Abstract
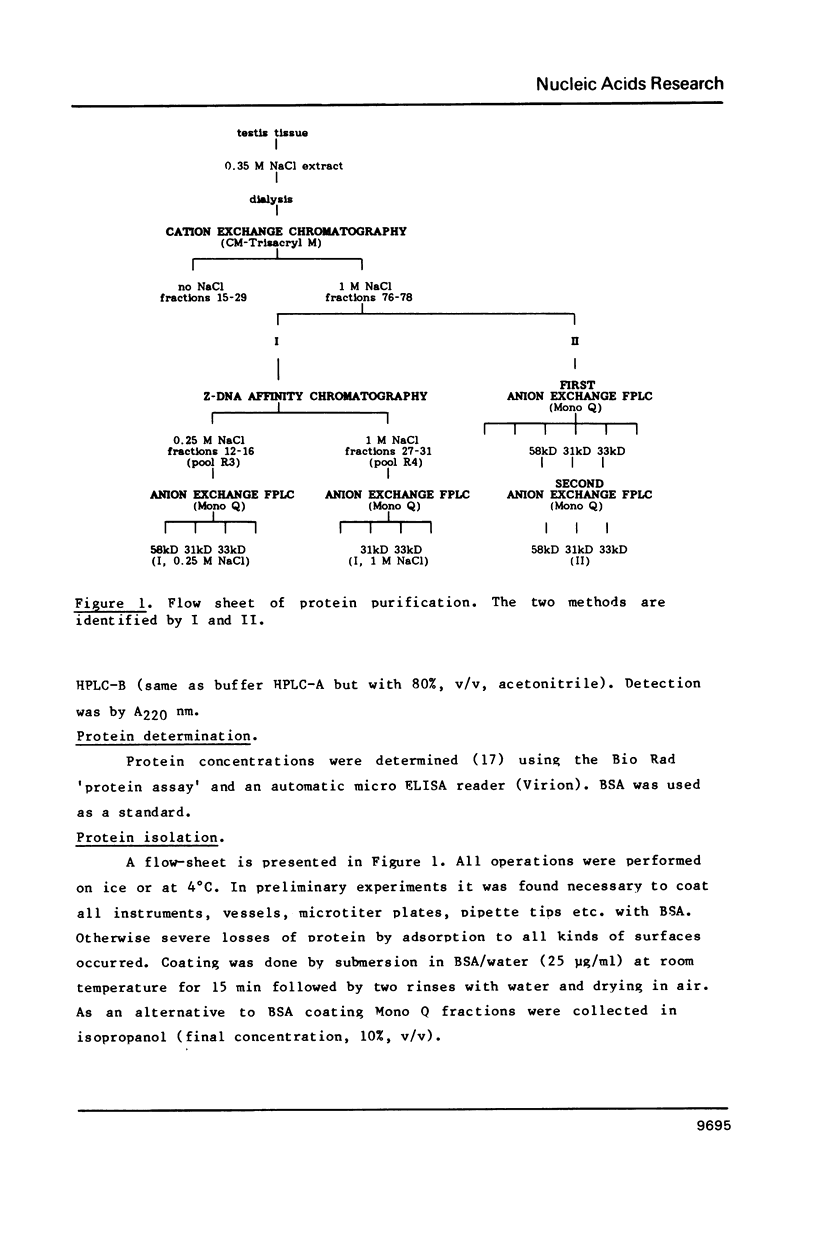
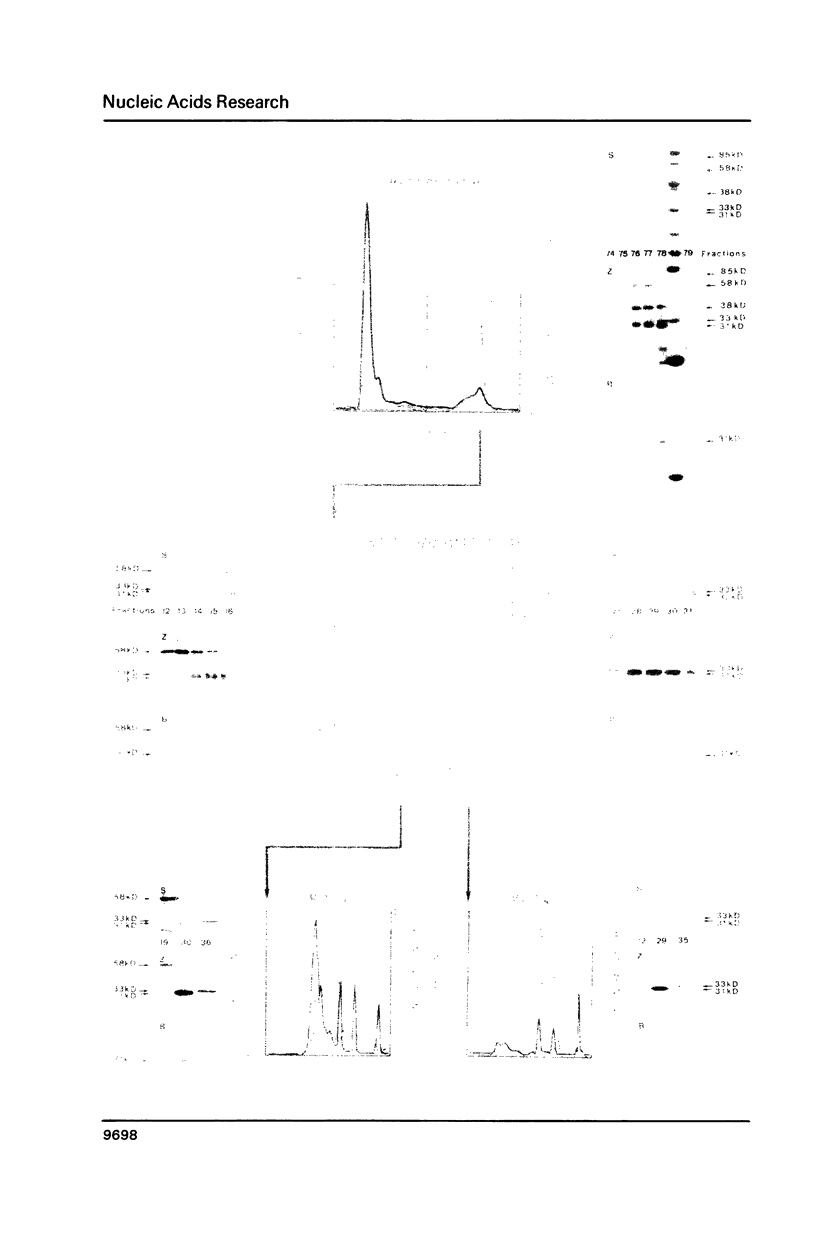
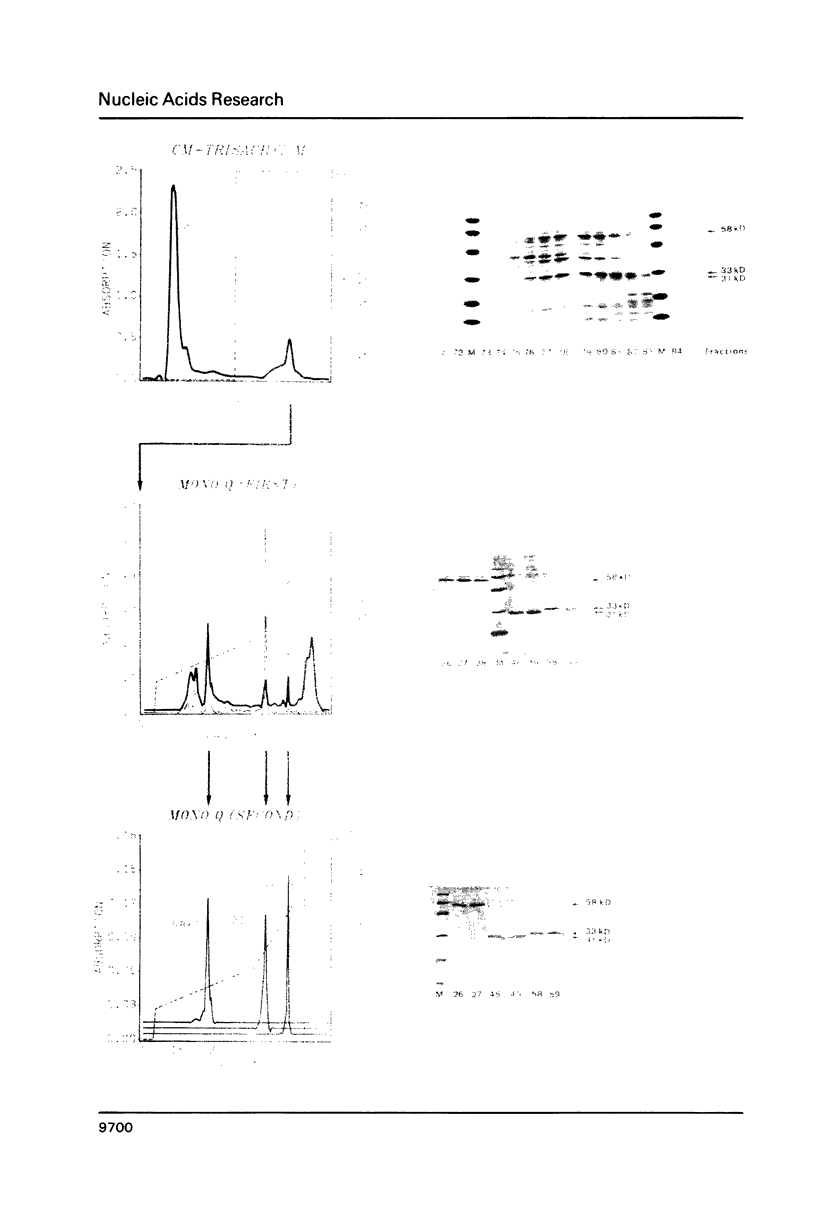
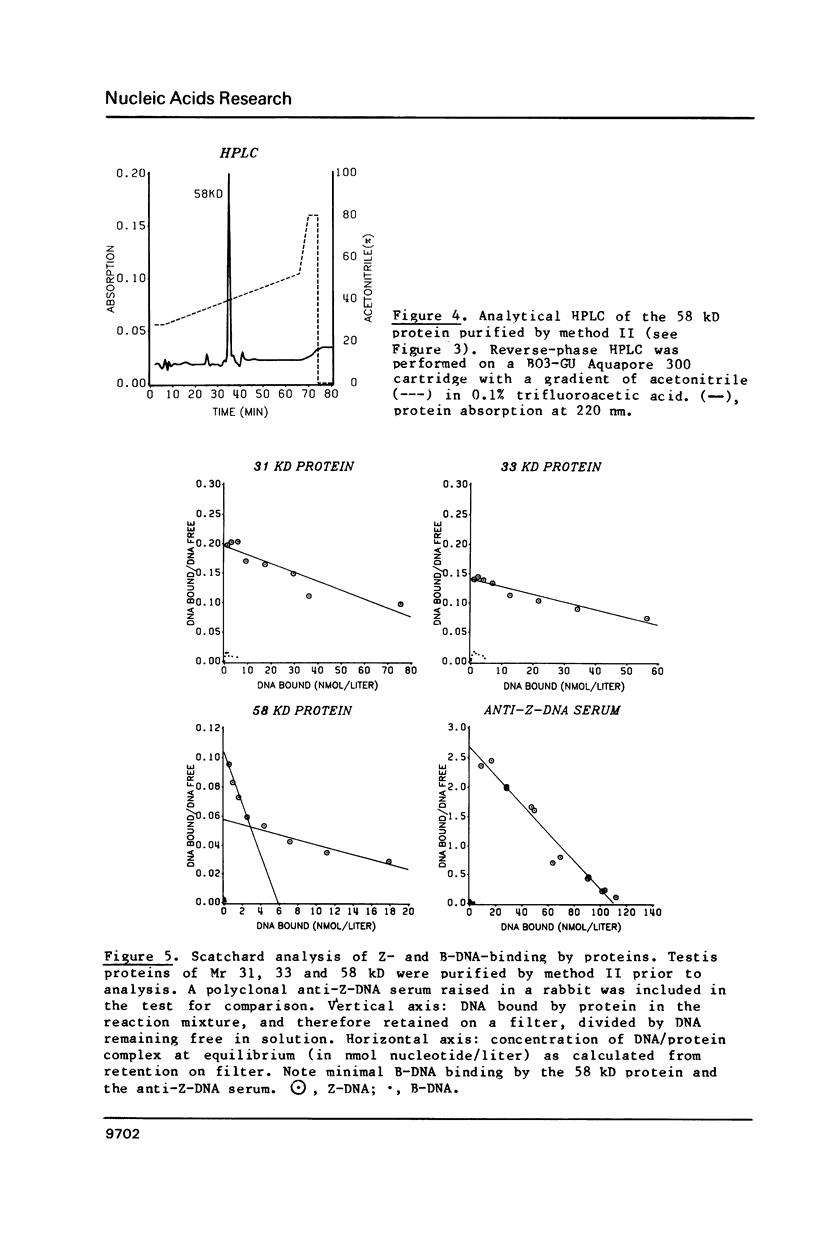
Three Z-DNA-binding proteins of Mr 31, 33 and 58 kD were isolated from mature bull testis. They were obtained in a native state suitable for binding studies. These are the first examples of Z-DNA-binding proteins from a mammalian tissue. Purification involved tissue extraction with 0.35 M NaCl, cation exchange chromatography on CM-Trisacryl M and two consecutive anion exchange FPLC runs on Mono Q. The proteins appeared virtually homogeneous by anion exchange FPLC, SDS polyacrylamide gel electrophoresis and reverse phase HPLC (58 kD protein only). Yields from 50 g of testis tissue were: 31 kD protein, 40 micrograms; 33 kD protein, 100 micrograms; and 58 kD protein, 150 micrograms. Z-DNA binding was determined by Scatchard analysis of filter binding data using brominated poly(dG-dC).poly(dG-dC) as a conformation-specific ligand. Dissociation constants (Kz, in mol nucleotide/liter) were: 31 kD protein, 7 X 10(-7) M; 33 kD protein, 8 X 10(-7) M; 58 kD protein, 6 X 10(-8) M (primary binding site) and 6 X 10(-7) M (secondary binding site). B-DNA binding to poly(dG-dC).poly(dG-dG) was too low for reliable determination under the conditions of assay. This attested to a high degree of conformational specificity of the three proteins. The 58 kD protein bound Z-DNA at the primary site with an affinity almost equivalent to that of a polyclonal anti-Z-DNA antiserum raised in a rabbit (Kz, 4 X 10(-8) M).
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorin F., Hahn R., Rich A. Restriction endonucleases can be used to study B-Z junctions in supercoiled DNA. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5714–5718. doi: 10.1073/pnas.81.18.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho J. A., Wells R. D. Left-handed Z-DNA binding by the recA protein of Escherichia coli. J Biol Chem. 1987 May 5;262(13):6082–6088. [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dzandu J. K., Deh M. E., Barratt D. L., Wise G. E. Detection of erythrocyte membrane proteins, sialoglycoproteins, and lipids in the same polyacrylamide gel using a double-staining technique. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S., Matsumura O., Masuda S. A Z-DNA binding protein isolated from D. radiodurans. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1294–1300. doi: 10.1016/0006-291x(85)91755-3. [DOI] [PubMed] [Google Scholar]
- Kmiec E. B., Angelides K. J., Holloman W. K. Left-handed DNA and the synaptic pairing reaction promoted by Ustilago rec1 protein. Cell. 1985 Jan;40(1):139–145. doi: 10.1016/0092-8674(85)90317-4. [DOI] [PubMed] [Google Scholar]
- Kuenzle C. C., Heizmann C. W., Hübscher U., Hobi R., Winkler G. C., Jaeger A. W., Morgenegg G. Chromatin changes accompanying neuronal differentiation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):493–499. doi: 10.1101/sqb.1983.048.01.054. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Sousa R., Rosen B., Hsu A., Rich A. Isolation and characterization of Z-DNA binding proteins from wheat germ. Biochemistry. 1985 Sep 10;24(19):5070–5076. doi: 10.1021/bi00340a017. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Leng M. Antiserum to Z-DNA. FEBS Lett. 1981 Sep 14;132(1):45–48. doi: 10.1016/0014-5793(81)80424-3. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Z-DNA: still searching for a function. Science. 1985 Nov 15;230(4727):794–796. doi: 10.1126/science.2997919. [DOI] [PubMed] [Google Scholar]
- Morgenegg G., Celio M. R., Malfoy B., Leng M., Kuenzle C. C. Z-DNA immunoreactivity in rat tissues. Nature. 1983 Jun 9;303(5917):540–543. doi: 10.1038/303540a0. [DOI] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Kozlowski S. A., Patel D. J., Rich A. Bromination stabilizes poly(dG-dC) in the Z-DNA form under low-salt conditions. Biochemistry. 1984 Jan 3;23(1):54–62. doi: 10.1021/bi00296a009. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]





