Abstract
Objectives:
In this study, we quantified the profiles of phasic activity in respiratory muscles (diaphragm, genioglossus and external intercostal) and non-respiratory muscles (neck and extensor digitorum) across REM sleep. We hypothesized that if there is a unique pontine structure that controls all REM sleep phasic events, the profiles of the phasic twitches of different muscle groups should be identical. Furthermore, we described how respiratory parameters (e.g., frequency, amplitude, and effort) vary across REM sleep to determine if phasic processes affect breathing.
Methods:
Electrodes were implanted in Wistar rats to record brain activity and muscle activity of neck, extensor digitorum, diaphragm, external intercostal, and genioglossal muscles. Ten rats were studied to obtain 313 REM periods over 73 recording days. Data were analyzed offline and REM sleep activity profiles were built for each muscle. In 6 animals, respiratory frequency, effort, amplitude, and inspiratory peak were also analyzed during 192 REM sleep periods.
Results:
Respiratory muscle phasic activity increased in the second part of the REM period. For example, genioglossal activity increased in the second part of the REM period by 63.8% compared to the average level during NREM sleep. This profile was consistent between animals and REM periods (η2 = 0.58). This increased activity seen in respiratory muscles appeared as irregular bursts and trains of activity that could affect rythmo-genesis. Indeed, the increased integrated activity seen in the second part of the REM period in the diaphragm was associated with an increase in the number (28.3%) and amplitude (30%) of breaths. Non-respiratory muscle phasic activity in REM sleep did not have a profile like the phasic activity of respiratory muscles. Time in REM sleep did not have an effect on nuchal activity (P = 0.59).
Conclusion:
We conclude that the concept of a common pontine center controlling all REM phasic events is not supported by our data. There is a drive in REM sleep that affects specifically respiratory muscles. The characteristic increase in respiratory frequency during REM sleep is induced by this drive.
Citation:
Fraigne JJ; Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. SLEEP 2011;34(4):425-434.
Keywords: REM phasic event, diaphragm, genioglossus, external intercostal, rat
INTRODUCTION
All rapid eye movement (REM) sleep phasic events are purportedly controlled by a single pontine structure.33 This idea is supported by correlations between ponto-geniculo occipital (PGO) waves and rapid eye movements,33 rapid eye movements and muscle twitches,9 rapid eye movements and middle-ear muscle activity (MEMA),27 MEMA and motor activity of the extremities and the head.32 However, these correlations are weak, and there is evidence to suggest that phasic events have independent control. Hence, it is unclear if there is a unique center that controls the phasic events during REM sleep.
Many studies have focused on the inhibitory process13 that affects the respiratory motor system during REM sleep. However, the respiratory system is also subjected to excitatory processes,25,26 and these might explain the erratic respiratory pattern and the increase in breathing frequency characteristic of REM sleep.1 Several studies have demonstrated the existence of an endogenous excitatory drive to the respiratory system during REM sleep.25,26 Inspiratory, expiratory and diaphragmatic motor neurons are stimulated simultaneously or individually by this drive.26 When animals are mechanically ventilated to apnea and mechanical and chemical stimuli are maintained at a constant level, this drive develops after a delay, peaks in the second part of the REM period, and wanes towards the end of the REM period. A study comparing the activity of the tongue and the neck muscles shows that the genioglossus (GG) presents an activity profile similar to the one described above whereas the neck muscles do not.19 This study indicates that upper airway motor neurons may be affected by the same excitatory process as other respiratory neurons and that this process does not affect non-respiratory muscles.
We reasoned that if the respiratory excitation is the result of phasic REM sleep activity, and all phasic REM sleep events are controlled by a common center, then twitches of somatic muscles and the activity of respiratory muscles should present the same profile of activity. We reasoned also that evidence to the contrary would support the idea that the excitatory process is specific to the respiratory system. Thus, we quantified the electromyographic (EMG) activity of muscles which receive respiratory inputs (diaphragm, external intercostal, and genioglossus muscles) and muscles that do not (extensor digitorum and neck muscles) by dividing REM periods into 20 equal subdivisions and integrating EMG activity in each subdivision for each REM period. Furthermore, we analyzed breathing frequency, effort, amplitude and inspiratory peak activity to describe the effect of the excitatory drive on the respiratory system during REM sleep.
MATERIALS AND METHODS
Subjects
Ten adult male Wistar rats (325-600 g) were prepared for recordings of electroencephalographic (EEG), genioglossal (EMGG), external intercostal (EMGI), nuchal (EMGN), extensor digitorum (EMGL), and diaphragmatic electromyographic(EMGD) activity. Headcaps containing a connector for EEG and EMG electrodes were attached to the animals' skulls. The animals recovered from surgery for one week before experimentation. The Animal Care and Use Committee of Texas Tech University Health Sciences Center School of Medicine approved all surgical and experimental procedures.
Surgical Procedures
Sterile surgery was performed under anesthesia (0.5%-2% isoflurane). A single injection of atropine (0.04 mg/kg) was administered subcutaneously. The skull was exposed by a midline incision, and electrodes and electrode wires were tunneled subcutaneously to their target areas from that incision. The animals were placed on a heated surgical table in a dorsal recumbent position. An incision was made from the xiphoid process caudally along the linea alba to expose the abdominal cavity. Two EMG electrodes (Teflon-coated multistranded stainless steel Cooner wires) were implanted within the crural and semitendinous regions of the right diaphragm. The right intercostal muscles were exposed via an oblique incision and dissection of the latissimus dorsi muscle. Two EMG electrodes were sutured into the external intercostal muscles. The muscles of the right foreleg were exposed, and 2 EMG electrodes were sutured into the extensor digitorum. The ventral surface of GG was exposed via a submental incision and dissection of the geniohyoid and mylohyoid muscles. Two EMG electrodes were placed bilaterally, under direct vision into GG, and placements were confirmed by tongue protrusion in response to electrical stimulation. Submental, intercostal, foreleg, and abdominal incisions were closed with non-absorbable sutures. The rat was then turned to a prone position and placed in a stereotaxic apparatus. The dorsal midline incision over the skull was enlarged from the occipital muscle margin to the frontal area between the eyes. The exposed skull was cleaned with hydrogen peroxide and the periosteum was removed with a curette. Four holes were drilled in the skull for placement of EEG electrodes (00 1/16 inch stainless steel screws attached to multistranded stainless steel Teflon-coated Cooner wires), one in the left frontal area rostral to bregma, one in the left parietal area 6 mm caudal to bregma, one in the right parietal area 3mm caudal to bregma, and one in the left occipital area above the cerebellum. The EEG electrodes were tapped into the holes in the skull. Two EMG electrodes were placed bilaterally in the nuchal muscle. Two keyhole-shaped holes were drilled in the skull, and the heads of 00 3/16 inch stainless steel screws were slipped under the skull along the slot of each keyhole with the threads projecting upward. These screws served as anchors for the headcap. A base layer of a dental bounding agent (C&B Metabond, Parkell Bio-materials) was applied on the skull, EEG electrodes, and anchor screws. The electrode connector (19-pin gold HDMI connector, Pacific Custom Cable Inc) was cemented to the skull with dental acrylic. Finally, skin margins were sutured with 4-0 polyethylene suture. Immediately after surgery, a single injection of antibiotic, penicillin (0.25 mL), was administered subcutaneously. Postoperative analgesia was maintained with Buprenorphine (0.05 mg/kg) administered subcutaneously every 8-12 h for the first 24 h postoperatively. The animals were allowed to recover from surgery for at least 4 days before habituation to the recording apparatus and 7 days before recordings were taken.
Recording Procedures and Experimental Protocol
Animals were placed in a recording chamber 1 h before each 3-h recording session. The electrode connector was attached to the recording system with a light weight cable. The animals were recorded in a freely moving condition during the light phase of the light-dark cycle between 12:00 and 15:00. Rats were recorded for 2 weeks to obtain 30 (± 5) REM sleep periods. Muscle activities were amplified with Grass P511 amplifiers set to pass frequencies from 0.3 to 30 kHz. For the EEG, the amplified bandwidth was set between 1.6 Hz and 30 Hz. All recordings were digitized and stored on computer using CED Spike2 v 4.10 software. EMG and EEG signals were sampled at 10,000 Hz and 1,000 Hz sampling rates, respectively.
Sleep Data Analysis
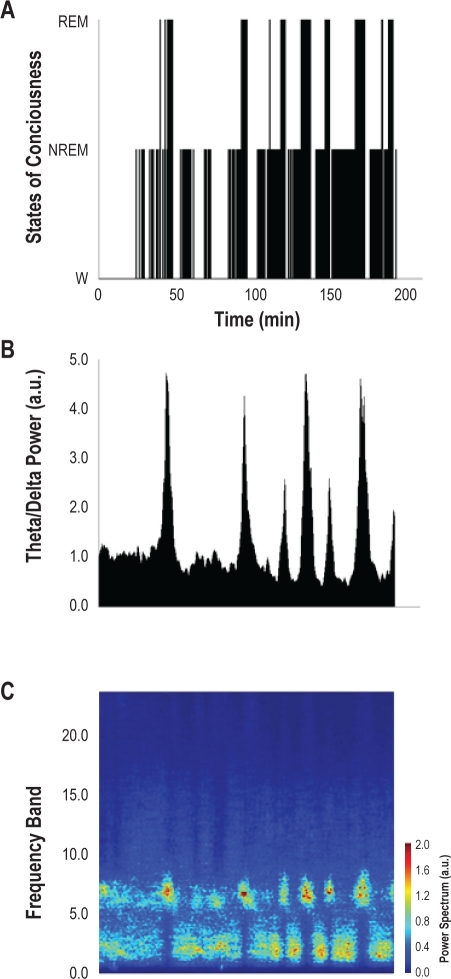
Sleep-wake states were defined according to standard EEG and nuchal EMG criteria (Figure 1). Sleep scoring was done visually by epochs of 30 s with the help of a spectral analysis of EEGs using a fast Fourier transform of 1020 sampling points. As shown in Figure 2, we verified the accuracy of our visual scoring by computing the ratio of theta to delta power in the EEG and the power spectrum of EEG frequency bands. We defined frequency bands as: delta (δ) 0.5-4 Hz, theta (θ) 5-8 Hz, alpha (α) 9-14 Hz, and beta (β) 15-40 Hz. Non-rapid eye movement (NREM) sleep was scored as such if the characteristic δ activity was sustained for > 50% of an epoch (30 s). REM sleep was scored as such if the EEG became desynchronized with characteristic θ activity and the nuchal EMG presented loss of muscle tone > 50% of an epoch. A hypnogram was constructed from the scored record. Only REM sleep periods > 30 s were selected for EMG analysis. The full duration of the REM periods, from the onset of REM sleep (desynchronized EEG with characteristic θ activity, accompanied by loss of nuchal activity) to wakefulness onset (return of nuchal activity), were used for EMG analysis.
Figure 1.
Continuous recording of genioglossal muscle activity (EMGGG), nuchal muscle activity (EMGN), and EEG during NREM sleep, REM sleep, and wakefulness.
Figure 2.
Sleep and wakefulness in a 3-h session in one animal (MST7). (A) Hypnogram; (B) Theta power to delta power ratio as a function of time for the same session as in A; (C) Power spectrum of various frequency bands (absolute values) as a function of time for the same session as in A.
EMG Analysis
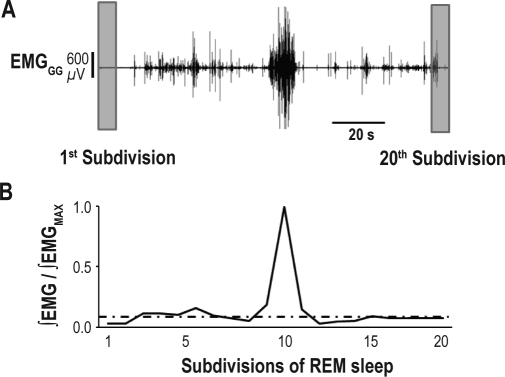
For each muscle and each REM period, the EMG signal was full-wave rectified, divided into 20 equal subdivisions from the onset to the end of the REM period, and integrated within each of these 20 subdivisions (Figure 3). This allowed us to account for the variation in duration of individual REM period. Then for each REM period, the integrated values were normalized to the highest value for that REM period. The EMG signals of the previous NREM sleep period was analyzed in the same manner and the values were expressed as a fraction of the highest value of the corresponding REM period. For the NREM sleep EMG data, a variable number of subdivisions, each having the same duration as the subdivisions of the subsequent REM period, was used for analysis. The number of subdivisions in this case depended on the length of the NREM period. For each animal, the 20 subdivisions obtained for each REM period were averaged across REM periods, and then all averaged subdivisions were compiled in weighted averages across animals to build an activity profile for the population.
Figure 3.
Analysis of rectified EMG in 20 subdivisions. (A) Recording of genioglossal (EMGGG) muscle activity during a REM period. (B) EMGGG is rectified then integrated for each of the 20 subdivisions. The activity is expressed as a ratio of the integrated EMG over the maximum activity during the REM period. Average ∫EMG level during the preceding NREM sleep period is indicated as an intermittent line. The integrated activity across all 20 subdivisions, with repeated measurements over all REM periods, was analyzed then with an ANOVA (not shown).
Diaphragmatic Activity Analysis
A breath-by-breath analysis was written using Spike2 software to quantify breathing frequency (F), amplitude, inspira-tory duration, inspiratory peak, and respiratory effort based on diaphragmatic EMG. The signal was full-wave rectified and smoothed (τ = 0.05) to obtained a moving time average. After selecting a zero line and determining the start of inspiration and expiration for each breath, breathing amplitude was obtained by integrating the rectified smoothed EMGD. Inspiratory peak was defined as the highest activity during each inspiration. Finally, respiratory effort was defined as the ∫EMGD divided by the inspiratory duration for each breath. As described above, each REM period was divided into 20 subdivisions, and each parameter was averaged in each subdivision. Then, these average values were normalized to the highest value for that REM period. NREM sleep periods were analyzed similarly by averaging each respiratory parameter and expressing the averages as a fraction of the highest value of the corresponding REM period. All averaged values of each animal were compiled in weighted averages to build a profile for each respiratory parameter.
Statistical Analysis
A one-way repeated-measures ANOVA was used for statistical analysis of the effect of time (represented by 20 subdivisions) on EMG activity, breathing frequency, amplitude, inspiratory duration, inspiratory peak, and respiratory effort. Results were considered significant when P < 0.05. Using the F ratio obtained from the ANOVA, η2 were calculated as follows:
With dfb: degree of freedom between subjects; and dfw: degree of freedom within subjects.
This effect size statistic was applied for either the whole duration of REM periods (20 subdivisions) or for the first 19 subdivisions (Table 1). This differential statistic was performed to clarify a confounding effect of the last subdivisions, where for all parameters the value dropped systematically. A one-way repeated-measures ANOVA was used to compare each subdivision during REM sleep to the average values during the previous NREM sleep period.
Table 1.
Effect size statistic (η2) of time in REM sleep on muscles activity and respiratory parameters
| Muscles | 20 subdivisions |
19 subdivisions |
||||
|---|---|---|---|---|---|---|
| P | F | η2 | P | F | η2 | |
| Genioglossus | < 0.0001 | 13.07 | 0.58 | < 0.0001 | 13.22 | 0.58 |
| Diaphragm | < 0.0001 | 8.04 | 0.46 | < 0.0001 | 6.43 | 0.40 |
| Intercostal | < 0.0001 | 3.36 | 0.39 | < 0.005 | 2.44 | 0.32 |
| Neck | < 0.0001 | 4.94 | 0.34 | 0.5875 | N/A | N/A |
| Extensor Digitorum | < 0.0001 | 17.40 | 0.80 | < 0.05 | 1.79 | 0.29 |
| Respiratory parameters | ||||||
| Frequency | < 0.0001 | 7.36 | 0.58 | < 0.005 | 2.61 | 0.33 |
| Amplitude | < 0.0001 | 5.00 | 0.49 | < 0.005 | 2.32 | 0.31 |
Data are P values, F ratio, and η2 obtained from one-way repeated measure ANOVAs when considering either the complete duration of REM periods (20 subdivisions) or when omitting the last part of REM periods (19 subdivisions). The null hypothesis is that time in REM sleep does not affect muscles activity or respiratory parameters. η2 values < 0.3 are considered to be a small effect size and values > 0.8 to be a large effect size.
RESULTS
Results reported here were obtained from 10 adult male Wi-star rats during 219 hours of recording in 73 sessions. The 313 REM periods used for analysis had a 1.93 (± 0.05 SEM) min average duration.
Respiratory Muscle Activity Profile during REM Sleep
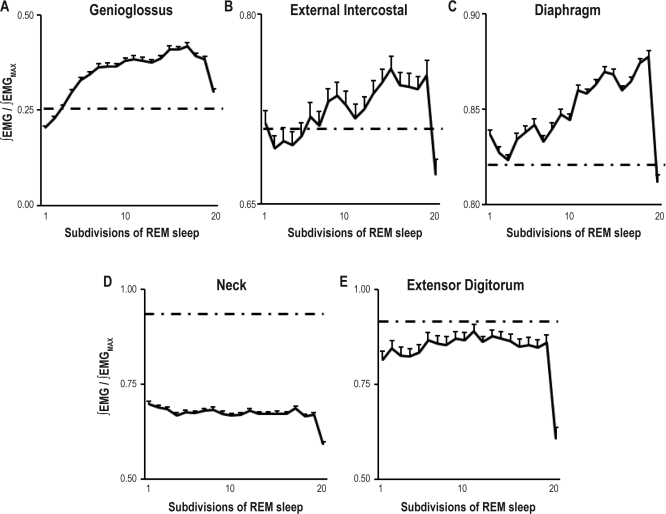
The activity profile in REM sleep of the diaphragm, external intercostal, and genioglossal muscles was a unimodal negatively skewed curve with a rise to a peak in the second half of the REM period and a waning phase toward the end of the period (Figure 4). Activities in respiratory muscles were significantly higher during REM sleep than during the preceding NREM sleep periods (Figure 4).
Figure 4.
Genioglossus (A), external intercostal (B), diaphragm (C), neck (D), and extensor digitorum (E) muscle activity across 313 REM periods divided into 20 subdivisions from 10 Wistar rats. Activity is expressed as a ratio of activity to the REM sleep peak activity for each muscle and each REM period. Average ∫EMG level during the preceding NREM sleep period is indicated as an intermittent line. Values are weighted averages ± SEMs (N = 10).
Results presented here are weighted averages of integrated muscle activity for REM periods divided into 20 subdivisions. In all respiratory muscles, time in REM sleep had a significant effect on muscle activity (Table 1). The diaphragm maintained its respiratory activity during REM sleep. Still, a significant increase of activity was detected in the integrated diaphragmatic EMG (EMGD) in the second part of the REM period compared to the average level of activity of the preceding NREM period. The peak activity was 6.2% higher than the average level during NREM sleep (Figure 4). Similarly, the external intercostal muscle maintained respiratory (inspiratory) activity during REM sleep. The peak activity of the external intercostal muscle as seen in the rectified integrated EMG was 4.4% higher than the average level during the preceding NREM period (Figure 4). Inspiratory activity, evident in the genioglossus during NREM sleep, stopped at the transition between NREM and REM sleep (Figure 1). In the first part of the REM period, the genioglossus was silent. This first phase had an average duration of 11.6 s. Then muscle activity developed to a peak in the second part of the REM period with a value 63.8% higher than the average level during the preceding NREM sleep period (Figure 4). This activity was not respiratory in nature. In all respiratory muscles, activity decreased toward NREM sleep values at the end of the REM period.
Non-Respiratory Muscle Activity Profile
The combined data of 10 and 5 animals for the neck and extensor digitorum muscles respectively presented a flat profile (Figure 4). Motor activity was equally probable during any subdivision of REM sleep except the last, a subdivision when activity was least (Figure 4). Time in REM sleep did not have a significant effect on nuchal activity when considering the first 19 subdivisions before EMG activity dropped prior to waking (P = 0.59). In these first 19 subdivisions, the extensor digitorum activity presented a weak pattern (η2 = 0.29) characterized by a peak activity in the middle of the REM period. Neck muscles were more affected by motor atonia than the extensor digitorum. The average level of activity of the nuchal EMG during REM sleep was 27.4% less than the average level of muscle activity during NREM sleep. The extensor digitorum, which under the conditions of this study was less active than the neck muscle, only decreased its average level of activity by 3.2% in REM sleep compared to the average level of activity during NREM sleep.
Breathing Frequency, Effort, Amplitude, and Inspiratory Peak
Respiratory parameters of 6 male Wistar rats were analyzed over 192 REM periods (Figures 5–7). The average duration of the REM periods was 1.69 min. The results described are weighted averages of breaths taken during each REM and NREM periods.
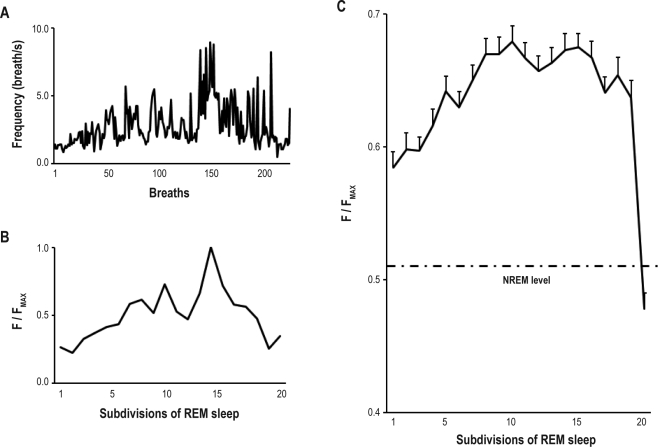
Figure 5.
Analysis of respiratory frequency across REM sleep. (A) Breath-by-breath frequency value (breaths/s) during one REM period in one animal (MQ15); (B) Normalized frequency (F) value as function of time in REM sleep expressed in 20 subdivisions. This is the same session as in A; (C) Combined respiratory frequency as a function of time in REM sleep across 180 REM periods in 6 animals. Average breathing frequency during the preceding NREM sleep period is indicated as an intermittent line. Values are weighted averages ± SEMs (N = 6). Time in REM sleep (20 subdivisions) had a significant effect (P < 0.05) on breathing frequency.
Figure 6.
Analysis of respiratory amplitude across REM sleep. Combined respiratory amplitude as a function of time in REM sleep across 180 REM periods in 6 animals. Average respiratory amplitude during the preceding NREM sleep period is indicated as an intermittent line. Values are weighted averages ± SEMs (N = 6). Time in REM sleep (20 subdivisions) had a significant effect (P < 0.05) on respiratory amplitude.
Figure 7.
Interpretation of diaphragmatic muscle activity profile during REM sleep. Product of frequency and amplitude expressed as a function of time in REM sleep for the same condition as in Figure 5 and 6, respectively. Values are weighted averages (N = 6). Both amplitude and frequency contribute to the diaphragmatic activity profile seen in Figure 4.
Using rectified smoothed diaphragmatic EMG, breathing frequency was analyzed breath-by-breath and averaged in each of the 20 subdivisions (Figure 5). During REM sleep, frequency increased to a peak in the middle of the REM period. This peak had a value 28.3% higher than the average frequency level during NREM sleep. In individual REM periods, the frequency could reach values of 500 breaths/min. The frequency decreased towards NREM sleep values toward the end of the REM period.
The combined data of 6 animals showed a 30.0% decrease in respiratory amplitude in the first part of the REM period compared to the average amplitude levelduring NREM sleep (Figure 6). Amplitude levels increased toward NREM sleep values in the second part of the REM periods and were above NREM sleep values prior to waking.
Amplitude, effort, and inspiratory peak all presented similar profiles, with a decrease during the first part of the REM period and an increase in the second part of the REM period. Effort and amplitude correlated strongly and positively with inspiratory peak activity during REM sleep. The correlation coefficients with peak activity were 0.9492 and 0.7593 for respiratory effort and amplitude, respectively.
As shown in Figure 7, when respiratory frequency and amplitude values were combined for each subdivision, the profile became similar to the integrated diaphragmatic EMG (Figure 4).
DISCUSSION
Respiratory muscles in rats present a specific profile of activity during REM sleep that is similar to the activity profile seen in the diaphragm and respiratory neurons during mechanically induced hypocapnic apnea in REM sleep in cats.25,26 Increased activity was seen in the second part of the REM period, and this activity waned just prior to waking. The greatest change in activity during REM sleep compared to NREM sleep was seen in the genioglossal muscles. Both the diaphragm and the external intercostal muscle maintained their respiratory activity in REM sleep. The increased integrated activity seen in the second part of the REM period in the diaphragm was due to both an increase in the number and amplitude of breaths. On the other hand, the phasic activity of non-respiratory muscles did not have a profile similar to that of the respiratory muscles. Moreover, in the case of the neck muscles, no significant pattern was observed during the majority of the REM period. Thus, the idea of a common pontine generator that controls all phasic events was not supported by our data. However our data support the idea that a REM-specific excitatory process affects the respiratory system after a short delay from the onset of REM sleep and that this process maximally increases its activity in the second part of the REM period.25,2
Excitatory Endogenous Drive Affects the Respiratory System during REM Sleep
It has been shown previously that there is an endogenous excitatory drive to the respiratory system during REM sleep.25,26 Mechanical ventilation studies in cats during REM sleep showed that, on a background of apnea, respiratory neurons and muscles become active and this activity has a specific profile with a peak in the second part of the REM period. This drive can affect simultaneously inspiratory and expiratory neurons as well as respiratory motor neurons, and therefore it may disorganize breathing.26 The drive caused respiratory neurons to discharge during apnea at 65% of the mean eupneic discharge rate.
Upper airway muscles, such as the genioglossus, are reported to be atonic during REM sleep. Sleep apnea patients may fare worse during REM sleep presumably because of the loss of tone in these muscles.6 Many studies13 have focused on the neuro-pharmacology of REM sleep atonia because of its possible role in sleep apnea. However, there is evidence inconsistent with the idea of a prevailing atonia in REM sleep. Our data showed that activity of the genioglossus muscle is greater during REM sleep than during NREM sleep—supporting the earlier finding of Lu et al.19 Sahin et al.29 showed in dogs that hypoglossal neural activity is increased during REM sleep. Both respiratory activity and tonic (non-respiratory) activity were increased. In cats, Richard and Harper27 found that hypoglossal neurons discharged at rates in REM sleep that were similar to wakefulness. Finally, in humans, some studies16,35 have reported that alea nasi and genioglossus EMG activity were similar in REM sleep and NREM sleep, and that phasic cricothyroid muscle activity was greater in REM sleep than in NREM sleep.
Accessory respiratory muscles, such as intercostal muscles, are reported to be atonic during REM sleep in humans. However, there is evidence that these muscles can be recruited and resume their activity during REM sleep when the diaphragm is paralyzed.2,31 In the rat, external intercostal muscles reportedly maintain their activity during REM sleep.21 Our data showed that in addition to respiratory drive, a REM-specific drive excites the activity of this muscle in the second part of the REM period.
The diaphragm may be spared the atonia of REM sleep. The diaphragm maintains, and may increase, its respiratory-related activity during REM sleep. In eupneic conditions in cats, the activity of the diaphragm has a greater rate of rise during REM sleep than during NREM sleep.24 Also, large diaphragmatic motor units are recruited earlier during REM sleep, which produces a square airflow profile.24 During ventilator-induced apnea in cats, bursts of phasic diaphragmatic activity develop to a peak in the second part of the REM period. This activity is rarely related to breathing, and recognizable behaviors such as purring or meowing are rare. On the other hand, other studies indicate that there is inhibition of the diaphragm in REM sleep. Hendricks and colleagues11 have shown, following lesions of pontine tegmental structures that cause REM atonia, an increase in the rate of rise of diaphragmatic activity, and Lovering et al.18 showed that post-inspiratory activity, which is common in NREM sleep, does not occur in REM sleep. Our data confirm earlier results reporting that the diaphragm is excited during REM sleep,25 but they show also an inhibition of diaphragmatic activity in the first part of the REM period. The integrated EMG activity peaked in the second part of the REM period. This increase of activity was due to both an increase in frequency and amplitude. Increased breathing frequency has been a hallmark of REM sleep since the first description of the state by Aserinsky and Kleitman.1 However, this is the first report to quantify the variation in respiratory frequency throughout a REM period. This change followed a unimodal profile with a peak in the middle of the REM period. Lazarenko et al. showed that isoflurane increases respiratory rate via neurons of the retrotrapezoid nucleus (RTN) in a CO2-independent manner.17 They concluded that RTN neurons provide an excitatory drive to the respiratory system even when other elements of the system are depressed. It is possible that the change in respiratory frequency observed during REM sleep is also mediated by RTN neurons.
In our study, the diaphragm was also subject to an inhibition that affected amplitude, respiratory effort and inspiratory peak in the first part of the REM period. In the second part of the REM period, these parameters increased towards NREM sleep values. In this case, either the inhibition decreased or an excitatory process stimulated the diaphragm and overcame the inhibition in the later part of the REM period. It is possible that the secondary excitation seen in amplitude was a response to the hypoventilation of the first part of the REM period. However, cats ventilated to apnea presented this excitatory process in the second part of the REM period. In these animals, chemical stimuli (PCO2) were maintained constant.25 Furthermore end-tidal CO2 values in cats breathing spontaneously drop during REM sleep—indicating hyperventilation.25 When we combined the change in frequency profile with the change in amplitude, we obtained a profile almost identical to the integrated EMG profile.
Neural Control of REM Phasic Events
It has been suggested that a caudoventral tegmental pontine group controls the generation of all phasic events during REM sleep.32 This idea has been supported by evidence of correlation between PGO waves and rapid eye movements, rapid eye movements and muscle twitches,9 rapid eye movements and MEMA,27 and MEMA and motor activity of the extremities and head.32 However, these correlations appear to be weak. De Gennaro and Ferrara4 showed in humans that, following sleep deprivation, MEMA and rapid eye movements become dissociated, with rapid eye movements decreasing while MEMA stayed constant. Also, there is a weak positive correlation between PGO-wave activity and diaphragmatic activity during mechanically-induced apnea in cats25 and between PGO-wave and respiratory neuronal activity.23 We have shown that irregularities of breathing and PGO-wave activity can be dissociated during hypercapnic stimulation in cats.8 In that study, the coefficient of variation of the tidal volume decreased significantly with increasing inspired CO2, indicating a more steady breathing, while PGO density tended to increase. However, in this case it is possible that the chemical drive overpowered the state-specific inputs to the respiratory system but did not affect other REM phasic events. Finally, the activity profile of MEMA and PGO waves differs from the respiratory muscle profile. Their activity appears at the onset of and prior to the REM period respectively, and MEMA activity is greatest at the beginning of the REM period.27 In this study, we have shown that the activity profile of respiratory muscles presented a specific shape with a peak in the second part of the REM period whereas non-respiratory muscle (i.e., neck and extensor digitorum) activity did not. Twitches of neck muscles could appear at any time during the REM period whereas respiratory muscle activity increased specifically in the second part. Only during the last REM period subdivisions, did we observe a correlation between respiratory and non-respiratory motor activity. Prior to waking, all activity decreased significantly.
Origin of the REM Excitatory Drive Affecting the Respiratory System
The origin of the REM sleep excitation is unknown. Motor activity has been linked to dream content, which would suggest that the excitation might arise from cortical structures. This idea is supported by the dream enactment of patients suffering from REM sleep behavior disorder20 and similarly by the behavior of oneiric experimental animals who have REM sleep without muscle atonia.30
There is conflicting evidence of the importance of dreams in the pattern of breathing in REM sleep. One study reports that breathing rates are high and variable in association with dreams of physical activity and high emotional content.12 However, other data are less convincing. Hauri and Van de Castle10 reported that respiratory rate was related to emotionality and dream intensity, but there was no significant relation to physical activity in the dream. Also, neuronal and muscular respiratory activity in experimental animals ventilated to apnea in REM sleep is generally not recognizable as a behavioral act (e.g., purring).25 Respiratory neurons of different types (e.g., inspiratory and expiratory neurons) were sometimes active simultaneously during this condition.26 Moreover, the characteristic irregularity of breathing during REM sleep is still present in neonatal and pontine animals as well as in decorticates humans,15 none of whom presumably have dream content. This would suggest that rather than having a cortical origin, the excitatory drive may originate from brainstem structures that are active in REM sleep. REM-specific neurons of the gigantocellular and lateral medullary tegmental fields22 discharge in relation to respiratory frequency and present a profile of discharge similar to the respiratory muscle profile described in cats in other studies and in rats in this study, and they are therefore candidates for the origin of the REM-specific excitatory process of the respiratory system.
Potential Clinical Relevance
We, as others before us have, hypothesize that excitatory processes in REM sleep are involved in disease. In some cases, these processes may benefit the patient. Children suffering from chronic hypoventilation syndrome (CHS) require ventilatory support in NREM sleep but breathe during REM sleep without mechanical ventilation.7 Huang and colleagues14 reported less central sleep apnea and hypoventilation during REM sleep in CHS children—presumably due to the excitation of the respiratory system in that state. Also, it has been suggested that REM sleep might protect newborns from sudden infant death syndrome by limiting the apneic events during that state.34 In other cases, these processes may contribute to hypoventilation in REM sleep. Chronic obstructive pulmonary disease patients become more hypoxemic during REM sleep. Douglas5 suggested that excitation of the respiratory system in REM sleep leads to erratic breathing and causes the worsening of hypoxemia in these patients.
CONCLUSION
In this study, we demonstrated in rat that the respiratory muscles are excited in REM sleep. We quantified this process and demonstrated that the excitation has a specific profile with a peak in the second part of the REM period. This profile may be the result of interaction between inhibitory (i.e., REM sleep atonia) and excitatory processes (Figure 8). The increased respiratory frequency characteristic of REM sleep may be induced by this drive. This REM excitation seems to be specific to the respiratory system and does not affect other motor pools. Our results do not support the idea that a single pontine structure generates simultaneously phasic REM sleep events. A REM-specific excitatory process has been hypothesized to be responsible for the first respiratory movements in utero.3 Thus, the excitatory activity, described in this study, may be a remnant of an excitatory phenomenon necessary for the development of respiratory rhythmogenesis in utero.
Figure 8.
Representation of excitation and inhibition of the respiratory system in REM sleep. (A) Time course and amplitude of REM excitation and REM inhibition (i.e., atonia) during a REM sleep period. (B) Summation of the processes in A produces the effects seen on the respiratory system during REM sleep. After a delay, the activity profile rises to a peak in the second part of the REM sleep period.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank B. Tilton for care of the animals. Supported by grant NS46062 as part of the NSF/NIH Collaborative Research in Computational Neuroscience Program.
REFERENCES
- 1.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–4. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JR, Dunroy HM, Corfield DR. Respiratory muscle activity during REM sleep in patients with diaphragm paralysis. Neurology. 2004;62:134–7. doi: 10.1212/01.wnl.0000101719.84675.e0. [DOI] [PubMed] [Google Scholar]
- 3.Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972;220:119–43. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Gennaro L, Ferrera M. Sleep deprivation and phasic activity of REM sleep: independence of middle-ear muscle activity from rapid eye movements. Sleep. 2000;23:81–5. [PubMed] [Google Scholar]
- 5.Douglas NJ. Sleep in patients with chronic obstructive pulmonary disease. Clin Chest Med. 1998;19:115–25. doi: 10.1016/s0272-5231(05)70436-6. [DOI] [PubMed] [Google Scholar]
- 6.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–64. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming PJ, Cade D, Bryan MH, Bryan AC. Congenital central hypoventilation and sleep state. Pediatrics. 1980;66:425–8. [PubMed] [Google Scholar]
- 8.Fraigne JJ, Dunin-Barkowski WL, Orem JM. Effect of hypercapnia on sleep and breathing in unanesthetized cats. Sleep. 2008;31:1025–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Geisler P, Meier-Ewert K, Matsubayshi K. Rapid eye movements, muscle twitches and sawtooth waves in the sleep of narcoleptic patients and controls. Electroencephalogr Clin Neurophysiol. 1987;67:499–507. doi: 10.1016/0013-4694(87)90051-4. [DOI] [PubMed] [Google Scholar]
- 10.Hauri P, Van de Castle RL. Psychophysiological parallels in dreams. Psychosom Med. 1973;35:297–308. doi: 10.1097/00006842-197307000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hendricks JC, Kline LR, Davies RO, Pack AI. Effect of dorsolateral pontine lesions on diaphragmatic activity during REMS. J Appl Physiol. 1990;68:1435–42. doi: 10.1152/jappl.1990.68.4.1435. [DOI] [PubMed] [Google Scholar]
- 12.Hobson JA, Goldfrank F, Snyder F. Respiration and mental activity in sleep. J Psychiatr Res. 1965;3:79–90. doi: 10.1016/0022-3956(65)90017-8. [DOI] [PubMed] [Google Scholar]
- 13.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol. 2008;164:179–96. doi: 10.1016/j.resp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Colrain IM, Panitch HB, et al. Effect of sleep stage on breathing in children with central hypoventilation. J Appl Physiol. 2008;105:44–53. doi: 10.1152/japplphysiol.01269.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouvet M, Jouvet D. A study of the neurophysiological mechanisms of dreaming. Electroencephalogr Clin Neurophysiol. 1963;(Suppl 24):133. [PubMed] [Google Scholar]
- 16.Kuna ST, Smickley JS, Vanoye CR, McMillan TH. Cricothyroid muscle activity during sleep in normal adult humans. J Appl Physiol. 1994;76:2326–32. doi: 10.1152/jappl.1994.76.6.2326. [DOI] [PubMed] [Google Scholar]
- 17.Lazarenko RM, Fortuna MG, Shi Y, et al. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci. 2010;30:9324–34. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovering AT, Dunin-Barkowski WL, Vidruk EH, Orem JM. Ventilatory response of the cat to hypoxia in sleep and wakefulness. J App Physiol. 2003;95:545–54. doi: 10.1152/japplphysiol.01051.2002. [DOI] [PubMed] [Google Scholar]
- 19.Lu JW, Mann GL, Ross RJ, Morrison AR, Kubin L. Differential effect of sleep-wake states on lingual and dorsal neck muscle activity in rats. Respir Physiol Neurobiol. 2005;147:191–203. doi: 10.1016/j.resp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Mahowald MW, Schenck CH. REM sleep parasomnias. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine, 4th edition. Vol. 897. Elsevier Saunders; 2005. p. 916. [Google Scholar]
- 21.Megirian D, Pollard MJ, Sherrey JH. The labile respiratory activity of ribcage muscles of the rat during sleep. J Physiol. 1987;389:99–110. doi: 10.1113/jphysiol.1987.sp016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netick A, Orem JM, Dement W. Neuronal activity specific to REM sleep and its relationship to breathing. Brain Res. 1977;120:197–207. doi: 10.1016/0006-8993(77)90900-3. [DOI] [PubMed] [Google Scholar]
- 23.Orem J. Medullary respiratory neuron activity: relationship to tonic and phasic REM sleep. J Appl Physiol. 1980;48:54–65. doi: 10.1152/jappl.1980.48.1.54. [DOI] [PubMed] [Google Scholar]
- 24.Orem J, Anderson CA. Diaphragmatic activity during REM sleep in the adult cat. J Appl Physiol. 1996;81:751–60. doi: 10.1152/jappl.1996.81.2.751. [DOI] [PubMed] [Google Scholar]
- 25.Orem J, Lovering AT, Dunin-Barkowski W, Vidruk EH. Endogenous excitatory drive to the respiratory system in rapid eye movement sleep in cats. J Physiol. 2000;527:365–76. doi: 10.1111/j.1469-7793.2000.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orem JM, Lovering AT, Vidruk EH. Excitation of medullary respiratory neurons in REM sleep. Sleep. 2005;28:801–7. doi: 10.1093/sleep/28.7.801. [DOI] [PubMed] [Google Scholar]
- 27.Pessah MA, Roffwarg HP. Spontaneous middle ear muscle activity in man: a rapid eye movement sleep phenomenon. Science. 1972;178:773–6. doi: 10.1126/science.178.4062.773. [DOI] [PubMed] [Google Scholar]
- 28.Richard CA, Harper RM. Respiratory-related activity in hypoglossal neurons across sleep-waking states in cats. Brain Res. 1991;542:167–70. doi: 10.1016/0006-8993(91)91014-r. [DOI] [PubMed] [Google Scholar]
- 29.Sahin M, Durand DM, Haxhiu MA. Chronic recordings of hypoglossal nerve activity in a dog model of upper airway obstruction. J Appl Physiol. 1999;87:2197–2206. doi: 10.1152/jappl.1999.87.6.2197. [DOI] [PubMed] [Google Scholar]
- 30.Sastre JP, Jouvet M. Oneiric behavior in cats. Physiol Behav. 1979;22:979–89. doi: 10.1016/0031-9384(79)90344-5. [DOI] [PubMed] [Google Scholar]
- 31.Sherrey JH, Megirian D. After phrenicotomy the rat alters the output of the remaining respiratory muscles without changing its sleep-waking pattern. Respir Physiol. 1990;81:213–25. doi: 10.1016/0034-5687(90)90047-3. [DOI] [PubMed] [Google Scholar]
- 32.Slegel DE, Benson KL, Zarcone VP, Jr, Schubert ED. Middle-ear muscle activity (MEMA) and its association with motor activity in the extremities and head in sleep. Sleep. 1991;14:454–9. [PubMed] [Google Scholar]
- 33.Vanni-Mercier G, Debilly G. A key role for the caudoventral pontine tegmentum in the simultaneous generation of eye saccades in bursts and associated ponto-geniculo-occipital waves during paradoxical sleep in the cat. Neuroscience. 1998;86:571–85. doi: 10.1016/s0306-4522(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K, Inokuma K, Negoro T. REM sleep prevents sudden infant death syndrome. Eur J Pediatr. 1983;140:289–92. doi: 10.1007/BF00442665. [DOI] [PubMed] [Google Scholar]
- 35.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71:488–97. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]