administration, apoptotic nuclei were identified in glomeruli, tubules, and arterioles (arrows) (Magnification: ×400 counterstained with hematoxylin). Apoptosis was quantified as described previously.37 (D) Bar graphs summarize results of apoptosis obtained from five different mice in each group. HPF, high power field. (E) Bar graphs summarize results of caspase-3 activation obtained from kidney cortex lysates (n = 5 mice per group). (F) Quantitative analysis of serum creatinine and BUN (G) (n = 6 mice/group) in the I-R model of AKI. Data are shown as means ± SEM.
Next, we examined the effect of preconditioned nanofibers on the ischemia-reperfusion (I-R) model of kidney injury. As shown in Figure 5, F and G, in comparison with sham animals, mice that were subjected to I-R injury and were allocated to nonpreconditioned nanofibers showed a significant elevation of serum creatinine and BUN at 24 hours. However, mice that received preconditioned nanofibers intraperitoneally exhibited a marked improvement in serum creatinine (P < 0.05) and BUN (P < 0.05). Taken together, these findings suggest that preconditioned nanofibers protect against kidney injury in different models of AKI.
Effect of Preconditioned Nanofibers on LPS-Induced Rho Kinase Activation In Vivo
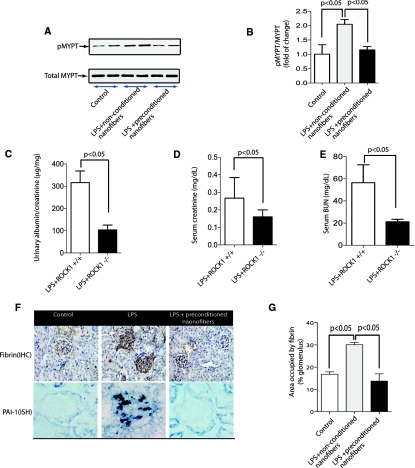
To address whether preconditioned nanofibers also modulate Rho kinase activation in vivo, we examined Rho kinase activity in kidney lysates. Rho kinase activity increased in the kidney lysates 24 hours after LPS administration (Figure 6, A and B). However, preconditioned nanofibers prevented the increase in Rho kinase activation.
Figure 6.
Preconditioned nanofibers inhibit Rho kinase activation in vivo. (A) Representative immunoblot of Rho kinase activity in whole kidney lysates. Rho kinase activation was examined by assessing phosphorylation of MYPT-1 at threonine 853. (B) Quantitative analysis of Rho kinase activation (n = 3 mice per group). (C) Quantitative analysis of albumin/creatinine ratio in response to LPS in ROCK1−/− and ROCK1+/+ mice (n = 5 mice per group). (D) and (E) Quantitative changes in serum creatinine and BUN in response to LPS in ROCK1−/− and ROCK1+/+ mice (n = 5 mice per group). (F) Fibrin deposits in kidney sections were evaluated by IHC staining (upper panel). ISH signal for PAI-1 mRNA in kidney sections (lower panel). IHC, immunohistochemical; ISH, in situ hybridization. (G) Quantitative analysis of staining with antifibrin antibody (n = 3 mice per group). Quantification was performed as described previously.67
To specifically examine the pathogenic role of Rho kinase activation in the LPS model of AKI in vivo, we used Rho kinase-1 knock out (ROCK1−/−) mice and analyzed their response to LPS injection. ROCK1−/− and wild-type ROCK1 (ROCK1+/+) mice were intraperitoneally injected with LPS (10 μg/g body wt), and their kidney function was then monitored for 24 hours. LPS challenge caused a significant increase in proteinuria, serum BUN, and creatinine in ROCK1+/+ mice (Figure 6, C through E). ROCK1−/− mice, however, showed significantly lower levels of proteinuria, and significant improvement in their kidney function compared with ROCK1+/+ mice, suggesting that Rho kinase activation plays a crucial role in the LPS model of AKI.
Because plasminogen activator inhibitor-1 (PAI-1), a downstream target of Rho kinase activation, has been suggested as a critical mediator of fibrin deposition and microvascular occlusion in the kidneys, leading to decreased kidney perfusion,42 we also examined the effect of preconditioned nanofibers on PAI-1 expression in LPS-induced AKI. Immunohistochemical analysis of kidney sections showed a robust increase in fibrin deposits in the glomerular and peritubular areas in LPS-treated animals [Figure 6, F(upper panel) and G]. However, in animals allocated to preconditioned nanofibers, fibrin deposition was significantly attenuated. Similarly, PAI-1 mRNA was also significantly decreased in treated animals with preconditioned nanofibers [Figure 6F (lower panel)]. These findings suggest that preconditioned nanofibers prevent LPS-induced generation of PAI-1 and fibrin formation, key mediators of decreased kidney perfusion and oxygenation.43
Anti-inflammatory Effect of Preconditioned Nanofibers
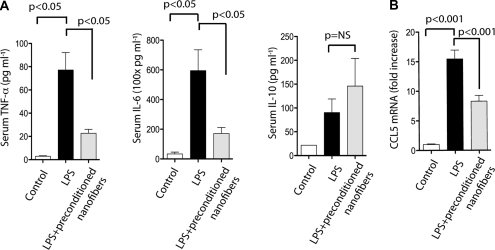
Our findings indicate that preconditioned nanofibers modulate Rho kinase activation, a master regulator of the inflammatory response. This feature prompted us to investigate the effect of preconditioned nanofibers on LPS-induced inflammatory cascade. Previously published experimental data suggest that TNF-α, IL-6, and IL-10 play central roles in LPS-induced inflammatory response.43,44 In our experimental model, LPS also induced a significant increase in serum TNF-α and IL-6 concentrations, which were significantly reduced 6 hours after LPS administration in mice allocated to preconditioned nanofibers (Figure 7A). However, preconditioned nanofibers did not significantly alter serum levels of IL-10. Because expression of chemokine CCL5 in the kidney has been suggested as a key mediator of LPS-induced inflammatory response,45 we also investigated the effect of preconditioned nanofibers on CCL5 mRNA expression in the kidney. As shown in Figure 7B, CCL5 was highly expressed in the kidneys of mice injected with LPS. However, significantly lower levels of CCL5 mRNA expression were detected in mice treated with preconditioned nanofibers. These results corroborate the notion that preconditioned nanofibers exert anti-inflammatory properties.
Figure 7.
Preconditioned nanofibers exert anti-inflammatory effects. (A) Serum levels of TNF-α (left panel), IL-6 (middle panel), and IL-10 (right panel) after treatment with preconditioned nanofibers. (B) RT-qPCR of CCL5 mRNA obtained from whole kidneys (n = 3 mice per group).
Analysis of Secretory Proteome from Preconditioned Nanofibers
ESCs have been shown to secrete a broad spectrum of cytokines, chemokines, and growth factors.46 We performed a semiqualitative cytokine antibody array analysis to assess the secretome from preconditioned nanofibers. To this end, preconditioned nanofibers were transferred to a solution of PBS. The PBS solution was then removed and replaced with fresh PBS periodically to assess the time course release of secreted proteins. As control, nonconditioned nanofibers were also placed in PBS for 24 hours. Overall, a total of 36 secreted proteins were differentially increased in conditioned PBS (ratio >1.5) (Figure 8A). These results indicate that preconditioned nanofibers can retain and gradually release paracrine/endocrine factors from ESCs.
Figure 8.
Secreted protein expression profiling of preconditioned nanofibers. (A) Cytokine array profiling was performed on secretomes obtained from nonconditioned and preconditioned nanofibers using a biotin-labeled cytokine protein array system (RayBio L-308 Mouse Array). A total of four separate samples using 1× concentration of the conditioned PBS samples were tested. Each square represents a mean of measurements performed in triplicate. The color in each square indicates the ratio of fluorescence intensity on the color bar. The fluorescence intensities were analyzed by using the QuantArray software. (B) Simultaneous knockdown of three secreted proteins (follistatin, adiponectin, and SLPI) showing the reduction at the mRNA levels. (C) Quantification of albumin/creatinine ratio in mice treated with preconditioned nanofibers after simultaneous triple knockdown (n = 7 mice per group). (D) Representative immunoblot of Rho kinase activation from kidney lysates. (E) Effect of individual knockdown of follistatin, adiponectin, and SLPI on Rho kinase activation in the kidney. Recombinant Rho kinase 2 served as a positive control. (F) Quantitative analysis of urine albumin/creatinine ratio 24 hours after intraperitoneal administration of LPS with ESC-CM (n = 7 per group). (G) Western blot analysis of OPN cleavage by MMP-3 using anti-OPN antibodies. Recombinant mouse OPN was incubated in the presence and absence of MMP-3. Note the absence of low molecular weight bands in sample from preconditioned nanofibers.
To dissect the contribution of differentially expressed secreted proteins in the renoprotective effects of preconditioned nanofibers, we targeted proteins that were previously reported to play critical roles in the LPS model of organ injury. A survey of the literature prompted us to target follistatin, adiponectin, and secretory leukoprotease inhibitor (SLPI) as our initial targets.47–49 Because we expected that the effects of preconditioned nanofibers might stem from multiple secreted proteins, in addition to designing siRNA oligos directed against the individual genes, we also used a combinatorial siRNA strategy for their simultaneous knockdown. To this end, before preconditioning of nanofibers, ESCs were transfected with siRNAs for individual or multiple genes simultaneously. Nanofibers were then preconditioned as described previously. We found that triple simultaneous knockdown of follistatin, adiponectin, and SLPI in ESCs prevented the renoprotective effect of preconditioned nanofibers in vivo (Figure 8, B and C). With use of a combinatorial knockdown of these three genes, strong Rho kinase activation also remained unchanged in the kidneys of mice treated with LPS even in the presence of preconditioned nanofibers (Figure 8D). This suggests a critical role for follistatin, adiponectin, and SLPI as well as for Rho kinase activation as the underlying molecular mechanism by which preconditioned nanofibers exert their renoprotective effect. Interestingly, individual knockdown of these genes failed to prevent the effect of preconditioned nanofibers on Rho kinase activation (Figure 8E). We also evaluated the effect of conditioned medium (ESC-CM) on our experimental model of AKI. Mice were injected intraperitoneally with conditioned medium (200 μl per mouse) 1 hour after injection of LPS. Interestingly, no significant benefit was observed with ESC-CM on albuminuria (Figure 8F). This observation suggests that our designer nanofiber facilitates the uptake of proteins from secretome, a feature not surprising considering that nanofibers exhibit extremely high surface area-to-volume ratio.50
Finally, we asked whether the use of preconditioned nanofibers would protect the secretome from proteolysis. We characterized the effect of preconditioned nanofiber on the degradation of osteopontin (OPN), a secreted glycoprotein, which was significantly upregulated in the secretome. We used purified recombinant OPN and active matrix metalloproteinase 3 (MMP3) to perform a cleavage assay. In the presence of MMP3, OPN was cleaved to generate two additional fragments (40 and 32 kD) (Figure 8G). However, in the presence of preconditioned nanofibers, the pattern of cleavage of OPN was very similar to the control sample with the absence of low molecular weight bands, supporting the notion that preconditioned nanofibers provide a protease resistant environment.
DISCUSSION
We report a novel observation that may prompt a new approach to stem cell therapy for a wide range of applications. To our knowledge, this is the first report describing the use of nanofibers for stem cell therapy in an experimental model of organ injury in vivo. Our findings indicate that preconditioned nanofibers deliver secretome from ESCs both in vitro and in vivo, exhibit cytoprotective and anti-inflammatory properties, and ameliorate LPS-induced AKI. Our results also identify follistatin, adiponectin, and SLPI as the key peptides, and Rho kinase activation as a critical molecular mechanism by which preconditioned nanofibers exert their modulatory effects. On the basis of these findings, we propose that preconditioned nanofibers serve as a novel acellular delivery platform for a broad spectrum of stem cells applications whereby beneficial effects of stem cells are preserved, whereas many limitations of stem cell therapy are circumvented.
Coupling of peptide nanofibers with ESCs presents several advantages: (1) it provides an acellular platform for delivery of secretome from stem cells; (2) it offers an opportunity for a wider preclinical and clinical use of ESCs after organ injury because it circumvents the potential formation of teratomas; (3) by modifying the designer peptide motifs, it is possible to pursue a more targeted therapy; (4) it may extend the biologic activity of cytokines and chemokines secreted from ESCs; (5) it can be used to dissect the paracrine/endocrine effects of stem cells on gene expression or cell-signaling processes; and (6) peptide nanofibers can break down into natural amino acids, which are nontoxic. Thus, peptide nanofibers may be useful as a bio-reabsorbable source of delivery of secretome for kidney repair. A main advantage of using preconditioned nanofibers, which can potentially explain the lack of tissue protective effects with ESC-CM, is the extremely high surface area-to-volume ratio of nanofibers that could facilitate the uptake and release of proteins delivered by the nanofibers.50 The combined topographical and biochemical signaling from peptide nanofibers may also play a role in enhancing biologic activities of paracrine/endocrine factors secreted from ESCs.
Another important finding of this study is the observation that the simultaneous secretion of follistatin, adiponectin, and SLPI is critical for the renoprotective effects of preconditioned nanofibers in vivo. Although several published studies have previously reported the individual effect of these proteins in the LPS model of acute organ injury,47–49 our findings indicate that simultaneous secretion of these peptides are necessary to prevent LPS-induced AKI in our experimental model. Our findings also suggest that Rho kinase inhibition is a key molecular mechanism by which preconditioned nanofibers exert their renoprotective effects. Our group and others have previously shown a crucial role for Rho kinase activation in endothelial cell hyperpermeability,39 and we have recently proposed that Rho kinase may constitute a novel targeted therapy for kidney diseases.51 Furthermore, pharmacologic inhibition of Rho kinase activation in the LPS model of AKI has also been reported to exert a protective effect.45,52 In this study, we used a genetic approach to examine the effect of Rho kinase knockout in this model. Our findings indicate that targeted deletion of ROCK1 protects against LPS model of AKI. Although Rho kinase inhibition may not be the only mechanism responsible for the beneficial effects of preconditioned nanofibers, our results reveal that the renoprotective effect of preconditioned nanofibers correlated with Rho kinase inhibition both in vitro and in vivo.
In summary, we describe the first demonstration of peptide nanofiber efficacy as a novel platform for delivery of secretome released from stem cells. Our findings illustrate the utility of preconditioned nanofibers in developing alternatives to conventional stem cell therapy for a diverse set of diseases, including those involving kidney injuries.
CONCISE METHODS
LPS (Escherichia coli 0111:B4) was obtained from List Laboratories (Campbell, CA). Antifibrin antibody was purchased from Nordic Immunological Laboratories (Tilburg, The Netherlands). Polyclonal anti-phospho myosin phosphatase targeting subunit 1 (MYPT-1) (T853) antibody, recombinant MYPT-1, and recombinant Rho kinase 2 protein were from Upstate Biotechnology (Lake Placid, NY). Goat polyclonal antiosteopontin (OPN) antibody was from Abcam (Cambridge, MA), and anti-OPN rabbit polyclonal antibodies were obtained from Alexis Biochemicals (San Diego) and Rockland Immunochemicals (Gilbertsville, PA). Recombinant rat VEGF-A164 and human recombinant osteopontin (huOPN) were acquired from R&D Systems (Minneapolis). The catalytic domain of the matrix metalloproteinase (MMP)-3 was obtained from Alexis Biochemicals (San Diego). Cell culture media and supplies were from Invitrogen (Carlsbad, CA).
Self-assembled Peptide Nanofibers
Nanofibers were prepared through the self-assembly of a short, synthetic peptide with the sequence EESLSLSLSLSLSLEEGRGDS. The N-terminus was acetylated and the C-terminus was amidated. The peptide was prepared by standard automated solid-phase synthesis on an APEX 396 Multipeptide Synthesizer. It was purified by selective precipitation followed by dialysis against deionized water and lyophilized. Purified peptide was characterized by MALDI-TOF mass spectrometry. The lyophilized peptide powder was dissolved in deionized water and pH-adjusted to 7.0 with the addition of NaOH to make a viscous solution of 2% by weight. Gels were formed by mixing equal volumes of 2 wt% peptide solution with cell culture medium containing 1.2 μl of 1 M MgCl2 solution per 100 μl of gel.
Cell Culture
Conditionally immortalized renal microvascular endothelial cells were kindly provided by Dr. Robert Langley (University of Texas, M.D. Anderson Cancer Center).53 Conditionally immortalized mouse renal podocytes were provided by Dr. Peter Mundel (University of Miami).54,55 Mouse embryonic stem cell line (ESC-D3) was obtained from the Center for Stem Cell and Regenerative Medicine (Baylor College of Medicine) and derived from 129/Sv+/+ mouse as described previously and according to previously reported methods.56 HK2 cells (ATTC, Manassas, VA) were grown in complete keratinocyte serum-free media. Mouse embryonic fibroblasts (MEF) were obtained from 13-day-old CF-1 mouse embryos (Charles River) and according to previously reported methods.57 Cells were released by trypsin/EDTA digestion for 30 minutes and harvested in 75-cm2 culture flasks at a density of 3 × 105 per ml in MEM-α supplemented with 10% FBS (Life Technologies, USA). Subcultures were prepared after the cells reached a confluence of >90% in the same medium.
Preparation of Preconditioned Nanofibers and Conditioned Media
Preconditioning of nanofibers was performed by exposing nanofibers (100 μl) to ESCs (2 × 105 cells) for 24 hours in 24-well inserts (0.4-μm pore size) of a two-compartment transwell coculture system (BD Biocoat, MA) in which nanofibers were placed in the lower compartment, whereas ESCs were seeded in the upper compartment in the presence of serum-free medium (knockout DMEM).58,59 After 24 hours of conditioning, ESCs were removed and media replaced. To prepare for encapsulated nanofibers, 2 × 105 ESCs were mixed with 100 μl of nanofibers for 1 hour. Conditioned media from ESCs (ESC-CM) was prepared with 2 × 105 ESCs incubated in the presence of serum-free knockout DMEM for 24 hours in an incubator at 37°C with 5% CO2 in 95% air. The ESC-CM was then collected and concentrated by ultrafiltration using centrifugal filters with a 3-kD molecular weight cutoff (Amicon Ultra-PL 3; Millipore, Bedford, MA).
Transendothelial 125I-BSA Permeability Measurements
Two-compartment transwell coculture system (BD Biocoat) was used to determine the permeability of 125I-BSA (bovine serum albumin) across confluent endothelial cell monolayers as described previously.36
Animal Models of AKI
All animals were cared for at Baylor College of Medicine and according to the AIACU and Principles of Laboratory Animal Care of the National Institute of Health. Generation of Rock1−/− mice was previously described.60 Adult male C57BLKS mice (8 to 10 weeks old) were obtained commercially (Jackson Laboratory, Bar Harbor, ME). Mice received a single injection with 10 μg/g body wt of LPS (E. coli; List Laboratories, Campbell, CA) or sterile normal saline intraperitoneally.29,30,32 For LPS-induced model of AKI, groups of 6 to 11 mice were randomly allocated to individual groups. One group of mice was used as the control group. All other groups received a single intraperitoneal injection of LPS (10 μg/g body wt). In LPS-treated animals, mice were injected with 0.8 ml of isotonic saline intraperitoneally to prevent hypovolemia after 12 hours.29 In experiments where the effect of preconditioned nanofibers was assessed, animals were given an injection of nanofibers (200 μl) intraperitoneally 1 hour after LPS injection. Mice were sacrificed 24 hours after LPS injection. Urine albumin and creatinine were determined using Albuwell M and Creatinine Assay (Exocell, Philadelphia). Kidney ischemia reperfusion (I-R) was performed in male C57BL/6 mice, aged 8 to 10 weeks, obtained from Jackson Laboratory.61,62 Briefly, mice were anesthetized with intraperitoneal ketamine, had abdominal incisions, and then have both renal pedicles bluntly dissected. A microvascular clamp (Roboz) was placed on both renal pedicles for 30 minutes while the animal was kept at a constant temperature (37°C) and well hydrated. After ischemia, the clamps were removed, the wounds were sutured, and the animal was allowed to recover. Mice were randomly allocated into the following groups (n = 6): (1) sham; (2) I-R+preconditioned nanofibers (100 μl) injected intraperitoneally 1 hour before induction of ischemia and at the time of reperfusion; and (3) I-R+nonconditioned nanofibers (100 μl) injected intraperitoneally 1 hour before induction of ischemia and at the time of reperfusion. Mice were administered with 0.5 ml of isotonic saline intraperitoneally (preheated to 37°C) immediately after closure of abdomen, and sacrificed 24 hours after reperfusion. Serum creatinine and BUN were measured on obtained blood samples.
Small Interfering RNAs (siRNAs)
siRNAs were purchased from Dharmacon (Thermo Scientific Dharmacon, Lafayette, CO) using ON-TARGETplus. Transfection was performed with HiPerfect transfection reagent (Qiagen, Valencia, CA). The following sequences were used: follistatin: GACUACAGCUUUCCUAUCU; adiponectin: GCAGAUAACGUCAACGACU; and SLPI: GACCAGGGUUACUGUUUUA. Transfected ESCs with nontarget siRNAs (Dharmacon) served to establish basal mRNA expression levels. ESCs were cocultured with nanofibers for 24 hours using transwell coculture system. To validate the efficiency of siRNA targeting, semiquantitative PCR was performed using the following primers: Adiponectin: forward: GTTGCAAGCTCTCCTGTTCC; reverse: GCTTCTCCAGGCTCTCCTTT. Follistatin: forward: TCCACTTGTGTGGTGGATCAGACCA; reverse: CAAAGGCTATGTCAACACTGAACAT. SLPI: forward: TGCTTAACCCTCCCAATGTC; reverse: GAATGCTGAGCCAAAAGGAG. β-Actin: forward: AGAGGGAAATCGTGCGTGA; reverse: CACACTGTGTTGGCATAGA GGTC.
Rho Kinase Activation Assay
The activity of Rho kinase was evaluated by measuring the phosphorylation of a Rho kinase substrate, MYPT-1, at threonine 853 (Upstate Biotechnology) as described previously.51
Detection of Apoptosis
Apoptotic cells were determined using an Apoptag Fluorescein In Situ Apoptosis Detection Kit (TUNEL assay; Chemicon, Billerica, MA), and by quantifying cytoplasmic histone-associated DNA fragments detection (Cell Death Detection ELISA; Roche, Indianapolis), according to the manufacturers' instructions. Caspase-3 activation was assessed by caspase-3 activation assay (BD Biosciences, San Jose, CA).
Serum Cytokines Assay
Serum TNF-α, IL-6, and IL-10 were assessed using the Milliplex MAP Mouse Cytokine Kit Assay as per manufacturer's specifications (Millipore, Chicago).
In Situ Hybridization
In situ hybridization with an antisense PAI-1 riboprobe was performed as described previously.63,64 Total RNA from mouse lung was reverse-transcribed into cDNA using Superscript reverse transcriptase (Clontech) and random hexamers (Sigma). PCR amplification was performed using T7-tagged forward and SP6-tagged reverse primers to amplify a 848-bp fragment representing bases 663-1510 of the coding region of the murine PAI-1 gene. The PCR-amplified cDNA template was used for in vitro transcription of digoxygenin-labeled riboprobe using T7 RNA polymerase for the antisense probe and SP6 for the sense control probe.
Scanning Electron Microscopy (SEM)
Cells were fixed with 3% glutaraldehyde plus 2% paraformaldehyde and 7.5% sucrose in 0.1 M cacodylate, postfixed with 1% cacodylate buffered osmium tetroxide plus 7.5% sucrose, dehydrated with graded series of hexamethyldisilazane-ethanol (HMDS-ethanol) series and air-dried overnight. Samples were examined with a JSM-5910 scanning electron microscope (JEOL USA, Peabody, MA) at an accelerating voltage of 5 kV.
Atomic Force Microscopy
Peptides were dissolved at 0.1 wt% in deionized water at pH 7. Two drops of peptide solution were added onto freshly cleaved mica while spinning on a Headway Research, Inc. photoresist spinner. The mica was then rinsed with deionized water and spun for an additional 9 minutes. AFM images were collected at ambient temperature, on a Digital Instruments Nanoscope IIIa atomic force microscope in tapping mode.
Transmission Electron Microscopy
Kidney cortical tissues were minced and fixed at room temperature with a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.3. After fixation, samples were washed, postfixed with 1% buffered osmium tetroxide, and stained en block with 1% Millipore-filtered uranyl acetate. Samples were dehydrated in increasing concentrations of ethanol, infiltrated, embedded in LX-112 medium, and polymerized in an oven for 2 days. Ultrathin sections were cut in a Leica Ultracut microtome (Leica, Deerfield, IL), stained with uranyl acetate and lead citrate in a Leica EM Stainer, and examined with a JEM 1010 transmission electron microscope (JEOL USA, Inc., Peabody, MA). Digital images were obtained using AMT Imaging System (Advanced Microscopy Techniques Corporation, Danvers, MA).
RT-qPCR
The mRNA expression was quantified using a DNA Engine Opticon Continuous Fluorescence Detector (MJ Research) with SYBR green as described previously.65 The primers used were as follows: CCL5: forward: 3′-GCAAGTGCTCCAATCTTGCA-5′; reverse: 3′-CTTCTCTG GGTTGGCACA CA-5′. β-Actin: forward: 3′-AGAGGGAAATCGTGCGTGA CA-5′; reverse: 3′-CACTGTGTT GGCATAGAGGTC-5′.
Immunohistochemistry
Staining was performed using the VectaStain ABC kit (Vector Laboratories, Burlingame, CA). Briefly, the tissue sections were deparaffinized, treated with 3% hydrogen peroxide, and incubated with 10% normal rabbit serum for 30 minutes. The slides were then incubated with primary antibodies at 4°C overnight, followed by sequential treatment with biotinylated-rabbit-anti-goat IgG, streptavidin-peroxidase conjugate, and aminoethylcarbazole chromogen. The slides were counterstained with Gill's modified hematoxylin and mounted on VectaMount (Vector Laboratories).
Enzyme Cleavage Assays
Human recombinant osteopontin (1 μg, huOPN) mixed with 20 μl of nanofibers were cleaved by MMP3 (50 ng) in equal volume of cleavage buffer (200 mM NaCl, 50 mM Tris-HCl, pH 7.6, 5 mM CaCl2) for 10 minutes at 37°C as described previously.66 The mixture of cleaved OPN fragments was separated on a 12.5% polyacrylamide gel under reducing conditions. The membrane was incubated in a combination of three primary anti-OPN antibodies, anti-OPN goat polyclonal antibody and two anti-OPN rabbit polyclonal antibodies, for 1 hour. Proteins were visualized with ECL Plus (GE Healthcare).
Statistical Analysis
All data are shown as mean ± SEM. Statistical significance was assessed by performing ANOVA followed by the Tukey-Kramer post hoc analysis for multiple comparisons using an α value of 0.05 in Graphpad Prism software (version 5.0; San Diego). Differences between paired means were assessed with the unpaired, two-tailed t test.
DISCLOSURES
None.
Acknowledgments
This work was supported by NIH grants DK067604 and DK078900, Seed funding from Baylor College of Medicine, and the IBBM Medical Innovations Award from Rice University.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Cells for Treating Organ Damage: How Long Will We Need Them?” on pages 590–592.
REFERENCES
- 1. Guo J-K, Cantley LG: Cellular maintenance and repair of the kidney. Annu Rev Physiol 72: 357–376, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G: Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int 72: 430–441, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Ikarashi K, Li B, Suwa M, Kawamura K, Morioka T, Yao J, Khan F, Uchiyama M, Oite T: Bone marrow cells contribute to regeneration of damaged glomerular endothelial cells. Kidney Int 67: 1925–1933, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG: Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P: Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 14: 1188–1199, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Maeshima A, Yamashita S, Nojima Y: Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 14: 3138–3146, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G: Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 15: 1794–1804, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Poulsom R, Forbes SJ, Hodivala-DilkeKairbaan, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Toby H, Alison M, Cook T, Pusey C, Wright NA: Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG: Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G: Insulin-Like Growth Factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol 18: 2921–2928, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C: Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C: Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT: Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A 103: 8155–8160, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsieh P, Davis M, Gannon J, MacGillivray C, Lee R: Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest 116: 237–248, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Branco MC, Pochan DJ, Wagner NJ, Schneider JP: Macromolecular diffusion and release from self-assembled beta-hairpin peptide hydrogels. Biomaterials 30: 1339–1347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R: Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A 103: 6315–6320, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC: Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci U S A 105: 2586–2591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S: Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci U S A 106: 4623–4628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong H, Paramonov SE, Hartgerink JD: Self-assembly of alpha-helical coiled coil nanofibers. J Am Chem Soc 130: 13691–13695, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Dong H, Paramonov SE, Aulisa L, Bakota E, Hartgerink JD: Self-assembly of multidomain peptides: Balancing molecular frustration controls conformation and nanostructure. J Am Chem Soc 129: 12468–12472, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Hartgerink JD, Beniash E, Stupp SI: Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 294: 1684–1688, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Paramonov SE, Jun H-W, Hartgerink JD: Self-assembly of peptide-amphiphile nanofibers: The roles of hydrogen bonding and amphiphilic packing. J Am Chem Soc 128: 7291–7298, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI: Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater 1: 387–397, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Galler KM, Cavender A, Yuwono V, Dong H, Shi S, Schmalz G, Hartgerink JD, D'Souza RN: Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng, Part A 14: 2051–2058, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Gelain F, Bottai D, Vescovi A, Zhang S: Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS One 1: e119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faul C, Donnelly M, Merscher-Gomez S, Chang Y, Franz S, Delfgaauw J, Chang J, Choi H, Campbell K, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei C, Moller C, Altintas M, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi M, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Zahedi K, Barone S, Kramer DL, Amlal H, Alhonen L, Janne J, Porter CW, Soleimani M: The role of spermidine/spermine N1-acetyltransferase in endotoxin-induced acute kidney injury. Am J Physiol Cell Physiol 299: C164–C174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR: Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Bannerman DD, Goldblum SE: Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol 284: L899–L914, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Bannerman DD, Sathyamoorthy M, Goldblum SE: Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem 273: 35371–35380, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ: Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Jho D, Mehta D, Ahmmed G, Gao X-P, Tiruppathi C, Broman M, Malik AB: Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res 96: 1282–1290, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN: Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol 15: 3093–3102, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J: Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3: 346–352, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Zeng L, Xu H, Chew T-L, Eng E, Sadeghi MM, Adler S, Kanwar YS, Danesh FR: HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. FASEB J 05-4240fje, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K: Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15: 2208–2216, 1996 [PMC free article] [PubMed] [Google Scholar]
- 41. Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita A: Involvement of Rho-kinase in hypertensive vascular disease: A novel therapeutic target in hypertension. FASEB J 15: 1062–1064, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto K, Loskutoff D: Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest 97: 2440–2451, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cohen J: The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Nemeth K, Leelahavanichkul A, Yuen P, Mayer B, Parmelee A, Doi K, Robey P, Leelahavanichkul K, Koller B, Brown J, Hu X, Jelinek I, Star R, Mezey E: Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15: 42–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyer-Schwesinger C, Dehde S, von Ruffer C, Gatzemeier S, Klug P, Wenzel UO, Stahl RAK, Thaiss F, Meyer TN: Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-{kappa}B p65 signaling. Am J Physiol Renal Physiol 296: F1088–F1099, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Van Hoof D, Passier R, Ward-Van Oostwaard D, Pinkse MWH, Heck AJR, Mummery CL, Krijgsveld J: A quest for human and mouse embryonic stem cell-specific proteins. Mol Cell Proteomics 5: 1261–1273, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Maeshima A, Zhang Y-Q, Nojima Y, Naruse T, Kojima I: Involvement of the activin-follistatin system in tubular regeneration after renal ischemia in rats. J Am Soc Nephrol 12: 1685–1695, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Nakamura A, Mori Y, Hagiwara K, Suzuki T, Sakakibara T, Kikuchi T, Igarashi T, Ebina M, Abe T, Miyazaki J, Takai T, Nukiwa T: Increased susceptibility to LPS-induced endotoxin shock in secretory leukoprotease inhibitor (SLPI)-deficient mice. J Exp Med 197: 669–674, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ohashi K, Iwatani H, Kihara S, Nakagawa Y, Komura N, Fujita K, Maeda N, Nishida M, Katsube F, Shimomura I, Ito T, Funahashi T: Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol 27: 1910–1917, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Cao H, Liu T, Chew SY: The application of nanofibrous scaffolds in neural tissue engineering. Adv Drug Deliv Rev 61: 1055–1064, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR: Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes 57: 714–723, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Prakash J, de Borst MH, Lacombe M, Opdam F, Klok PA, van Goor H, Meijer DK, Moolenaar F, Poelstra K, Kok RJ: Inhibition of renal rho kinase attenuates ischemia/reperfusion-induced injury. J Am Soc Nephrol 19: 2086–2097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ: Tissue-specific microvascular endothelial cell lines from H-2Kb-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res 63: 2971–2976, 2003 [PubMed] [Google Scholar]
- 54. Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Mundel P, Reiser J, Borja AZM, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM: Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336: 684–687, 1988 [DOI] [PubMed] [Google Scholar]
- 57. Ozolek JA, Jane EP, Krowsoski L, Sammak PJ: Human embryonic stem cells (HSF-6) show greater proliferation and apoptoses when grown on glioblastoma cells than mouse embryonic fibroblasts at day 19 in culture: Comparison of proliferation, survival, and neural differentiation on two different feeder cell types. Stem Cells Dev 16: 403–412, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Jun C, Amalia D, Aya T, Lisa G-B, Pamela LS: Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis 39: 100–104, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Hancock CR, Wetherington JP, Lambert NA, Condie BG: Neuronal differentiation of cryopreserved neural progenitor cells derived from mouse embryonic stem cells. Biochem Biophys Res Commun 271: 418–421, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Zhang Y-M, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L: Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J 20: 916–925, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Sean EK, Jonathan HE: Murine renal ischaemia-reperfusion injury (Methods in Renal Research Paper). Nephrology 13: 390–396, 2008 [DOI] [PubMed] [Google Scholar]
- 62. Kumar S, Allen DA, Kieswich JE, Patel NSA, Harwood S, Mazzon E, Cuzzocrea S, Raftery MJ, Thiemermann C, Yaqoob MM: Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol 20: 2412–2425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou Y-C, Eble TN, Patel A, Thaller C, Fang P, Van den Veyver IB: Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet 39: 836–838, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Jenkins ZA, van Kogelenberg M, Morgan T, Jeffs A, Fukuzawa R, Pearl E, Thaller C, Hing AV, Porteous ME, Garcia-Minaur S, Bohring A, Lacombe D, Stewart F, Fiskerstrand T, Bindoff L, Berland S, Ades LC, Tchan M, David A, Wilson LC, Hennekam RCM, Donnai D, Mansour S, Cormier-Daire V, Robertson SP: Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet 41: 95–100, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Long J, Wang Y, Wang W, Chang BHJ, Danesh FR: Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem 285: 23457–23465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L: Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 276: 28261–28267, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Klein J, Gonzalez J, Decramer S, Bandin F, Neau E, Salant DJ, Heeringa P, Pesquero J-B, Schanstra J-P, Bascands J-L: Blockade of the kinin B1 receptor ameloriates glomerulonephritis. J Am Soc Nephrol 21: 1157–1164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]