Abstract
Objective
Since nucleus pulposus cells reside in hypoxia, we determined if expression of ANK, a pyrophosphate transporter, is regulated by the HIF proteins.
Methods
Quantitative RT-PCR and Western blot were used to measure ANK expression in nucleus pulposus cells. Transfections were performed to determine the effect of HIF-1/-2 on ANK promoter activity.
Results
ANK was expressed in embryonic and mature rat disc. Oxygen dependent changes in ANK expression in nucleus pulposus cells were minimal. However, silencing of HIF-1α and HIF-2α resulted in increased ANK expression and upregulation of promoter activity. HIF mediated suppression of ANK was validated by measuring promoter activity in HIF-1β null embryonic fibroblasts. Compared with wild type cells, in hypoxia, there was induction of promoter activity in the null cells. We overexpressed HIF-1α and HIF-2α in nucleus pulposus cells and noted a significant suppression in ANK promoter activity. Since the ANK promoter contains two hypoxia response elements (HRE), we performed site-directed mutagenesis and measured promoter activity. We found that HIF-1 can bind to either of the HRE and suppress promoter activity. In contrast, HIF-2 was required to bind to both HRE to suppress activity. Finally, analysis of human nucleus pulposus tissue showed that while ANK was expressed in normal tissue, there was increased expression of ANK along with alkaline phosphatase in the degenerate state.
Conclusion
Both HIF-1 and HIF-2 serve as negative regulators of ANK expression in the disc. We propose that baseline ANK expression in the disc serves to prevent mineral formation under physiological conditions.
Keywords: intervertebral disc, nucleus pulposus, hypoxia, HIF, ANK, mineralization, disc degeneration
Introduction
Within the spine, the vertebrae are separated by a complex tissue, the intervertebral disc. At the disc periphery, a ligamentous tissue, the annulus fibrosus, encloses the proteoglycan-rich nucleus pulposus. Although details of the ontology of cells of the adult nucleus pulposus are obscure, it is known that they are derived from the notochord (1), an embryonic tissue with a limited blood supply. The superior and inferior boundaries of the intervertebral disc are formed by the cartilage endplates. A limited number of blood vessels infiltrate the endplates and the outer annulus fibrosus, but do not enter the nucleus pulposus (2, 3). For this reason, there is considerable support for the view that the nucleus pulposus cells reside in an hypoxic environment (4–6). Surprisingly, while the disc contains both fibrous proteins and a hydrated extracellular matrix, calcified deposits are absent in the nucleus pulposus and the annulus fibrosus in healthy state.
Deposition of mineral salt is regulated by ANK, a multipass transmembrane channel that controls the transport of inorganic pyrophosphate (PPi) a powerful inhibitor of mineralization (7). Several genetic studies have identified multiple mutations in ANK that result in autosomal dominant disorders causing abnormal mineralization in joints and bone (8–11). These disorders include familial chondrocalcinosis, characterized by excessive deposition of calcium pyrophosphate dihydrate crystals in affected joints, and cranial metaphyseal dysplasia, Jackson type, whose phenotype includes hyperostosis of craniofacial bones (8–11). In articular and growth plate cartilages, ANK expression is primarily localized to the superficial zone and the hypertrophic zone, respectively, suggesting that ANK expression is dependent on oxygen availability (12). Indeed, in a recent study Zaka et al have demonstrated that the oxemic status of chondrocytes influences ANK expression and its regulation was mediated by hypoxia inducible factor (HIF)-1α (13). The goal of the present investigation was threefold: to determine if ANK expression in cells of the intervertebral disc was dependent on the local oxygen tension, to determine the role of HIF in ANK regulation, and to investigate the relationship between disc degeneration and ANK expression. Using nucleus pulposus cells of the intervertebral disc and embryonic fibroblasts (MEF) from HIF-1β null mice, we show for the first time that HIF-1 and HIF-2 regulate ANK gene expression in a unique fashion. This finding lends further credence to the view that HIFs and ANK functionally adapt nucleus pulposus cells to the unique microenvironment of the disc.
Materials and Methods
Plasmids and Reagents
Expression plasmids were provided by Dr. Celeste Simon, University of Pennsylvania [pCA-HIF-2α with a triple mutation (P405A/P530A/ N851A)] (14), Dr. Eric Huang, National Cancer Research Institute, Bethesda [p(HA)HIF-11α(401Δ603) and pARNT]. HIF-2α siRNA and control SiRNA duplexes were purchased from Dharmacon (ON-TARGET plus SMARTpool). SiHIF-1α (PBS/pU6-HIF1α) plasmid developed by Dr. Connie Cepko was obtained from Addgene (#21103) (15). Construction of wild type and mutant ANK reporter plasmids has been reporter before (13) wild type reporter contains a 388 bp of proximal ANK promoter from −684 bp to −296 bp with respect to the translation initiation into the pGL4.10 luciferase reporter vector. As an internal transfection control, pRL-TK (Promega) containing Renilla reniformis luciferase genes was used. For Western blots, rabbit polyclonal antibodies directed against two different peptides sequences in mouse ANK were used (Ab36, aa 36–49: RGIAAVKEDAVEML, Ab218, aa 218–231: DIIPDRSGPEGGD).
Isolation of nucleus pulposus cells
Rat nucleus pulposus cells were isolated using an explant culture method reported earlier by Risbud et al. (16). Nucleus pulposus cells migrated out of the explant after 1 week. When confluent, the cells were lifted using a trypsin (0.25 %) EDTA (1 mM) solution and sub-cultured in 10 cm dishes. These cells expressed high levels of aggrecan and collagen type II and were used for the studies described below.
Human tissue collection and grading
Lumbar disc tissues were collected as surgical waste from individuals undergoing elective spinal surgical procedures (average age 54 years, ranging from 38–82 years). In line with Thomas Jefferson University’s Institutional Review Board guidelines, informed consent for sample collection was obtained for each patient. Assessment of the disease state was performed using the modified Thompson grading (17).
Cell culture in hypoxia
Nucleus pulposus cells, and MEF from HIF-1β wild type (+/+) and null (−/−) mice (kindly provided by Dr. Celeste Simon, University of Pennsylvania) were maintained in Dulbeccos Modified Eagles Medium (DMEM) and 10% fetal bovine serum (FBS) supplemented with antibiotics. Cells were cultured in an Hypoxia Work Station (Invivo2, Ruskinn, UK) with a mixture of 1% O2, 5% CO2, 93% N2 for 24 −48 h.
Immunohistological studies
Freshly isolated spines or whole embryos were immediately fixed in 4% paraformaldehyde in PBS and then embedded in paraffin. Transverse and coronal sections, 6–8 µ in thickness, were deparaffinized in xylene, rehydrated through graded ethanol and stained with alcian blue, eosin and hematoxylin. For localizing ANK, sections were incubated with the peptide directed rabbit polyclonal anti-ANK218 antibody in 2% bovine serum albumin in PBS at a dilution of 1:200 at 4 °C overnight. After thoroughly washing the sections, the bound primary antibody was incubated with Alexa fluor-488 conjugated anti-rabbit secondary antibody (Invitrogen), at a dilution of 1:100 for 45 min at room temperature. Sections were mounted in mounting medium containing DAPI and visualized using a fluorescence microscope (Olympus, Japan).
Western blotting
Cells were placed on ice immediately following treatment and washed with ice-cold HBSS. All the wash buffers and final re-suspension buffer included 1X protease inhibitor cocktail (Roche), NaF (5 mM) and Na3VO4 (200 µM). Total cell proteins were resolved on 8–12 % SDS-polyacrylamide gels and transferred by electroblotting to PVDF membranes (Bio-Rad, CA). The membranes were blocked with 5% non-fat dry milk in TBST (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% tween 20) and incubated overnight at 4 °C in 3% non-fat dry milk in TBST with the anti-ANK (1:1500) or anti-β-tubulin antibody (1:3000, DSHB). Immunolabeling was detected using the ECL reagent (Amersham Biosciences). Blot intensity was determined by densitometric analysis using Kodak 1D 3.6 software (Kodak, Rochester, NY)
Real time RT-PCR analysis
Total RNA was extracted from nucleus pulposus cells using RNAeasy mini columns (Qiagen). Before elution from the column, RNA was treated with RNase free DNAse I (Qiagen). For human samples, total RNA was isolated from 100 to 300 mg of nucleus pulposus tissue. Tissue was homogenized in Trizol (Invitrogen) on ice using Omni TH Homogenizer (Omni International). Following Trizol extraction, RNA was passed through the RNAEasy mini columns. The purified, DNA-free RNA was converted to cDNA using Superscript III Reverse Transcriptase (Invitrogen). Template cDNA and gene specific primers were added (Rat ANK F: 5’ atgggctggcgtattctctgatga 3’, Human ANK F: 5’ caacaaactggtgagcacgagcaa 3’) to Fast SYBR Green master mix (Applied Biosystems) and mRNA expression was quantified using the 7900HT Fast Real-Time PCR System (Applied Biosystems). 18S and GAPDH were used to normalize the expression. Melting curves were analyzed to verify the specificity of the RT-PCR reaction and the absence of primer dimer formation. Each sample is analyzed in duplicate and included a template-free control. All the primers used were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Immunofluorescence microscopy
Cells were plated in flat bottom 96 well plates (4 × 103/ well) and cultured in hypoxia (1% O2) for 24 h. After incubation, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% triton-X 100 in PBS for 10 min, blocked with PBS containing 5% FBS, and incubated with antibodies against ANK (1:200), at 4° C overnight. As a negative control, cells were reacted with isotype IgG under similar conditions. After washing, the cells were incubated with Alexa fluor-488 conjugated anti-rabbit secondary antibody (Invitrogen), at a dilution of 1:50 for 45 min at room temperature. Cells were imaged using a laser scanning confocal microscope (Olympus Fluoview, Japan).
Transfections and dual luciferase assay
Cells were transferred to 24-well plates at a density of 4 × 104 cells/well one day before transfection. To investigate the effect of HIF-1/2 over expression on ANK promoter activity, cells were cotransfected with 100–300 ng of CA-HIF-1α or CA-HIF-2α or backbone vectors pcDNA3.1 and 100 ng pARNT with 300 ng ANK reporter and 300 ng pRL-TK plasmid. For silencing experiments, we used 100–300 ng of ShHIF-1α or SiHIF-2α or respective control SiRNA with 400 ng of ANK reporter with 300 ng pRL-TK plasmid. LipofectAMINE 2000 (Invitrogen, CA) was used as a transfection reagent. For each transfection, plasmids were premixed with the transfection reagent. Twenty four hours after transfection, the cells were transferred to a hypoxia work station (1% O2) or maintained in normoxia (21% O2) for 24− 48 h. Cells were harvested and a Dual-Luciferase™ reporter assay system (Promega) was used for sequential measurements of firefly and Renilla luciferase activities. Quantification of luciferase activities and calculation of relative ratios were carried out using a luminometer (TD-20/20, Turner Designs, Sunnyvale, CA, USA). At least three independent transfections were performed, and all analysis were carried out in triplicate.
Statistical analysis
All measurements were performed in triplicate, data is presented as mean ± S.D. Differences between groups were analyzed by the student t test; *p< 0.05.
Results
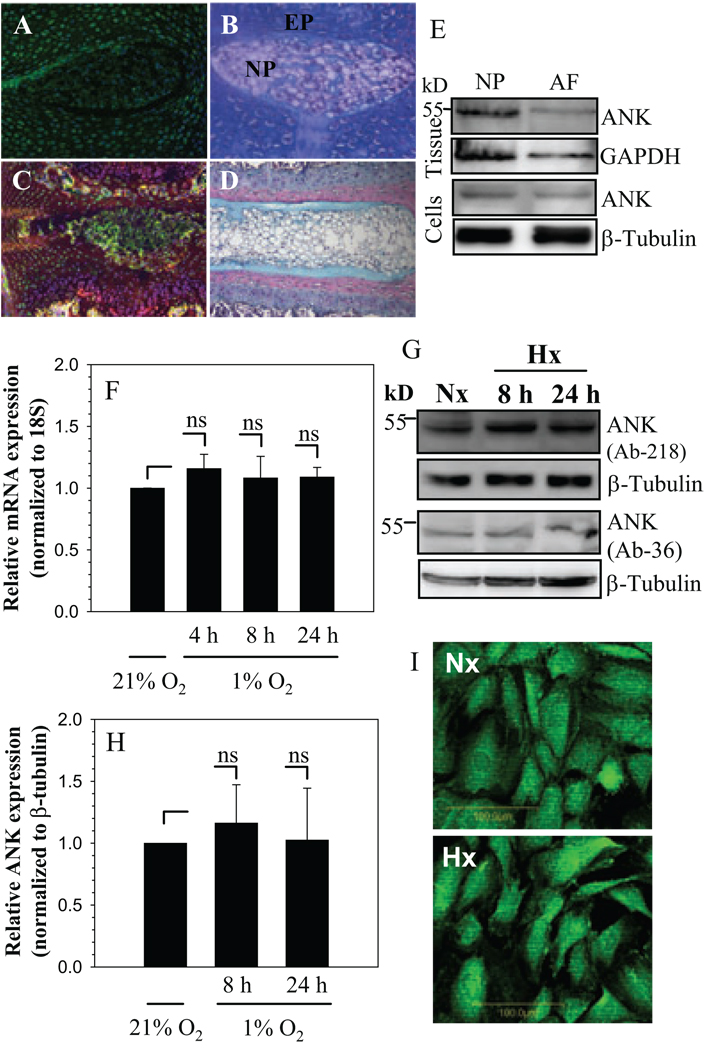
Saggital sections of the neonatal (Fig. 1 A, B) and skeletally mature rat discs (Fig. 1 C,D) were stained with an antibody to ANK (Fig. 1A, C) or counter stained with H&E and alcian blue (Fig. 1B, D). ANK is expressed by cells of the nucleus pulposus, annulus fibrosus and cartilaginous endplate (Fig. 1A, C). In all cases, staining is localized to the membrane and cytosol (Fig. 1A, C). Expression of ANK in disc tissues and cultured cells was studied using Western blot analysis. Fig. 1E indicates that nucleus pulposus and annulus fibrosus tissues express a prominent 54.4 kDa ANK band. Moreover, there is a robust expression of ANK in nucleus pulposus and annulus fibrosus cells (Fig. 1E).
Figure 1.
A–D. Sagittal sections of the intervertebral disc of embryonic (A and B) and a mature (C and D) animal. Sections were treated with ANK antibody (A and C), or counterstained with hemotoxylin, eosin and alcian blue (B and D). ANK was expressed in nucleus pulposus (NP) of the adult and the neonate (see arrow). Annulus fibrosus (AF) and endplate cartilage (EP) (Mag. X 20). E. Western blot of ANK expression in rat disc tissues and cultured cells. F. Real-time RT-PCR analysis of ANK expression by NP cells in hypoxia (1% O2). There was no significant change in ANK mRNA expression in hypoxia. G. Western blot analysis of ANK expression in NP cells in hypoxia using two ANK antibodies. A prominent ANK band (54.4 kD) was detected. H. Densitometric analysis of ANK expression on multiple Western blots. Note, there was no significant change in ANK expression in hypoxia. I, Immunofluorescent analysis of NP cells cultured in hypoxia. There was a similar level of ANK expression in normoxia and hypoxia, Mag X 20. Values shown are mean ± SE from three independent experiments, * p < 0.05, ns = non significant.
We examined the expression of ANK in the nucleus pulposus, in relationship to its oxemic status. Figure 1F shows that when nucleus pulposus cells are cultured in hypoxia, there is no induction of ANK mRNA (Fig. 1F). We then studied the effect of hypoxia on ANK protein expression in nucleus pulposus cells using Western blot analysis (Fig. 1G, H) and immunofluorescence microscopy (Fig. 1I). Two peptide directed antibodies against ANK were used for the Western blotting. Again, there is no significant difference between normoxic and hypoxic expression of ANK protein (Fig.1G–I).
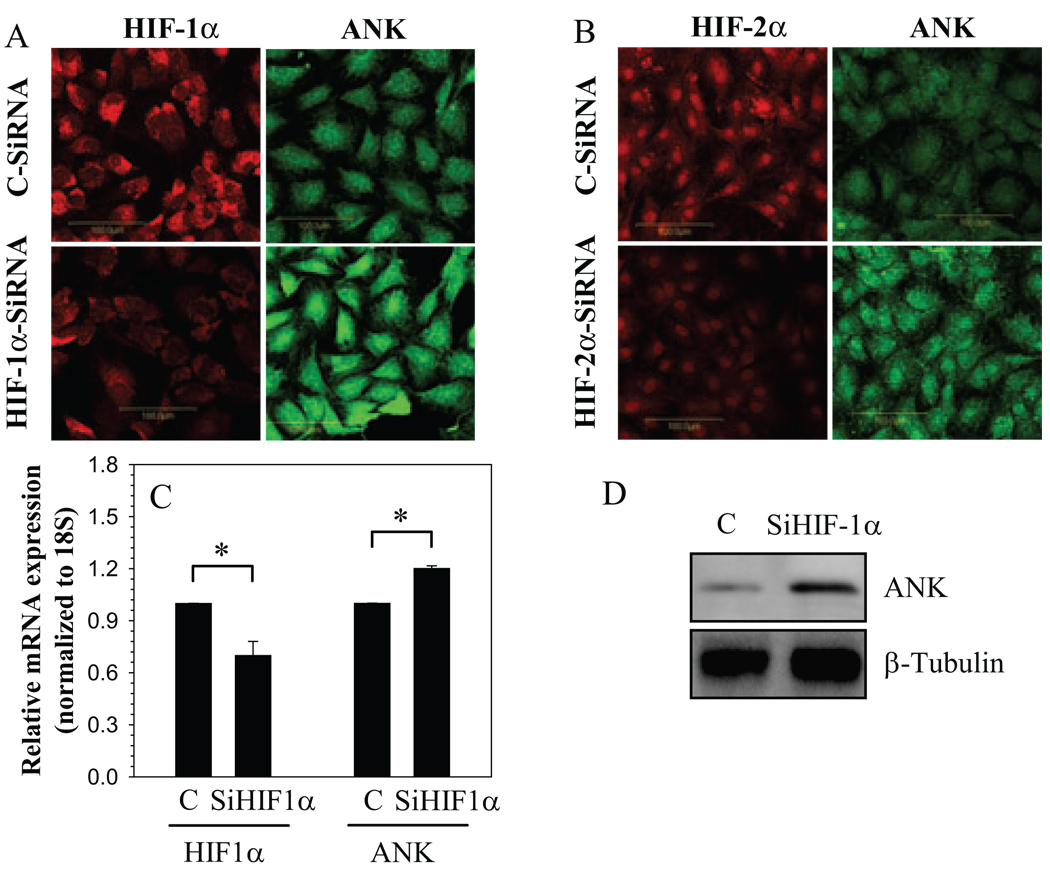
To investigate if HIF-1 and HIF-2 played a role in regulation of ANK expression in nucleus pulposus cells, we evaluated ANK expression in HIF silenced nucleus pulposus cells in hypoxia (Fig. 2A–C). As expected, HIF-1α and HIF-2α silenced cells evidence a decrease in protein and mRNA expression of HIF-1α (Fig. 2A, C) and HIF-2α protein (Fig. 2B) when compared with cells transfected with control SiRNA. In addition, we confirmed that suppression of HIF-1α lowered its target gene expression. When HIF-1α is silenced, in both normoxia and hypoxia, there is significant suppression of enolase-1 promoter activity, a known HIF-1α target gene in nucleus pulposus cells (not shown). Moreover, in hypoxia, silencing of either HIF-1α (Fig. 2A, C) or HIF-2α (Fig. 2B) results in increased ANK protein (Fig.2A, B, D) and mRNA (Fig.2C) expression when compared to cells transfected with control SiRNA.
Figure 2.
HIF regulation of ANK expression. Cells were transfected with A, C and D) HIF-1α-SiRNA (SiHIF-1α) B) HIF-2α-SiRNA (SiHIF-2α) or control siRNA (C) and cultured in hypoxia. Immunofluorescent staining for A) HIF-1α and ANK B) HIF-2α and ANK in HIF silenced cells in hypoxia. Note, the increase in ANK staining in HIF silenced cells when compared with controls. As expected silenced cells show decreased staining for respective HIFs. C) Silencing of HIF-1α results in increased ANK mRNA expression. D) Western blot analysis of ANK in SiHIF-1α transfected cells. Higher expression of ANK protein is evident in HIF-1 silenced cells compared to control cells. Values shown are mean ± SE from three independent experiments, * p < 0.05.
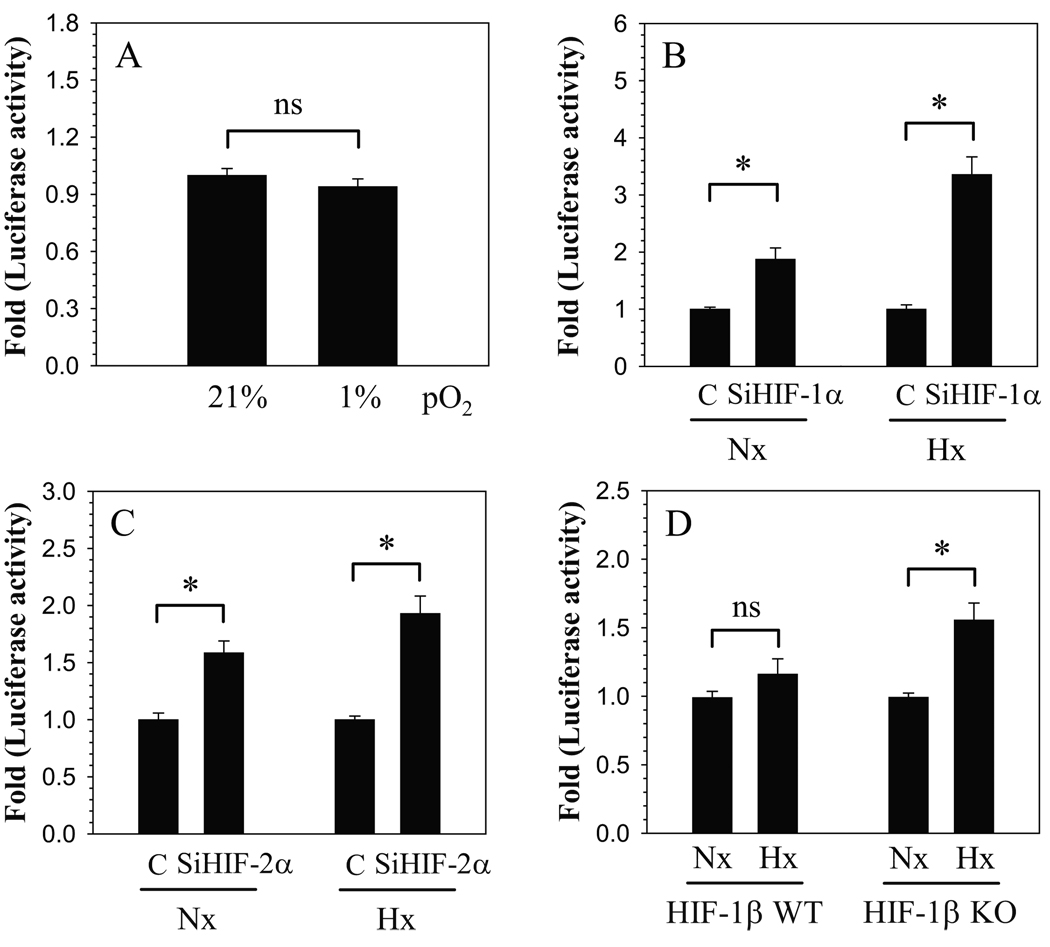
To further evaluate relationship between ANK transcription and oxygen tension, we measured the activity of ANK proximal promoter under normoxic and hypoxic conditions. Figure 3A shows that, in nucleus pulposus cells, ANK promoter activity is similar in both hypoxia and normoxia. In addition, to confirm the role of HIF-1 and HIF-2 in transcriptional regulation of ANK expression, promoter activity was measured by performing loss of function studies. When HIF-1α expression was silenced using siRNA, ANK promoter activity is induced under both normoxic and hypoxic conditions (Fig. 3B). However, induction of activity is more pronounced in hypoxia. To confirm the role of HIF-2 in regulation of ANK, we co-transfected nucleus pulposus cells with HIF-2α SiRNA. Figure 3C shows that HIF-2α silencing results in induction in promoter activity, independent of oxemic status. Again, hypoxic silencing of HIF-2α causes a profound induction of promoter activity. The role of HIF-1 and HIF-2 in the regulation of ANK promoter activity was further examined using wild type and null MEF derived from HIF-1β homozygous null (−/−) and wild type (+/+) mice. Wild type MEFs do not show a significant change in ANK promoter activity in hypoxia (Fig. 3D). On the other hand, null cells exhibit a significant hypoxic induction of ANK promoter activity (Fig. 3D).
Figure 3.
Effect of oxygen tension and HIF on ANK promoter activity in nucleus pulposus (NP) cells. A. NP cells were transfected with ANK reporter constructs and luciferase reporter activity was measured in normoxia (21% O2) or hypoxia (1% O2). NP cells did not evidence hypoxic change in ANK promoter activity. Cells were transfected with B) HIF-1α-SiRNA (SiHIF-1α) C) HIF-2α-SiRNA (SiHIF-1α) and cultured in normoxia (Nx) and hypoxia (Hx). Control cells were transfected with control siRNA (C). Silencing of HIF-1α or HIF-2α resulted in a significant induction of ANK promoter activity. Induction was more pronounced in hypoxia. D) Effect of hypoxia on ANK promoter activity in HIF-1β wild type (WT) and null (KO) MEFs. In contrast to wild type cells, hypoxia induced ANK promoter activity in null cells. Values shown are mean ± SE from three independent experiments, * p < 0.05.
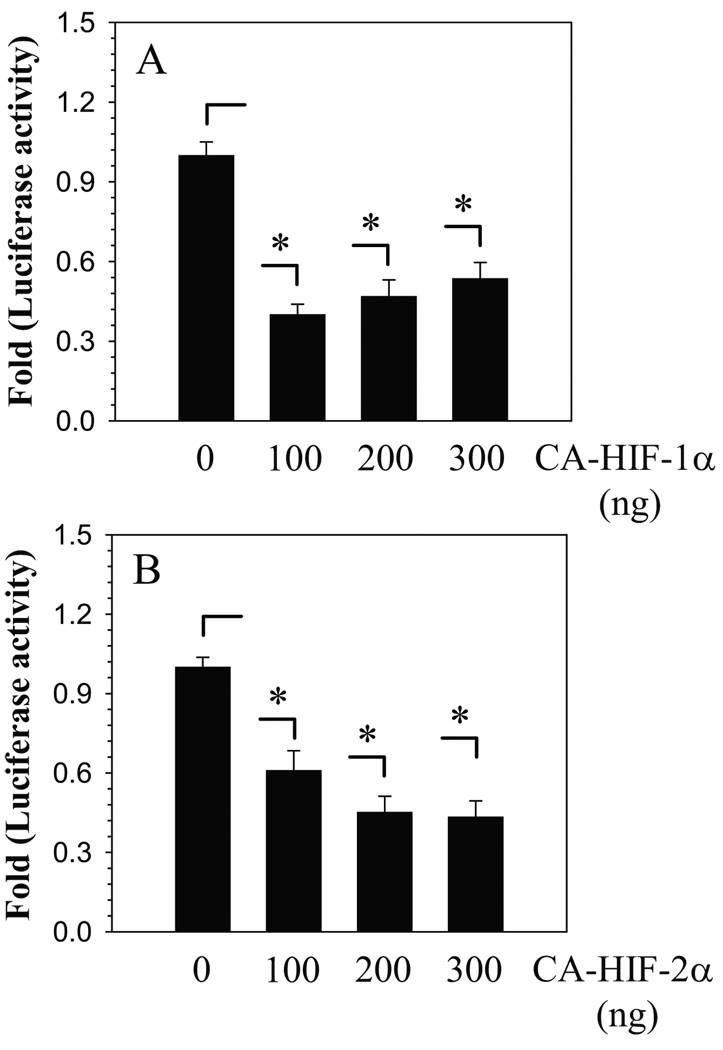
To further validate whether ANK promoter activity is responsive to HIF signaling, we co-transfected nucleus pulposus cells with plasmids encoding CA-HIF-1α or CA-HIF-2α, which are insensitive to oxidative degradation (Fig. 4A, B). Transfection with CA-HIF-1α as well as CA-HIF-2α significantly suppresses ANK promoter activity (Fig. 4A, B). The inhibitory effect of CA-HIF-1α on ANK promoter activity is such that the level of inhibition remained constant when the concentration of plasmid is decreased from 300 to 100 ng (Fig. 4A). For CA-HIF-2α, while suppression of promoter activity is evident at 100 ng, the effect is further enhanced when the concentration of plasmid is increased from to 200 ng (Fig. 4B).
Figure 4.
Effect of A) CA-HIF-1α and B) CA-HIF-2α expression on ANK promoter activity in nucleus pulposus cells. Note the significant suppression in ANK reporter activity even when transfected with 100 ng of HIF plasmid. Values shown are mean ± SE, of 3 independent experiments; *p<0.05, ns = non significant.
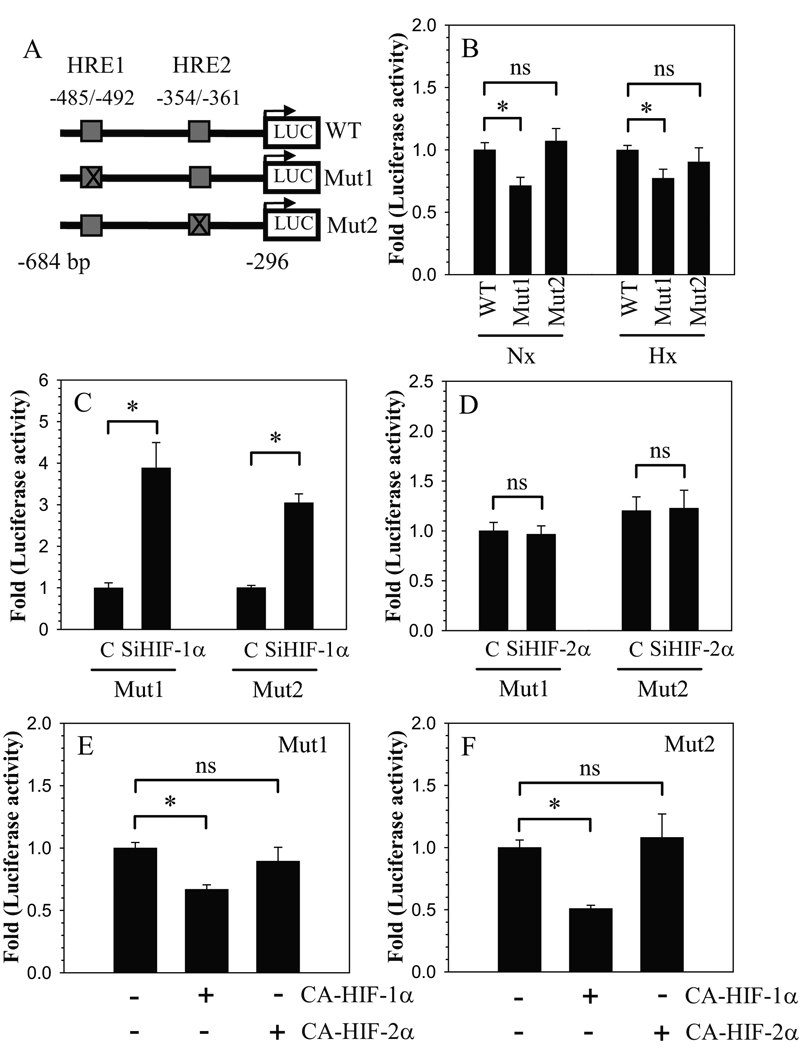
We evaluated the interaction of HIFs with hypoxia response elements (HRE) in the ANK promoter, using reporters containing mutation in either HRE1 (CGCGTGCC to CGTATGCC) or HRE2 (CGCGTGCT to CGTATGCT) (Fig. 5A). Regardless of oxemic tension, compared to the wild type reporter, a mutation in HRE1 results in decreased basal activity, whereas a mutation in HRE2 has no effect on basal activity (Fig. 5B). We examined the effect of HIF silencing on the activity of HRE mutants in hypoxia. The results clearly show that HIF-1α silencing leads to increased activation of both HRE1 and HRE2 mutant reporters (Fig. 5C). On the other hand, activity of both HRE mutant reporters is unresponsive to HIF-2α silencing (Fig. 5D). Moreover, overexpression of CA-HIF-1α results in suppression of activity of both HRE1 and HRE2 mutant reporters (Fig. 5E). In contrast, CA-HIF-2α fails to suppress activity of either of the mutant reporters (Fig. 5F).
Figure 5.
Interaction of HIFs with HREs in the ANK promoter. A) Schematic of different ANK reporter constructs (WT: wild type; Mut1: HRE1 mutant; Mut2: HRE2 mutant) used in transfections. B. Relative luciferase activity of cells transfected with WT, Mut1, and Mut2 ANK reporter constructs measured in normoxia (Nx) and hypoxia (Hx). Effect of silencing of C) HIF-1α (SiHIF-1α) and D) HIF-2α (SiHIF-2α) on mutant ANK reporters. SiHIF-1α caused induction in activity of both Mut1 and Mut 2 compared to cells transfected with control SiRNA (C). HIF-2α Silencing had no effect on activity of either Mut1 or Mut2. Effect of E) CA-HIF-1α and F) CA-HIF-2α co-expression on mutant ANK reporter activity. HIF-1α suppressed activity of both Mut1 or Mut2, whereas either mutant was unresponsive to HIF-2α Values shown are mean ± SE from three independent experiments, * p < 0.05; ns = non significant.
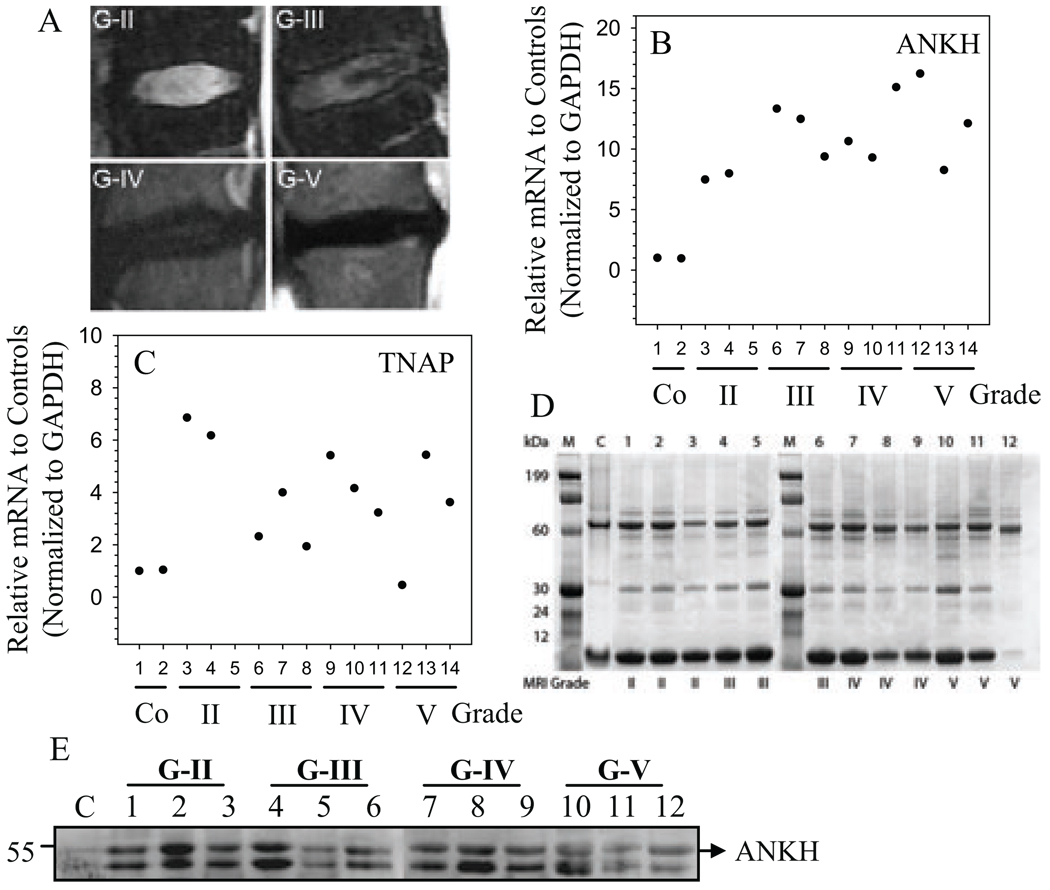
Finally, we evaluated ANK and tissue non-specific alkaline phosphatase (TNAP) expression in Thompson graded degenerate human disc tissues (Fig. 6A). Real-time RT-PCR analysis shows that there is a trend in the increase in expression of ANK (Fig. 6B) as well as TNAP (Fig. 6C) mRNA in degenerate tissues when compared with the normal control tissues. We extracted proteins from the same tissue samples and confirmed their integrity by gel electrophoresis. All tissue extracts show discrete protein bands with minimal sample deterioration (Fig. 6C). Western blot analysis was performed on equal amount of these tissue proteins using anti-ANK antibody. Figure 6D indicates that there is a discrete ANK protein band (54.4 kD) in all the samples, with a lower level of expression evident in the normal control tissue. Inspection of the combined data set suggests a trend of increased ANK expression with degeneration. Not surprisingly, patient to patient variation within the same grade is also evident.
Figure 6.
Expression of ANK in human degenerate NP. A. Representative MRI images of patients with degenerative disc disease. (From the top left, clockwise: Grade II,III,IV,V) B and C. Real time RT-PCR analysis of ANK (B) and TNAP (C) mRNA expression in multiple human NP tissue samples. Samples from three different patients were used for each degenerative grade. With increased severity of disc degeneration there is a relative increase in the expression of ANK and TNAP mRNA. Mean expression of normal controls (c) was set at 1.0. C. Human NP protein extracts were subjected to SDS gel electrophoreses and stained with coomasie blue. The distinct bands in each sample verifies the quality of the protein samples. D. Western blot analysis of ANK expression in human degenerate NP samples. ANK expression is increased in all the degenerate samples compared to control.
Discussion
The goal of this investigation was to examine the expression of ANK in the intervertebral disc and to test the hypothesis that oxygen tension regulated ANK expression; in addition, we assessed whether ANK expression was dependent on HIF, activity. We examined the expression and localization of ANK in the intervertebral disc of animals at two different stages of skeletal maturity. Our results indicated that there was significant expression of ANK in the nucleus pulposus with mature animals exhibiting a higher level of expression than the neonate. In light of a previous report from our laboratories demonstrating that ANK expression is sensitive to oxygen tension (13), and in recognition of the fact that in the adult disc, the nucleus pulposus is completely devoid of vasculature and the oxygen tension is low, the observed high levels of ANK expression were unanticipated. We were not surprised, however, to observe that, analogous to the growth plate, ANK expression was high in the calcified zone of the endplate, a region rich in vascular supply. Our observations suggested the existence of intrinsic differences in ANK regulation between the nucleus pulposus and the growth plate and led us to explore in more detail the effect of oxygen on ANK expression in the nucleus pulposus.
In accord with our previous studies of constitutively high HIF expression in the nucleus pulposus in a manner that was independent of oxygen tension, we observed that ANK expression was also not responsive to pO2. Since HIF-1 and to some extent HIF-2 levels in nucleus pulposus cells are refractory to changes in oxygen tension (6, 16, 18–20), it is difficult to show that ANK is regulated by HIF. For this reason, we used gain and loss of function studies with the promoter sequences of the murine ANK gene containing two conserved HRE motifs: a proximal motif at − 354 bp (HRE2) and a distal motif at − 485 bp (HRE1). When we silenced the expression of HIF-1α or HIF-2α in the cells, we found that ANK expression was induced in hypoxia at both the mRNA and protein levels, suggesting that ANK expression is regulated in a HIF-dependent manner. Furthermore, forced expression of HIF-1α or HIF-2α caused suppression in ANK reporter activity in nucleus pulposus cells. This finding was further confirmed by site directed mutagenesis studies. Interaction of HIF-1 to either wild type HRE1 or HRE2 suppressed promoter activity; however, mutation of either HRE1 or HRE2 abolished the responsiveness of the ANK promoter to changes in HIF-2 levels, indicating that binding of HIF-2 to both HREs is likely required for suppression of ANK promoter activity. In addition, observation that mutation in HRE1 resulted in decreased basal promoter activity suggests that HIF’s binding to this motif is likely required for recruitment of a regulatory factor that maintains baseline promoter activity. Importantly, these experiments highlighted differences between two HIF isoforms; compared to HIF-2, HIF-1 robustly influences ANK promoter activity even in normoxia. Interestingly, despite similar level of HIF proteins in normoxia and hypoxia, silencing of both HIF isoforms in hypoxia results in significantly higher induction in ANK reporter suggesting that hypoxia and HIF may exert opposing effect on ANK transcription. In support of this notion, unlike wild type cells, hypoxia induced ANK promoter activity in HIF-1β null cells that are effectively null with respect to HIF-1α and HIF-2α activity. Based on these complimentary findings, it is postulated that in nucleus pulposus cells, hypoxia may also regulate ANK promoter activity in an HIF-independent fashion.
Significantly, this study lends further strength to the recent observations of Zaka et al that ANK is a HIF-1 target gene (13). However, in contrast to the observed low levels of ANK expression in hypoxic regions of the growth plate, our studies of ANK in the nucleus pulposus demonstrates robust ANK expression despite the hypoxic nature of the tissue. Under normal physiological conditions, local control of mineralization in the nucleus pulposus is necessary to prevent ectopic mineralization of the disc (21). Although the precise function of ANK in the intervertebral disc is unknown, we suggest that, in part, control of mineralization is facilitated by the ability of ANK to transport the mineralization inhibitor PPi (22, 23). Therefore, it is likely that the factors that limit the expression of ANK in hypoxia in the growth plate do not apply to the expression of ANK in the nucleus pulposus. Zaka et al proposed that the hypoxic repression of ANK expression in the growth plate may be mediated by recruitment of a secondary transcription factor that may interfere with the binding of HIF-1 to the responsive HRE in the proximal promoter of ANK (13). Further studies are currently underway in our laboratory to determine the precise nature of HIF-mediated regulation of ANK expression in the nucleus pulposus. Nevertheless, we conclude that in the hypoxic intervertebral disc, the expression of HIF-1 and HIF-2 serves to maintain basal ANK expression. Furthermore, we hypothesize that the expression of ANK in the nucleus pulposus cells represents an adaptational response to its unique microenvironmental niche and metabolic status within the intervertebral disc (18, 19).
Calcification of intervebral discs is associated with disease and aging (24, 25). Indeed, studies by Gruber et al. and Melrose et al. indicate that calcified deposits are evident in the human as well as animal discs (26, 27). Since ANK is known to be involved in local control of mineralization in tissues such as bone and the calcified zone of the growth plate, protein expression is elevated in articular cartilage during osteoarthritis and CPPD deposition disease (28, 29) we chose to evaluate levels of expression of ANK in the degenerate human nucleus pulposus. While the number of control tissues were very limited (due to practical difficulties in acquiring normal human discs), measurement of both mRNA and protein expression levels suggested that as the nucleus became degenerate, there was a rise in expression of the ANK. In addition to the transcriptional regulation of ANK expression by HIF proteins, ANK expression has also been shown to respond to a variety of other stimuli, including TGFβ (30). Indeed, we have recently demonstrated that ANK expression is up-regulated in response to treatment with TGFβ in nucleus pulposus cells (unpublished data). It is therefore not unreasonable to assume that in the degenerate disc dysregulation of HIF activity, as well as elevated levels of factors such as TGFβ, may contribute to increased ANK levels (30). From a functional perspective, we conclude that basal expression of ANK is required to prevent dystrophic mineral formation in the normal intervertebral disc. On the other hand, in the degenerate disc, high ANK expression, raised ePPi levels in conjunction with inorganic pyrophosphate-generating ectoenzymes such as PC-1 and CILP, and increased elaboration of tissue non-specific alkaline phosphatase could promote hydroxyapatite deposition (29, 31). Future studies are aimed to investigate this hypothesis.
Acknowledgements
This work was supported by a grant from the National Institutes of Health R01AR050087, R01AR055655 (to MVR and IMS) and R01 AR052619 (CJW)
Abbreviations
- ANK
progressive ankylosis
- HIF
hypoxia inducible factor
- PPi
pyrophosphate
Contributor Information
Renata Skubutyte, Email: Renata.Skubutyte@jefferson.edu.
Dessislava Markova, Email: Dessislava.Markova@jefferson.edu.
Theresa A. Freeman, Email: Theresa.Freeman@jefferson.edu.
D. Greg Anderson, Email: greg.anderson@rothmaninstitute.com.
Arnold S. Dion, Email: Arnold.Dion@jefferson.edu.
Charlene J. Williams, Email: charlene.williams@jefferson.edu.
Irving M. Shapiro, Email: irving.shapiro@jefferson.edu.
Makarand V. Risbud, Email: makarand.risbud@jefferson.edu.
References
- 1.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 2.Hassler O. The human intervertebral disc. A micro-angiographical study on its vascular supply at various ages. Acta Orthop Scand. 1969;40:765–772. doi: 10.3109/17453676908989540. [DOI] [PubMed] [Google Scholar]
- 3.Rudert M, Tillmann B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993;64:37–40. doi: 10.3109/17453679308994524. [DOI] [PubMed] [Google Scholar]
- 4.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 5.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal A, Gajghate S, Smith H, Anderson DG, Albert TJ, Shapiro IM, Risbud MV. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008;58:3798–3808. doi: 10.1002/art.24073. [DOI] [PubMed] [Google Scholar]
- 7.Zaka R, Williams CJ. Role of the progressive ankylosis gene in cartilage mineralization. Curr Opin Rheumatol. 2006;18:181–186. doi: 10.1097/01.bor.0000209432.36355.6e. [DOI] [PubMed] [Google Scholar]
- 8.Nürnberg P, Thiele H, Chandler D, Höhne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28:37–41. doi: 10.1038/ng0501-37. [DOI] [PubMed] [Google Scholar]
- 9.Pendleton A, Johnson MD, Hughes A, Gurley KA, Ho AM, Doherty M, Dixey J, Gillet P, Loeuille D, McGrath R, Reginato A, Shiang R, Wright G, Netter P, Williams C, Kingsley DM. Mutations in ANKH cause chondrocalcinosis. Am J Hum Genet. 2002;71:933–940. doi: 10.1086/343054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, Baur ST, Shiang R, Grange DK, Beighton P, Gardner J, Hamersma H, Sellars S, Ramesar R, Lidral AC, Sommer A, Raposo do Amaral CM, Gorlin RJ, Mulliken JB, Olsen BR. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet. 2001;68:1321–1326. doi: 10.1086/320612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams CJ, Pendleton A, Bonavita G, Reginato AJ, Hughes AE, Peariso S, Doherty M, McCarty DJ, Ryan LM. Mutations in the amino terminus of ANKH in two US families with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2003;48:2627–2631. doi: 10.1002/art.11133. [DOI] [PubMed] [Google Scholar]
- 12.Sohn P, Crowley M, Slattery E, Serra R. Developmental and TGF-beta-mediated regulation of Ank mRNA expression in cartilage and bone. Osteoarthritis Cartilage. 2002;10:482–490. doi: 10.1053/joca.2002.0810. [DOI] [PubMed] [Google Scholar]
- 13.Zaka R, Dion AS, Kusnierz A, Bohensky J, Srinivas V, Freeman T, Williams CJ. Oxygen tension regulates the expression of ANK (progressive ankylosis) in an HIF-1-dependent manner in growth plate chondrocytes. J Bone Miner Res. 2009;24:1869–1878. doi: 10.1359/JBMR.090512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Cepko CL. HDAC4 regulates neuronal survival in normal and diseased retinas. Science. 2009;323:256–259. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 17.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Risbud M, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell function: from Niche to Notch. Am J. Pathology. 2010 Feb 4; doi: 10.2353/ajpath.2010.090734. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–C631. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22:1851–1861. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 21.Gurley KA, Chen H, Guenther C, Nguyen ET, Rountree RB, Schoor M, Kingsley DM. Mineral formation in joints caused by complete or joint-specific loss of ANK function. J Bone Miner Res. 2006;21:1238–1247. doi: 10.1359/jbmr.060515. [DOI] [PubMed] [Google Scholar]
- 22.Johnson K, Goding J, Van Etten D, Sali A, Hu SI, Farley D, Krug H, Hessle L, Millán JL, Terkeltaub R. Linked deficiencies in extracellular PP(i) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J Bone Miner Res. 2003;18:994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Xu J, Du B, Kirsch T. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol Cell Biol. 2005;25:312–323. doi: 10.1128/MCB.25.1.312-323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai LY, Ye H, Qian QR. The natural history of cervial disc calcification in children. J Bone Joint Surg. 2004;86-A:1467–1472. doi: 10.2106/00004623-200407000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Chanchairujira K, Ching CB, Kim JY, Papakonstantinou O, Lee MH, Clopton P, Resnick D. Intervertebral disk calcification of the spine in an elderly population: radiographic prevalence, location, and distribution and correlation with spinal degeneration. Radiology. 2004;230:499–503. doi: 10.1148/radiol.2302011842. [DOI] [PubMed] [Google Scholar]
- 26.Gruber HE, Norton HJ, Sun Y, Hanley EN., Jr Crystal deposits in the human intervertebral disc: implications for disc degeneration. Spine J. 2007;7(4):444–450. doi: 10.1016/j.spinee.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Melrose J, Burkhardt D, Taylor TK, Dillon CT, Read R, Cake M, Little CB. Calcification in the ovine intervertebral disc: a model of hydroxyapatite deposition disease. Eur Spine J. 2009;18:479–489. doi: 10.1007/s00586-008-0871-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirose J, Ryan LM, Masuda I. Up-regulated expression of cartilage intermediate-layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum. 2002;46:3218–3229. doi: 10.1002/art.10632. [DOI] [PubMed] [Google Scholar]
- 29.Johnson K, Terkeltaub R. Upregulated ank expression in osteoarthritis can promote both chondrocyte MMP-13 expression and calcification via chondrocyte extracellular PPi excess. Osteoarthritis Cartilage. 2004;12:321–335. doi: 10.1016/j.joca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Cailotto F, Bianchi A, Sebillaud S, Venkatesan N, Moulin D, Jouzeau JY, Netter P. Inorganic pyrophosphate generation by transforming growth factor-beta-1 is mainly dependent on ANK induction by Ras/Raf-1/extracellular signal-regulated kinase pathways in chondrocytes. Arthritis Res Ther. 2007;9:R122. doi: 10.1186/ar2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki S, Kawaguchi Y, Chiba K, Mikami Y, Kizawa H, Oya T, Mio F, Mori M, Miyamoto Y, Masuda I, Tsunoda T, Kamata M, Kubo T, Toyama Y, Kimura T, Nakamura Y, Ikegawa S. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607–612. doi: 10.1038/ng1557. [DOI] [PubMed] [Google Scholar]