Abstract
Inflammation contributes to the pathogenesis of ischemic acute kidney injury (AKI), and T cells mediate the early phase of ischemia-reperfusion injury (IRI). The Fas/Fas ligand (FasL) pathway modulates the balance of T cell subsets in the peripheral circulation as well as multiple inflammatory responses, suggesting that FasL may mediate ischemic AKI. Here, we induced bilateral renal IRI in mice bearing a loss-of-function mutation of FasL (the gld mutation) and in wild-type mice. Compared with wild-type mice, serum creatinine was lower in gld mice (1.4 ± 0.9 mg/dl versus 2.6 ± 0.4) at 24 hours after IRI (P < 0.05). In addition, gld mice had fewer TNF-α-producing T lymphocytes in the kidneys and renal lymph nodes. Furthermore, pharmacologic blockade of FasL protected the kidneys of wild-type mice from IRI. Analysis of bone marrow chimeric mice suggested that the pathogenic effect of FasL involves leukocytes; reconstitution of wild-type mice with gld splenocytes attenuated IRI. In contrast, reconstitution of gld mice with wild-type splenocytes enhanced IRI. These data demonstrate that FasL, particularly on leukocytes, mediates ischemic AKI.
Renal ischemia/reperfusion injury (IRI) is a leading cause of acute kidney injury (AKI) in both allografts and native kidneys.1 Despite advances in medical care, the mortality and morbidity associated with AKI after IRI still remains high,2 and specific therapy is not available. Therefore, many studies have focused on elucidating the pathophysiology of renal IRI with the goal of identifying new therapeutic targets.
Inflammation has been demonstrated to contribute to the pathogenesis of renal IRI, with a role for leukocytes, adhesion molecules, and cytokines.3,4 Fas ligand (FasL) is a member of the TNF family that induces apoptosis by cross-linking its Fas receptor.5 Fas is known to be involved in various forms of renal injury such as IRI6 and tubular injury in glomerulonephritis.7,8 FasL is also believed to have a role in inflammation through recruitment of inflammatory cells,9,10 and induction of cytokine production.11 Previous studies have demonstrated diverse results regarding FasL effects on various inflammatory processes,12–15 which may be related to the pleiotropic effects of FasL and expressing cell types.16,17 Besides lymphocytes, FasL is frequently expressed on epithelial and endothelial cells18 and is critical for regulating T cell homeostasis.19 A double negative (DN) T cell (CD3+CD4−CD8−) population that is normally rare in the lymphoid tissues gradually accumulates in lymph nodes and spleens of mice bearing spontaneous loss-of-function point mutation of FasL (referred to as the gld mutation). In spite of accumulation of DN T cells and activated CD4+ and CD8+ T cells in mutant mice, FasL deficiency confers protection rather than exacerbation of organ-specific T cell–mediated autoimmune diseases.20,21 T lymphocytes are important mediators of IRI of kidney and other organs, and FasL could have a role in IRI through modulation of T cells homeostasis. Although various effects of FasL on IRI have been reported,22–25 little is known about the role of FasL in renal IRI, particularly from the angle of leukocytes.
In this report, we demonstrated using FasL-deficient (gld) mice and FasL-blocking mAb that inhibition of FasL significantly attenuated kidney IRI in mice. FasL deficiency induced decreased inflammatory cytokine production by T lymphocytes isolated from postischemic kidneys both in gld mice and wild-type (WT) mice treated with FasL-blocking antibody. Attenuation of IRI as assessed by functional and histologic parameters in WT chimeric mice reconstituted with gld splenocytes as compared with WT mice reconstituted with WT splenocytes demonstrated that the pathogenic effect was mediated predominantly by FasL of leukocyte origin.
RESULTS
FasL Deficiency Attenuated Renal Injury after IRI
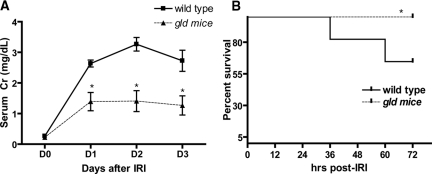
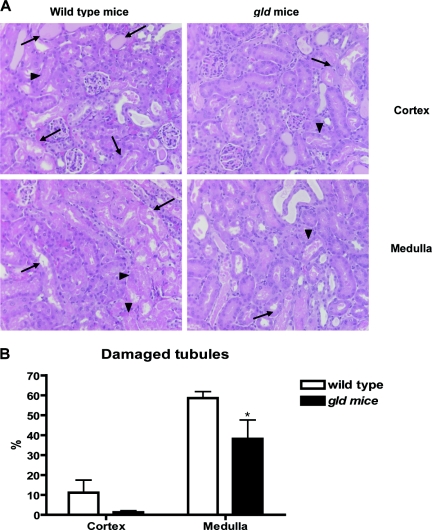
Renal dysfunction was evaluated by measuring serum creatinine on 3 consecutive days after IRI (Figure 1A). The gld mice demonstrated lower serum creatinine compared with wild-type (WT) control group after IRI. (WT versus gld: day 1, 2.6 ± 0.4 mg/dl versus 1.4 ± 0.3; day 2, 3.3 ± 0.2 versus 1.4 ± 0.3; day 3, 2.7 ± 0.3 versus 1.3 ± 0.3; P < 0.05). FasL deficiency also improved survival after IRI. On day 3 after IRI, the mortality was higher in WT than in gld (40 versus 0%, P = 0.02; Figure 1B). When renal tubular injury scores were compared, gld mice had reduced tubular damage. There were significantly more damaged tubules in the outer medulla of WT than gld mice (58.6 ± 3.3 versus 38.2 ± 9.4% of damaged tubules, P < 0.05; Figure 2). Immunohistochemical staining with caspase 3 on day 3 after IRI showed decreased apoptosis in the tubules of gld than in WT mice (0.0037 ± 0.0008 versus 0.0018 ± 0.0007% of the total area, P < 0.05; Supplemental Text 1).
Figure 1.
Improved renal function and survival after renal IRI in FasL deficient (gld) mice compared to wild type (WT). Serum creatinine in gld mice was lower compared with WT after bilateral renal pedicle clamping (n = 11 for each group, panel A), and survival in gld mice was significantly higher at 3 days after IRI (B). *P < 0.05 compared with WT.
Figure 2.
Reduced tubular damage of post ischemic kidneys in FasL deficient (gld) mice compared to wild type (WT). On day 3 after IRI (D3), postischemic kidneys of gld mice showed decreased tubular damage compared with WT in medulla. Arrows indicate necrotic tubules and arrowheads indicate necrotic debris. Magnification: ×200. *P < 0.05 compared with WT.
FasL Deficiency Reduced TNF-α Production in T Cells after IRI in Kidney and Renal Lymph Node
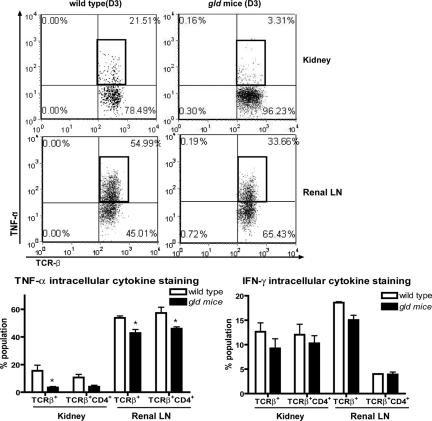
When baseline characteristics of different leukocytes were compared between WT and gld mice, there was significantly higher frequency of CD3+CD4−CD8− double negative (DN) T cells of spleens and blood in gld than in WT mice (Supplemental Text 2). However, no significant difference of their frequency in the kidneys was observed between the two genotypes, although the kidney normally contains a large population of DN T cells. In postischemic kidneys at day 3 after IRI, the NK cell population was reduced in gld as compared with that in WT mice (WT versus gld: 13.1 ± 1.6 versus 9.0 ± 0.8% population; P < 0.05), whereas percentages of other leukocyte subsets did not change (Table 1). Because FasL is known to modulate T cells homeostasis, we measured production of proinflammatory cytokines by T cells from ischemic kidneys of WT and gld mice using intracellular cytokine staining. TNF-α production in T cells from kidneys was slightly lower in gld than in WT mice 1 day after IRI (WT versus gld; 11.5 ± 1.8 versus 6.9 ± 1.1% population; P = 0.06), but became significantly different on day 3 after IRI (WT versus gld; 15.5 ± 4.0 versus 3.4 ± 0.7% population; P < 0.05). T cells isolated from renal lymph nodes in gld also showed lower production of TNF-α compared with those in WT (WT versus gld; 53.7 ± 1.4 versus 42.8 ± 2.6 for TCRβ+ cells and 57.3 ± 4.1 versus 45.9 ± 1.3 for TCRβ+CD4+ cells % population at day 3 after IRI; P < 0.05). IFN-γ production by T cells from gld mice was also lower on day 1 after IRI (WT versus gld; 23.0 ± 0.7 versus 8.6 ± 1.6 for TCRβ+ cells and 22.4 ± 1.9 versus 9.6 ± 1.8 for TCRβ+CD4+ cells % population at day 1 after IRI; P < 0.05), but it was not significantly different between groups on day 3 after IRI (Figure 3, Supplemental Text 3).
Table 1.
Comparison of populations of T cells, B cells, NKT cells, NK cells, and macrophages in the postischemic kidneys between groups (day 3 after IRI)
| Wild Type | gld | |
|---|---|---|
| Cell counts/ml | 3.5 ± 0.9 × 105 | 4.1 ± 1.7 × 105 |
| % population | ||
| CD4 T cells (CD4+TCRβ+) among TCRβ+ cells | 47.2 ± 2.8 | 48.4 ± 1.9 |
| activated CD4 T cells(CD69+CD4+ TCRβ+) among CD4+TCRβ+cells | 19.3 ± 0.8 | 21.2 ± 3.7 |
| CD8 T cells (CD8+ TCRβ+) among TCRβ+cells | 27.6 ± 1.5 | 33.7 ± 2.3 |
| activated CD8 T cells (CD69+CD8+ TCRβ+) among CD8+TCRβ+cells | 18.3 ± 3.5 | 22.6 ± 5.5 |
| double negative T cells (CD4−CD8− TCRβ+) among TCRβ+cells | 22.8 ± 2.4 | 14.0 ± 5.6 |
| TCRγδ cells (TCRγδ+) among gated KMNC | 1.73 ± 0.3 | 1.6 ± 0.3 |
| B cells (CD19+) among gated KMNC | 36.7 ± 10.9 | 22.3 ± 7.2 |
| differentiated B cells (MHCII+CD19+) among CD19+ cells | 3.8 ± 1.5 | 3.3 ± 0.6 |
| IgM+CD5+ B cells (IgM+CD5+CD19+) among CD19+ cells | 2.9 ± 0.2 | 1.9 ± 0.3 |
| NK cells (NK1.1+ TCRβ−) among gated KMNC | 13.1 ± 1.6 | 9.0 ± 0.8a |
| NKT cells (NK1.1+ TCRβ+) among gated KMNC | 4.6 ± 1.0 | 3.4 ± 0.5 |
| CD1d+ NKT cells (CD1d+NK1.1+ TCRβ+) among NK1.1+ TCRβ+ cells | 23.8 ± 3.7 | 24.8 ± 1.5 |
| macrophage (F4/80highCD11clow) among gated KMNC | 2.9 ± 0.5 | 1.7 ± 0.5 |
Except for NK cells, which were lower in gld mice, there were no significant differences between groups. KMNC, kidney mononuclear cells.
aP < 0.05 compared to WT.
Figure 3.
TNF-α and IFN-γ production from T cells in kidneys and renal lymph nodes after IRI was decreased in FasL deficient (gld) mice compared to wild type (WT). TNF-α production in T cells, particularly in TCRβ+CD4+ T cells from postischemic kidneys and renal lymph nodes (LN), at 3 days after IRI was lower in gld mice. *P < 0.05 compared with WT.
FasL-Blocking Antibody Attenuated Renal Injury after IRI
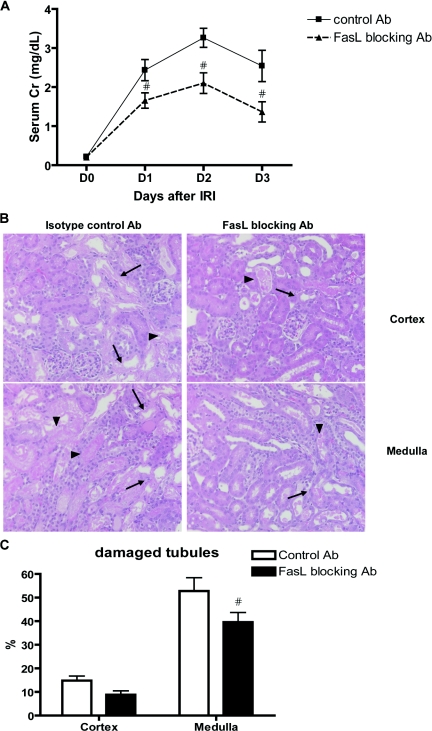
Increase in serum creatinine after IRI was attenuated in mice treated with FasL-blocking monoclonal antibody (Ab) compared with isotype control hamster IgG-treated group (control versus FasL-blocking Ab: day 1, 2.4 ± 0.3 mg/dl versus 1.7 ± 0.2; day 2, 3.3 ± 0.2 versus 2.1 ± 0.3; day 3, 2.5 ± 0.4 versus 1.4 ± 0.3; P < 0.05; Figure 4A). Histologic assessment revealed reduced tubular damage in the medulla of mice treated with FasL-blocking Ab as compared with isotype control group (control versus FasL-blocking Ab; 52.8 ± 5.6 versus 39.6 ± 4.1% of damaged tubules, P < 0.05; Figure 4, B and C).
Figure 4.
Improved kidney function and tissue damage after IRI with FasL blocking Ab compared to isotype control Ab. Serum creatinine was lower in FasL-blocking Ab–treated group after IRI compared with mice treated with isotype control Ab (n = 7 for each group, panel A). On day 3 after IRI, post ischemic kidneys of FasL-blocking Ab–treated group showed decreased tubular damage compared with mice treated with isotype control Ab in medulla (panels B and C). Arrows indicate necrotic tubules and arrow-heads indicate necrotic debris. Magnification: ×200. #P < 0.05 compared with isotype control Ab group.
FasL-Blocking Antibody Reduced Inflammatory Cytokine Production in T Lymphocytes after IRI
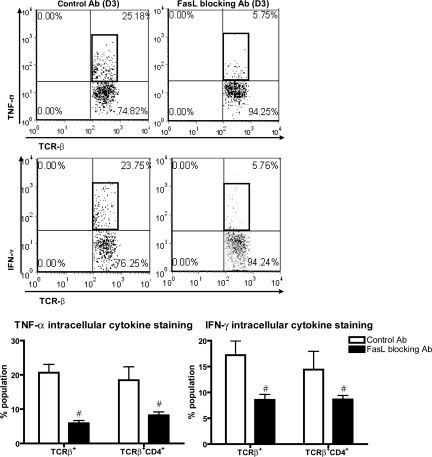
We also analyzed the effects of FasL-blocking Ab treatment on cytokine production in kidney-infiltrating leukocytes. TNF-α and IFN-γ production from total renal T lymphocytes at day 3 after IRI was markedly reduced in mice treated with FasL-blocking Ab compared with mice in the control group (control versus FasL-blocking Ab: 20.7 ± 2.5 versus 5.9 ± 0.9 for TNF-α and 17.2 ± 2.8 versus 8.6 ± 1.1 for IFN-γ % population; P < 0.05). Cytokine production from TCRβ+CD4+ T lymphocytes at 3 days after IRI was also markedly reduced in kidneys of FasL-blocking Ab–treated mice than in the control group (Figure 5).
Figure 5.
Decreased TNF-α and IFN-γ production by kidney T cells during IRI in mice treated with FasL blocking Ab compared to isotype control Ab. TNF-α and IFN-γ production in T cells from postischemic kidneys at 3 days after IRI was lower in FasL-blocking Ab–treated mice compared with isotype control Ab—treated group. #P < 0.05 compared with isotype control Ab group.
FasL Deficiency in Splenocytes Conferred Renal Protection after IRI
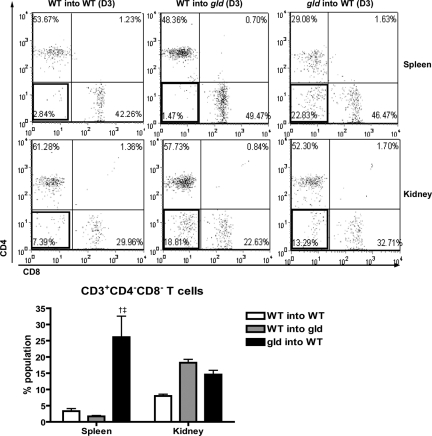
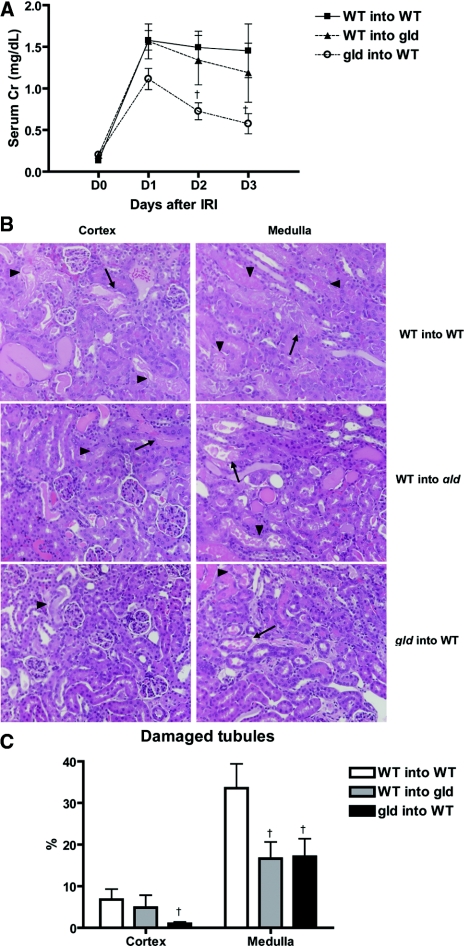
To determine the source of FasL effect on renal IRI, we generated three groups of partial bone marrow chimeric mice developed by adoptive transfer of donor splenocytes into sublethally irradiated hosts. Partial chimerism was verified with the population of CD3+CD4−CD8− double negative (DN) T cells in spleens, with the fact that the proportion of DN T cells in spleens was significantly different between wild-type (WT) and gld mice. As shown in Figure 6, in gld mice reconstituted with WT splenocytes (WT into gld), the DN T cell population in spleen was decreased to the same level as in WT mice reconstituted with WT splenocytes (WT into WT), whereas the DN T cell population significantly increased in WT mice reconstituted with gld splenocytes (gld into WT). Proportions of DN T cell population in spleens of WT into WT, WT into gld, and gld into WT were 3.3 ± 0.8 versus 1.7 ± 0.2 versus 26.2 ± 6.5% population, respectively (P < 0.05). Percentages of DN T cells in kidneys of different groups were similar. After IRI, WT into gld mice showed no differences in serum creatinine compared with WT into WT. By contrast, in gld into WT chimera, serum creatinine was lower at both day 2 and day 3 as compared with WT into WT after IRI (WT into WT versus gld into WT: day 2, 1.5 ± 0.2 mg/dl versus 0.7 ± 0.1; day 3, 1.5 ± 0.4 versus 0.6 ± 0.2; P < 0.05; Figure 7A). Upon histologic assessment, gld into WT chimera showed less tubular damage in both the cortex and medulla than WT into WT chimera (WT into WT versus gld into WT: cortex, 7.6 ± 2.7 versus1.0 ± 0.4; medulla, 37.0 ± 5.3 versus 17.1 ± 4.3% of damaged tubules; P < 0.05; Figure 7, B and C). WT into gld mice showed reduced tubular damage in the medulla as compared with WT into WT control (WT into WT versus WT into gld: 37.0 ± 5.3 versus 16.7 ± 4.0% of damaged tubules; P < 0.05; Figure 7, B and C).
Figure 6.
Increased numbers of double negative T cells (CD3+CD4−CD8−) in spleen of wild type recipients reconstituted with gld splenocytes compared to wild type splenocytes (WT into WT) or gld recipient reconstituted with wild type splenocytes (WT into gld). Splenic DN T cells of gld recipient reconstituted with wild-type splenocytes (WT into gld) decreased to the same level with wild-type mice reconstituted with wild-type splenocytes (WT into WT), whereas wild-type recipient reconstituted with gld splenocytes (gld into WT) showed significant increase of DN T cells in spleens. †P < 0.05 compared with WT into WT; ‡P < 0.05 compared with WT into gld.
Figure 7.
Wild type mice reconstituted with gld splenocytes (gld into WT) show significant functional and histologic renal protection after IRI compared to wild type reconstituted with wild type splenocytes (WT into WT). Serum creatinine was significantly lower in wild-type mice reconstituted with gld splenocytes (gld into WT) at day 2 and day 3 after IRI compared with wild-type receiving wild-type splenocytes (WT into WT). Serum creatinine of gld mice receiving wild-type splenocytes (WT into gld) was not different from other groups (n = 8 for each group; panel A). In histologic examination (B), tubular damage was significantly lower both in cortex and in medulla of gld into WT compared with WT into WT. WT into gld showed reduced tubular damage in medulla compared with WT into WT (Figure 7C). Arrows indicate necrotic tubules and arrowheads indicate necrotic debris. Magnification: ×200. †P < 0.05 compared with WT into WT.
FasL Deficiency Effect on Myeloperoxidase Activity in Postischemic Kidneys
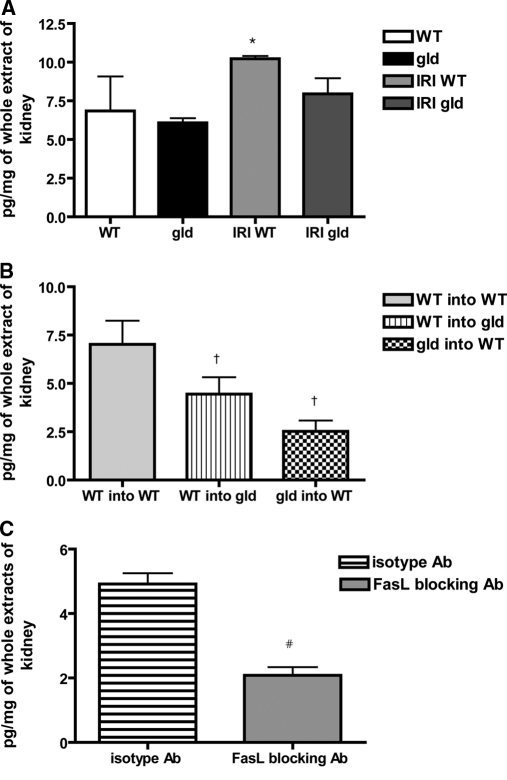
Myeloperoxidase (MPO) activity was measured in kidney extracts after IRI to assess the effect of FasL deficiency on neutrophil (also macrophages) infiltration. MPO activity, which was increased after IRI in WT, however, was not significantly different between gld and WT mice at day 3 after IRI (IRI WT versus IRI gld: 10.2 ± 0.4 versus 7.9 ± 2.4 pg/mg of kidney extracts; P = 0.07; Figure 8A). Among chimeric mice, WT mice reconstituted with WT splenocytes showed significantly higher MPO activity compared with other groups (WT into WT versus WT into gld versus gld into WT: 7.1 ± 2.1 versus 4.4 ± 2.0 versus 2.5 ± 1.3 pg/mg of kidney extracts; P < 0.05; Figure 8B). MPO level was significantly lower in mice treated with FasL-blocking Ab as compared with mice in the isotype control group (isotype versus FasL-blocking Ab: 4.9 ± 0.7 versus 2.1 ± 0.6 pg/mg of kidney extracts; P < 0.05; Figure 8C).
Figure 8.
Reduced myeloperoxidase activity in post ischemic kidneys in mice treated with FasL blocking Ab compared to isotype control Ab. Myeloperoxidase, which was increased at 3 days after IRI in WT compared with nonoperated WT mice, was not different in postischemic kidneys of gld compared with WT, but showed a trend to be lower (P = 0.07; panel A). In the partial chimeric mice, WT mice reconstituted with WT splenocytes showed higher MPO activity compared with other groups after IRI (B). MPO level after IRI was significantly lower in FasL-blocking Ab–treated mice compared with isotype control Ab–treated mice (C). WT and gld: kidneys from nonoperated mice; IRI WT and IRI gld: postischemic kidneys at 3 days after IRI. *P < 0.05 compared with WT, †P < 0.05 compared with WT into WT, and #P < 0.05 compared with isotype control Ab group.
FasL Deficiency Reduced Inflammatory Cytokine Expression in Postischemic Kidneys
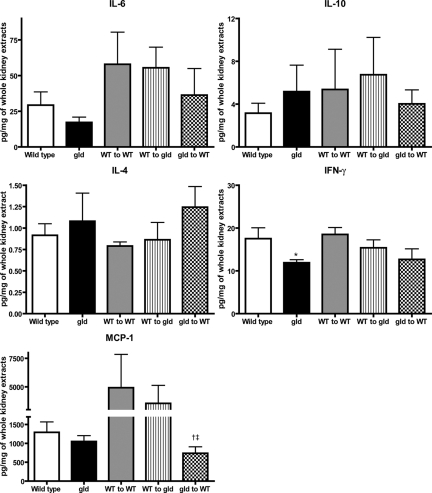
Renal cytokines and chemokines were measured with protein samples extracted from postischemic kidneys of WT and gld mice at day 3 after IRI. IFN-γ production in kidneys was lower in gld than in WT mice (WT versus gld: 17.5 ± 2.5 versus 11.9 ± 0.9 pg/mg of kidney extracts; P < 0.05; Figure 9). Among chimeric mice, MCP-1 production in kidneys of gld into WT group was lower than that in the other two groups (WT into WT versus WT into gld versus gld into WT: 4927.2 ± 2917.9 versus 3554.4 ± 1578.2 versus 736.2 ± 171.7 pg/mg; P < 0.05; Figure 9).
Figure 9.
Decreased IFN-γ expression in post-ischemic kidneys of FasL deficient (gld) mice compared to wild type. Level of IFN-γ in postischemic kidneys was lower in gld mice compared with that in WT at day 3 after IRI. In partial chimeric mice, level of MCP-1 in postischemic kidneys of gld into WT was significantly lower compared with those in other groups. *P < 0.05 compared with WT, †P < 0.05 compared with WT into WT, and ‡P < 0.05 compared with WT into gld.
DISCUSSION
In this study, we demonstrated that FasL deficiency conferred protection against AKI after IRI, and reduced production of inflammatory cytokines in T cells. Furthermore, our results implicated FasL on leukocytes rather than on nonhemopoietic tissues in kidney IRI.
Fas/FasL interaction is known to induce cell death through activation of the caspase signaling cascade, leading to cleavage of chromatin and loss of structural integrity.26 FasL, whose expression is normally highly restricted, is frequently expressed on different cell types in the kidney including mesangial cells, tubular epithelial cells, fibroblasts, endothelial cells, and leukocytes.18 Increased expression of Fas and FasL has been documented in various renal diseases and this pathway is believed to have a role in promoting renal injury through induction of apoptosis in glomerular and tubular cells.27 However, manipulation of FasL expression has showed inconsistent results in renal injury,18 suggesting diverse roles for this molecule. FasL is a major regulator of clonal expansion and peripheral homeostasis of T cells.19,26 Thus, it was expected that FasL deficiency would lead to T cell–mediated autoimmune diseases and increase of activated T cells. However, despite persistence of autoreactive T cells, FasL deficiency prevents autoimmune diabetes.21 Moreover, FasL cross-linking leads to recruitment of neutrophils and increased inflammation,12 and FasL deficiency attenuates inflammatory cell infiltration and cytokine secretion.11,22 However, overexpression of FasL in dendritic cells decreased allergen-specific T cells and airway inflammation.15,25 Studies on the effects of FasL in IRI have also produced conflicting results.22–25 FasL expression was increased in infiltrating neutrophils and T lymphocytes after renal IRI;28 however, little is known about their role in AKI after IRI.
We hypothesized that FasL would exert an important role in renal IRI through mediating inflammation. In our current study, FasL deficiency attenuated renal injury after IRI and improved survival. Renal protective effect from IRI in FasL-deficient mice (gld mice) was accompanied by reduced production of inflammatory cytokines by T cells isolated at different time points from kidneys of gld mice after IRI, which likely contributed to the attenuation of IRI. Previous studies have shown that TNF-α production synergistically enhance Fas-FasL expression and cellular apoptosis.29 FasL is also important in mediating the cytotoxic function of T cells,30,31 and FasL deficiency confers beneficial effects on T cell–mediated corneal rejection.32 We found that level of IFN-γ in postischemic kidneys of gld mice was lower than that in WT mice, which could also help explain attenuation of tissue injury in gld mice. Decreased production of the TNF-α and IFN-γ inflammatory cytokines by T lymphocytes isolated from postischemic kidneys was also seen after treatment of WT mice with FasL-blocking Ab. Thus, both genetically and pharmacologically, loss of FasL function is associated with reduced production of inflammatory cytokines by the kidney-infiltrating T cells.
FasL deficiency could also lead to reduced neutrophil infiltration. This possibility was assessed indirectly by measuring MPO activity in postischemic kidneys. FasL deficiency resulted in a trend toward lower MPO level after IRI as compared with WT. In addition, the MPO level was significantly decreased by FasL blockade. Trafficking of NK cells into the kidney after IRI was different between WT and gld, but similar in mice treated with FasL-blocking Ab compared with mice treated with control Ab (data were not shown). The possible role for NK cells in mediating FasL effect on renal IRI is yet to be fully determined.
Recent studies have demonstrated Fas and FasL expression after IRI,33 and the effect of this interaction was dependent on the cell types.17,34 To determine the pathogenic source of FasL, we generated three different types of partial chimeric mice. Chimerism was verified by studying splenic T cells. The proportion of DN T cells in blood and spleens of adult gld mice is usually significantly higher than that in WT mice. The frequency of DN T cell population in splenocytes of recipients was dependent on the genotype of donors. WT mice reconstituted with gld splenocytes showed more DN T cells in spleens than gld mice reconstituted with WT splenocytes. Importantly, renal protection was observed in WT mice that received gld splenocytes (gld into WT), as indicated by lower serum creatinine at day 2 and day 3 after IRI, and less tubular damage both in the cortex and in the medulla than WT mice reconstituted with WT splenocytes (WT into WT). In contrast, serum creatinine after IRI in gld mice reconstituted with WT splenocytes (WT into gld) was not different from that of WT into WT, although tubular damage in medulla was milder in WT into gld. This implies that FasL on leukocytes rather than on nonhemopoietic cells is the major mediator of the pathogenic effect of FasL seen during kidney IRI.
The increased proportion of CD3+CD4− CD8− DN T lymphocytes in blood and spleens of gld mice has been previously described.21 Consistent with our previous study,35 our current data demonstrated that normal kidney contained a large number of DN T cells. The proportion of DN T cells in kidneys, however, was not different between WT and gld mice. Similarly, the gld mutation does not change the frequency of intraepithelial DN T cell population.36 Although an increasing body of evidence revealed the effect of DN T cells in immune response,37,38 their role in kidney diseases is not well known and a fertile topic for future studies.
In summary, FasL deficiency conferred renal protection after IRI, likely through reduced production of inflammatory cytokines by T lymphocytes. The importance of FasL in ischemic AKI was confirmed by complementary genetic and pharmacologic approaches using gld mutant mice and FasL-blocking mAb, respectively. Blockade of FasL on leukocytes may represent a novel therapeutic strategy for preventing ischemic AKI.
CONCISE METHODS
Animal and Experimental Protocols
Wild-type (C57BL/6J) and FasL-deficient (gld) mice on the same background (B6Smn.C3-Faslgld/J) were purchased from the Jackson Laboratory (n = 11 for each group). All mice were 8-week-old males and were housed in a specific pathogen-free barrier facility. The Johns Hopkins University Animal Care and Use Committee approved all studies. Mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (75 mg/kg). After abdominal midline incision, both renal pedicles were bluntly dissected and clamped with a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) for 30 minutes. During the procedure, mice were kept well hydrated with warm sterile saline at a constant temperature (37°C). After the clamps were removed, the wounds were sutured and the mice allowed to recover with free access to chow and water. Cohorts of mice were sacrificed on day 1 and day 3 after surgery.
Assessment of Renal Function
Blood samples were obtained from the tail vein before and at 24, 48, and 72 hours after renal IRI. Serum creatinine (mg/dl) was measured with a Cobas Mira plus autoanalyzer (Roche Diagnostics Corp., Indianapolis, IN).
Tissue Histologic Analysis
Mice were sacrificed at 72 hours after renal IRI. All kidneys were harvested after full exsanguination. Tissue samples were fixed with 10% buffered formalin followed by paraffin embedding, and then renal sections were stained with hematoxylin and eosin (H&E). Renal tubular damage was scored in a blinded fashion by a renal pathologist.
Caspase 3 Immunohistochemistry
Cleaved p17 caspase-3 is the active fragment of pro-caspase-3, which targets the key modulator of the apoptotic pathway and therefore represents a good marker for apoptosis. The staining was performed on formalin-fixed kidney sections (4 μm), after deparaffinization and rehydration. Cleaved caspase-3 (17/19 kDa) was stained using rabbit anti-mouse polyclonal Ab (Cell Signaling Technology, Inc., Danvers, MA), following the manufacturer's protocol, with biotinylated secondary Ab to rabbit IgG (Jackson ImmunoResearch, West Grove, PA). Ten randomly chosen, nonoverlapping fields from the same section of renal cortex and outer medulla were captured (×200, Olympus BX51, Japan). The area of positive staining (dark brown color) was calculated with a computer program, Image-Pro Plus (Media Cybernetics, Silver Spring, MD), and it was expressed as the proportion of stained area over the entire field.
Flow Cytometric Analysis of Kidney-Infiltrating Mononuclear Cells (KMNCs)
KMNCs were isolated according to a method previously described.39 Briefly, decapsulated kidneys were immersed in RPMI buffer (Mediatech, Manassas, VA) containing 5% fetal bovine serum (FBS) and disrupted mechanically using Stomacher 80 Biomaster (Seward, UK). Samples were strained, washed, and resuspended in 36% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) followed by gentle overlaying onto 72% Percoll. After centrifugation at 1000g for 30 minutes at room temperature, KMNCs were collected from the Percoll interface, washed twice, and counted on a hemocytometer using trypan blue exclusion. Staining for surface markers of KMNCs was then performed as described previously.39 Isolated KMNCs were preincubated with anti-CD16/CD32 Fc receptor–blocking antibody for 10 minutes to minimize nonspecific antibody binding. Cells were then incubated with anti-mouse anti-CD1d, CD3, CD4, CD5, CD8, CD19, CD69, IgM, TCR-β, TCR-γδ, and NK1.1 (all from BD Biosciences) for 25 minutes at 4°C, washed with FACS buffer, and fixed 1% paraformaldehyde solution. Four-color immunofluorescence staining was analyzed using a FACSCalibur instrument (BD Biosciences) and FCS Express V3 (De Novo Software, Los Angeles). Each assay included at least 10,000 gated events.
Intracellular Cytokine Staining
One million KMNCs were suspended in RPMI 1640 medium with 10% FBS, l-glutamine, and penicillin/streptomycin and incubated at 37°C in the presence of phorbol myristate acetate (5 ng/ml), ionomycin (500 ng/ml; both Sigma-Aldrich), and monensin (BD Biosciences). After a 5-hour culture, cells were washed and stained for surface markers using anti–CD4-PerCP and anti–TCR-β-allophycocyanin Abs. These cells were then permeabilized in Cytofix/Cytoperm solution (BD Biosciences) for 20 minutes and washed twice with perm/wash buffer. Cells were then incubated with anti–TNF-α-FITC and anti–IFN-γ-PE Abs for 20 minutes, washed with perm/wash buffer, and analyzed with a FACSCalibur instrument (BD Biosciences).
In Vivo FasL Blockade
Neutralizing anti-FasL monoclonal IgG (MFL4) is previously described.40 Anti-FasL MFL4 antibody (Ab) or control hamster IgG (n = 7 for each group) were injected intraperitoneally (500 μg per mouse). Bilateral renal pedicle clamping was performed 24 hours after Ab administration, and booster injection at half dose was administered into the peritoneal cavity immediately after reperfusion during the procedure. Functional and histologic parameters were assessed.
Comparison of Renal Function after IRI in Mice Receiving Sublethal Irradiation and Adoptive Transfer of Splenocytes
To determine the relative importance of leukocytes or organ resident cells on renal protection of FasL, we prepared partial bone marrow chimeric mice by adoptive transfer of splenocytes after sublethal irradiation. Single-cell suspension prepared from spleens of donors was injected intravenously (2.5 × 107 cells) into sublethally irradiated recipients (800 cGy). Three types of partial chimera were developed. The first group was WT recipients receiving WT splenocytes and the second was WT recipients receiving splenocytes from gld mice, whereas the third group was gld mice receiving WT splenocytes (n = 8 for each group). Bilateral renal pedicle clamping was performed in recipient mice 2 days after adoptive transfer. Functional and histologic parameters were assessed.
Measurement of Myeloperoxidase Activity by ELISA
Myeloperoxidase level in postischemic kidneys was measured in whole-kidney protein extracts in each experimental group at day 3 after IRI by ELISA (Hycult biotech, The Netherlands) according to the manufacturer's recommended protocol.
Bioplex Protein Array
A panel of cytokines was measured in whole kidney protein extracts using the Bioplex Protein Array system (Bio-rad, Hercules, CA), which is a multiplexed, particle-based, flow cytometric assay that utilizes anticytokine monoclonal antibodies linked to microspheres incorporating distinct properties of two fluorescence dyes. Our assay was designed to detect and quantify IL-4, IL-6, IL-10, IFN-γ, and monocyte chemotactic protein-1 (MCP-1). Each cytokine value was normalized by dividing the raw cytokine concentration (pg/ml) by the kidney protein concentration (mg/ml) measured by the Bradford assay.
Statistical Analysis
All data were expressed as mean ± SEM). Group means were compared using Mann-Whitney analysis and ANOVA followed by Newman-Keuls post hoc analysis in GraphPad Prism version 4 (GraphPad Software, LA Jolla, CA). Survival rate was compared using Kaplan-Meier method in GraphPad Prism version 4. Statistical significance was determined as a P value <0.05.
DISCLOSURES
None.
Acknowledgments
G. J. Ko was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (E00029). H.R. was supported by the US National Institutes of Health, National Kidney Foundation and by Mr. Rogelio Miro of Panama.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Perico N, Cattaneo D, Sayegh MH, Remuzzi G: Delayed graft function in kidney transplantation. Lancet 364: 1814–1827, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M: Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 115: 3451–3459, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. : Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373: 441–444, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J: Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci U S A 101: 14883–14888, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erkan E, Garcia CD, Patterson LT, Mishra J, Mitsnefes MM, Kaskel FJ, Devarajan P: Induction of renal tubular cell apoptosis in focal segmental glomerulosclerosis: Roles of proteinuria and Fas-dependent pathways. J Am Soc Nephrol 16: 398–407, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Del Rio M, Imam A, DeLeon M, Gomez G, Mishra J, Ma Q, Parikh S, Devarajan P: The death domain of kidney ankyrin interacts with Fas and promotes Fas-mediated cell death in renal epithelia. J Am Soc Nephrol 15: 41–51, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Dupont PJ, Warrens AN: Fas ligand exerts its pro-inflammatory effects via neutrophil recruitment but not activation. Immunology 120: 133–139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cruise MW, Melief HM, Lukens J, Soguero C, Hahn YS: Increased Fas ligand expression of CD4+ T cells by HCV core induces T cell-dependent hepatic inflammation. J Leukoc Biol 78: 412–425, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, Liles WC: Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 170: 6209–6216, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Gregory MS, Koh S, Huang E, Saff RR, Marshak-Rothstein A, Mukai S, Ksander BR: A novel treatment for ocular tumors using membrane FasL vesicles to activate innate immunity and terminate immune privilege. Invest Ophthalmol Vis Sci 46: 2495–2502, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Dekel B, Bocher WO, Marcus H, Yussim A, Reisner Y: Acute cellular rejection of human renal tissue by adoptive transfer of allogeneic human peripheral blood mononuclear cells into chimeric rats: Sequential gene expression of cytokines, chemokines and cytolytic effector molecules, and their regulation by CTLA-4-Ig. Int Immunol 11: 1673–1683, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Modiano JF, Sun J, Lang J, Vacano G, Patterson D, Chan D, Franzusoff A, Gianani R, Meech SJ, Duke R, Bellgrau D: Fas ligand-dependent suppression of autoimmunity via recruitment and subsequent termination of activated T cells. Clin Immunol 112: 54–65, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Chuang YH, Suen JL, Chiang BL: Fas-ligand-expressing adenovirus-transfected dendritic cells decrease allergen-specific T cells and airway inflammation in a murine model of asthma. J Mol Med 84: 595–603, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Boonstra JG, van der Woude FJ, Wever PC, Laterveer JC, Daha MR, van Kooten C: Expression and function of Fas (CD95) on human renal tubular epithelial cells. J Am Soc Nephrol 8: 1517–1524, 1997 [DOI] [PubMed] [Google Scholar]
- 17. An S, Hishikawa Y, Koji T: Induction of cell death in rat small intestine by ischemia reperfusion: Differential roles of Fas/Fas ligand and Bcl-2/Bax systems depending upon cell types. Histochem Cell Biol 123: 249–261, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Ortiz A, Lorz C, Egido J: The Fas ligand/Fas system in renal injury. Nephrol Dial Transplant 14: 1831–1834, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Hamad AR, Schneck JP: Antigen-induced T cell death is regulated by CD4 expression. Int Rev Immunol 20: 535–546, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Miyagawa J, Namba M, Makino S, Nagata S, Kono N, Matsuzawa Y: Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med 186: 613–618, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohamood AS, Guler ML, Xiao Z, Zheng D, Hess A, Wang Y, Yagita H, Schneck JP, Hamad AR: Protection from autoimmune diabetes and T-cell lymphoproliferation induced by FasL mutation are differentially regulated and can be uncoupled pharmacologically. Am J Pathol 171: 97–106, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiraishi H, Toyozaki T, Tsukamoto Y, Saito T, Masuda Y, Hiroshima K, Ohwada H, Kobayashi N, Hiroe M: Antibody binding to fas ligand attenuates inflammatory cell infiltration and cytokine secretion, leading to reduction of myocardial infarct areas and reperfusion injury. Lab Invest 82: 1121–1129, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Nogae S, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, Nakane PK, Nakanishi Y, Koji T: Induction of apoptosis in ischemia-reperfusion model of mouse kidney: Possible involvement of Fas. J Am Soc Nephrol 9: 620–631, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Tekin D, Xi L, Kukreja RC: Genetic deletion of fas receptors or Fas ligands does not reduce infarct size after acute global ischemia-reperfusion in isolated mouse heart. Cell Biochem Biophys 44: 111–117, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Yang J, Jones SP, Suhara T, Greer JJ, Ware PD, Nguyen NP, Perlman H, Nelson DP, Lefer DJ, Walsh K: Endothelial cell overexpression of fas ligand attenuates ischemia-reperfusion injury in the heart. J Biol Chem 278: 15185–15191, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Krueger A, Fas SC, Baumann S, Krammer PH: The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev 193: 58–69, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez-Cuadrado S, Lorz C, Garcia del Moral R, O'Valle F, Alonso C, Ramiro F, Ortiz-Gonzalez A, Egido J, Ortiz A: Agonistic anti-Fas antibodies induce glomerular cell apoptosis in mice in vivo. Kidney Int 51: 1739–1746, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Miyazawa S, Watanabe H, Miyaji C, Hotta O, Abo T: Leukocyte accumulation and changes in extra-renal organs during renal ischemia reperfusion in mice. J Lab Clin Med 139: 269–278, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Sayers TJ, Brooks AD, Lee JK, Fenton RG, Komschlies KL, Wigginton JM, Winkler-Pickett R, Wiltrout RH: Molecular mechanisms of immune-mediated lysis of murine renal cancer: Differential contributions of perforin-dependent versus Fas-mediated pathways in lysis by NK and T cells. J Immunol 161: 3957–3965, 1998 [PubMed] [Google Scholar]
- 30. Nagata S, Golstein P: The Fas death factor. Science 267: 1449–1456, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Legge KL, Braciale TJ: Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity 23: 649–659, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Hegde S, Beauregard C, Mayhew E, Niederkorn JY: CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: Role of Fas-induced apoptosis. Transplantation 79: 23–31, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nakajima H, Mizuta N, Fujiwara I, Sakaguchi K, Ogata H, Magae J, Yagita H, Koji T: Blockade of the Fas/Fas ligand interaction suppresses hepatocyte apoptosis in ischemia-reperfusion rat liver. Apoptosis 13: 1013–1021, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Pinkoski MJ, Brunner T, Green DR, Lin T: Fas and Fas ligand in gut and liver. Am J Physiol Gastrointest Liver Physiol 278: G354–G366, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, Soloski MJ, Rabb H: Normal mouse kidneys contain activated and CD3+CD4- CD8- double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohamood AS, Bargatze D, Xiao Z, Jie C, Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP, Hamad AR: Fas-mediated apoptosis regulates the composition of peripheral alphabeta T cell repertoire by constitutively purging out double negative T cells. PLoS One 3: e3465, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, Schneck JP: B220+ double-negative T cells suppress polyclonal T cell activation by a Fas-independent mechanism that involves inhibition of IL-2 production. J Immunol 171: 2421–2426, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L: Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med 6: 782–789, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H: Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Kayagaki N, Yamaguchi N, Nagao F, Matsuo S, Maeda H, Okumura K, Yagita H: Polymorphism of murine Fas ligand that affects the biological activity. Proc Natl Acad Sci U S A 94: 3914–3919, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]