Abstract
Although endothelin-receptor antagonists reduce albuminuria in diabetic nephropathy, fluid retention limits their use. Here, we examined the effect of atrasentan, a selective endothelin A receptor (ETAR) antagonist, on albuminuria in a randomized, double-blind, placebo-controlled trial of subjects with diabetic nephropathy already receiving stable doses of renin-angiotensin system (RAS) inhibitors. We randomly assigned 89 subjects with eGFR >20 ml/min per 1.73 m2 and a urinary albumin-to-creatinine ratio (UACR) of 100 to 3000 mg/g to placebo or atrasentan (0.25, 0.75, or 1.75 mg daily) for 8 weeks. Compared with placebo, atrasentan significantly reduced UACR only in the 0.75- and 1.75-mg groups (P = 0.001 and P = 0.011, respectively). Compared with the 11% reduction in the geometric mean of the UACR from baseline to final observation in the placebo group during the study, the geometric mean of UACR decreased by 21, 42, and 35% in the 0.25-, 0.75-, and 1.75-mg atrasentan groups (P = 0.291, P = 0.023, and P = 0.073, respectively). In the placebo group, 17% of subjects achieved ≥40% reduction in UACR from baseline compared with 30, 50, and 38% in the 0.25-, 0.75-, and 1.75-mg atrasentan groups, respectively (P = 0.029 for 0.75 mg versus placebo). Peripheral edema occurred in 9% of subjects receiving placebo and in 14, 18, and 46% of those receiving 0.25, 0.5, and 1.75 mg atrasentan, respectively (P = 0.007 for 1.75 mg versus placebo). In summary, atrasentan, at the doses tested, is generally safe and effective in reducing residual albuminuria and may ultimately improve renal outcomes in patients with type 2 diabetic nephropathy.
Diabetic nephropathy (DN) continues to be the most common cause of ESRD, despite attempts at rigorous control of hyperglycemia and hypertension.1,2 The addition of renin-angiotensin system (RAS) inhibitors to reduce the deleterious effects of excessive renal angiotensin receptor activation has been the only kidney-specific therapy developed for DN during the past 10 years. Although treatment with RAS inhibitors shows reductions in albuminuria in association with delays in chronic kidney disease (CKD) progression,3,4 there remains a significant unmet need to develop therapies that completely prevent progression to ESRD or even induce regression of glomerular pathology.5
The endothelin (ET) system is chronically activated in patients with diabetes and in preclinical models as evidenced by elevated circulating levels of endothelin-1 (ET-1),6 enhanced kidney ET-1 concentrations,7 and increased renal and systemic endothelin A receptor (ETAR) activation.8 Glomerular ETAR, but not ETBR, activation promotes podocyte and mesangial cell dysfunction, leading to proteinuria and glomerulosclerosis.9 A recent clinical trial with avosentan, an endothelin receptor antagonist that likely blocked both ETAR and ETBR, reduced albuminuria in patients with macroalbuminuria and type 2 diabetes, although significant safety concerns related to fluid retention resulted in early trial termination.10
Atrasentan is a highly selective ETAR antagonist with an approximate 1800:1 selectivity for ETAR to ETBR.11 Such ETAR, as opposed to ETBR, selectivity may be ideal for targeting the ET pathogenicity in DN. The purpose of this randomized, double-blind, placebo-controlled clinical trial was to prospectively evaluate the efficacy and safety of atrasentan for the reduction of residual albuminuria in subjects with type 2 DN who were receiving stable doses of angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs).
RESULTS
The disposition of study subjects is shown in Figure 1. Of the 239 subjects screened, 89 subjects comprised the intent-to-treat population and were randomly assigned to one of four treatment groups: placebo (n = 23), atrasentan 0.25 mg daily (n = 22), 0.75 mg daily (n = 22), or 1.75 mg daily (n = 22).
Figure 1.
Disposition of subjects during the study. Subjects may have had more than one reason for discontinuation.
Patient Characteristics
Baseline demographic, clinical and biochemical characteristics, and concomitant therapies were balanced between the four groups (Table 1). At baseline, 27% of subjects had 30 to 200 mg/g creatinine, 72% of subjects had >200 mg/g creatinine, and 26% of subjects had an estimated GFR >60 ml/min per 1.73 m2. The majority of subjects (87%) were white, and the mean age of the study population was 64 years.
Table 1.
Subject demographics and baseline characteristics
| Variable | Placebo (n = 23) | Atrasentan |
||
|---|---|---|---|---|
| 0.25 mg (n = 22) | 0.75 mg (n = 22) | 1.75 mg (n = 22) | ||
| Gender, n (%) | ||||
| female | 4 (17%) | 9 (41%) | 8 (36%) | 6 (27%) |
| male | 19 (83%) | 13 (59%) | 14 (64%) | 16 (73%) |
| Race, n (%) | ||||
| white | 22 (96%) | 19 (86%) | 18 (82%) | 18 (82%) |
| black | 0 | 3 (14%) | 2 (9%) | 2 (9%) |
| other | 1 (4%) | 0 | 2 (9%) | 2 (9%) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 13 (57%) | 14 (64%) | 14 (64%) | 11 (50%) |
| no ethnicity | 10 (44%) | 8 (36%) | 8 (36%) | 11 (50%) |
| Age, years, n (%) | ||||
| <65 | 12 (52%) | 13 (59%) | 7 (32%) | 11 (50%) |
| ≥65 | 11 (48%) | 9 (41%) | 15 (68%) | 11 (50%) |
| Age, years | ||||
| mean (SD) | 61 (8) | 63 (12) | 67 (9) | 64 (13) |
| Weight, kg | ||||
| mean (SD) | 99 (20) | 84 (13) | 96 (19) | 97 (20) |
| Body mass index, kg/m2 | ||||
| mean (SD) | 34 (5) | 31 (4) | 34 (6) | 33 (5) |
| UACR, mg/g creatinine | ||||
| median (Q1 to Q3) | 515 (170 to 1477) | 350 (194 to 1226) | 360 (209 to 726) | 433 (157 to 998) |
| Estimated GFR, ml/min/BSA | ||||
| mean (SD) | 52 (25) | 50 (24) | 61 (25) | 48 (20) |
| Serum creatinine, mg/dl | ||||
| Mean (SD) | 1.6 (0.6) | 1.5 (0.6) | 1.3 (0.5) | 1.8 (0.8) |
| SBP, mmHg | ||||
| mean (SD) | 138 (14) | 134 (14) | 137 (15) | 135 (11) |
| DBP, mmHg | ||||
| mean (SD) | 78 (8) | 75 (8) | 74 (8) | 75 (9) |
| Hemoglobin, g/dl | ||||
| mean (SD) | 13 (1) | 12 (1) | 13 (2) | 13 (1) |
| Hemoglobin A1c, % | ||||
| mean (SD) | 7.4 (0.9) | 7.6 (1.0) | 7.6 (1.2) | 7.3 (1.1) |
Primary and Secondary Outcomes
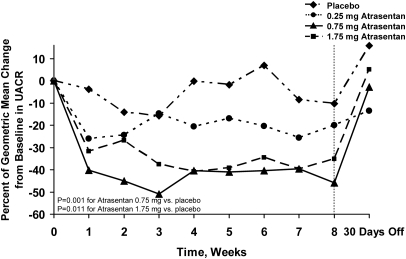
The primary efficacy analysis, comparing treatment group differences between each atrasentan group and placebo for change from baseline to each postbaseline assessment (after a log transformation) using a repeated-measures analysis showed that urinary albumin-to-creatinine ratio (UACR) was significantly reduced during the course of the 8-week treatment period in the 0.75- and 1.75-mg groups (P = 0.001 and P = 0.011 versus placebo, respectively; Figure 2). For the 0.75-mg group, a significant treatment effect was seen as early as week 1 (P = 0.005) and was sustained to the last treatment visit (week 8) of the study (P = 0.008). The modest UACR reduction in the 0.25-mg group was not significant (P = 0.150).
Figure 2.
Atrasentan treatment significantly reduces albuminuria. Effect of atrasentan on change in UACR from baseline. Significant reductions in UACR were seen with the 0.75-mg dose (P = 0.001 versus placebo by repeated measures analysis) and 1.75-mg dose (P = 0.011 versus placebo by repeated-measures analysis). UACR returned toward baseline values after 30 days from drug discontinuation.
Multiplicity adjustments were not made among the three pairwise comparisons for the primary efficacy analysis because this was an exploratory phase 2a study. However, if a Bonferroni adjustment is made post hoc to adjust for multiplicity of comparisons among three pairs, the study can still claim success because the significance level of 0.017 (0.05/3) was achieved by the prespecified primary efficacy analysis in the 0.75 mg (P = 0.001 versus placebo) and 1.75 mg groups (P = 0.011 versus placebo).
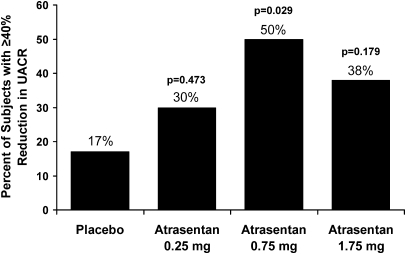
The geometric mean reduction from baseline to final UACR was significantly greater in the 0.75-mg group (42% reduction) compared with placebo (11% reduction, P = 0.023). For the 1.75-mg group, the effect did not quite meet significance (35% reduction, P = 0.073), whereas the reduction by the 0.25-mg group (21% reduction, P = 0.291) was not significant compared with placebo. A significantly greater proportion of subjects in the 0.75-mg group achieved at least a 40% reduction from baseline to final UACR compared with placebo (50 versus 17%, P = 0.029; Figure 3). The proportion achieving ≥40% reduction in UACR in the 1.75- and 0.25-mg groups was 38 (P = 0.179) and 30% (P = 0.473), respectively. The proportion of subjects achieving ≥25% reduction was not significantly different compared with placebo (39%) for any of the treatment groups: 0.25 mg, 40% (P = 0.999); 0.75 mg, 68% (P = 0.075); 1.75 mg, 62% (P = 0.227).
Figure 3.
Atrasentan treatment significantly increases the percentage of subjects achieving ≥ 40% reduction in UACR compared to placebo.
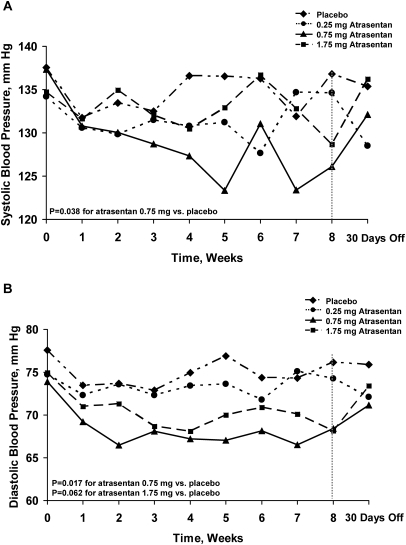
There was an early and sustained reduction in systolic BP (SBP) in the 0.75-mg group (P = 0.038 by repeated-measures analysis versus placebo) as shown in Figure 4A. The mean change from baseline to week 8 of treatment SBP was −0.3 mmHg in the 0.25-mg group (P = 0.834 versus 0.7 mmHg in placebo), −8.8 mmHg (P = 0.049 versus placebo) in the 0.75-mg group, and −7.6 mmHg (P = 0.086 versus placebo) in the 1.75-mg group. There was also an early and sustained decrease in diastolic BP (DBP; Figure 4B) in the same treatment groups (Figure 4B), where the mean change from baseline to week 8 of treatment was −0.5 mmHg in the 0.25-mg group (P = 0.753 versus −1.4 mmHg in placebo) −5.8 mmHg in the 0.75-mg group (P = 0.132 versus placebo), and −7.4 mmHg in the 1.75-mg group (P = 0.042 versus placebo).
Figure 4.
Atrasentan affects longitudinal measures of BP by repeated-measures analysis. Systolic BP was reduced in the 0.75-mg dose (P = 0.038 versus placebo by repeated-measures analysis). Diastolic BP was reduced in the 0.75-mg dose (P = 0.017 versus placebo by repeated-measures analysis). BP values returned toward baseline after 30 days from drug discontinuation.
Linear regression models (path analysis) to study the relationship between change in SBP and change in log UACR suggested that the SBP response was associated with only a minor portion of the UACR reduction (<21% of the total treatment effect). There were no significant differences in the mean change from baseline in estimated GFR (range, −2 to 2 ml/min per 1.73 m2 for all three dose groups across postbaseline visits) or body weight (range, −1.0 to 1.1 kg for all three dose groups across postbaseline visits; Figure 1; Appendix) between atrasentan dose groups and placebo. There were significant reductions in hemoglobin concentrations induced by atrasentan (Figure 2; Appendix), consistent with the known vasodilatory and thus hemodilutional effect of this class of compounds. The mean change from baseline to week 8 of treatment was −0.7 g/dl in the 0.25-mg group, −0.4 g/dl in the 0.75-mg group, and −0.9 g/dl in the 1.75-mg group compared with 0.1 g/dl for placebo (P < 0.001, P = 0.015, and P < 0.001, respectively).
All of the changes in UACR, BP, and hemoglobin returned toward baseline values at the 30-day follow-up visit in the atrasentan 0.75-mg and 1.75-mg groups.
All subjects received concomitant RAS inhibitors per the study inclusion criteria. Thirty-eight percent of subjects received the maximum dose as recommended by the product label. To evaluate whether concomitantly taking the maximum dose of RAS inhibitors could influence the effect of atrasentan on UACR, a post hoc analysis was conducted. Subjects were dichotomized by those who received maximum doses of RAS inhibitors versus those who did not; the treatment-by-subgroup interaction on log UACR for change from baseline to final observation was not significant (P = 0.816), indicating that the treatment effect of atrasentan was present regardless of the level of RAS inhibition (Figure 3; Appendix). In the same model for subgroup analysis, the effect for subgroup was not statistically significant.
Pharmacokinetic parameters for atrasentan on day 1 of treatment and the mean concentrations at each of the visits (weeks 2, 4, 6, and 8) are presented in Tables 2 and 3, respectively.
Table 2.
Atrasentan pharmacokinetic parameters on treatment day 1
| Pharmacokinetic Parameters (units) | Atrasentan |
|||
|---|---|---|---|---|
| 0.25 mg (n = 20) | 0.75 mg (n = 23) | 1.75 mg (n = 21) | ||
| Tmax | (h) | 2.19 ± 2.66 | 1.38 ± 1.98 | 1.12 ± 1.74 |
| Cmax | (ng/ml) | 0.28 ± 0.24 | 1.72 ± 1.34 | 4.12 ± 3.15 |
| AUC0–6 | (ng · h/ml) | 0.71 ± 0.91 | 5.31 ± 3.83 | 13.07 ± 9.74 |
Data presented as mean ± SD. Tmax, time to Cmax; Cmax, maximum plasma concentration; AUC0–6, area under the plasma concentration time curve from 0 to time to last sample.
Table 3.
Atrasentan trough plasma concentrations on treatment weeks 2, 4, 6, and 8
| Dose (mg) | Treatment Visits |
|||
|---|---|---|---|---|
| Week 2 | Week 4 | Week 6 | Week 8 | |
| 0.25 | 0.52 ± 0.28 | 0.63 ± 0.47 | 0.54 ± 0.48 | 0.46 ± 0.26 |
| 0.75 | 1.81 ± 1.83 | 2.06 ± 2.06 | 1.62 ± 1.27 | 1.61 ± 1.80 |
| 1.75 | 2.22 ± 1.05 | 2.28 ± 1.10 | 2.25 ± 1.06 | 2.39 ± 1.14 |
Data presented as mean ± SD.
Diuretic use (48 to 68%) was similar among the treatment groups throughout the study (11, 12, 15, and 11 subjects in the placebo and 0.25-, 0.75-, and 1.75-mg groups, respectively). Loop diuretics (furosemide or torsemide) were the most common type in 8, 5, 10, and 9 subjects, respectively (80 to 97% of treatment days), followed by distal diuretics (hydrochlorothiazide, chlorthalidone, or metolazone) in 5, 9, 6, and 2 subjects, respectively (85 to 100% of treatment days). Spironolactone was used in one placebo subject (all treatment days), and triamterene was used in three subjects (one in 0.25 mg for 76 days and two in 0.75-mg group for all treatment days).
Adverse Events
There was a significantly higher proportion of adverse events considered to be at least possibly related to study drug only in the 1.75-mg group (59 versus 22% in placebo, P = 0.016). Two subjects each from the 0.75- and 1.75-mg groups discontinued the drug prematurely because of one or more of the following adverse events: congestive heart failure and coronary artery disease (nonemergent cardiac catheterization) in the 0.75-mg group and hypotension, angioedema, headache, and peripheral edema in the 1.75-mg group. The most common adverse event was peripheral edema, which showed a dose–response relationship: placebo, 9%; 0.25 mg, 14%; 0.75 mg, 18%; 1.75 mg, 46% (P = 0.007 for placebo versus 1.75 mg). Peripheral edema was reported as mild in two subjects and moderate in two subjects in the 0.75-mg group and as mild in nine subjects and moderate in one subject in the 1.75-mg group. One subject was discontinued from the study because of angioedema (in the 1.75-mg group). Combining these two MedDRA preferred terms (peripheral edema and edema), the impact of edema on subjects' percentage change in UACR was examined (Figure 5). The analysis indicated that the treatment effect of atrasentan 0.75 mg was independent of the occurrence of edema.
Figure 5.
The effect of atrasentan on change in UACR from baseline to final visit is independent of edema occurrence during treatment.
Serious adverse events were observed in 0% of placebo, in 5% of the 0.25-mg group, in 14% of the 0.75-mg group, and in 5% of the 1.75-mg group (Table 4). Only one serious adverse event was considered to be possibly related to study drug, occurring in a subject in the 0.75-mg group who developed accelerated hypertension and diastolic heart failure approximately 3 weeks after starting treatment that was quickly reversed with BP control and diuretic therapy. This subject had a baseline N-terminal pro-brain type natriuretic peptide (NT-pro BNP) NT-pro BNP level that was >20-fold higher than normal before receiving atrasentan, which may have reflected subclinical diastolic heart failure. The protocol was amended shortly thereafter to exclude subjects with a NT-pro BNP of 500 pg/ml or greater.
Table 4.
Treatment-emergent adverse events in study subjects
| Subjects Experiencing, N (%) | Placebo (n = 23) | Atrasentan |
||
|---|---|---|---|---|
| 0.25 mg (n = 22) | 0.75 mg (n = 22) | 1.75 mg (n = 22) | ||
| Any adverse event | 13 (57%) | 16 (73%) | 16 (73%) | 19 (86%)a |
| Any adverse event at least possibly related to study drug | 5 (22%) | 8 (36%) | 6 (27%) | 13 (59%)b |
| Any severe adverse event | 0 | 1 (5%) | 0 | 1 (5%) |
| Any serious adverse event | 0 | 1 (5%) | 3 (14%) | 1 (5%) |
| Any adverse event leading to discontinuation of study drug | 0 | 0 | 2 (9%) | 2 (9%) |
| Deaths | 0 | 0 | 0 | 0 |
| Most commonly reported adverse effectsc | ||||
| peripheral edema | 2 (9%) | 3 (14%) | 4 (18%) | 10 (46%)d |
| diarrhea | 2 (9%) | 1 (5%) | 3 (14%) | 0 |
| dizziness | 0 | 3 (14%) | 2 (9%) | 1 (5%) |
| urinary tract infection | 1 (4%) | 0 | 2 (9%) | 3 (14%) |
| headache | 0 | 2 (9%) | 1 (5%) | 2 (9%) |
| cough | 1 (4%) | 1 (5%) | 2 (9%) | 0 |
| hypertension | 1 (4%) | 1 (5%) | 1 (5%) | 1 (5%) |
| hypoglycemia | 0 | 3 (14%) | 0 | 1 (5%) |
| hypotension | 1 (4%) | 0 | 1 (5%) | 2 (9%) |
aP = 0.047 versus placebo.
bP = 0.016 versus placebo.
cReported in ≥5% of subjects.
dP = 0.007 versus placebo.
There were two additional nonserious adverse cardiovascular events (atrioventricular block, coronary artery disease), one each in the 0.75-mg and 1.75-mg groups, which were considered by the investigator to not be related to the study drug. There were two nonserious adverse events of acute renal failure, both occurring in the 0.75-mg group. One occurred in a subject with a baseline serum creatinine of 2.0 mg/dl, rising to 3.3 mg/dl, 5 days after an increase in diuretic dose and 7 days after starting treatment (corresponding to a BP change from 127/73 to 115/60). Serum creatinine fluctuated between 3.3. and 2.4 through week 7 and then rose to 4.0 mg/dl at week 8 (BP 113/80). The 30-day post-treatment creatinine and BP were 1.9 mg/dl and 145/64, respectively. The other event was prerenal azotemia occurring after the subject, who had a history of percutaneous transluminal coronary angioplasty, was discontinued at study week 5 for nonemergent coronary artery bypass grafting.
There were no significant changes in serum sodium, hepatic enzymes, or bilirubin in any of the atrasentan treatment groups compared with placebo.
DISCUSSION
This study showed that atrasentan, a selective ETAR antagonist, is safe and efficacious in treating residual albuminuria over an 8-week period in subjects with type 2 DN who are on stable doses of RAS inhibitors. The UACR-lowering effect, which occurred early after drug initiation, was sustained throughout the treatment period for the 0.75- and 1.75-mg doses but was not significant in the lowest dose group. Although both of the effective doses were associated with significant lowering of BP, the major effect of atrasentan on UACR reduction was independent of the BP change. The finding that estimated GFR did not change during treatment suggests that ETAR antagonism of efferent vasoconstriction may not be the dominant mechanism for its albuminuria-lowering effect, in contrast to the action of compounds that reduce renal angiotensin receptor activation and decrease estimated GFR over a similar time frame.3,4 However, more definitive testing of changes in true GFR and renal plasma flow will be necessary to determine whether filtration fraction is favorably altered in response to atrasentan.
An acceptable safety profile of atrasentan treatment was observed in this trial, although the treatment period was relatively short. Only one serious adverse event related to fluid retention occurred in a subject with a history of cardiovascular disease. It is notable that this subject began treatment with a markedly elevated NT-pro-BNP that predicted an impending acute event,12 and it is therefore unclear what role atrasentan had in this case. Nevertheless, we believe that patients who are at high risk for developing congestive heart failure should be appropriately assessed for that risk before initiating ETAR antagonist therapy. Although it was expected that peripheral edema would constitute the majority of adverse events, it is reassuring that a clear dose–response relationship was seen, a low relative rate of edema occurred with the atrasentan dose that had the greatest effect on albuminuria reduction (0.75 mg), and most episodes were mild in severity. Although mild edema was most common, even with the highest dose studied, the frequency was significantly higher than observed with placebo, and therefore 1.75 mg of atrasentan may ultimately be a limiting therapeutic dose because greater efficacy was not established compared with the 0.75-mg group. We attribute the low incidence of edema to the doses studied and to atrasentan's chemical structure, which provides an approximate 1800 fold selectivity for ETAR:ETBR. However, concerns about fluid retention will continue to be an important management issue in patients taking ETR antagonists, and a better understanding of how both receptor systems are involved with sodium excretion continues to be elucidated. Current evidence supports using a highly selective ETAR antagonist because of the importance of ETBR in mediating sodium excretion. The ETBR, located in the collecting duct, is crucial for normal sodium excretion.13 Studies in mice with collecting duct-specific knockout of ETAR and/or ETBR have established that ETB receptors are of primary importance in mediating ET-1 inhibition of renal sodium reabsorption13–16; inhibition of ETBR is highly likely, therefore, to cause fluid retention. ETAR blockade is also associated with vasodilatation and may also promote fluid retention; however, the mechanisms responsible for this, if it does occur, are poorly understood.
The clinical relevance of fluid retention with the use of ETAR antagonists in patients with diabetes and CKD became apparent in recent studies that examined the effect of avosentan on albuminuria lowering17 and on CKD progression.10 In the dose ranging, 12-week study of albuminuria reduction, treatment-emergent edema occurred in all of the avosentan treatment groups (5, 10, 25, and 50 mg) in a dose-related manner (9 to 24% incidence) that was significantly greater than in the placebo group (4%).17 In the larger hard endpoint trial, 45 and 46% of subjects in the avosentan 25- and 50-mg groups, respectively, reported symptomatic edema compared with 31% in the placebo group (P < 0.0001) after a median treatment period of 4 months. These high rates of edema were associated with a higher incidence of chronic heart failure–related adverse events in the avosentan 25- (6%) and 50-mg (4%) groups compared with placebo (2%), which was a major reason for the study's premature termination.10 Although the mechanism of the edema formation is not fully understood, one likely possibility is that the relatively high doses of this ETAR antagonist may have induced partial blockade of renal ETBR despite its 50:1 selectivity of ETAR to ETBR binding.9 In contrast, the doses of atrasentan in this study may have limited the incidence of edema through incomplete ETAR blockade, allowing the endogenous ET-1 to bind at both ETAR and ETBR sites, limiting the systemic vasodilatory effects expected with unopposed ET-1 binding at ETBRs.
The finding that SBP and DBP were reduced was not unexpected because it is known that ETAR-mediated vasoconstriction may contribute to hypertension in CKD.18 However, the small sample size may have contributed to a larger mean change in SBP observed in this study. Moreover, this study was not designed to look for a BP effect using more rigorous monitoring conditions, such as ambulatory BP measurements. Future trials that use a run-in period to establish adequate BP control before baseline and include ambulatory BP monitoring will be critical for observing the true effect of atrasentan on change in BP.
ETAR antagonists likely act to reduce glomerular hypertension by decreasing efferent arteriolar vasoconstriction to reduce filtration fraction.19 ETAR antagonists may directly attenuate podocyte dysfunction through downregulation of TGFβ and inhibition of macrophage infiltration.20 In a recent study of a type 1 DN model,21 the addition of an ETAR antagonist to RAS blockade in animals with established DN resulted in reduced albuminuria and regression of glomerulosclerosis. These changes coincided with increased podocyte nephrin expression, decreased accumulation of TGFβ and collagen III, reduced macrophage infiltration, and increased expression of matrix metalloproteinase-9, the enzyme principally involved with matrix degradation.21
Despite the controlled and double-blinded design of this study, the limitations of small sample size, short duration of treatment, and absence of ambulatory BP readings require that a larger and longer study be performed for confirmation. The study was also limited by the absence of quantitative measures of renal hemodynamics to quantify potential changes in filtration fraction and renal vascular resistance. The short duration of observation also precludes identifying additional potential long-term safety concerns, such as liver enzyme elevation, which have been identified with this class of compounds.22 We expect, however, that hepatic abnormalities will not be a concern because atrasentan's chemical structure is not expected to induce hepatic enzymes given that it is a carboxylic acid derivative rather than a sulfonamide derivative, and there is a shown liver safety profile observed in long-term studies with patients taking larger doses of atrasentan.23
The results of this study are restricted to the population of patients with type 2 diabetes who have residual albuminuria while receiving RAS inhibitors and who have not had a history of heart failure. Several large clinical trials with drugs that intervene in the RAS have shown that reductions in albuminuria are associated with long-term renoprotective effects, and the observed protection from ESRD may be independent of changes in BP control.3,4 As a result, reductions in UACR have been proposed as a surrogate outcomes marker, and therefore, it is expected that the large magnitude of reductions in UACR observed in our study may translate into improved renal and potentially cardiovascular outcomes in hard endpoint trials, because a reduction in albuminuria by 30% of baseline within the first 6 to 12 months of treatment in patients with kidney disease has been shown to predict long-term renal24 and cardiovascular outcomes.25 These results will need to be confirmed in larger size, placebo-controlled, randomized clinical trials.
In conclusion, atrasentan may offer an additional therapeutic benefit to the current standard of care using RAS inhibition for albuminuria reduction in patients with type 2 DN. Outcome-driven clinical trials will ultimately be required to establish its long-term safety and efficacy in slowing or preventing the progression to ESRD.
CONCISE METHODS
Study Design
This was a randomized, double-blind, placebo-controlled clinical trial that enrolled subjects from 21 sites in the United States and Puerto Rico between June 2009 and June 2010. There were 239 subjects screened, and 89 subjects were randomly assigned to receive atrasentan or matching placebo for 8 weeks. Subjects were examined at baseline, every week during the treatment phase, and 30 days after treatment withdrawal. BP and pulse (measured twice at each visit, and the average was used for the analyses), adverse events, concomitant medications and adherence to medication regimens, and blood chemistry were assessed at each visit. For each subject, two first morning void urine specimens collected on 2 consecutive days before each scheduled visit were collected, and the geometric mean of two UACR measures was calculated and used as the UACR value for the visit to be included in data analysis.
Randomization was stratified into two strata defined by baseline UACR (thresholds of 1000 and >1000 mg/g). The randomization schedule was computer generated by the study sponsor, and the randomization was implemented using an Interactive Voice Response System. Subjects were assigned in a 1:1:1:1 ratio to placebo and atrasentan doses of 0.25, 0.75, or 1.75 mg daily within each stratum. Study medication was masked and packaged by the study sponsor and distributed to sites through the Interactive Voice Response System as masked study drug kits. The study investigator, study subjects, and study sponsor's personnel involved with analysis and collection of study data were completely blinded to the subject's treatment group assignment during the study. Subjects randomization assignments were not disclosed until all database issues had been resolved and the study database was locked.
Subjects
Eligible subjects were enrolled if they had type 2 diabetes and had been taking anti-diabetic medications for at least 1 year before screening and had received a stable dose of an ACE inhibitor or ARB for at least 2 months, had an estimated GFR >20 ml/min per 1.73 m2 by the abbreviated Modified Diet in Renal Disease formula, and a UACR between 100 and 3000 mg/g.26 Female subjects were postmenopausal for at least 1 year or were surgically sterile. The main exclusion criteria included a history of significant peripheral edema, heart failure, pulmonary edema, or loop diuretic therapy of >60 mg/d of furosemide (or equivalent) and recent coronary arterial disease. The full list of inclusion and exclusion criteria is found in Appendix 1.
Subjects received their usual care for diabetes and cardiovascular protection. If the subject's BP exceeded 130/80 mmHg, anti-hypertensive medication (not including RAS inhibitors) was increased or added to obtain acceptable BP control based on current guidelines. Dose alterations of ACE inhibitors or ARBs were not allowed after randomization. Diuretics were added for new onset or worsening edema at the discretion of the investigator according to the protocol-specified guidelines; subjects not taking a loop diuretic could receive a loop diuretic; subjects taking a loop diuretic could receive increased doses (50% increase suggested as the initial increment). The study protocol was approved by an independent ethics committee and local and central review boards, and all subjects provided written informed consent.
Efficacy Parameters
The protocol-specified primary efficacy measure was change from baseline to each postbaseline observation in UACR over the course of the treatment period. The secondary efficacy measures included the proportion of subjects achieving at least a 40 and 25% reduction from baseline in the last on-treatment UACR level and mean change in estimated GFR. The same endpoints (for efficacy or safety) were used to assess the changes 30 days after stopping treatment.
UACR was assessed by a central laboratory using an immunoturbidemetic method. Subjects were required to collect two consecutive first morning void urine samples before each scheduled visit. The geometric mean of the two samples was used as the UACR visit value for subjects included in the analyses.
Pharmacokinetic Evaluation
For all subjects in the study, blood samples for plasma atrasentan concentrations were collected before dosing (0 hours), at 0.25, 1, 2 hours, and anytime between 4 and 6 hours after dosing on treatment day 1. Additionally, for all subjects, one blood sample was collected before dosing (0 hours) at treatment weeks 2, 4, 6, and 8 of the treatment phase.
Pharmacokinetic Variables
Atrasentan pharmacokinetic parameters including maximum plasma concentration, time to maximum plasma concentration, and area under the plasma concentration time curve from 0 to time to last sample were calculated for concentration data on treatment day 1 using noncompartmental methods. In, addition atrasentan plasma trough concentrations were summarized for pharmacokinetic data on treatment weeks 2, 4, 6, and 8.
Safety Evaluation
Safety endpoints were evaluated weekly and 30 days after treatment withdrawal and included vital signs (e.g., SBP and DBP), body weight, liver enzymes, hemoglobin, and adverse events. Automated BP measurements were performed twice with the appropriate cuff size in the nondominant arm at 2-minute intervals after 5 minutes of rest while in the sitting position, and the mean value was recorded.
Statistical Analysis
The sample size of 20 per arm was planned to allow the study to have at least 85% power to detect a group difference of −0.42 in mean change from baseline to final observation in log-transformed UACR between an atrasentan group and the placebo group (this difference corresponds to a 34% between-group reduction of geometric mean change in UACR from baseline to final) at the one-sided significance level of 0.05. A common SD of 0.46 was assumed. The sample size was adjusted to allow for one to two subjects per group without any UACR data after randomization.
All analyses were performed on the intent-to-treat population, which was comprised of the data from all randomized subjects who had received at least one dose of study drug. The primary efficacy analysis was a mixed-effect, maximum likelihood, repeated-measures analysis for change from baseline to each postbaseline assessment of log UACR. The model contained the terms of treatment, visit, treatment-by-visit interaction, baseline measurements, and baseline-by-visit interaction with unstructured as the variance-covariance structure. The primary treatment group comparison was for the overall effect; however, the comparison at each time point was also performed. The secondary efficacy analysis for log UACR was an analysis of covariance of change from baseline to final observation. The model included the terms of treatment with baseline as the covariate. Similar statistical models were applied to evaluate treatment group differences in other continuous efficacy or safety variables, such as estimated GFR, SBP, DBP, and hemoglobin. Treatment group differences in binary random variables, such as the proportion of subjects who achieved at least 40% reduction on UACR and the incidence of treatment-emergent adverse events were evaluated using Fisher's exact test.
Path analysis27 was used to test whether atrasentan 0.75 or 1.75 mg had a direct effect on UACR reduction after controlling for its therapeutic effects of lowering SBP. Two regression models were used in the path analysis: (1) change in log UACR = a0 + a1 × treatment + a2 × change in SBP + a3 × baseline in log UACR + a4 × baseline SBP + e1 and (2) change in SBP = b0 + b1 × treatment + b2 × baseline SBP + b3 × baseline log UACR + e2.
In the analysis, a1 quantifies the direct effect and is tested by a t test; b1 × a2 represents the indirect effect and its significance is tested by a Sobel's t-ratio.28 The percentage of direct effect of the total effect (defined as a1 + b1 × a2) and the indirect effect of the total effect were calculated. The significance of the total effect can be tested by testing the treatment coefficient in a third regression model: change in log UACR = c0 + c1 × treatment + c3 × baseline of log UACR + c4 × baseline of SBP, when c1 = a1 + b1 × a2 holds true. All analyses were performed using SAS version 9.1.3 (SAS Institute) under the Unix operating system.
DISCLOSURES
D.K. is a consultant for Abbott Laboratories. M.M. is a consultant for Abbott Laboratories and receives research support from Eli Lilly & Co., Ipsen, Corcept, and Sanofi-Aventis. Y.P., S.W., P.A., and D.L.A. are employees of Abbott Laboratories, and Y.P., P.A., and D.L.A. own Abbott stock.
Acknowledgments
An abstract containing data from this study was submitted and accepted for presentation at the American Society of Nephrology 2010 meeting. This study was supported by Abbott Laboratories. We thank Michael Amdahl, an Abbott statistician, for his contribution to the protocol design and Bo Yan, PhD, also an employee of Abbott, for his assistance with implementation of the statistical analysis plan. Medical writing support was provided by Amanda Fein, PhD, on behalf of Abbott. The sponsor was involved in the design of the study, in the collection and analysis of the data, and in writing the report. All authors had access to study results, and the lead author vouches for the accuracy and completeness of the data reported. The lead author had the final decision to submit the publication. The study was overseen by a data review committee. The following investigators contributed to subject recruitment for this study: Hanna Abboud, MD; Mario Belledonne, MD; Diogo Belo, MD, FACP; Rafael Burgos-Calderon, MD; Jose L. Cangiano, MD; Douglas S. Denham, DO; Mohamed El-Shahawy, MD, MPH, MHA; George Z. Fadda, MD; FACP; Venu M. Kondle, MD; K. Jean Lucas, MD; Robert G. Perry, MD; Edgardo Pineiro, MD; Marc Rendell, MD; Jeffery Rosen, MD; Bhupinder Singh, MD, FASN; Mark Warren, MD, FACE; and Steven Zeig, MD.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Endothelin Antagonist as Add-on Treatment for Proteinuria in Diabetic Nephropathy: Is There Light at the End of the Tunnel?” on pages 593–595.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 16: 3736–3741, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kakizawa H, Itoh M, Itoh Y, Imamura S, Ishiwata Y, Matsumoto T, Yamamoto K, Kato T, Ono Y, Nagata M, Hayakawa N, Suzuki A, Goto Y, Oda N: The relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism 53: 550–555, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Minchenko AG, Stevens MJ, White L, Abatan OI, Komjati K, Pacher P, Szabo C, Obrosova IG: Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J 17: 1514–1516, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Kohan DE: Endothelin, hypertension and chronic kidney disease: New insights. Curr Opin Nephrol Hypertens 19: 134–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neuhofer W, Pittrow D: Endothelin receptor selectivity in chronic kidney disease: Rationale and review of recent evidence. Eur J Clin Invest 39[Suppl 2]: 50–67, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, Littke T, Viberti G: Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, Gehrke LJ, Hernandez L, Magnuson SR, Marsh KC, Novosad EI, Von Geldern TW, Wessale JL, Winn M, Wu-Wong JR: Pharmacological characterization of A-127722: An orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther 276: 473–481, 1996 [PubMed] [Google Scholar]
- 12. Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, Holmer S: Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension 46: 118–123, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Kohan DE: Biology of endothelin receptors in the collecting duct. Kidney Int 76: 481–486, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE: Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE: Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE: Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol 289: F692–F698, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Wenzel RR, Littke T, Kuranoff S, Jurgens C, Bruck H, Ritz E, Philipp T, Mitchell A: Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shindo T, Kurihara H, Maemura K, Kurihara Y, Ueda O, Suzuki H, Kuwaki T, Ju KH, Wang Y, Ebihara A, Nishimatsu H, Moriyama N, Fukuda M, Akimoto Y, Hirano H, Morita H, Kumada M, Yazaki Y, Nagai R, Kimura K: Renal damage and salt-dependent hypertension in aged transgenic mice overexpressing endothelin-1. J Mol Med 80: 105–116, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Dhaun N, Ferro CJ, Davenport AP, Haynes WG, Goddard J, Webb DJ: Haemodynamic and renal effects of endothelin receptor antagonism in patients with chronic kidney disease. Nephrol Dial Transplant 22: 3228–3234, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS: Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, Conti S, Rottoli D, Longaretti L, Remuzzi A, Remuzzi G, Benigni A: Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol 297: F1448–F1456, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE: Profile of past and current clinical trials involving endothelin receptor antagonists: The novel “-sentan” class of drug. Exp Biol Med (Maywood) 231: 653–695, 2006 [PubMed] [Google Scholar]
- 23. Raichlin E, Prasad A, Mathew V, Kent B, Holmes DR, Jr, Pumper GM, Nelson RE, Lerman LO, Lerman A: Efficacy and safety of atrasentan in patients with cardiovascular risk and early atherosclerosis. Hypertension 52: 522–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 25. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Lu Y: An application of path analysis in the design of clinical trials. Am Stat Assoc: 2576–2582, 2003 [Google Scholar]
- 28. Sobel M: Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models, San Francisco, Jossey-Bass, 1982 [Google Scholar]