Abstract
For the individual patient with primary IgA nephropathy (IgAN), it remains a challenge to predict long-term outcomes for patients receiving standard treatment. We studied a prospective cohort of 332 patients with biopsy-proven IgAN patients followed over an average of 13 years. We calculated an absolute renal risk (ARR) of dialysis or death by counting the number of risk factors present at diagnosis: hypertension, proteinuria ≥1 g/d, and severe pathologic lesions (global optical score, ≥8). Overall, the ARR score allowed significant risk stratification (P < 0.0001). The cumulative incidence of death or dialysis at 10 and 20 years was 2 and 4%, respectively, for ARR = 0; 2 and 9% for ARR = 1; 7 and 18% for ARR = 2; and 29 and 64% for ARR = 3, in adequately treated patients. When achieved, control of hypertension and reduction of proteinuria reduced the risk for death or dialysis. In conclusion, the absolute renal risk score, determined at diagnosis, associates with risk for dialysis or death.
Primary IgA nephropathy (IgAN) was first described by Jean Berger.1,2 One of the difficulties in this disease3–6 is to predict at the time of the initial diagnosis the very long-term (decade) prognosis in the individual patient. This has been approached since the 1990s by the multivariate Cox regression model, taking into account the time duration of follow-up (FU) or the time elapsed since disease onset to occurrence of the events chosen as secondary or primary end points, usually chronic kidney disease (CKD, stage 3+) and end-stage renal failure (ESRF) as strong markers of progression. The predictive risk factors (RF) identified can be classified in two groups: major and the others. The major independent consensual factors7–12 are: (1) the occurrence/presence of arterial hypertension (HT); (2) the amount of daily proteinuria with a usual cut-off over 1 g/d; and (3) the presence of severe lesions on initial renal biopsy (RB) such as crescents, abundant obsolescent glomeruli, focal and segmental hyalinosis, and also tubulointerstitial fibrosis. However, at that time, there was no international classification for renal pathology in IgAN, and different groups like ours7,13 have defined their own scoring systems.14–16 The other factors are numerous and controversial or not widely confirmed: age at disease onset,9,17 gender, overweight/obesity,18 hypertriglyceridemia/hyperuricemia,19 and different immunogenetic markers (HLA antigens, cytokines polymorphisms,20,21 candidate genes for hypertension, etc.).
In this paper, our goal was to use these three major risk factors to calculate a simple absolute renal risk (ARR) score allowing the accurate prediction of dialysis/death event at 10 and 20 years after disease onset, in adequately treated IgAN patients and in analogy to the well-known absolute cardiovascular (CV) risk of death/CV events at 10 years.22,23
RESULTS
Characteristics of the IgAN-STET-CO
The characteristics of the patients over the disease course are given in Table 1. The distribution of the primary and secondary end points over the disease course is given in Table 2; at the last FU the final number of events was 32 for dialysis plus 13 deaths before dialysis (causes were cardiovascular in six patients, cancer in three patients, infection in two patients, and other in two patients), i.e. 45 primary dialysis/death (D/D) events, and 99 for CKD3+.
Table 1.
Patient characteristics over the disease course
| Onset | Diagnosis | Last FU | |
|---|---|---|---|
| Age, years (mean [SD]) | 35.9 (15.4) | 41.4 (15.1) | 48.8 (15.5) |
| Gross hematuria, yes: (N [%]) | 64 (19.3) | 68 (20.5) | 94 (28.3) |
| Microhematuria, yes: (N [%]) | 97 (29.2) | 259 (78.0) | 261 (78.6) |
| Proteinuria, yes: (N [%]) | 187 (56.3) | 191 (57.5) | 124 (37.3) |
| Hypertension, yes: (N [%]) | 74 (22.3) | 120 (36.1) | 164 (49.4) |
| Renal failure, yes: (N [%]) | 39 (11.7) | 85 (25.6) | 99 (29.8) |
| ESRF/dialysis, yes: (N [%]) | 0 (0.0) | 4 (1.2) | 32 (9.6) |
| Death (before), yes: (N [%]) | 13 (3.9) |
Renal failure/insufficiency was defined as eGFR of <60 ml/mn per 1.73m2 S; acute or chronic at onset but chronic after (CKD-3+); n = 332; men = 237 patients (71.4%).
Table 2.
End points over the disease course
| End points | Diagnosis | Last FU | P |
|---|---|---|---|
| eGFR (ml/mn per 1.73 m2 S) | |||
| mean (SD) | 74.7 (28.3) | 68.0 (31.3) | P < 0.0001 |
| median (range) | 80 (5.6 to 157.1) | 72.5 (4.6 to 184.7) | |
| Stage N (%) | |||
| 1 (≥90) | 102 (30.7) | 80 (24.1) | |
| 2 (60 to 89) | 145 (43.7) | 153 (46.1) | |
| 3 (30 to 59) | 55 (16.6) | 50 (15.1) | P < 0.0001 |
| 4 (15 to 29) | 18 (5.4) | 15 (4.5) | χ2 = 344.2 |
| 5 (<15) | 12 (3.6) | 34 (10.2) | |
| CKD-3+ (eGFR < 60) | P < 0.0001 | ||
| yes (N [%]) | 85 (25.6) | 99 (29.8) | χ2 = 178.9 |
| Dialysis/death | 4 dialysis | 32 dialysis, 13 dead | P = 0.0003 |
| yes (N [%]) | 4 (1.2) | 45 (13.6) | χ2 = 18.9 |
CKD-3+ was defined as eGFR of <60 ml/mn per 1.73m2 S.
The Major Risk Factors
The details of the major risk factors are presented in Table 3.
Table 3.
Major risk factors evolution
| Risk Factors | Diagnosis | Last FU | P |
|---|---|---|---|
| Proteinuria | |||
| mean (SD) (g/d) | 0.97 (1.44) | 0.51 (1.15) | P < 0.0001 |
| median (g/d) | 0.41 | 0.15 | Paired t test |
| class: N [%] | |||
| 0 (<0.30) | 141 (42.5) | 208 (62.7) | P < 0.0001 |
| 1 (0.30 to 0.99) | 91 (27.4) | 63 (19.0) | χ2 = 147.0 |
| 2 (1.00 to 2.99) | 68 (20.5) | 39 (11.7) | |
| 3 (≥3.00) | 32 (9.6) | 22 (6.6) | |
| proteinuria ≥1 g/d, yes: N [%] | 100 (30.1) | 61 (18.4) | |
| Hypertension, yes: N [%] | 120 (36.1) | 164 (49.4) | |
| SBP in HT + ve (mean [SD]) | 150.9 (24.3) | 138.0 (16.5) | P < 0.0001 |
| DBP in HT + ve (mean [SD]) | 86.4 (14.2) | 81.9 (11.7) | χ2 = 192.5 |
| controlled HT, yes: (N [%]) | 77 (47.0) | ||
| Pathology | |||
| GOS (0 to 20) | |||
| mean (SD) | 7.00 (3.17) | ||
| med (range) | 6.50 (2 to 19) | ||
| GOS ≥8, yes: (N [%]) | 120 (36.1) | ||
| obsolescent, yes: N [%] | 30 (9.0) | ||
| FSGS, yes: N [%] | 60 (18.1) | ||
| crescents, yes: N [%] | 22 (6.6) | ||
| indices: m [SD], G = 3.25 (1.48) | V = 2.04 (0.93) | T = 0.58 (0.68) | I = 1.05(0.84) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; controlled HT, blood pressure ≤130/80; FSGS, focal segmental glomerular sclerosis.
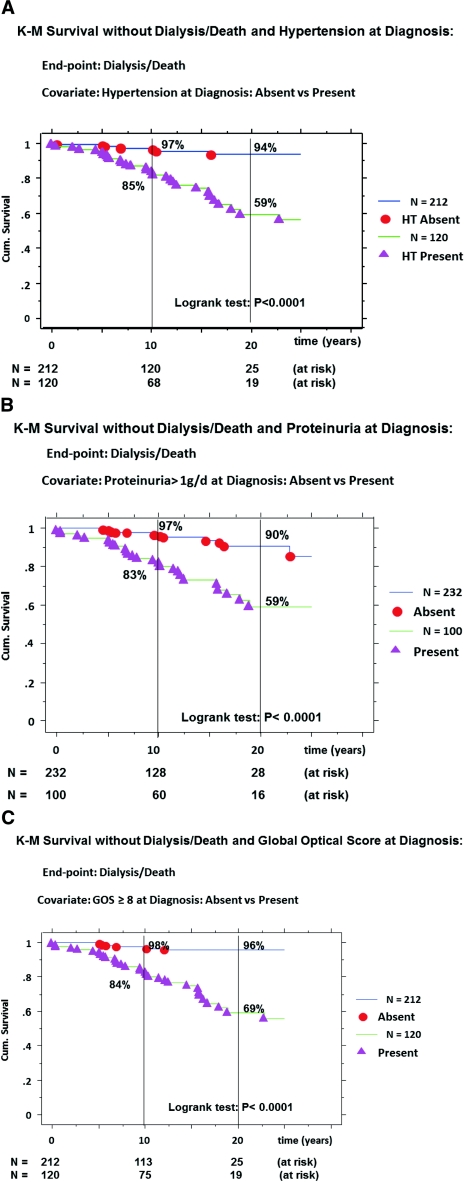
Hypertension was already present at the time of diagnosis in 120 (36.1%) patients. By univariate Cox regression analysis, the absence of HT at the time of diagnosis was significantly protective for occurrence of dialysis/death (Table 4A). By the Kaplan-Meier (K-M) method, the survival curve without D/D (Figure 1A) was significantly better in the absence of HT at diagnosis; the cumulative incidence rate of D/D at 10 and 20 years being, respectively, 3% (120 at risk) and 6% (25 at risk) in the absence of HT versus 15% (68 at risk) and 41% (19 at risk) if present (P < 0.0001).
Table 4.
Mono and multivariate Cox regression analyses with D/D (A) or with CKD-stage 3+ (B) as end points and using the risk factors (hypertension, proteinuria ≥1 g/d, and global optical score of ≥8) or the ARR scoring as covariates
| A. Cox regression analyses for D/D (n = 45) and risk factors | |||||||
|---|---|---|---|---|---|---|---|
| Items status | β | β/SE | χ2 | P | RR | 95% CI | RRR (%) |
| Univariate | |||||||
| HT: absence | −1.89 | −4.85 | 23.5 | <0.0001 | 0.15 | 0.07 to 0.32 | 85 |
| proteinuria ≥1 g/d: absence | −1.53 | −4.60 | 21.2 | <0.0001 | 0.22 | 0.11 to 0.42 | 78 |
| GOS ≥8: absence | −2.04 | −4.94 | 24.5 | <0.0001 | 0.13 | 0.06 to 0.29 | 87 |
| Multivariate | |||||||
| (1) GOS ≥8: absence | −1.21 | −2.65 | 7.02 | 0.008 | 0.30 | 0.12 to 0.73 | 70 |
| (2) HT: absence | −1.13 | −2.63 | 6.91 | 0.009 | 0.33 | 0.14 to 0.75 | 67 |
| (3) proteinuria ≥1 g/d: absence | −0.81 | −2.32 | 5.37 | 0.02 | 0.44 | 0.22 to 0.88 | 56 |
| Univariate | |||||||
| ARR = 0 | −2.81 | −5.21 | 27.2 | <0.0001 | 0.06 | 0.02 to 0.17 | 94 |
| ARR = 1 | −2.42 | −3.95 | 15.2 | <0.0001 | 0.09 | 0.03 to 0.30 | 91 |
| ARR = 2 | −1.24 | −3.32 | 11.0 | <0.0009 | 0.28 | 0.14 to 0.60 | 72 |
| ARR = 3 | 1.00 | ||||||
| ARR (category): overall | 41.8 | <0.001 | |||||
| B. Cox regression analyses for CKD-3+ (n = 99) and risk factors | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | |||||||
| HT: absence | −1.65 | −7.17 | 51.4 | <0.0001 | 0.19 | 0.12 to 0.30 | 81 |
| proteinuria ≥1 g/d: absence | −0.87 | −4.05 | 16.4 | <0.0001 | 0.42 | 0.28 to 0.64 | 58 |
| GOS ≥: absence | −1.61 | −7.06 | 49.9 | <0.0001 | 0.20 | 0.14 to 0.31 | 80 |
| Multivariate | |||||||
| (1) HT: absence | −1.14 | −4.46 | 19.9 | <0.0001 | 0.32 | 0.19 to 0.53 | 68 |
| (2) GOS ≥8: absence | −1.03 | −3.96 | 15.7 | <0.0001 | 0.36 | 0.22 to 0.60 | 64 |
| (3) proteinuria ≥1 g/d: absence | −0.21 | −0.94 | 0.88 | NS | |||
| Univariate | |||||||
| ARR = 0 | −2.35 | −7.25 | 52.6 | <0.0001 | 0.10 | 0.05 to 0.18 | 90 |
| ARR = 1 | −1.55 | −4.97 | 24.7 | <0.0001 | 0.21 | 0.11 to 0.39 | 79 |
| ARR = 2 | −0.61 | −2.51 | 6.30 | <0.0001 | 0.54 | 0.34 to 0.88 | 46 |
| ARR = 3 | 1.00 | ||||||
| ARR (category): overall | 63.4 | <0.01 | |||||
β/SE, weight and influence (negative, protective; positive, deleterious) of the covariate; RRR, relative risk reduction if protective.
Figure 1.
Kaplan-Meier survival curves without D/D according to the presence/absence at diagnosis of: HT (A), proteinuria ≥1 g/d (B), and GOS of ≥8 (C); D/D (primary event); comparison of curves by the log-rank test; cumulative incidence of event = (1 − survival).
Proteinuria was measured as g/d. Overall, 100 (30.1%) patients presented with severe proteinuria (≥1 g/d) at diagnosis. By Cox univariate analysis, the amount of 24-hour proteinuria was a significant predictive factor for ultimate D/D events: n = 45; β = 0.48; β/SE = 7.31; χ2 = 53.5; P < 0.0001; relative risk (RR) = 1.62 (confidence interval [CI], 1.42 −1.84) per g of proteinuria. Similar data were obtained with the four classes of proteinuria, the absence of proteinuria being the most protective for D/D: events = 45; β = −0.63; β/SE = −4.97; χ2 = 24.7; P < 0.0001; RR = 0.04 (CI, 0.01 to 0.15); RR reduction rate = 96%. With the use of proteinuria as a dichotomous variable (<1 versus ≥1 g/d), the absence of proteinuria ≥1 g/d was also protective for D/D (Table 4A). The survival curve without D/D (Figure 1B) was significantly better for the subgroup without proteinuria ≥1 g/d; the cumulative incidence rates for D/D being at 10 and 20 years, 3% (128 at risk) and 10% (28 at risk), respectively, in patients with low/absent proteinuria versus 17% (60 at risk) and 41% (16 at risk), respectively, in patients with proteinuria ≥1 g/d (P < 0.0001).
The severity of the renal lesions was appreciated by the global optical score (GOS) on initial RB (Table 3). One hundred twenty patients (36.1%) presented with a severe score (≥8 units) at diagnosis. By Cox univariate analysis, GOS as continuous variable was a strong predictor for D/D events: n = 45; β = 0.33; β/SE = 8.36; χ2 = 70.0; P < 0.0001; RR = 1.39 (CI, 1.29 −1.50) per unit of scoring. When GOS was used as dichotomous, the low score (<8 units) was also significantly protective for D/D (Table 4A). The survival curve without D/D (Figure 1C) was better for the low score subgroup; the cumulative incidence rates for dialysis/death, at 10 and 20 years, respectively, being 2% (113 at risk) and 4% (25 at risk) in patients with GOS <8 as compared with 16% (75 at risk) and 31% (19 at risk) in the others (P < 0.0001). Similar results were obtained for each of these three risk factors in the prediction of the secondary end point, CKD3+, presented in Table 4B, but the curves are not shown.
Independence and Weight of These Three Factors in the Prediction of the Primary Event
These simplified dichotomous RF were found independent predictors for ultimate dialysis/death in the multivariate Cox regression analysis (Table 4A). The respective weights of the RFs, appreciated by both the β/SE value in the different Cox regression analyses (Table 4A) and the classical measurements of accuracy and predictability (Table 5), were in fact quite similar.
Table 5.
Respective value of the risk factors at diagnosis (absent or present) to predict on long term the primary event (D/D)
| Items at Diagnosis (n = 332) | GOS ≥8 Present in 120 Patients | HT Present in 120 Patients | Proteinuria ≥1 g/d Present in 100 Patients | All Three Together Present in 47 Patients |
|---|---|---|---|---|
| Se | 0.844 | 0.822 | 0.711 | 0.622 |
| Sp | 0.714 | 0.711 | 0.763 | 0.934 |
| Se + Sp | 1.558 | 1.533 | 1.474 | 1.556 |
| Pos PV | 0.317 | 0.308 | 0.320 | 0.596 |
| Neg PV | 0.967 | 0.962 | 0.944 | 0.940 |
| Pretest probability event | 0.136 | 0.136 | 0.136 | 0.136 |
| Pretest OR Event | 0.157 | 0.157 | 0.157 | 0.157 |
| LLR if present | 2.951 | 2.844 | 3.000 | 9.424 |
| LLR if absent | 0.218 | 0.250 | 0.379 | 0.405 |
| Post-test OR if present | 0.463 | 0.447 | 0.471 | 1.480 |
| Post-test OR if absent | 0.034 | 0.039 | 0.060 | 0.064 |
| Probability event if present | 0.316 | 0.308 | 0.320 | 0.597 |
| Probability event if absent | 0.033 | 0.038 | 0.057 | 0.060 |
Se, sensitivity; Sp, specificity; Pos PV, positive predictive value; Neg PV, negative predictive value; LLR, likelihood ratio. The most remarkable numbers are bolded.
The addition of gender and age (as continuous/category variables) at diagnosis in this model did not have a significant effect, and this was explained by a significantly different distribution of these RF (Table 6). We observed the same effect for overweight/obesity with worse distribution of ARR (χ2 = 24.8, P < 0.0001). We do not have sufficiently precise data on smoking for this cohort.
Table 6.
The ARR score distribution according to gender and age at diagnosis (with three categories: <35 years, 35 to 54.9 years, and ≥55 years)
| ARR | All (332 Patients) | Men (237 Patients) | Women (95 Patients) | Age at Diagnosis |
||
|---|---|---|---|---|---|---|
| <35 Years | 35 to 54.9 Years | ≥55 Years | ||||
| 0 | 151 (45.4) | 96 (40.5) | 55 (57.9) | 75 (62.5) | 64 (44.1) | 12 (17.9) |
| 1 | 69 (20.8) | 52 (21.9) | 17 (17.9) | 19 (15.8) | 41 (28.3) | 9 (13.4) |
| 2 | 65 (19.6) | 49 (20.7) | 16 (16.8) | 13 (10.8) | 25 (17.2) | 27 (40.3) |
| 3 | 47 (14.2) | 40 (16.9) | 7 (7.4) | 13 (10.8) | 15 (10.3) | 19 (28.4) |
| χ2 | 9.88 | 57.7 | ||||
| P | 0.02 | <0.0001 | ||||
The Evaluation of the ARR at Time of Diagnosis
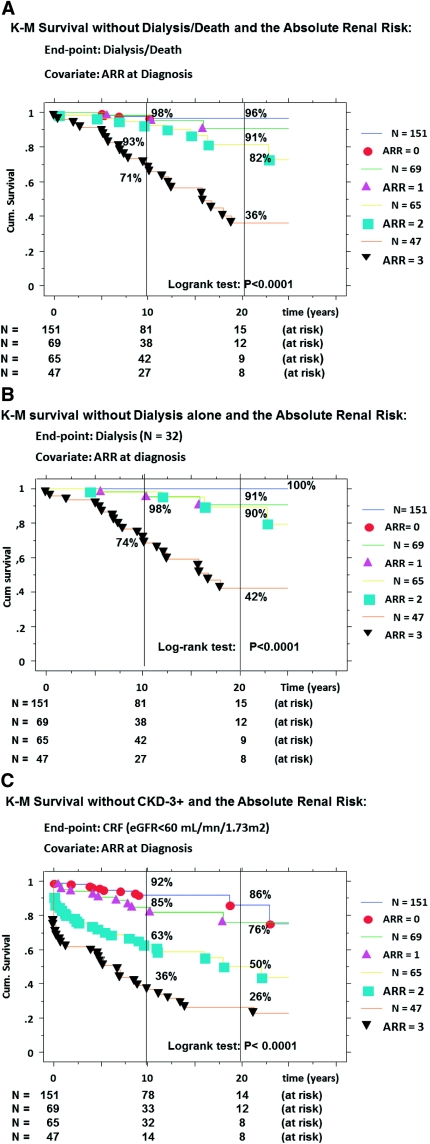
The ARR results are given in Table 7. By Cox regression, the ARR scoring was confirmed as a strong predictor for D/D (Table 4A). The survival curves without D/D (Figure 2A) were nicely stratified with a worsening survival rate from ARR = 0 to ARR = 3. Finally, the cumulative incidence rates of D/D, respectively at 10 and 20 years, were 2% (81 at risk) and 4% (15 at risk) for ARR = 0, 2% (38 at risk) and 9% (12 at risk) for ARR = 1, 7% (42 at risk) and 18% (9 at risk) for ARR = 2, and 29% (27 at risk) and 64% (8 at risk) for ARR = 3 (P < 0.0001). Similar results were obtained when we analyzed progression to dialysis alone (Figure 2B) or to CKD3+ (Figure 2C).
Table 7.
Distribution of the ARR score at diagnosis and cumulative incidence rate of event at 10 and 20 years post-onset
| Number of RF Present | ARR Score (Risk Level) | Number of Patients (%) | Number of D/D Events (%) | CKD-3 + Incidence |
D/D Incidence |
||
|---|---|---|---|---|---|---|---|
| 10 years | 20 years | 10 years | 20 years | ||||
| 0 | 0 (very low) | 151 (45.5) | 4 (2.6) | 8% | 14% | 2% | 4% |
| 1 | 1 (low) | 69 (20.8) | 3 (4.3) | 15% | 24% | 2% | 9% |
| 2 | 2 (high) | 65 (19.6) | 10 (15.4) | 37% | 50% | 7% | 18% |
| 3 | 3 (very high) | 47 (14.1) | 28 (59.6) | 64% | 74% | 29% | 64% |
CKD-3+ was defined as eGFR <60 ml/mn per 1.73 m2 S (secondary event).
Figure 2.
Kaplan-Meier survival curves without: D/D (A), dialysis alone (B), and CKD-stage 3+ (C), according to the ARR scoring at diagnosis; D/D (primary event); CKD-stage 3+ = chronic kidney disease stage 3 and up, defined as eGFR <60 ml/mn per 1.73 m2 S (secondary event); comparison of curves by the log-rank test; cumulative incidence of event = (1 − survival).
The Effect on Primary Outcome of Controlling These RF
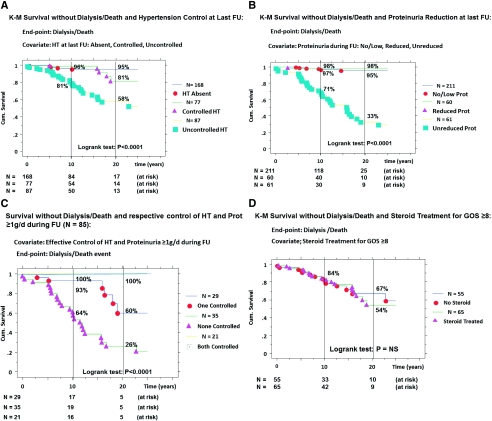
The effective control of BP (Table 3). At the last FU, the number of HT patients had increased to 164, and among them 77 (49%) had reached target BP (≤130/80) for at least 2 years. The respective mean (SD) BP values were 124.4 (10.8) over 76.1 (8.8) in the 168 non-HT versus 124.6 (8.2) over 75.4 (7.6) in the controlled HT subgroup (P = NS). For HT patients at diagnosis, we achieve target BP in the same proportion 57/120 = 47.5%. The K-M survival curves without D/D (Figure 3A) for the three subgroups of patients demonstrated better survival with effective HT control; the cumulative incidence rates for Dialysis/Death at 10 and 20 years being, respectively, 4% (84 at risk) and 5% (17 at risk) for never HT at the last FU, 1% (54 at risk) and 19% (14 at risk) for controlled HT, and 19% (50 at risk) and 42% (13 at risk) for uncontrolled HT during FU (P < 0.0001).
Figure 3.
Kaplan-Meier survival curves without D/D according to: the control of hypertension (absence, controlled, and uncontrolled) (A), the reduction of proteinuria ≥1 g/d (low/absent, reduced, and unreduced) (B), the control of both HT and proteinuria (both, none, and only one controlled) (C), and steroid treatment for severe renal lesions (no or yes) at last follow-up (D); D/D (primary event); comparison of curves by the log-rank test; cumulative incidence of event = (1 − survival).
The effective reduction of proteinuria is shown in Table 3. At diagnosis, 100 patients had proteinuria ≥1 g/d with an additional 21 patients over the disease course. At the last FU, we achieved an effective reduction (<1 g/d) in 60 patients for at least 2 years, labeled “reduced” proteinuria subgroup, whereas 61 had persistent proteinuria ≥1 g/d, labeled “unreduced,” and 211 patients had final low/absent proteinuria. The K-M survival curves without D/D (Figure 3B) for these three subgroups showed improvement with effective reduction of proteinuria; the cumulative incidence rates for dialysis/death at 10 and 20 years being, respectively, 3% (118 at risk) and 5% (25 at risk) for no/low proteinuria, 2% (40 at risk) and 2% (10 at risk) for reduced proteinuria, and 29% (30 at risk) and 67% (9 at risk) for the unreduced/high proteinuria at the last FU (P < 0.0001).
It should be noted that effective control of both HT and proteinuria ≥1 g/d had an additive effect on survival improvement (Figure 3C). For instance in the subgroup of patients with ARR = 3 (n = 47), failure of controlling HT and proteinuria ≥1 g/d in 23 patients led to D/D in 21 (91%) compared with 7 (64%) in 11 patients with one RF controlled and compared with none in 13 (0%) with dual control.
The control of severe pathologic lesions is difficult to estimate from our cohort in the absence of repeated biopsies. Among the 120 patients with a score of ≥8 at diagnosis, 65 (54%) have received at least one steroid treatment (usually high-dose methyl prednisolone every other day followed by oral prednisolone tapered over 6 months; the cumulative dose received by a 70-kg patient was about 6 g). The K-M survival curves without D/D (Figure 3D) for the steroid-treated and not-treated subgroups failed to demonstrate any difference (P = NS). Similar results were obtained in the prediction of secondary outcome (CKD3+) with the control of each of these RF, but the data are not shown.
The Internal Validation of Our Prognostic Model
For our retrospective IgAN cohort (1980 to 1989), we applied exactly the same protocol and confirmed that the ARR score permitted a similar stratification of the D/D events (χ2 = 34.5, P < 0.0001): the cumulative incidence rates of D/D, respectively, at 10 and 20 years were 1% (92 at risk) and 4% (55 at risk) for ARR = 0 (n = 130); 9% (37 at risk) and 22% (17 at risk) for ARR = 1 (n = 58); 15% (26 at risk) and 35% (15 at risk) for ARR = 2 (n = 34); and 18% (23 at risk) and 49% (13 at risk) for ARR = 3 (n = 28).
DISCUSSION
To our knowledge, we are the first to publish about the absolute renal risk of dialysis/death and to propose comprehensive scoring with an important clinical application. Using this ARR score, we could predict early, at diagnosis, the 10- and 20-year incidence cumulative rate of dialysis/death with a nice stratification of the results. We think we are in a situation similar to the well-known absolute cardiovascular risk of death/CV events that emerged from the Framingham study with permanent refinements.22,23
In this study, we selected abbreviated Modification of Diet in Renal Disease formula over adjusted Cockcroft-Gault for estimated GFR (eGFR) calculation24; this did not affect the number of D/D events, despite the fact that the number of patients with CKD3+ was slightly different (99 versus 95, respectively).
We have selected the date of disease onset over the date of renal biopsy as time zero, because this study was a prospective observational study and not a randomized controlled trial. The overall duration of the disease in an individual patient, from onset to D/D, might also reflect the potential of its progression.
In our study, the primary end point was either renal death (dialysis) or patient death before reaching dialysis; the choice of CKD-stage 3+ as a secondary end point corresponded in fact to an intermediate end point between normal GFR and dialysis, and we have considered the reduction in eGFR as a continuum in the disease progression. For this reason, in the prediction model, we did not include a direct or indirect marker of renal function at diagnosis (eGFR value/staging or presence of CKD3+), and we have been cautious not to confuse risk factors and end points. Nevertheless, we have tested this parameter, but the addition of CKD3+ at diagnosis (present or absent) as a fourth covariate in the model sorted out as the only independent risk factor, substituting to the other three risk factors.
In this study, we have used our own pathologic classification7,13 developed in 1990 that integrates all elementary renal lesions present; it already demonstrated its usefulness in a retrospective cohort.7 The Oxford pathologic classification for IgAN is now published25,26 but was built from patients with a proteinuria of at least 1 g/d at diagnosis in adults and excluding the rapidly progressive rare cases; so the two tails of the spectrum of IgAN were excluded. Four elementary lesions were highly predictive of progression (decreased eGFR over 5-year FU): mesangial hypercellularity (M score), endocapillary hypercellularity (E score), segmental glomerulosclerosis (S score), and tubular atrophy/interstitial fibrosis (T score). Overall, the final MEST result is complex: M(0/1) + E(0/1) + S(0/1) + T(0/1/2) and may be not easy to use in a study like ours (MEST, 0 to 5). All of these predictive lesions were integrated in our quantitative GOS.
Our definition of HT was the classical World Health Organization definition; however, our results are in favor of changing this definition for IgAN patients to a more restrictive one such as >130/80 mmHg, which will allow earlier introduction of antihypertensive treatment and hopefully faster and better HT control, keeping the same BP target. This concern is part of the prehypertension problem27 also discussed in the general population.
Many studies7–13,15–17,28–32 have focused on predicting outcomes in IgAN, but direct comparisons are difficult because of differences in end points, risk factors, or methodology. For example, hypertension was not included by Beukhof et al.28
Rauta et al.29 found, like us, that integration of CKD3+ among the risk factors substituted to HT and proteinuria and for this reason proposed a model for patients with normal renal function at diagnosis. The Toronto group (Bartosik et al.11) did not provide an a priori outcome prediction at the time of diagnosis but rather a prediction on the basis of years of FU after diagnosis (5 years seemed optimal, but a prediction could be safely derived after 3 years) with the computation of mean BP and daily proteinuria data over time. In their study, the histopathological grade did not play a significant role, whereas it was predominant for Radford et al.,9 Manno et al.,16 and Walsh et al.31 Our results are close to the Japanese multicenter experience published by Goto et al.30 Our ARR scoring could be refined as soon as new RF arose such as HT or proteinuria ≥1 g/d during FU after diagnosis. In this respect, the quantitation of proteinuria and BP as proposed by the Toronto group might be more precise and eventually more adapted; finally our prediction formulas are not opposite but more complementary. All of the proposed predictive calculations/formulas, including ours, are lacking validation in another external cohort except for the Toronto formula.33 We think our ARR scoring at diagnosis is the simplest, does not require sophisticated computer calculation like in a recent paper,32 and is readily worldwide applicable with some adaptation of the pathology score (for instance substitution of GOS ≥8 by Haas grade III/IV or Oxford-MEST score of 3 to 5).
Our model was validated in our retrospective 1980 to 1989 cohort with also a significant stratification of the risk of dialysis/death: the results obtained in the two cohorts are not exactly similar but are of the same magnitude, especially in the two opposite subgroups: patients with ARR = 0 definitely have a very low risk (4% at 20 years) and patients with ARR = 3 have a very high risk (over 50% at 20 years).
We demonstrated and confirmed in this study that control of HT in this disease was a major step with an important improvement in survival of patients with BP at target.34 However, survival without D/D (Figure 3A) for non-HT and controlled HT subgroups remained identical only for about 10 years but diverged afterwards; this was due in fact to the absence of concomitant reduction of proteinuria (Figure 3C).
Concerning the reduction of proteinuria over time, the problem is more complex. It is already well known that in the presence of HT, there is an increase in proteinuria with subsequent significant reduction after optimal BP control. In our study, we clearly demonstrated and confirmed the major effect on end points of reducing significantly/persistently the amount of proteinuria.10,35 In fact, the survival curves without D/D for no/low proteinuria and reduced proteinuria at the last FU remained similar over 20 years (Figure 3C). With the control of both HT and proteinuria ≥1 g/d, the final survival was similar to patients without these RF. The majority of patients with HT and proteinuria ≥1 g/d were receiving longterm angiotensin I converting enzyme inhibitors and/or angiotensin II receptor blockers (see Concise Methods).
In contrast, we could not demonstrate a direct effect of steroid treatment on progression toward dialysis/death; however, steroid treatment alone or in association36 helped to control severe proteinuria and may have had an indirect positive effect. In our cohort, there was no systematic repeated biopsy, and it was thus not possible to evaluate directly the effect of steroids on the elementary pathologic lesions. This negative finding does not impact the previously published results in a specifically designed randomized controlled trial.37,38
The three risk factors used to establish our ARR turned out to have similar weight in the prediction; their respective absence was similarly protective for the ultimate outcome (Table 4A), and in the accuracy parameters (Table 5), they had an individual negative predictive value around 0.95 but a low positive predictive value around 0.30. We could have used the concordance (c) statistics with area under the receiving operator characteristic curves to evaluate each RF (calibration of our model), but it seems more appropriate for diagnostic than prognostic models39; nevertheless, we have used ROC analysis to validate a posteriori the cut-off of 1 g/d for proteinuria (area under the curve = 0.810).
Overall, the a priori probability of long-term evolution to dialysis/death was 60% in patients with all three RF present (ARR = 3) and by contrast only 6% in their absence (ARR = 0), but a posteriori was heavily dependent on the effective control of both HT and proteinuria; for instance, in patients with ARR = 3 at diagnosis, the final prevalence of D/D culminated to 91% (21 of 23) in those with uncontrolled HT/proteinuria, and this might represent the true worse natural history prognosis.
Finally, our ARR scoring at diagnosis permitted us to establish a priori and early the final risk of dialysis/death in adequately treated IgAN patients. This is important in designing future prospective trials in IgAN with inclusion of patients strictly limited to those with predefined RF and study duration appropriate to the objective. Major randomized controlled trials should be done with inclusion of patients with ARR = 3 and a minimal/optimal study duration of 5 years; unfortunately, this has been rarely done, and we are still lacking a definite demonstration of drug efficiency in this disease.
CONCISE METHODS
The IgAN Patients
The cohort of IgAN patients from the area of Saint-Etienne, France (IgAN-STET-CO) enrolled prospectively all locoregional patients with a renal biopsy performed at our institution, from January 1, 1990 to December 31, 1999, and disclosing the diagnosis of IgAN with subsequent regular clinical/biologic follow-up. IgAN was defined by the presence, on immunofluorescent microscopy, of at least 1+ IgA mesangial deposits as dominant or codominant immunoglobulins. We restricted inclusion to primary forms and excluded 25 patients with different ethnic backgrounds, such as patients from North Africa (Maghreb) who have also lower income and diminished access to regular medical follow-up. Finally, we ended up with a homogenous cohort of 332 patients. Onset of the disease was defined as the first occurrence/discovery of the different renal signs: gross hematuria, and/or microhaematuria, and/or proteinuria, and at a later stage HT and/or CRF. The time interval between disease onset to RB was: mean (SD) = 5.7 (8.5) years and median (extremes) = 2.5 (0.1 to 46.9) years. The total exposure time from onset to either last FU or the primary event was: mean (SD) = 12.9 (9.5) years and median (extremes) = 11.3 (0.01 to 56.0); 44 patients were censored at 20 years plus 38 having reached the primary end point within 20 years. Progression of the disease was based on occurrence of the following events: the primary end point was composite including either renal death (dialysis start) or patient death (if occurring before dialysis), and the secondary end point was the start of chronic renal insufficiency/failure defined according to Kidney Disease Outcomes Quality Initiative recommendations as an eGFR (calculated by Modification of Diet in Renal Disease) below 60 ml/mn per 1.73 m2 body surface area and corresponding to CKD, stage 3 and up. In this study, ESRF/dialysis was defined as the need for chronic dialysis, with all patients being in CKD stage 5 and eGFR values below 10 ml/mn per 1.73 m2.
The major risk factors studied were: (1) hypertension defined as BP over 140/90 mmHg at different clinical examinations and the need for antihypertensive medication including diuretics; the variable was used as a category: presence or absence; (2) the amount of proteinuria was measured on 24-hour urine collection and expressed in g/d; the covariate was used either as a continuous variable or as a category variable using the Kidney Disease Outcomes Quality Initiative classification: <0.30 g/d = absent or traces; 0.30 to 0.99 g/d = moderate; 1.0 to 3.49 g/d = severe; and >3.00 g/d = heavy; for simplification, we have used this item as dichotomous: <1 g/d versus ≥1 g/d; and (3) the presence of severe pathologic renal lesions, appreciated by the GOS previously described,7,13 integrating all elementary lesions and validated in a large retrospective cohort of 282 IgAN patients. The GOS (range, 0 to 20) was the sum of glomerular (0 to 6), vascular (0 to 5), tubular (0 to 4), and interstitial (0 to 5) indices with an additional glomerular score (0 to 2) when the percentage of affected glomeruli by crescents and/or hyalinosis and/or obsolescence was over 25%. GOS was established on the RB, which permitted the diagnosis of IgAN. This covariate was used as a continuous variable and then simplified as a dichotomous variable: <8 versus ≥8; this cut-off value was previously calculated by ROC analysis for dialysis alone (area under the curve = 0.910).
The ARR score was simply the number of these simplified dichotomous RF present at diagnosis with four classes: 0 for none, 3 for their simultaneous presence, and the intermediate scores 1 and 2 for the presence of any one or two of these factors. This ARR evaluation was then used as a unique categorical covariate.
It should be emphasized that this cohort does not represent the natural history of primary IgAN. After the diagnosis, all of these patients were optimally treated when indicated. At the last FU, 153 patients were receiving long-term angiotensin I converting enzyme inhibitors and/or angiotensin II receptor blockers40–42 to treat 164 patients with HT (76%, plus other antihypertensive agents with a BP target of ≤130/80), to reduce proteinuria ≥1 g/d in 121 patients (73%) if not already treated, or to target both in 85 patients (84%). Among patients with severe pathologic lesions, i.e. with a GOS of ≥8, 72.4% had received at least one steroid treatment36–38 composed of high-dose pulses followed by oral doses tapered over about 3 months. None have received fish oil treatment.43
The internal validation of our prognostic model was done on a retrospective cohort of 250 IgAN patients (180 men, 72%) with a diagnostic RB performed between January 1, 1980 and December 31, 1989. The mean age at diagnosis was 33.9 (SD = 15.1) years, and the total exposure time to the primary risk was 17.55 (SD = 10.82) years. The total number of events occurring within 20 years after onset was 38 (15.2%) with 31 dialysis and 7 deaths.
The Methods
The end points chosen for this study are really time dependent, so we have used both the Kaplan-Meier survival (without the event) method and the Cox regression analysis to isolate the really prognostic/predictive factors44 with their respective weight. For both methods, time zero was set up at the onset of the disease, and the end points were the start of CKD3+ (secondary end point) and chronic dialysis start or patient death (composite primary end point). For each patient, we established the dates of disease onset, of each risk factor, of end points, and of last follow-up. For the survival curves construction, we have limited the time to 25 years and stopped the analysis when the number of patients at risk went below 10 to 15% of the time zero number. The Kaplan-Meier method allows only the testing of one categorical or dichotomous covariate, whereas Cox regression permits the analysis of continuous or categorical variables one by one (univariate) or of all significant covariates at the same time to check independency (multivariate). Comparisons of K-M survival curves were done by the log-rank test. The Cox regression provided for each covariate tested the following items: regression coefficient (β); β/SE, which represents the weight of the factor; χ2; probability (P), RR with its 95% CI; and the RR reduction rate, if any.
Other Statistical Analyses
Comparison of continuous variables was done by t test paired or unpaired accordingly. Comparison of distributions was done by a contingency table with x columns and y lines resulting in χ2 and P values. To appreciate the respective power of each RF to predict accurately the ultimate primary event, we used the classical accuracy measurements such as sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, pretest probability of event, pretest odds ratio (OR) of event, post-test OR if RF present, probability of event if RF present, post-test OR if RF absent, and probability of event if RF absent.
DISCLOSURES
None.
Acknowledgments
We are pleased to thank all of the patients who made regular visits to our clinic over the years and all of the staff members of the nephrology department who helped in the completion of our IgAN cohorts.
François Berthoux, Hesham Mohey, Aida Afiani, and Christophe Mariat took care of these patients since 1990; François Berthoux, Hesham Mohey, and Aida Afiani have maintained the IgAN-STET-CO clinical database since 1990; Blandine Laurent and Lise Thibaudin reviewed all renal biopsies with our own scoring and updated the biological database with collection of sera and DNA from these patients with their consent; and François Berthoux established this prospective cohort in 1990, was responsible for the concept of this study, and, with Christophe Mariat, wrote and discussed the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Berger J, Hinglais N: Intercapillary deposits of IgA-IgG. J Urol Nephrol 74: 694–695, 1968 [PubMed] [Google Scholar]
- 2. Berger J: IgA glomerular deposits in renal disease. Transplant Proc 1: 939–944, 1969 [PubMed] [Google Scholar]
- 3. Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 28: 4–9, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Feehally J: Predicting prognosis in IgA nephropathy: editorials. Am J Kidney Diseases 38: 881–883, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F: Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis 18: 12–19, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Koyama A, Igarashi M, Kobayashi M: Natural history and risk factors for immunoglobulin A nephropathy in Japan: Research Group on Progressive Renal Diseases. Am J Kidney Dis 29: 526–532, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Radford MG, Donadio JV, Bergstralh EJ, Grande JP: Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol 8: 199–207, 1997 [DOI] [PubMed] [Google Scholar]
- 10. D'Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Li PK, Ho KK, Szeto CC, Lu Y, Lai FM: Prognostic indicators of IgA nephropathy in the Chinese: Clinical and pathological perspectives. Nephrol Dial Transplant 17: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Alamartine E, Sabatier JC, Berthoux FC: Comparison of pathological lesions on repeated renal biopsies in 73 patients with primary IgA glomerulonephritis: Value of quantitative scoring and approach to final prognosis. Clin Nephrol 34: 45–51, 1990 [PubMed] [Google Scholar]
- 14. Haas M: Histologic subclassification of IgA nephropathy: A clinicopathological study of 244 cases. Am J Kidney Dis 29: 829–842, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, Park SY, Han JS, Kim S, Lee JS: Histological grading of IgA nephropathy predicting renal outcome: Revisiting H. S. Lee's glomerular grading system. Nephrol Dial Transplant 20: 342–348, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Manno C, Strippoli GF, D'Altri C, Torres D, Rossini M, Schena FP: A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis 49: 763–775, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Donadio JV, Bergstralh EJ, Grande JP, Rademcher DM: Proteinuria patterns and their association with subsequent end-stage renal disease in IgA nephropathy. Nephrol Dial Transplant 17: 1197–1203, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Bonnet F, Deprele C, Sassolas A, Moulin P, Alamartine E, Berthezène F, Berthoux F: Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis 37: 720–727, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Syrjänen J, Mustonen J, Pasternack A: Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 15: 34–42, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Panzer U, Schneider A, Steinmetz OM, Wenzel U, Barth P, Reinking R, Becker JU, Harendza S, Zahner G, Fischereder M, Krämer BK, Schlöndorff D, Ostendorf T, Floege J, Helmchen U, Stahl RA: The chemokine receptor 5 Delta32 mutation is associated with increased renal survival in patients with IgA nephropathy. Kidney Int 67: 75–81, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Berthoux FC, Berthoux P, Mariat C, Thibaudin L, Afiani A, Linossier MT: CC-chemokine receptor five gene polymorphism in primary IgA nephropathy: The 32 bp deletion allele is associated with late progression to end-stage renal failure with dialysis. Kidney Int 69: 565–572, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kannel WB, McGee D, Gordon T: A general cardiovascular risk profile: The Framingham study. Am J Cardiol 38: 46–51, 1976 [DOI] [PubMed] [Google Scholar]
- 23. D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB: General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation 117: 743–753, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Delanaye P, Mariat C, Cavalier E, Krzesinski JM: Errors induced by indexing glomerular filtration rate for body surface area: Reduction ad absurdum. Nephrol Dial Transplant 24: 3593–3596, 2009 [DOI] [PubMed] [Google Scholar]
- 25. A working group of the international IgA nephropathy network, the renal pathology society: Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, D'Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 26. A working group of the international IgA nephropathy network, the renal pathology society: Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D'Agati V, D'Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Pimenta E, Oparil S: Prehypertension: Epidemiology, consequences and treatment. Nat Rev Nephrol 6: 21–30, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Beukhof J, Kardaun O, Schaafsma W, Poortema K, Donker AJ, Hoedemaeker PJ, van der Hem GK: Towards individual prognosis of IgA nephropathy. Kidney Int 29: 549–556, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Rauta V, Finne P, Fagerudd J, Rosenlof K, Tornroth T, Gronhagen-Riska C: Factors associated with progression of IgA nephropathy are related to renal function: A model for estimating risk of progression in mild disease. Clin Nephrology 58: 85–94, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H., Manns B, Hemmelgarn B: Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol 5: 425–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goto M, Kawamura T, Wakai K, Ando M, Endoh M, Tomino Y: Risk stratification for progression of IgA nephropathy using a decision tree induction algorithm. Nephrol Dial Transplant 24: 1242–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacKinnon B, Fraser EP, Cattran D, Fox JG, Geddes CC: Validation of the Toronto formula to predict progression in IgA nephropathy. Nephron Clin Pract 109: c148–c153, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Kanno Y, Okada H, Saruta T, Suzuki H: Blood pressure reduction associated with preservation of renal function in hypertensive patients with IgA nephropathy: A 3-year follow-up. Clin Nephrol 54: 360–365, 2000 [PubMed] [Google Scholar]
- 35. Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry: Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Cheng J, Zhang X, Zhang W, He Q, Tao X, Chen J: Efficacy and safety of glucocorticoids therapy for IgA nephropathy: A meta analysis of randomized controlled trials. Am J Nephrol 30: 315–322, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Cook NR: Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 115: 925–935, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Praga M, Gutiérrez E, Gonzalez E, Morales E, Hernandez E: Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J Am Soc Nephrol 14: 1578–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Russo D, Minutolo R, Pisani A, Esposito R, Signoriello G, Andreucci M, Balletta MM: Coadministration of losartan and enalapril exerts additive antiproteinuric effect in IgA nephropathy. Am J Kidney Dis 38: 18–25, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Dillon JJ; Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for IgA nephropathy. Semin Nephrol 24: 218–224, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Donadio JV, Jr, Grande JP, Bergstralh EJ, Dart RA, Larson TS, Spencer DC: The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial: Mayo Nephrology Collaborative Group. J Am Soc Nephrol 10: 1772–1777, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Tripepi G, Jager KJ, Dekker FW, Zoccali C: Testing for causality and prognosis: Etiological and prognostic models. Kidney Int 74: 1512–1515, 2008 [DOI] [PubMed] [Google Scholar]