Abstract
Extended-release dipyridamole plus low-dose aspirin (ERDP/ASA) prolongs primary unassisted graft patency of newly created hemodialysis arteriovenous grafts, but the individual contributions of each component are unknown. Here, we analyzed whether use of aspirin at baseline associated with primary unassisted graft patency among participants in a randomized trial that compared ERDP/ASA and placebo in newly created grafts. We used Cox proportional hazards regression, adjusting for prespecified baseline comorbidities and covariates. Of all participants, 43% reported use of aspirin at baseline; of these, 82% remained on nonstudy aspirin (i.e., excluding ERDP/ASA) at 1 year. After 1 year of follow-up, the incidence of primary unassisted patency among participants using aspirin at baseline was 30% (95% CI: 24 to 35%) and among those not using aspirin was 23% (95% CI: 18 to 27%). Use of aspirin at baseline associated with a dose-dependent prolongation of primary unassisted graft patency that approached statistical significance (adjusted HR, 0.83; 95% CI: 0.68 to 1.01; P = 0.06). Use of aspirin at baseline did not associate with prolongation of cumulative graft patency or participant survival. In conclusion, use of aspirin associates with a trend toward longer primary unassisted patency of newly placed hemodialysis grafts similar to that observed for ERDP/ASA.
A well-functioning vascular access is essential for maintenance hemodialysis. However, vascular access failure occurs frequently and results in substantial morbidity for patients treated with hemodialysis.1 Loss of vascular access function is also costly.1 Vascular access for hemodialysis may be provided by an autogenous arteriovenous fistula, an arteriovenous graft, or a central venous catheter. The arteriovenous graft is considered a secondary choice for vascular access when a fistula cannot be created.2 Although a graft does not require maturation, as is the case with fistulas, it has a high rate of thrombosis requiring frequent and costly interventions to maintain patency.1,3,4 Angiographic studies have identified vascular stenosis, typically at the venous anastomosis, as the most common underlying etiology of thrombosis.5 A safe and cost-effective therapy to prevent access stenosis and subsequent thrombosis would greatly improve the usefulness of grafts.
Previously, we showed in a multicenter placebo-controlled clinical trial that extended release dipyridamole plus low dose aspirin (ERDP/ASA) had a significant but modest effect on prolonging primary unassisted graft patency (hazard ratio [HR] = 0.82; 95% confidence interval [CI] = 0.68 to 0.98; P = 0.03).6 ERDP was selected for that trial because it was believed to directly inhibit the vascular smooth muscle cell proliferation underlying access stenosis.7 Because the only approved form of ERDP available in the United States contained aspirin, we tested this drug combination in our trial. Aspirin use was not an exclusion criterion for the ERDP/ASA trial, and participants could take aspirin unrelated to the trial intervention after randomization (referred to below as nonstudy aspirin). In this report, we describe a secondary cohort analysis of our trial to determine whether baseline aspirin use, which also reflected nonstudy use of aspirin during the follow-up period of the clinical trial, was associated with improved primary unassisted graft patency and two secondary outcomes: cumulative graft patency and all-cause mortality.
RESULTS
Participant Characteristics
The demographic and clinical characteristics of participants stratified by baseline use of aspirin are shown in Table 1. Participants who reported taking aspirin at baseline were significantly older, had a lower diastolic BP, had a higher prevalence of diabetes, and had a higher prevalence of vascular disease, but had less current use of tobacco.
Table 1.
Baseline characteristics of the participantsa
| Characteristic | On Aspirin at Baseline (n = 279) | Not on Aspirin at Baseline (n = 370) |
|---|---|---|
| Age, years | 62.5 ± 12.2b | 55.1 ± 14.9 |
| Male, % | 39.1 | 40.0 |
| Black, % | 69.9 | 72.4 |
| Body mass index, kg/m2 | 30.4 ± 8.1 | 30.8 ± 8.5 |
| Systolic BP, mmHg | 143.8 ± 24.6 | 142.6 ± 24.5 |
| Diastolic BP, mmHg | 76.0 ± 13.8b | 79.0 ± 15.3 |
| Diabetes mellitus, % | 74.2b | 54.3 |
| Cardiovascular disease,c % | 53.8b | 31.1 |
| Cerebrovascular disease,d % | 24.0b | 9.5 |
| Peripheral arterial disease,e % | 19.7b | 13.0 |
| Venous thromboembolic disease,f % | 1.1 | 3.5 |
| Current tobacco use, % | 11.8b | 18.6 |
| Hemoglobin, g/dl | 11.7 ± 1.7 | 11.8 ± 1.7 |
| Serum albumin, g/dl | 3.7 ± 0.5 | 3.7 ± 0.5 |
| Hemodialysis initiated before graft creation, % | 71.3 | 74.1 |
| Time on hemodialysis, years | 3.2 ± 2.2 | 3.5 ± 2.2 |
Values are means ± SD. Conversion to SI units: to convert hemogloblin to g/L, multiply by 10. To convert serum albumin to g/L, multiply by 10.
aComparisons were made using t tests for continuous variables and c2 tests for categorical variables.
bP < 0.05.
cIncludes history of myocardial infarction, angina, coronary artery angioplasty, coronary artery bypass surgery, or congestive heart failure.
dIncludes history of stroke, transient ischemic attack, or carotid endarterectomy.
eIncludes history of nontraumatic amputation, lower extremity angioplasty or bypass surgery, or claudication.
fIncludes history of deep venous thrombosis or pulmonary embolism.
Use of Nonstudy Aspirin
Forty-three percent of participants reported taking aspirin at baseline. During follow-up, 94, 89, 84, and 82% of participants who were on aspirin at baseline and did not reach the primary outcome remained on nonstudy aspirin at 3, 6, 9, and 12 months after randomization, respectively. For participants not on aspirin at baseline, initiation of nonstudy aspirin occurred in 6, 13, 16, and 19% of participants who had not reached the primary outcome at 3, 6, 9, and 12 months after randomization, respectively.
At baseline, 53% of participants taking aspirin received a dose of 81 mg of aspirin per day and 38% received a dose of 325 mg of aspirin per day. The remaining 12% used doses of aspirin ranging from 12 to 800 mg/d. The dose of aspirin in one participant was unknown. The overall average daily dose of aspirin at baseline was 221 ± 188 (SD) mg and was not significantly different between the two randomized treatment groups (ERDP/ASA = 230 ± 190 mg/d; placebo = 212 ± 186 mg/d). The average daily dose of nonstudy aspirin in follow-up did not differ significantly from that used at baseline or between the two randomized groups at 3, 6, 9, or 12 months.
Outcomes
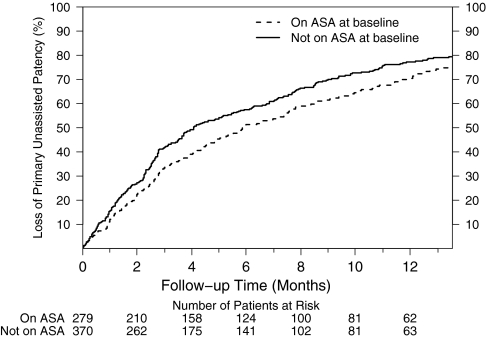
As shown in Figure 1, the loss of primary unassisted patency in participants on aspirin at baseline tended to be less than that observed among participants not taking aspirin. After 1 year of follow-up, the loss of primary unassisted patency in participants on aspirin at baseline was 70% compared with 77% for participants not on aspirin at baseline. After adjusting for randomization group, differences in baseline covariates (age, diabetes history, cardiovascular disease, cerebrovascular disease, peripheral arterial disease, smoking status, diastolic BP) and prespecified factors (albumin and use of angiotensin converting enzyme inhibitor and/or angiotensin receptor blockers), baseline aspirin use was associated with a 17% lower rate of loss of primary unassisted graft patency compared with nonuse that did not reach predefined criteria for statistical significance (HR = 0.83, 95% CI = 0.68 to 1.01; P = 0.06; Table 2).
Figure 1.
Primary unassisted patency is prolonged in patients on aspirin at baseline. Cumulative incidence of loss of primary unassisted graft patency for baseline aspirin users (dashed line) and nonusers (solid line). The median patency in the baseline aspirin users and nonusers was 5.8 (95% CI, 4.8 to 7.4) and 4.1 months (95% CI, 3.5 to 5.3; P = 0.13), respectively.
Table 2.
Unassisted patency and secondary outcomes based on use of aspirin at baseline
| Unassisted Patency | On Aspirin at Baseline (n = 279) | Not On Aspirin at Baseline (n = 370) | Hazard Ratio (95% Confidence Interval)a,b |
|---|---|---|---|
| Overall loss of primary unassisted patency, % | 225 (81%) | 305 (82%) | 0.83 (0.68, 1.01) |
| Thrombosis, %b | 103 (37%) | 163 (44%) | 0.76 (0.58, 1.01) |
| Thrombosis with stenosis ≥50%, % | 64 (23%) | 84 (23%) | 0.90 (0.62, 1.31) |
| Thrombosis with stenosis <50%, % | 3 (1%) | 5 (1%) | 0.73 (0.15, 3.73) |
| Thrombosis, no diagnostic procedure, % | 36 (13%) | 74 (20%) | 0.60 (0.38, 0.95) |
| Angioplasty, stenosis 350% no thrombosis, % | 93 (33%) | 103 (28%) | 0.89 (0.64, 1.24) |
| Angioplasty, stenosis <50% no thrombosis, % | 2 (1%) | 3 (1%) | NA |
| Infection, % | 16 (6%) | 19 (5%) | 1.41 (0.63, 3.15) |
| Failure to use graft by 12 weeks in prevalent patient, % | 4 (1%) | 8 (2%) | 0.60 (0.14, 2.56) |
| Procedure for other reason, %c | 7 (3%) | 9 (2%) | 0.34 (0.18, 1.83) |
| Stenosis ≥50% with or without thrombosis, % | 157 (56%) | 187 (51%) | 0.90 (0.70, 1.14) |
| Secondary outcomes | |||
| cumulative graft failure, % | 143 (51%) | 191 (52%) | 0.91 (0.71, 1.16) |
| Death, % | 110 (39%) | 110 (28%) | 1.04 (0.78, 1.40) |
| Cumulative graft failure or death, % | 185 (66%) | 241 (65%) | 0.87 (0.70, 1.08) |
aHazard ratio was obtained from a Cox model that adjust for randomization group and baseline variables including age, diabetes history, diastolic BP, cardiovascular disease, cerebrovascular disease, peripheral arterial disease, smoking status, albumin, and use of angiotensin converting enzyme inhibitor and/or angiotensin receptor blocker. The model was also stratified by access location and clinical center.
bP > 0.05 for the interaction effect between intervention and use of aspirin at baseline for all endpoints.
cProcedures done for other reasons. For the group on aspirin at baseline: access ligation for hand ischemia (four patients), angioplasty, or access ligation for central vein stenosis (one patient), surgical revision for peudoaneursym without stenosis (one patient), and ligation for uncontrolled bleeding without angiogram reported (one patient). For the group not on aspirin at baseline: access ligation for hand ischemia (three patients), angioplasty or access ligation for central vein stenosis (three patients), surgical revision for peudoaneursym without stenosis (two patients), and ligation for uncontrolled bleeding without angiogram reported (one patient).
The frequency of occurrence of individual components of the primary outcome is shown in Table 2. Overall, the primary outcome occurred in 81% of participants on aspirin at baseline compared with 82% of participants not on aspirin at baseline. Thrombosis was the most common cause of graft failure, occurring in 37% of participants on aspirin at baseline and 44% of participants not on aspirin at baseline. Treatment with aspirin at baseline was associated with a 24% reduction in the rate of thrombosis compared with participants not on aspirin at baseline that did not reach predefined criteria for statistical significance (HR = 0.76, 95% CI = 0.58 to 1.01, P = 0.06). There was no statistically significant interaction between aspirin use at baseline and the randomized study intervention for any component outcome.
Four different subgroups of aspirin exposure existed: patients who were users or nonusers of aspirin at baseline outside of the clinical trial (the “observational comparison” in the first panel of Table 3) and patients exposed to ERDP/ASA or placebo as part of the clinical trial (the “randomized comparison” in the second panel of Table 3). Only one of these four subgroups was not exposed to any aspirin at randomization (i.e., nonusers of aspirin at baseline who were assigned to placebo). Table 3 examines the relationship between the primary patency outcome for the overall comparison and within the subgroups of aspirin exposure in each of the two main treatment comparisons (first two panels). The hazard ratio for the interaction term between subgroups of aspirin exposure within each comparison group was not statistically significant (P = 0.42 for both the comparison of subgroups in the “observational” and the “randomized” comparison).
Table 3.
Relationship of primary unassisted patency with combinations of randomized treatment assignment and baseline aspirin use
| Type of Treatment Comparison | Subgroup | Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Observational comparisons | ||
| baseline aspirin use versus no baseline aspirin use | All patients | 0.83 (0.68 to 1.01) |
| Patients assigned to the placebo arm | 0.73 (0.55 to 0.97) | |
| Patients assigned to the ERDP/ASA arm | 0.90 (0.67 to 1.22) | |
| Randomized comparisons | ||
| assignment to ERDP/ASA versus assignment to placebo | All patients | 0.82 (0.68 to 0.99) |
| Patients not taking aspirin at baseline | 0.75 (0.59 to 0.96) | |
| Patients taking aspirin at baseline | 0.89 (0.66 to 1.19) | |
| Randomized/observational comparison | ||
| patients randomized to ERDP/ASA or receiving aspirin at baseline (“any aspirin”) versus patients randomized to placebo and not receiving aspirin at baseline (“no aspirin”) | — | 0.75 (0.61 to 0.93) |
Shown are HRs for the overall comparison and each subgroup of aspirin exposure in the observational trial (aspirin use vs. nonuse at baseline, first panel) and in the randomized trial (ERDP/ASA vs placebo, second panel) as well as a post hoc comparison of patients randomized to ERDP/ASA or receiving aspirin at baseline (“any aspirin”) vs. patients randomized to placebo and not receiving aspirin at baseline (“no aspirin”; third panel). The p-value for the overall observational comparison between aspirin users and nonusers was 0.06 (first panel), and for the overall comparison of randomized treatment groups was 0.036 (second panel). The HRs for the interaction term comparing the two randomized subgroups (ERDP/ASA vs placebo, first panel) did not differ significantly between aspirin users and nonusers in the observational trial (P = 0.42). Similarly, the HRs for the interaction term comparing the subgroups of aspirin users vs nonusers at baseline (second panel) did not differ significantly between the two randomized groups in the randomized trial (P = 0.42).
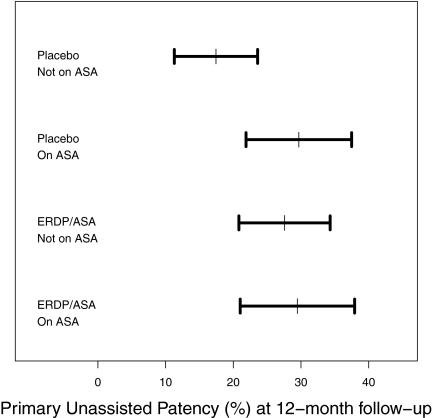
The bottom panel of Table 3 shows the result of the post hoc analysis of the primary unassisted patency outcome for the subgroup of patients with no aspirin exposure at randomization to the combined three subgroups of patients with some aspirin exposure at randomization derived from the combined observational and randomized studies. The rate of loss of primary unassisted patency was 25% lower in patients exposed to any aspirin at randomization (i.e., patients who were either randomized to ERDP/ASA or were aspirin users at baseline) than in those patients not exposed to aspirin at randomization (i.e., randomized to placebo and were not aspirin users at baseline, HR = 0.75, 95% CI = 0.61 to 0.93, P = 0.02). As shown in Figure 2, in this post hoc analysis, the point estimate and 95% CI for 1-year primary unassisted patency was 17.4% (11.3 to 23.6%) for the subgroup with no aspirin exposure at randomization (placebo and no ASA at baseline), whereas it was 29.7 (21.9 to 37.4%), 27.6 (20.8 to 34.3%), and 29.5% (21 to 37.9%) for each of the three subgroups with some aspirin exposure at randomization (placebo and on aspirin at baseline, ERDP/ASA and no aspirin at baseline, and ERDP/ASA and on aspirin at baseline, respectively).
Figure 2.
Primary unassisted graft patency is prolonged for all groups exposed to aspirin. Primary unassisted graft patency and 95% confidence intervals at 1 year are shown for the four groups of aspirin exposure: patients randomized to either ERDP/ASA or placebo and patients who were users or nonusers of aspirin at baseline.
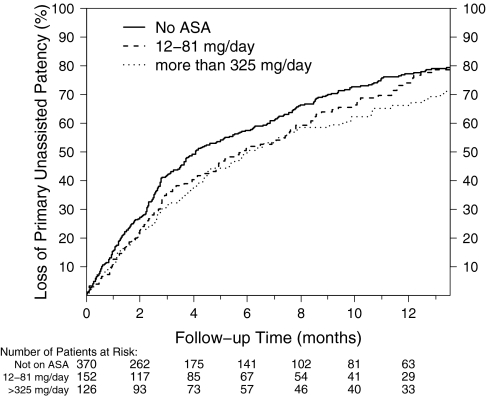
In a separate analysis, study participants were categorized into the following groups based on self-reported baseline aspirin use: no aspirin (370 participants), a dose of 12 to 81 mg (n = 152 participants), or a dose ≥325 mg of aspirin (n = 126 participants) per day. The reduction in loss of primary unassisted patency for participants on baseline aspirin at doses of 12 to 81 mg/d was 11% (HR = 0.89, 95% CI = 0.70 to 1.12, P = 0.30), whereas participants on ≥325 mg/d of baseline aspirin experienced a 24% (HR = 0.76, 95% CI = 0.59 to 0.99, P = 0.04) reduction in loss of primary unassisted patency compared with participants not on aspirin at baseline (Figure 3).
Figure 3.
Prolongation of primary unassisted graft patency is associated with a higher baseline dose of aspirin. Cumulative incidence of loss of primary unassisted graft patency in patients stratified into three levels of self-reported baseline aspirin use: no aspirin use (solid line) or 12 to 81 mg/d (dashed line) or ≥325 mg/d (dotted line) of aspirin. Patients using 12 to 81 mg/d and ≥ 325 mg/d of aspirin at baseline had an 11 (HR = 0.89, 95% CI = 0.70 to 1.12, P = 0.30) and 24% (HR = 0.76, 95% CI = 0.59 to 0.99, P = 0.04) reduction in loss of primary unassisted patency compared with participants on no aspirin at baseline, respectively.
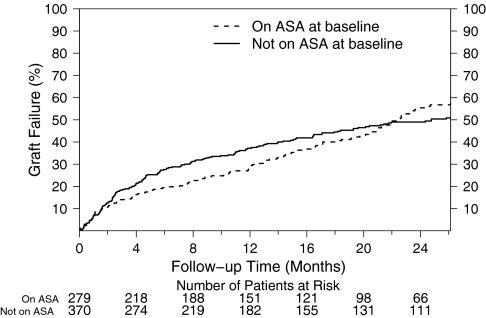
The cumulative graft failure according to baseline aspirin use is shown in Figure 4. Although there was an early trend that appeared to favor aspirin use at baseline for cumulative graft failure, overall there was no statistically significant association between baseline aspirin use and any of the secondary endpoints of cumulative graft failure, death, or the composite of these two outcomes (Table 2).
Figure 4.
Cumulative graft patency is not prolonged by baseline aspirin use. Cumulative incidence of loss of cumulative graft patency in baseline aspirin users (dashed line) and nonusers (solid line).
Adverse Events
Serious adverse events during follow-up occurred in 56% of participants on aspirin at baseline (1.34 events per participant-year) and in 53% not on aspirin at baseline (1.30 events per participant-year; HR for aspirin at baseline = 0.90; 95% CI = 0.71 to 1.13). As shown in Table 4, the risk of bleeding, death, hospitalization, or vascular access events was not statistically significantly increased among participants on aspirin at baseline.
Table 4.
Adverse events based on use of aspirin at baseline
| Event | On Aspirin at Baseline (n = 272) |
Not On Aspirin at Baseline (n = 377) |
Hazard Ratio (95% Confidence Interval)a | ||
|---|---|---|---|---|---|
| Number of Patients (%) | First Event Rate per Patient-Yearb | Number of Patients (%) | First Event Rate per Patient-Yearb | ||
| Any serious adverse event | 155 (56%) | 1.34 | 194 (53%) | 1.30 | 0.90 (0.71 to 1.13) |
| Bleedingc | 37 (13%) | 0.24 | 40 (11%) | 0.20 | 1.31 (0.80 to 2.17) |
| intermediate/minor bleeding | 24 (9%) | 0.15 | 25 (7%) | 0.13 | |
| major bleeding | 4 (1%) | 0.02 | 11 (3%) | 0.05 | |
| life threatening | 9 (3%) | 0.06 | 8 (2%) | 0.04 | |
| Hospitalization | 151 (54%) | 1.30 | 192 (52%) | 1.29 | 0.89 (0.70 to 1.12) |
| ischemic heart disease | 23 (8%) | 0.15 | 14 (4%) | 0.07 | |
| congestive heart failure | 17 (6%) | 0.11 | 22 (6%) | 0.11 | |
| Arrhythmia | 6 (2%) | 0.04 | 12 (3%) | 0.06 | |
| cerebrovascular disease | 5 (2%) | 0.03 | 3 (1%) | 0.02 | |
| peripheral vascular disease | 8 (3%) | 0.05 | 11 (3%) | 0.06 | |
| vascular access event: study access | 42 (15%) | 0.27 | 68 (18%) | 0.35 | 0.79 (0.52 to 1.20) |
| vascular access event: nonstudy access | 9 (3%) | 0.06 | 20 (5%) | 0.10 | 0.74 (0.32 to 1.73) |
| death | 18 (7%) | 0.11 | 12 (3%) | 0.06 | 1.52 (0.68 to 3.39) |
aHazard ratio, time to first event per patient. P > 0.27 for each category of serious adverse effect.
bEvent rate was a patient-level analysis calculated as the ratio of the number of first serious adverse effects to the total patient-years of follow-up until the first serious adverse effect of this type in each patient.
cIntermediate or minor bleeding events were those that were not classified as major, life threatening, or fatal. Major bleeding was defined as a confirmed retroperitoneal, intra-articular, intraocular, or intracranial bleed or any bleed that led to a drop in hemoglobin by 2 g/dl and required hospitalization or the need for a transfusion. Life-threatening bleeding was defined as any bleed leading to a drop in hemoglobin of ≥5 g/dl, required emergency surgical intervention, caused a symptomatic intracranial hemorrhage, or required a transfusion of more than 4 units of packed red blood cells or whole blood. Fatal bleeding was any bleed that caused or precipitated death.
DISCUSSION
Use of aspirin at entry into the clinical trial of ERDP/ASA was associated with a 17% lower risk of loss of primary unassisted graft patency independent of treatment assignment in the clinical trial that did not meet predefined criteria for statistical significance. This effect seemed to be dose dependent, with an 11 and 24% risk reduction in loss of primary graft patency for participants on a baseline aspirin dose of 12 to 81 and ≥325 mg per day, respectively. The magnitude of the reduction in loss of primary unassisted graft patency associated with use of aspirin at baseline was similar to the observed 18% reduction in the rate observed for the randomized comparison of ERDP/ASA versus placebo in the clinical trial.6 In contrast, as observed for ERDP/ASA in the randomized clinical trial, there was no statistically significant association between baseline aspirin use and the secondary outcomes of cumulative graft patency or participant survival.
A small randomized clinical trial reported that dipyridamole with or without aspirin, but not aspirin alone, reduced graft thrombosis.8 However, larger studies of aspirin alone or in combination with another anti-platelet agent have reported trends for aspirin to improve graft patency.9–11 The Dialysis Outcomes and Practice Patterns Study (DOPPS) found that treatment with aspirin was associated with a 16% (P = 0.069) reduction in the adjusted relative risk for loss of primary graft patency and a 30% (P < 0.001) lower risk of loss of cumulative graft patency.10 A retrospective analysis of the U.S. Renal Data System Mortality and Morbidity Wave II study found that use of aspirin was associated with a 22% reduction in the hazard ratio for graft failure (HR = 0.78, 95% CI = 0.48 to 1.26).11 A randomized double-blind placebo controlled trial of aspirin plus clopidogrel reported a 19% reduction in the hazard ratio for loss of primary unassisted graft patency (HR = 0.81, 95% CI = 0.49 to 1.40, P = 0.45), but was terminated early because of increased risk of bleeding.9 Several smaller studies have also reported a beneficial effect of aspirin or clopidogrel on hemodialysis graft patency.12–14 Compared with these prior vascular access studies, this study had the largest number of participants on aspirin and the reduction in the adjusted hazard ratio for loss of primary unassisted graft patency (17%) was similar to the prior studies (16 to 22%).9–11 Collectively, these studies suggest that aspirin may be effective in prolonging primary patency of hemodialysis grafts. However, as noted for ERDP/ASA in our randomized clinical trial, the overall effect is clinically modest.6
The hypothesis for the clinical trial was that ERDP directly acts on vascular smooth muscle to inhibit the progression of venous stenosis, leading to graft thrombosis.7 However, aspirin works primarily as an anti-platelet agent. The observation in this study that ERDP/ASA and nonstudy aspirin prolong primary unassisted graft patency to a similar degree raises the question whether the effect of both drugs is mediated primarily by an action on platelets and not vascular smooth muscle cells. Consistent with this hypothesis, a recent study in swine showed that direct application of dipyridamole to the periadventitial surface of the graft-vein anastomosis in a concentration that inhibits vascular smooth muscle proliferation in vitro failed to prevent the development of neointimal hyperplasia.15
Whether the combination of ERDP/ASA is better than aspirin for hemodialysis graft patency remains uncertain. Two large trials in patients with cerebrovascular disease have shown that ERDP/ASA is more effective than aspirin alone for secondary prevention of stroke or vascular death.16,17 However, in this study, the observed reduction in the rate of loss of primary unassisted patency with aspirin alone was similar to that observed for ERDP/ASA in the clinical trial.6 Moreover, the primary unassisted patency among participants randomized to ERDP/ASA, but not taking aspirin at baseline appeared very similar to that among participants taking aspirin at baseline regardless of treatment with ERDP/ASA (Figure 2). A randomized controlled trial comparing ERDP/ASA to aspirin alone for hemodialysis graft patency would be needed to definitely answer this question and address whether any added benefit of ERDP would exceed its additional cost.
In this study, there was no statistically significant association between baseline aspirin use and cumulative graft patency. In contrast, the Dialysis Outcomes and Practice Patterns Study (DOPPS) found a statistically significant association of baseline aspirin use with prolongation of cumulative graft survival.10 The average follow-up for cumulative patency in the Dialysis Outcomes and Practice Patterns Study was only 8.5 months compared with 16.9 months in this study. The longer follow-up in this study may have allowed for more cross-over in aspirin use between groups that might have obscured an association between cumulative graft patency and aspirin exposure. Further study is needed to determine whether aspirin prolongs cumulative graft patency.
The risk of bleeding is generally increased in the hemodialysis population, which raises concerns about the safety of using anti-platelet agents in this population.18 A previous trial of clopidogrel plus aspirin for hemodialysis graft patency was stopped early because of increased risk of bleeding.9 In our trial of ERDP/ASA, we did not observe an increased risk of bleeding or other adverse events compared with placebo.6 In this study, treatment with aspirin at baseline was associated with a higher rate of bleeding, but this was not statistically significant. The overall rate of adverse events was not greater among participants on aspirin at baseline. These findings suggest that treatment with aspirin was relatively well tolerated. However, patients at high risk of bleeding were excluded from the trial, and the median follow-up for adverse events was only about 6 months. The risk of bleeding while on aspirin is likely to be higher in the general hemodialysis population, and the cumulative risk will likely increase with longer use.
This study has several strengths. It represents the largest study of aspirin's effect on hemodialysis graft patency to date. As part of a prospective clinical trial of ERDP/ASA on graft survival, baseline data including medication use was carefully assessed, and participants had close follow-up, enabling the recording of vascular access outcomes, medication use, and adverse events. In particular, specific focus was given to determining the use and dose of aspirin at each follow-up study visit.
A limitation, common to retrospective analyses, is the problem of confounding by indication. Aspirin was most likely prescribed for secondary prevention of vascular disease. Consistent with this, participants on baseline aspirin were older, had a statistically significant higher prevalence of diabetes and vascular disease, and had a lower likelihood of current use of tobacco. Age, diabetes, and vascular disease have been reported to be associated with decreased vascular access patency in some studies. Hence, the risk of graft failure may have been higher in the aspirin-treated population, but this would potentially create a bias against finding an association of aspirin with improved graft patency.
A second limitation was the use of baseline aspirin exposure to predict future vascular access outcomes. Although this is a common study design, it does not address changes in nonstudy aspirin use during follow-up. This can lead to misclassification bias that might obscure the true relationship between aspirin use and graft patency. Moreover, as per the clinical trial study design, collection of information on medication use including aspirin was stopped 1 month after the occurrence of the primary outcome. This precluded using aspirin exposure as a time-dependent variable in the analysis of aspirin's effect on cumulative graft patency.
An additional potential limitation is that nonstudy aspirin use was based on self-report. Possible resultant misclassification of exposure would tend to diminish the strength of an association.
Finally, the clinical trial did not reach its original enrollment goal of 1056 patients, which may limit study power for subsequent secondary analyses.
In conclusion, this secondary cohort analysis of the ERDP/ASA clinical trial suggests that aspirin alone may modestly prolong primary unassisted patency of newly created hemodialysis grafts in a dose-dependent fashion. The point estimate for the rate of loss of primary patency was similar to that observed for ERDP/ASA in the clinical trial. Whether ERDP/ASA is more effective than aspirin alone is unknown and the effect of aspirin on cumulative graft patency remains uncertain. However, the absolute magnitude of using either ERDP/ASA or aspirin on unassisted graft patency compared with those receiving no anti-platelet agent seems greater than that originally reported in the randomized trial. Aspirin may be a cost-effective alternative to prolong unassisted patency in patients receiving a new synthetic hemodialysis vascular access graft.
CONCISE METHODS
Study Design
We performed a secondary cohort analysis of participants (n = 649) enrolled in a randomized double-blind placebo-controlled clinical trial of ERDP/ASA, which permitted the use of nonstudy aspirin, to determine the association of baseline aspirin use with hemodialysis graft patency.
Details of the trial design and major outcomes have been previously published.6,7 Persons were ineligible for the trial if they were pregnant or breastfeeding, had an increased risk of bleeding or a known bleeding disorder, had active esophagitis, gastritis, or peptic ulcer disease, had a platelet count <75,000/μl, had advanced liver disease, or required an anti-coagulant or anti-platelet agent other than aspirin.
Participants were randomized within 2 days after successful access surgery (i.e., a patent graft) to either ERDP/ASA (Aggrenox, containing 200 mg ERDP and 25 mg aspirin) or an identical looking placebo taken orally twice daily. Treatment continued until the occurrence of the primary outcome.
Participants were followed monthly after graft surgery to examine the access; record access-related complications, adverse drug reactions, and hospitalizations; determine the use and dose of nonstudy aspirin, measure access blood flow, and assess ERDP/ASA compliance by pill count. Follow-up was continued for a period of 1 month after the occurrence of the primary outcome.
As in the clinical trial, the primary outcome for this study was loss of primary unassisted graft patency, defined as the first occurrence of graft thrombosis or an access procedure performed to correct a stenosis ≥50% of the diameter of the adjacent normal vessel or other surgical modification of the graft (e.g., for infection). Participants were referred for angiography if the access blood flow declined to <600 ml/min or declined >25% from baseline and was <1000 ml/min.7 Among participants undergoing regular hemodialysis using a catheter, failure to use the graft by 12 weeks after placement was considered graft failure.
The major secondary outcomes, consistent with the clinical trial, were cumulative access patency, defined as the time from randomization to complete loss of the access site for dialysis, death from any cause, and a combined outcome including death from all causes and cumulative patency, each of which was ascertained until the administratively defined end of the study.
Statistical Analysis
Standard descriptive statistics were used to compare baseline demographic and clinical characteristics between aspirin users and nonusers at baseline. The frequency of use and dose of nonstudy aspirin were analyzed at 3-month intervals up to 1 year after randomization for participants who remained in the study and who had not reached the primary unassisted patency outcome.
Cumulative incidence curves comparing rates of loss of primary unassisted patency and the secondary outcomes between aspirin users and nonusers were prepared using the Kaplan-Meier method. Cox proportional hazards regression was used to jointly relate the primary and secondary outcomes to randomized treatment assignment and to an indicator variable for aspirin use at baseline after controlling for baseline covariates. The baseline covariates included seven factors (age, diabetes history, cardiovascular disease, cerebrovascular disease, peripheral arterial disease, smoking status, diastolic BP) implicated in graft survival that were significantly unbalanced between baseline aspirin users and nonusers (P < 0.05) and two additional baseline factors (serum albumin and use of an angiotensin converting enzyme inhibitor and/or angiotensin receptor blocking anti-hypertensives) (19,20) that were prespecified as covariates in the trial protocol for comparisons of patency outcomes between the randomized treatment groups. The baseline hazard function was stratified by vascular access location and clinical center.6 The proportional hazard assumption was assessed by checking Martingale residuals.21 The functional forms of each continuous covariate were assessed by a supremum test.21 Subgroup analysis was performed by applying the same model to the corresponding subset of the data.
In further analyses, an interaction term between baseline aspirin use and randomized ERDP/ASA assignment was added to the basic Cox model. To assess dose response, the analysis was repeated after grouping participants into three clinically relevant levels of daily aspirin use: none, low dose (12 to 81 mg per day), and higher dose (≥325 mg per day). (No participants were on a dose of 82 to 324 mg of aspirin per day.) Follow-up time for the primary and secondary outcome was handled as per the randomized clinical trial.6 All P values are two-sided and were not adjusted for multiple testing.
DISCLOSURES
Dr. Dixon reports receiving consulting fees from Proteon Therapeutics, Pervasis Therapeutics, and Shire Pharmaceuticals and receives grants from Proteon Therapeutics, Novartis Pharmaceuticals, and Cardiokine. Dr. Beck reports receiving grant support from Boehringer Ingelheim Pharmaceuticals. Dr. Delmez reports receiving consulting fees from Abbott Laboratories and Genzyme and grant support from Genzyme and Proteon Therapeutics. Dr. Allon reports receiving consulting fees from Angiotech. Dr. Dember reports receiving research support from Proteon Therapeutics, consulting fees from Shire Pharmaceuticals and Bristol-Myers Squibb, an d grant support from Neurochem. Dr. Himmelfarb reports receiving consulting fees from Boehringer Ingelheim Pharmaceuticals. Dr. Feldman reports receiving consulting fees from GE Healthcare for providing expert testimony and grant support from Amgen–RTI and GE–Healthcare.
Acknowledgments
This clinical trial was supported by cooperative agreements U01DK058981, U01DK058982, U01DK058973, U01DK058985, U01DK058978, U01DK058968, U01DK058986, and U01DK058966 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Boehringer-Ingelheim Pharmaceuticals, Ridgefield, CT, provided support, ERDP/ASA, and placebo. We are indebted to all the Dialysis Access Consortium investigators, study coordinators and patients who participate in the trial.
Boston University Medical Center—L. Dember, J. Kaufman, M. Hawley, A. Lauer, P. LeSage, R. Nathan, E. Holmberg; Baystate Medical Center—G. Braden, M. Ryan, A. Berkowitz; Charleston Area Medical Center—A. Rahman, B. Lucas Jr, R. Santos, B. Reyes; Duke University Medical Center—A. Greenberg, M. Berkoben, E. Kovalik, J. Lawson, J. Middleton, S, Schwab, D. Schumm, S. Adams, K. Gitter, T. Cantaffa, A. Quarles; Emory University—J. Work, S. Rhodes; Maine Medical Center—J. Himmelfarb, J. Whiting, J. Kane, S. Freedman, R. Violette, H. Cyr-Alves, K. Garrison; St. Louis University—K. Martin, P Schmitz, V. Jenkins; Tyler (Texas) Nephrology Associates—J. Cotton Jr., E. Husband; University of Alabama at Birmingham—M. Allon, M. Robbin, M. Lockhart, B. Casey, J. Newsome; University of Iowa, Iowa City, IA—B. Dixon, B. Franzwa, L. Hunsicker, J. Hoballah, D. Katz, W. Sharp, T. Kresowik, Y. Wu, S Rayhill; Renal Core Associates (Peoria, IL) —T. Pflederer, K. DuPage, K. Welch, F. Darras, A. Banqero, B. Ketel, A. Wounded Arrow, C. Grant, J. Deeb, L. Pyszka, Covenant Medical Center (Waterloo, IA) —M. Slavin, D. Wedeking; University of Texas Southwestern—M. Vazquez, I. Davidson, R. Toto, L. Littmon, C. Ying, T. Lightfoot, H. Quinones, R. Saxena, P. Clagett, J. Valentine, B. Dolmatch, J. Thompson; Baylor University Medical Center—A. Fenves, G. Pearl; Vanderbilt University Medical Center—A. Ikizler, P. Egbert; Vascular Surgery Associates (Baton Rouge, LA.) —J. McNeil, D. Holmes, W. Freiberger; Washington University in St. Louis—J. Delmez, D. Windus, D. Coyne, M. Rothstein, S. Shenoy, R. Creaghan, B. Lluka; National Institute of Diabetes and Digestive and Kidney Diseases—J. Kusek, C. Meyers; Steering Committee Chair—H. Feldman (U. Pennsylvania); Data Coordinating Center (Cleveland Clinic Foundation) —G. Beck, J. Gassman, T. Greene, B. Hu, S. Bi, A. Liu, M. Radeva, L. Tuason, B. Weiss; Data and Safety Monitoring Board (DSMB) —N. Levin (Chair), A. Besarab, G. Chertow, M. Diener-West, T. Louis, W. McClellan, C. Stehman-Breen.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Aspirin and Arteriovenous Graft Thrombosis in Hemodialysis: Just What the Doctor Ordered?” on pages 595–597.
REFERENCES
- 1. Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Besarab A, Work J: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO: Vascular access survival and incidence of revisions: a comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg 34: 694–700, 2001 [DOI] [PubMed] [Google Scholar]
- 4. U.S. Renal Data System: 2008 Annual Data Report: Atlas of End-stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: 2008 [Google Scholar]
- 5. Kanterman RY, Vesely TM, Pilgram TK, Guy BW, Windus DW, Picus D: Dialysis access grafts: Anatomic location of venous stenosis and results of angioplasty. Radiology 195: 135–139, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr, Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW, Feldman HI, Group, DACS: Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dixon BS, Beck GJ, Dember LM, Depner TA, Gassman JJ, Greene T, Himmelfarb J, Hunsicker LG, Kaufman JS, Lawson JH, Meyers CM, Middleton JP, Radeva M, Schwab SJ, Whiting JF, Feldman HI: Design of the dialysis access consortium (DAC) aggrenox prevention of access stenosis trial. Clin Trials 2: 400–412, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Sreedhara R, Himmelfarb J, Lazarus JM, Hakim RM: Anti-platelet therapy in graft thrombosis: Results of a prospective, randomized, double-blind study. Kidney Int 45: 1477–1483, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Kaufman JS, O'Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, Goldfarb DS, Peduzzi PN: Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol 14: 2313–2321, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Saran R, Dykstra DM, Wolfe RA, Gillespie B, Held PJ, Young EW: Association between vascular access failure and the use of specific drugs: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 40: 1255–1263, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Yevzlin AS, Conley EL, Sanchez RJ, Young HN, Becker BN: Vascular access outcomes and medication use: A USRDS study. Semin Dial 19: 535–539, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Domoto DT, Bauman JE, Joist JH: Combined aspirin and sulfinpyrazone in the prevention of recurrent hemodialysis vascular access thrombosis. Thromb Res 62: 737–743, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Trimarchi H, Young P, Forrester M, Schropp J, Pereyra H, Freixas E: Clopidogrel diminishes hemodialysis access graft thrombosis. Nephron Clin Pract 102: c128–c132, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Harter HR, Burch JW, Majerus PW, Stanford N, Delmez JA, Anderson CB, Weerts CA: Prevention of thrombosis in patients on hemodialysis by low-dose aspirin. N Engl J Med 301: 577–579, 1979 [DOI] [PubMed] [Google Scholar]
- 15. Kuji T, Masaki T, Goteti K, Li L, Zhuplatov S, Terry CM, Zhu W, Leypoldt JK, Rathi R, Blumenthal DK, Kern SE, Cheung AK: Efficacy of local dipyridamole therapy in a porcine model of arteriovenous graft stenosis. Kidney Int 69: 2179–2185, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A: European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 143: 1–13, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A: Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): Randomised controlled trial. Lancet 367: 1665–1673, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hiremath S, Holden RM, Fergusson D, Zimmerman DL: Antiplatelet medications in hemodialysis patients: A systematic review of bleeding rates. Clin J Am Soc Nephrol 4: 1347–1355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gradzki R, Dhingra RK, Port FK, Roys E, Weitzel WF, Messana JM: Use of ACE inhibitors is associated with prolonged survival of arteriovenous grafts. Am J Kidney Dis 38: 1240–1244, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Miller PE, Carlton D, Deierhoi MH, Redden DT, Allon M: Natural history of arteriovenous grafts in hemodialysis patients. Am J Kidney Dis 36: 68–74, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Lin DY, Wei LJ, Ying Z: Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 80: 557–572, 1993 [Google Scholar]