Abstract
Study Objectives:
Excessive daytime sleepiness (EDS) is associated with increased mortality in older adults, yet sleep disordered breathing (SDB), a common cause of sleepiness, has not been shown to increase mortality in older adults. This study examined the relationship between daytime sleepiness, SDB, self-report sleep parameters, and mortality in older adults.
Design:
Longitudinal cohort study.
Setting:
Clinical and Translational Research Center, at-home testing.
Participants:
289 study participants (age > 65, no dementia or depression at the time of enrollment) classified as having EDS (n = 146) or not (n = 143).
Measurements and Results:
Study participants underwent in-lab polysomnography and multiple sleep latency testing at cohort inception. Survival analysis was conducted, with an average follow-up of 13.8 years. Excessive daytime sleepiness was associated with an unadjusted mortality hazard ratio of 1.5 (95% CI 1.1-2.0). The unadjusted mortality hazard ratio for study participants with both EDS and SDB (apnea-hypopnea index ≥ 20 events/h) was 2.7, 95% CI: 1.8-4.2. These findings persisted with an adjusted mortality hazard ratio of 2.3, 95% CI: 1.5-3.6 in the final model that included other covariates associated with increased mortality (sleep duration > 8.5 h, self-reported angina, male gender, African American race, and age).
Conclusion:
The presence of SDB is an important risk factor for mortality from excessive daytime sleepiness in older adults. In the presence of SDB at an AHI ≥ 20 events/h, EDS was associated with an increased all-cause mortality risk in older adults, even when adjusting for other significant risk factors, such as prolonged sleep duration. In older patients who had SDB without EDS, or EDS without SDB, there was no increased all-cause mortality rate.
Citation:
Gooneratne NS; Richards KC; Joffe M; Lam RW; Pack F; Staley B; Dinges DF; Pack AI. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. SLEEP 2011;34(4):435-442.
Keywords: Sleep disordered breathing, daytime sleepiness, mortality, longitudinal, aged
INTRODUCTION
Excessive daytime sleepiness affects up to 10% to 33% of the elderly.1–3 Older adults and health care providers often view excessive daytime sleepiness (EDS) as a normal aspect of aging. However, EDS has been associated with significant consequences, including an increased incidence of functional impairment,4,5 falls,6 cognitive deficits,7 and mortality.8
Excessive daytime sleepiness can be a result of sleep disorders, such as sleep disordered breathing (SDB).1,9 The prevalence of SDB itself may increase with age to a prevalence of approximately 20% in older patients relative to 5% to 10% in younger populations.10 SDB has also been linked to mortality.11–13 These studies have included adult subjects from a broad age range. Research studies in older subjects have paradoxically been more equivocal,14 with only certain high-risk populations, such as female nursing home patients, showing increased rates of mortality associated with SDB.15 Others have suggested that mortality related to SDB may decline with age.16,17 This has created significant uncertainty as to the appropriate threshold of SDB severity that warrants treatment in older adults.
Some studies have observed that excessive daytime sleepiness symptoms may be an important element for understanding the clinical significance of SDB.18,19 Interestingly, most studies that have examined EDS and mortality have not addressed whether SDB modifies the mortality risk from EDS. Furthermore, most past research examining long-term outcomes of daytime sleepiness has relied on the self-report claim of daytime sleepiness; little is known about long-term consequences of patients with objective evidence of physiological daytime sleepiness in addition to subjective complaints.
To better understand the relationship between EDS, SDB, and mortality, we conducted a longitudinal study using a baseline cohort which enrolled 2 groups: older adults with self-reported EDS (n = 146) and those without (n = 143). We examined mortality risk as a function of self-reported EDS and polysomnographically determined SDB status. Additional objective measures collected at cohort inception included the multiple sleep latency test for assessing EDS physiologically. Our specific study hypothesis was that EDS increases the risk of long-term mortality in older adults, and that the presence of SDB may influence this risk. In studying this relationship, we also considered demographic factors, self-report sleep parameters, and other medical comorbidities to determine whether they modified the risk of mortality from EDS and SDB. Understanding the long-term consequences of EDS and SDB in older adults is crucial for identifying at risk patients and developing targeted interventions.
METHODS
Study Design and Subjects
A longitudinal observational cohort study design was used in which study participants were identified at cohort inception as having self-reported EDS (the exposure of interest) or not. Conditions for study eligibility included age > 65 years, the absence of dementia or depression, and meeting criteria for the presence or absence of self-reported EDS. Potential study participants with depression were excluded during the screening process because depression overlaps with daytime sleepiness and sleep disorders (hypersomnia and insomnia, for example, are diagnostic criteria for depression), and it is strongly related to mortality, thus creating significant confounding effects with several variables of interest and the outcome variable.
The criteria for the presence or absence of EDS were based on an EDS questionnaire (described below). Study participants with EDS consisted of individuals who self-identified as having daytime sleepiness as a problem ≥ 3-4 times per week. Study participants without EDS were those who reported not to have fallen asleep in active or passive situations more than once or twice a month. Individuals who did not meet either criterion were considered indeterminate and were excluded from the study.
The Institutional Review Board of the University of Pennsylvania Medical Center approved the study protocol. All participating subjects provided written informed consent at the time of data collection during the establishment of the initial cohort.
Subjects (n = 293) in this study were recruited from the independent-living complexes of retirement communities throughout the Philadelphia greater metropolitan area, with additional recruitment of community-dwelling African American patients to ensure adequate representation of minorities. Study participants were thus drawn from a community sample, and were not obtained from a convenience sample at a specific sleep clinic site. They were recruited from 9/1993 to 5/1998. Additional details regarding the subject source population are described elsewhere.20 Due to the low percentage of non-Caucasian and non–African American study participants (Asian race was endorsed by one subject and Other race by three), the analysis was limited to Caucasian and African American study participants (n = 289).
Baseline Assessment
After subjects provided informed consent, 3 screening instruments were administered: Folstein Mini-Mental Status Examination, Geriatric Depression Scale, and the EDS screening questionnaire. The first 2 instruments were used to exclude study participants with dementia (Short Blessed Examination Score ≥ 7) and depression (Geriatric Depression Score ≥ 10), respectively.20–22
The EDS questionnaire was then used to divide the subjects into an exposed group and a non-exposed group based on the presence or absence of excessive daytime sleepiness, respectively. The presence of excessive daytime sleepiness was based on self-report and not on objective criteria. We chose to use a self-report determination to maximize the clinical utility of the study findings since most clinicians will also rely on self-report during their initial evaluation of patients. The initial question was “Do you have a problem with sleepiness (feeling sleepy or struggling to stay awake) during the daytime?” The response choices were “nearly every day”, “3-4 times a week,” “1-2 times a week,” “1-2 times per month,” “hardly ever,” or “never.” The exposed group consisted of individuals who self-identified as having daytime sleepiness as a problem ≥ 3-4 times per week. The non-exposed group included subjects who denied they had a problem with daytime sleepiness, and confirmed this by responding “less than 1-2 times per month” to the following 2 additional questions: “During the past month, how often have you fallen asleep during active situations?” and “During the past month, how often have you fallen asleep during passive situations?” If control subjects cited a frequency ≥ 1 to 2 times per month, they were excluded from analysis because they were felt to have an indeterminate case/control status. Both subjective (compared to the Epworth Sleepiness Scale) and objective (compared to the multiple sleep latency test) validation of this methodology has been presented elsewhere and has shown statistically significant differences using these 2 measures between subjects with complaints of daytime sleepiness and those without.20
Medical history was obtained using a medical history form. Patients were asked about their current and past status for 22 medical conditions in several categories: diabetes, emphysema, chronic bronchitis, high blood pressure, angina, heart attack, arrhythmia, heart failure, stroke, sinus disease, hay fever, deviated septum, thyroid disease, depression, and arthritis. Other history elements included self-reported sleep parameters (sleep duration, sleep latency, and sleep efficiency for the prior month), demographic factors such as age, sex, and race, as well as alcohol consumption (> 7 alcoholic drinks per week) and smoking history (history of past or current use). A body mass index (kg/ m 2) was calculated for each subject.
Objective Measures
All subjects underwent 2 nights of nocturnal polysomnography at baseline.20 Research study participants had their sleep measured by an in-lab 16-channel polysomnography which included electroencephalogram, electrooculogram, electrocardiogram, snoring, chin and limb electromyelogram, chest/abdominal respiratory belts (piezoelectric), finger oximetry, and airflow monitoring with nasal and oral thermistors. Sleep records were manually scored in 30-sec epochs according to standard criteria.23 The first night was used to adapt subjects to the sleep laboratory due to the potential for a first-night effect that could lead to reduced total sleep time.24,25 Data from the first and second nights were not combined. Only the second night data were used for this analysis. To determine the distribution of respiratory events, apnea (cessation of airflow > 10 sec) and hypopneas (defined as a clearly discernible decrease in airflow associated with a 4% desaturation, arousal, or both) were used to calculate an apnea-hypopnea index (AHI, events/h).26 Apneas were classified as obstructive if they were associated with continued chest or abdominal movement, or central if there was a cessation of chest and abdominal movement. If the study participant had an AHI ≥ 15 events/h and more than 50% of the events were central in nature, they were considered to have predominantly central sleep apnea.27 Both obstructive and central apneas were included in the final calculation of the AHI; hence we refer to patients with respiratory events as having SDB.
Daytime sleepiness was also objectively measured physiologically with the multiple sleep latency test (MSLT).28 It was performed the morning after the second overnight sleep study. Research study participants were allowed to have four nap opportunities, each lasting 20 min, between 09:00 and 15:00. The sleep latency was calculated as the time from lights out to the onset of stage 2 sleep. Lower latencies to sleep onset represent greater propensity to fall asleep. A criterion of ≤ 8 min was used to define objective daytime sleepiness, consistent with clinical recommendations.29 The PSG and MSLT methodology antedated more recent scoring and assessment recommendations.26,30
Mortality Outcomes Assessment
To assess the survival status of subjects, the social security death index was queried. The social security number was entered and searched. If the subject's name or a name with a similar spelling appeared and the date of birth matched the subject's dates of birth, it was considered a positive identification that the individual was deceased. If both the subject's name and date of birth did not match the information listed for that social security number, it was assumed that the individual was still alive.
Statistical Analysis
The primary study outcome was death from any cause identified from the social security death index. A series of planned time-to-event analyses were performed to examine the risk factors for death in older adults. Follow-up for subjects ended on September 1, 2009; subjects who had not died before that date were considered censored. Since there can be a several month lag in updating the social security death index, data were censored after Sept 1, 2009 in order to provide a greater than 6-month window to ensure entry of the subject's survival status into the social security death index.
Associations between groups for various demographic and medical factors were assessed using t-test or ANOVA for mean values, and frequency comparisons (χ2 test) for dichotomous variables. Survival by group was estimated using the Kaplan-Meier method and was used in unadjusted comparisons. Different thresholds for AHI were initially considered, ranging from 5 events/h up to 30 events/h in increments of 5 events/h to determine the optimal AHI threshold for SDB for further comparisons. Cox proportional hazards analysis was used to determine hazard ratios and corresponding 95% confidence intervals for the unadjusted association of EDS (present/absent) and SDB status (present/absent) with mortality. There are 4 possible combinations of self-reported EDS and SDB: EDS+/SDB+, EDS+/SDB-, EDS-/SDB+ and EDS-/SDB-. Dummy variables were created for each combination, allowing for the calculation of hazard ratios, using EDS-/SDB- as the reference group.
We adjusted for confounding of the effect of SDB/EDS by other factors by including those factors in adjusted proportional hazards models. The potential confounders included demographic factors (age, gender, and race), smoking, alcohol (> 7 alcoholic beverages per week), body mass index, habitual sleep parameters (self-reported sleep duration, sleep latency, sleep efficiency), polysomnography sleep parameters (sleep duration, sleep latency, wakefulness after sleep onset, sleep efficiency), oxyhemoglobin desaturation (nadir in REM and NREM sleep during polysomnography), and the 22 medical conditions mentioned earlier. If a covariate was significant at the P < 0.05 level, then an interaction term was considered. Covariates and interaction terms whose association with the outcome were signifi-cant at the P < 0.05 level were added to the main effects model to derive adjusted estimates of the effect of EDS and SDB status on mortality. All reported P values are 2-sided. All statistical analyses were performed with the use of SAS 9.1 (SAS Institute, Cary, North Carolina).
RESULTS
Study participant baseline characteristics are presented in Table 1. The Epworth Sleepiness Scale, as expected, differed considerably between groups with higher scores indicative of more sleepiness in cases; values > 10 are considered to suggest signifi-cant daytime sleepiness (11.1 ± 4.7 versus 5.1 ± 3.7, P < 0.0001).
Table 1.
Baseline sample characteristics
| Parameter | Excessive daytime sleepiness, n = 146, mean (SD) | No excessive daytime sleepiness, n = 143, mean (SD) | P-value |
|---|---|---|---|
| Demographic | |||
| Age, years | 78.0 (6.3) | 77.9 (6.4) | 0.9 |
| Gender, percent female | 71.9% | 76.2% | 0.4 |
| Race, percent Caucasian | 74.0% | 74.1% | 0.9 |
| Education, percent with high school or less | 37.2% | 34.5% | 0.6 |
| Marital status, percent married | 38.6% | 41.3% | 0.6 |
| BMI, kg/m2 | 27.0 (4.6) | 25.8 (4.7) | 0.02 |
| Sleep parameters* | |||
| Habitual sleep duration, self-report, h | 6.5 (1.4) | 6.8 (1.4) | 0.08 |
| Total sleep time (polysomnography sleep duration), h | 5.8 (1.2) | 6.1 (1.0) | 0.09 |
| Slow wave sleep, percent | 6.4% (9.1) | 6.9% (8.3) | 0.6 |
| REM sleep, percent | 17.5% (7.2) | 16.6% (6.2) | 0.3 |
| Sleep onset latency, min | 18.1 (35.6) | 13.7 (17.1) | 0.2 |
| Wakefulness after sleep onset, min | 99.8 (63.1) | 87.3 (52.0) | 0.07 |
| REM latency, min | 101.2 (71.7) | 113.1 (79.6) | 0.2 |
| Sleep efficiency, percent | 74.9 (13.8) | 78.3 (11.4) | 0.03 |
| Apnea-hypopnea index (events/h) | 17.2 (21.2) | 11.9 (16.2) | 0.02 |
| Arousal index (arousals/h) | 23.8 (16.8) | 20.6 (11.9) | 0.06 |
| NREM oxygen saturation nadir, percent | 86.8 (8.9) | 88.2 (7.0) | 0.1 |
| REM oxygen saturation nadir, percent | 85.4 (9.5) | 88.3 (6.6) | 0.003 |
| Central sleep apnea, percent | 4.8% | 2.1% | 0.2 |
Sleep parameters were derived from polysomnography unless otherwise noted.
Study participants' mortality status was ascertained until September 1, 2009, for an average of 13.8 years after initial enrollment (SD: 1.1, range: 11.3-15.9). A total of 160 study participants died during this period (55.4%).
In unadjusted analysis, a significant association was observed between EDS complaints at baseline and risk of subsequent mortality: hazard ratio 1.5; 95 percent CI 1.1- 2.0; P = 0.02. The association was somewhat attenuated and no longer statistically significant when daytime sleepiness was defined as both the subjective complaint of EDS and an objective MSLT criterion of sleep onset latency ≤ 8 min relative to those without either criterion (HR 1.4; 95% CI 0.9-2.0, P = 0.20).
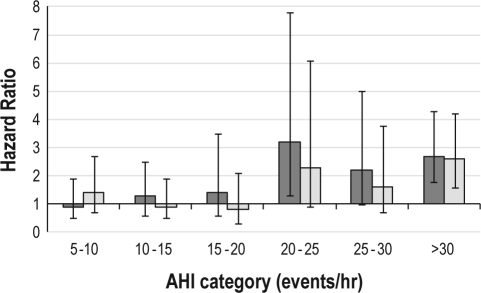
To examine the relationship between SDB, EDS, and mortality, we first examined mortality risk associated with various categories of AHI using increments of 5 events/h (Figure 1). The AHI ranges from 20-25 events/h and above were associated with noticeably higher mortality hazard ratio relative to the baseline category of AHI 0-5 events/h; the estimated hazard ratios for the 20-25 events/h and above categories exceeded 2.0. Thus the AHI threshold for SDB for future analyses was set at an AHI ≥ 20 events/h. Using this criteria, 28.8% of study participants in the EDS group had SDB versus 16.8% in those without EDS (P = 0.02). When categorizing into groups based on both EDS and SDB, the AHI for each of these 4 groups was as follows: EDS with SDB (EDS+/SDB+) 45.3 ± 19.2 events/h; EDS without SDB (EDS+/SDB-) 5.8 ± 5.7 events/h; no EDS but SDB present (EDS-/SDB+) 40.9 ± 19.5 events/h; and no EDS or SDB (EDS-/SDB-) 6.0 ± 5.9 events/h. Comparison across all 4 groups showed a significant difference in the mean AHI (df = 3, F-value 221.5, P < 0.0001); however, the mean AHI did not differ between the 2 groups with SDB (EDS+/SDB+ versus EDS-/SDB+), nor the 2 groups without SDB (EDS+/SDB- versus EDS-/SDB-).
Figure 1.
Hazard ratios for mortality from combined EDS and SDB at various levels of SDB (as defined by the apnea-hypopnea index, [AHI, events/h]). The reference category is an AHI < 5 events/h. Bars represent 95% confidence intervals. Dark gray columns indicate unadjusted analyses. Light gray columns indicate adjusted analyses that include covariates from the final model shown in Table 2.
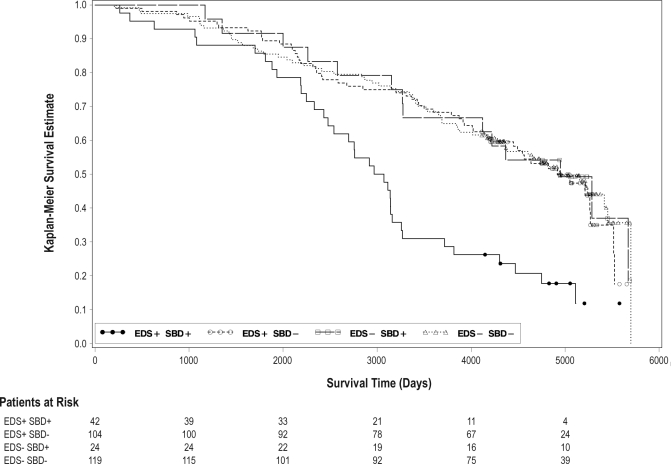
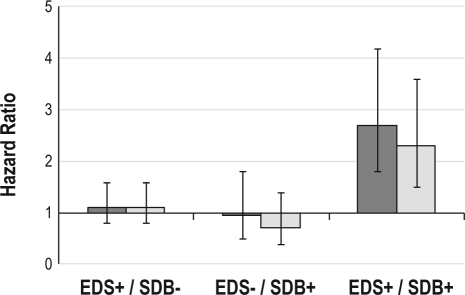
We then examined mortality risk associated with these 4 possible combinations of EDS (EDS) and SDB (SDB) status using this AHI criterion (Figure 2). A statistically significant hazard ratio for increased mortality was noted only in the EDS+/SDB+ group relative to the EDS-/SDB- reference group, HR 2.7, 95% CI: 1.8-4.2 (Figure 3). For the group defined as EDS-/SDB+, there was no increased mortality noted relative to the EDS-/ SDB- reference group. For the group defined as EDS+/SDB-, there was also no increased mortality relative to the EDS-/SDB-reference group.
Figure 2.
Survival curve as a function of excessive daytime sleepiness (EDS) and sleep disordered breathing (SDB) status. Solid line: EDS+/SDB+. Dashed line: EDS-/SDB+. Long-short dashed line: EDS+/SDB-. Dotted line: EDS-/SDB-. Dark circles: censored EDS+/SDB+. Open circles: censored EDS+/SDB-. Open squares: censored EDS-/SDB+. Open triangles: censored EDS-/SDB-.
Figure 3.
Hazard ratios for different categories of EDS (EDS) and SDB (SDB, defined as an AHI ≥ 20 events/h). The reference group is the EDS-/SDB- category. Bars represent 95% confidence intervals. Dark gray columns indicate unadjusted analyses. Light gray columns indicate adjusted analyses that include covariates from the final multivariate model.
Potential covariates that may be related to mortality were considered (as listed in the Statistical Analysis section). Of the demographic parameters considered, age, male gender, and African American race were each individually associated with increased mortality rates in unadjusted analyses. Self-reported history of angina, cardiac arrhythmia, emphysema, habitual sleep duration > 8.5 h, oxyhemoglobin desaturation (NREM and REM), polysomnographic sleep efficiency, and polysomnographic wakefulness after sleep onset were also each associated with an increased mortality risk in unadjusted analyses. All associations were in the expected direction.
Given the potential for interaction between these factors, daytime sleepiness and SDB, interaction terms were also considered; as none was significant at the P < 0.05 level, none was included in the final model. Cardiac arrhythmia, emphysema, oxyhemoglobin desaturation (NREM and REM), polysomnographic sleep efficiency and polysomnographic wakefulness after sleep onset were also no longer significant in the adjusted model at the P < 0.05 level, and they were removed from the final model. After adjustment in this final model, EDS+/SDB+ status remained associated to a statistically significant degree with increased mortality, although the association was somewhat attenuated compared to the unadjusted model (unadjusted: HR 2.7, 95% CI 1.8-4.2 vs adjusted: HR 2.3, 95% CI 1.5-3.6). The final model consisted of age, male gender, African American race, history of angina, habitual self-reported sleep duration > 8.5 h and EDS+/SDB+ status (final model χ2 = 88.8, df = 8, P < 0.0001, Table 2). When excluding study participants with central sleep apnea (approximately 4%), there was no meaningful change in the parameter coefficients for the final model. The hazard ratios by specific AHI categories (in increments of 5 events/h) after adjustment for these covariates are also presented in Figure 1. The hazard ratios noted in the adjusted analysis were attenuated, but paralleled those of the unadjusted analysis and confirmed the use of an AHI ≥ 20 events/h criterion for SDB+ status based on the increase in the mortality hazard ratio between the AHI 15-20 and AHI 20-25 groups.
Table 2.
Fully adjusted proportional hazards model for mortality
| Parameter | Hazard Ratio | P-value | 95% CI |
|---|---|---|---|
| EDS+/SDB+ | 2.28 | 0.0003 | 1.46–3.57 |
| EDS+/SDB- | 1.11 | 0.6 | 0.75–1.63 |
| EDS-/SDB+ | 0.74 | 0.3 | 0.39–1.38 |
| Habitual self-report sleep duration > 8.5 h | 2.11 | 0.03 | 1.06–4.22 |
| Race (African American) | 1.70 | 0.03 | 1.05–2.74 |
| Angina | 1.67 | 0.01 | 1.12–2.50 |
| Gender (male) | 1.53 | 0.02 | 1.08–2.17 |
| Age (per one year increment) | 1.09 | < 0.0001 | 1.06–1.12 |
EDS, Excessive daytime sleepiness; SDB, Sleep disordered breathing (apnea-hypopnea index ≥ 20 events/h). Other categories of EDS and SDB are included in the model even though nonsignificant because they constitute the primary exposure variables. Reference category is EDS-/SDB-
DISCUSSION
While EDS in older adults has been associated with an increased mortality risk, the role of SDB as a mortality risk factor in older adults has been controversial.1,31,32 The current study extends findings from previous work by including a rigorous assessment of EDS as well as SDB in a longitudinal cohort of 289 older adults followed for an average of 13.8 years. We observed that self-reported EDS in older adults was associated with a mortality hazard ratio of 1.5. When considering SDB (AHI ≥ 20 events/h), a common etiology of daytime sleepiness, the mortality hazard ratio increased to 2.3 for those with both conditions; study participants with EDS alone or SDB alone no longer had a significant increase in their mortality hazard ratio. This all-cause mortality risk seen in those with both EDS and SDB persisted in a multivariate model adjusting for several well-known mortality risk factors, including sleep duration greater than 8.5 hours. These findings lend support to the hypothesis that SDB, in the setting of symptoms of EDS, is a risk factor for increased mortality in older adults.
The mortality risk associated with SDB is a seminal issue for the field of sleep medicine, but has been difficult to determine in older adults (see Bliwise and Ancoli-Israel for a detailed review).31,32 Marti et al. noted that there was an increased mortality risk in those with untreated SDB relative to the general population mortality rates, but this risk was attenuated in older adults compared to younger subjects.33 Prospective analysis of the Sleep Heart Health Study cohort did not identify an increased mortality rate in older adults.11 Others have suggested a lower mortality rate in older adults with moderate sleep apnea.16 In select populations, such as institutionalized nursing home females, an increased mortality rate has been observed.15 One of the largest studies to address this question with an extended follow-up period (average 9.5 years) was a population-based study of 426 community-dwelling elderly conducted by Ancoli-Israel and colleagues.14 They noted a relative risk of death of 1.54 for study participants with a respiratory disturbance index (RDI) > 30 events/h (compared to the reference group with an RDI < 15 events/h) and a statistically significant reduction in survival time in that group. Their multivariate model found a trend towards significance when age was not included (P = 0.089) that was, as expected, further attenuated when age was included (P = 0.31). The final risk factor model from that study included many factors that were similar to those we also found to be significant: age, cardiovascular disease, and gender. Another long-term follow-up study, in this case from a sleep clinic sample with an average age of 58.7 to 60.9 years, also found an increased risk of death in those the sleep apnea (HR 1.7), but this too was attenuated in the fully adjusted model (P = 0.09).12
What explains the apparent differences between our findings and previous studies in this context? First, studies based on clinic populations may be influenced by a referral bias.12,17 Second, a shorter duration of follow-up will result in a lower event rate, which impacts on the statistical significance of the findings. Further, given the multiple risk factors for mortality in older adults, any potential risk from SDB may require a longer period of time in which to manifest. Third, our study sample included older subjects than most prior research (mean age was 78.0, ± 6.4, 66-98 years). Fourth, the severity of SDB in our study cohort at inception was higher than most prior research, perhaps as a result of the older age of our subjects. Fifth, andperhaps most import, few studies have included symptoms such as EDS in their assessment. A polysomnography-derived apneahypopnea index is a key measure of SDB and is recommended as part of the evaluation of suspected cases.29 The majority of research examining long-term mortality related to SDB has thus understandably focused on the apnea-hypopnea index to identify the condition. We included daytime sleepiness symptoms, and had a high threshold requiring that it be a problem at least 3 to 4 times per week; not surprisingly, our study participants with EDS had Epworth Sleepiness Scale scores in a range consistent with pathological sleepiness (mean value of 11.1), while our controls had much lower values (mean value of 5.1).
The findings from the current study have several clinical ramifications. Most important of these is that the presence of SDB in the setting of EDS appears to be a significant mortality risk factor even in older adults. The prior absence of a strong link between SDB and mortality in fully adjusted models in older adult populations in contrast to stronger mortality links in younger populations has raised questions regarding the appropriate management strategy for sleep-related breathing disorders in older adults. Our findings imply that the presence of clinical symptoms may substantially alter the significance of an elevated apnea-hypopnea index in older adults. Ancoli-Israel has suggested that SDB in the setting of EDS or decreased daytime functioning is of significant concern and warrants treatment.32 We concur with this recommendation based on our study results. An elevated apnea-hypopnea index can also occur in the absence of symptoms in older adults, highlighting the importance of symptom assessment in addition to the apneahypopnea index.31
The mortality risk manifested above an AHI of 20 events/h only in older adults with EDS. This threshold is higher than current clinical recommendations that identify sleep apnea syndrome as occurring in patients with an AHI ≥ 5 and clinical symptoms, such as daytime sleepiness.29 It is important to note, however, that while the mortality risk may become most prominent at an AHI ≥ 20 events/h, other significant morbidity from SDB may conceivably manifest at lower AHI thresholds. Our study did not address these other potential outcomes. Furthermore, risk can vary considerably by patient, thus a tailored approach is warranted that takes into consideration other comorbidities, such as cardiovascular disease. Overall, these findings are generally supportive of our prior recommendations when discussing treatment guidelines for SDB: treatment is not necessarily warranted for mild disease in the absence of sleepiness, but that for patients with moderate disease, those with excessive sleepiness should be treated.34 For patients with severe disease in the absence of symptoms, further research is necessary to clarify if there are other sequelae aside from mortality risks alone that would warrant treatment.
SDB in older adults has been suggested as representing a different process relative to SDB in younger subjects; the term “geriatric sleep apnea” has been used for this reason.35 In particular, SDB may exist as both an age-dependent process that is relatively benign and an age-related process that is associated with significant sequelae.31 Our finding of an increased mortality risk only in the setting of EDS supports this theory. Furthermore, it suggests that EDS may be one of the key hallmarks of pathologic SDB. In contrast with SDB in older adults, this may not necessarily apply for younger patient populations. The Wisconsin cohort, in an 18-year follow-up of subjects with an average age at cohort inception of 48 years, did not observe a significant effect of EDS in subjects with SDB on mortality risk relative to non-sleepy patients with SDB.13 These investigators noted an adjusted mortality hazard ratio of 3.8 for an AHI ≥ 30 events/h with or without daytime sleepiness.
The mechanism linking SDB to mortality in the setting of EDS is yet to be clearly determined. Others have noted that daytime sleepiness may be an important marker of increased mortality risk in patients with nocturnal sleep complaints.36 SDB has been associated with higher levels of fatigue causing hormones such as tumor necrosis factor-α and interleukin-6.37 Furthermore, the risk of having other adverse medical consequences of SDB, such as hypertension, has been linked to the presence of EDS.18 It is thus conceivable that patients who present with EDS and SDB may have higher levels of inflammatory cytokines than those with SDB alone, and thus may be at higher risk of mortality. Future research that includes biomarkers of inflammation would be worthwhile to explore this further.
In addition to SDB, we noted that habitual self-report sleep duration greater than 8.5 hours was a risk factor for mortality. This has been observed previously.38,39 Sleep duration as a risk factor persisted even in the fully adjusted model that included SDB, suggesting that prolonged sleep duration, even when caused by conditions other than SDB, remains a risk factor for mortality.
Our study has several limitations. The exposed group consisted of patients with significant levels of daytime sleepiness: it was defined as occurring at least 3 to 4 times per week, whereas controls reported not falling asleep in active or passive situations more than once or twice a month. Thus our finding of increased mortality in the setting of SDB and sleepiness only applies for those with significant daytime sleepiness, not mild levels of daytime sleepiness. When assessed in a more heterogeneous population, the study findings may be attenuated. While this is a prospective study suggesting a causal association between EDS + SDB and mortality, that does not necessarily mean that treatment of SDB will reduce this excess mortality. We did not obtain any data on the treatment status of the study participants in regards to their SDB. Study participants were given copies of their research sleep studies, but we do not know if they subsequently pursued further clinical evaluation and treatment. It is reasonable to assume, though, that if they had received treatment, it would have reduced the mortality risk that we observed, thus our study may underestimate the mortality risk; indeed, other studies have noted that excluding individuals treated with CPAP have resulted in higher mortality hazard ratios.13 Other sleep disorders may modify the relationship between EDS + SDB and mortality. These include nocturnal cardiac dysrhythmias, and obesity-hypoventilation syndrome. Our study sample was not sufficiently large enough for us to address these specific sub-group analyses. Similarly, we could not examine the impact of central sleep apnea since only 4% of the study participants had predominantly central sleep related breathing disorder. The equipment and scoring criteria we used for sleep staging and SDB antedated current sleep apnea scoring criteria: it is possible that use of the newer guidelines in future studies may lead to different findings. We did not calculate a total sleep time with oxyhemoglobin saturation below 90% (TST90) as part of our original dataset and instead considered the oxyhemoglobin desaturation nadir in REM and NREM; others have noted that TST90 may be related to mortality in younger but not older study participants.11 We could not reassess the severity of SDB over time to determine if it changed, nor could we reassess the level of EDS. Most studies suggest mild worsening even in those with stable weight, thus we do not anticipate that any individual's sleep apnea would necessarily resolve significantly.40,41 We used a single night assessment to identify the AHI, but there can be variability in this measure in older adults.42 However, this reflects current clinical practice where most patients only receive a single night study as well to characterize the presence or absence of SDB. While BMI is traditionally considered a mortality risk factor in younger populations, our study did not observe an increased mortality rate from elevations in BMI. This is not surprising: a lower BMI has been associated with increased mortality rates in older subjects, with lower mortality rates noted in those with a higher BMI.43 The findings from our study parallel those from Ancoli-Israel et al., who similarly did not see an increased mortality rate at higher BMI in their longitudinal study of mortality and SDB.14 Our study excluded subjects with dementia or depression, thus these findings cannot necessarily be extended to patients with EDS with either of these conditions. The baseline assessment did not measure functional activities of daily living. It is possible that one mechanism by which EDS increases mortality is through reduced activity; future prospective research examining the relationship of EDS, daily activity and mortality would be warranted to address this question. Lastly, the study sample had a high percentage (74%) of female participants. This most likely reflects the gender distribution of older adults in the Philadelphia metropolitan area, where approximately 63% of adults age > 65 years and 71% of adults age > 80 years are female.
The increased mortality in older adults with EDS and SDB emphasizes the importance of obtaining a sleep evaluation as part of an EDS work-up in older adults. EDS is often a multi-factorial process in older adults, with other significant risk factors being depression, insomnia, and other comorbid illnesses.1 Unfortunately, impaired sleep is often viewed as a common and natural aspect of aging, thus relatively few older adults undergo a sleep evaluation.1,44,45 In addition, the finding that older adults with isolated SDB and no daytime sleepiness did not have an increased mortality rate suggests that the treatment of SDB should not be with the sole intent of decreasing the AHI, but that treatment should also seek to decrease daytime sleepiness. Future research should be conducted to confirm these findings in more diverse populations, consider the potential interaction from other sleep disorders, and examine the morbidity and mortality effects in older adults who had resolution of their daytime sleepiness symptoms with SDB treatment compared to those who did not.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Gooneratne has received research support from Takeda and an unrestricted educational grant from Respironics, Inc. Dr. Dinges has received research support from Merck; has consulted for Sanofi-Aventis, Eli Lilly, Johnson & Johnson, and Arena; and is Scientific Advisor to Mars, Inc.
ACKNOWLEDGMENTS
Funding support for this project has been provided by the following NIH grants: 1) NIA R01 AG14155 (Drs. Dinges and Pack), 2) NIA K23 AG01021 (Dr. Gooneratne), 3) NIA R21 AG031390 (Dr. Gooneratne) and 4) NCRR Clinical and Translation Science Award UL1 RR024134. Support for the biostatistical analyses was provided by the Center for Sleep and Circadian Neurobiology at the University of Pennsylvania.
Footnotes
A commentary on this article appears in this issue on page 413.
REFERENCES
- 1.Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–89. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation “2003 Sleep in America” Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 3.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 4.Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–9. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Bliwise DL, Ansari FP, et al. Daytime sleepiness and functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15:620–6. doi: 10.1097/JGP.0b013e3180381521. [DOI] [PubMed] [Google Scholar]
- 6.Onen F, Higgins S, Onen SH. Falling-asleep-related injured falls in the elderly. J Am Med Dir Assoc. 2009;10:207–10. doi: 10.1016/j.jamda.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 8.Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40:1219–24. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- 9.Cochen V, Arbus C, Soto ME, et al. Sleep disorders and their impacts on healthy, dependent, and frail older adults. J Nutr Health Aging. 2009;13:322–9. doi: 10.1007/s12603-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 11.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 13.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19:277–82. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Klauber MR, Kripke DF, Parker L, Cobarrubias M. Sleep apnea in female patients in a nursing home. Increased risk of mortality. Chest. 1989;96:1054–8. doi: 10.1378/chest.96.5.1054. [DOI] [PubMed] [Google Scholar]
- 16.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18:397–403. doi: 10.1111/j.1365-2869.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 17.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–20. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 18.Kapur VK, Resnick HE, Gottlieb DJ. Sleep disordered breathing and hypertension: does self-reported sleepiness modify the association? Sleep. 2008;31:1127–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg E, Berne C, Franklin KA, Svensson M, Janson C. Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women--a population-based study. Respir Med. 2007;101:1283–90. doi: 10.1016/j.rmed.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Pack AI, Dinges DF, Gehrman PR, Staley B, Pack FM, Maislin G. Risk factors for excessive sleepiness in older adults. Ann Neurol. 2006;59:893–904. doi: 10.1002/ana.20863. [DOI] [PubMed] [Google Scholar]
- 21.Davis PB, Morris JC, Grant E. Brief screening tests versus clinical staging in senile dementia of the Alzheimer type. J Am Geriatr Soc. 1990;38:129–35. doi: 10.1111/j.1532-5415.1990.tb03473.x. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardization terminology: techniques and scoring system for sleep stages of human subjects. Los Angeles, California: Brain Information Services/Brain Research Institute, University of California at Los Angeles; 1968. [Google Scholar]
- 24.Riedel BW, Winfield CF, Lichstein KL. First night effect and reverse first night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med. 2001;2:125–33. doi: 10.1016/s1389-9457(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 25.Riedel BW, Lichstein KL. Objective sleep measures and subjective sleep satisfaction: how do older adults with insomnia define a good night's sleep? Psychol Aging. 1998;13:159–63. doi: 10.1037//0882-7974.13.1.159. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 27.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 28.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 30.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 31.Bliwise D. Normal aging. In: Kryger MH, Roth T, Dement W, editors. Principles and practice of sleep medicine. Vol. 26. Philadelphia: WB Saunders; 2000. p. 42. [Google Scholar]
- 32.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 33.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/ hypopnoea syndrome patients: impact of treatment. Eur Respir J. 2002;20:1511–8. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- 34.Pack AI, Platt AB, Pien GW. Does untreated obstructive sleep apnea lead to death? Sleep. 2008;31:1067–8. A commentary on Young et al. Sleep 2008;31:1071-8 and Marshall et al. Sleep 2008;31:1079-85. [PMC free article] [PubMed] [Google Scholar]
- 35.Berry DT, Phillips BA, Cook YR, et al. Geriatric sleep apnea syndrome: a preliminary description. J Gerontol. 1990;45:M169–74. doi: 10.1093/geronj/45.5.m169. [DOI] [PubMed] [Google Scholar]
- 36.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 38.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96. [PMC free article] [PubMed] [Google Scholar]
- 39.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 40.Bliwise D, Carskadon M, Carey E, Dement W. Longitudinal development of sleep-related respiratory disturbance in adult humans. J Gerontol. 1984;39:290–3. doi: 10.1093/geronj/39.3.290. [DOI] [PubMed] [Google Scholar]
- 41.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the sleep heart health study. Arch Intern Med. 2005;165:2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 42.Bliwise DL, Benkert RE, Ingham RH. Factors associated with nightly variability in sleep-disordered breathing in the elderly. Chest. 1991;100:973–6. doi: 10.1378/chest.100.4.973. [DOI] [PubMed] [Google Scholar]
- 43.Stessman J, Jacobs JM, Ein-Mor E, Bursztyn M. Normal body mass index rather than obesity predicts greater mortality in elderly people: the Jerusalem longitudinal study. J Am Geriatr Soc. 2009;57:2232–8. doi: 10.1111/j.1532-5415.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 44.Haponik EF. Sleep disturbances of older persons: physicians' attitudes. Sleep. 1992;15:168–72. doi: 10.1093/sleep/15.2.168. [DOI] [PubMed] [Google Scholar]
- 45.Littner M, Alessi C. Obstructive sleep apnea: asleep in our consciousness no more. Chest. 2002;121:1729–30. doi: 10.1378/chest.121.6.1729. [DOI] [PubMed] [Google Scholar]