Abstract
Objective:
The critical pressure (PCRIT), a measurement of upper airway collapsibility, is a determinant of the severity of upper airway obstruction during sleep. We examined the performance characteristics of the passive and active PCRIT by examining both within-night and between-night variability in the measurements.
Methods:
We studied 54 sleep apnea patients (39 men, 15 women) and 34 normal subjects (20 men, 14 women) on either 1 or 2 nights during sleep. The PCRIT was measured during relative hypotonia (“passive” state) or during periods of sustained upper airway obstruction used to recruit upper airway neuromuscular responses (“active” state) within- and between-nights. In a subgroup of 10 normal subjects, we performed repeated measurements during hypnotic-induced sleep. Bland-Altman analyses were used to determine the within-night and between-night reliability of the PCRIT measurements.
Results:
There were no significant within-night or between-night differences for the mean passive PCRIT. The active PCRIT was ∼1 cm H2O more collapsible on the second night than on the first night. The limits of agreement, which bound the passive and active PCRIT, was ∼ ± 3 cm H2O and was reduced to ∼ ± 1 cm H2O for the passive PCRIT with hypnotic-induced sleep.
Conclusion:
Passive and active PCRIT measurements are reasonably reliable within and between nights. An approximately 3 cm H2O change in passive or active PCRIT appears to represent the minimally significant change in PCRIT necessary to assess the effect of an intervention (e.g., positional therapy, surgical interventions, oral appliance effects, and pharmacotherapy) on upper airway mechanical loads or neuromuscular responses.
Citation:
Kirkness JP; Peterson LA; Squier SB; McGinley BM; Schneider H; Meyer A; Schwartz AR; Smith PL; Patil SP. Performance characteristics of upper airway critical collapsing pressure measurements during sleep. SLEEP 2011;34(4):459-467.
Keywords: Pharyngeal collapsibility, obstructive sleep apnea, sleep disordered breathing, neuromuscular compensation, upper airway mechanics
INTRODUCTION
Obstructive sleep apnea (OSA) is an increasingly common disorder associated with comorbid conditions, including cardiovascular disease and metabolic disorders.1–5 OSA is characterized by recurrent episodes of complete or partial upper airway obstruction during sleep. The primary defect is one of increased collapsibility during sleep, which increases susceptibility and severity to obstructive sleep apnea. The critical pressure (PCRIT), a measurement of upper airway collapsibility, is a determinant of the severity of upper airway obstruction during sleep.6–11 PCRIT also describes a continuum of pharyngeal collapsibility from health to varying degrees of upper airway obstruction including snoring, hypopneas, and apneas.9,12 In normal subjects, the PCRIT is markedly negative compared with sleep apnea patients in whom the PCRIT is closer to atmospheric pressure or above.
Current evidence suggests that defects in upper airway mechanical (passive) and neuromuscular (active) control play a role in the pathogenesis of obstructive sleep apnea. Methods to quantify PCRIT during sleep have been adapted to assess the relative contribution of these properties towards upper airway collapse.8,13–28 Specifically, measurements of PCRIT during the passive state (relative hypotonia) assess the contribution of mechanical loads on upper airway collapsibility. Measurements of PCRIT during the active state represent the combination of mechanical loads and neural reflex responses. Investigators have increasingly utilized measurements of passive and active PCRIT to elucidate the pathogenesis of obstructive sleep apnea.7,8,10,13–39 In particular, studies have been conducted to manipulate passive and active upper airway properties with treatments such as stimulation of upper airway dilator muscles,40 upper airway surgery,41 exogenous surfactant,42 or weight loss43 to effect reductions in PCRIT and disease severity.
Physiologic protocols have been established to assess upper airway pressure-flow relationships during sleep and derive measurements of PCRIT under the passive and active conditions. Establishing the reliability of PCRIT would guide clinical investigators in deploying these measurements in physiologic studies and treatment trials. The major goal of the current study was to examine the performance characteristics of the passive and active PCRIT by examining both within- and between-night reliability in the measurements.
METHODS
Subjects
A total of 54 sleep apnea subjects (39 men, 15 women) and 34 subjects without sleep apnea (20 men, 14 women; for both control and sedation groups; see Table 1) from the Johns Hopkins Sleep Disorders Center and the general community (Tables 1A-C) were included in this retrospective analysis of previously collected studies of upper airway collapsibility. Sleep apnea was defined as a NREM respiratory disturbance index (RDI) > 10 events/h. Subjects were excluded if they had a history of a concurrent sleep disorder or other confounding medical condition (e.g., narcolepsy, restless legs syndrome, previous upper airway surgery, or significant pulmonary disease). The protocol was approved by the Johns Hopkins Institutional Review Board, and informed written consent was obtained from each subject.
Table 1.
Subject demographics
| (A) Within-night repeatability of passive PCRIT and RUS | |||
| Control | OSA | Hypnotic | |
| (n = 14) | (n = 18) | (n = 10) | |
| Age (years) | 40 ± 7 | 45 ± 11 | 27 ± 8* |
| BMI (kg/m2) | 41 ± 12 | 40 ± 12 | 24 ± 2* |
| Men:Women (n) | 5:9 | 10:8 | 6:4 |
| NREM AHI (events/h) | 3 ± 2 | 55 ± 20* | 2 ± 2 |
| REM AHI (events/h) | 9 ± 5 | 68 ± 37* | 6 ± 8 |
| (B) Between-night passive PCRIT and RUS measurement reproducibility | |||
| Control | OSA | ||
| (n = 7) | (n = 26) | ||
| Age (years) | 40 ± 12 | 47 ± 11* | |
| BMI (kg/m2) | 28 ± 5 | 34 ± 8* | |
| Men:Women (n) | 6:2 | 21:5 | |
| NREM AHI (events/h) | 2 ± 1 | 46 ± 5* | |
| REM AHI (events/h) | 11 ± 9 | 51 ± 32* | |
| (C) Between-night active PCRIT and RUS measurement reproducibility | |||
| Control | OSA | ||
| (n = 3) | (n = 10) | ||
| Age (years) | 50 ± 7 | 44 ± 12 | |
| BMI (kg/m2) | 25 ± 1 | 30 ± 7 | |
| Men:Women (n) | 3:0 | 8:2 | |
| NREM AHI (events/h) | 3 ± 1 | 48 ± 16* | |
| REM AHI (events/h) | 11 ± 8 | 44 ± 35 | |
OSA, obstructive sleep apnea; BMI, body mass index; AHI, apnea/hypopnea index; P values are OSA or Hypnotic vs Control.
P < 0.05.
Experimental Procedures
Baseline polysomnographic measurements
All subjects slept in the sleep laboratory at the Johns Hopkins Sleep Disorders Center or the Clinical Research Unit at the Johns Hopkins Bayview Medical Center. Standard polysomnographic recording techniques were employed using a full montage acquisition system (Embla N7000, Somnologica, Medcare, Buffalo, NY). The signals acquired included: electroencephalograms (C3-A2, C3-O1, F3-A2), left and right electroculograms, electrocardiogram, submental electromyogram, oxyhemoglobin saturation, airflow via a nasal cannula and oronasal thermistor. Thoracic and abdominal movements were monitored by inductance plethysmography, and posture was continuously recorded using a body position monitor and confirmed via infrared video camera.
Pressure-airflow measurements
To determine the passive and active critical collapsing pressure (PCRIT), nasal pressure was manipulated to induce or alleviate upper airway obstruction and responses in airflow were recorded via a nasal mask as previously described.8 Airflow was measured using a pneumotachograph (Hans Rudolph model #4830, Hans Rudolph, Kansas City, MO) attached to pneumotachograph amplifier (Hans Rudolph model #1, Series 1110) in series with a nasal continuous positive airway pressure (CPAP) mask (Profile Lite Nasal Gel Mask, Respironics, Murraysville, PA). Nasal pressure (PN) was measured using a pressure transducer (Embla N7000 Patient Unit Pressure Sensor, Medcare, Buffalo, NY) attached via tubing to a pressure port on the nasal mask. Pressure was delivered to the nasal mask throughout the night from a remotely controlled modified continuous positive airway pressure device (ResMed Ltd, Bella Vista, NSW, Australia) capable of delivering negative and positive pressures over a range of −20 to +20 cm H2O.
Experimental Protocols
Protocols for assessment of upper airway collapsibility
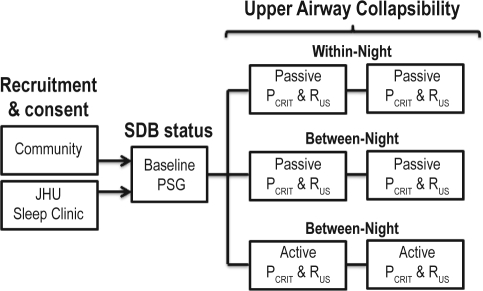
Each subject underwent baseline polysomnogram (PSG) assessment of sleep disordered breathing status followed by an additional 1 or 2 nights in the sleep laboratory for upper airway characterization (Figure 1). We measured PCRIT during conditions of hypotonia (passive PCRIT) and increased neuromuscular activity (active PCRIT). Passive PCRIT measurements were made within the same night and between nights. Active PCRIT measurements could only be made between nights. Measurements were obtained during periods when subjects slept in the supine position with one pillow underneath their head.
Figure 1.
Study design. The sleep apnea status of subjects recruited from the clinic or community was confirmed with a baseline polysomnographic (PSG) study. Additionally, each subject underwent either 1 or 2 nights in the sleep laboratory for determination of passive and/or active upper airway collapsibility (PCRIT) and up-stream resistance (RUS). During stable, NREM (stage 2) sleep, measurements were conducted during periods of relative hypotonia (“passive” state) or following extended periods of stable flow-limitation and upper airway muscle activity (“active” state). Repeated measurements were made either within-nights or between nights.
Passive upper airway critical collapsing pressure (passive PCRIT)
Participants were titrated to a holding PN at which inspiratory airflow limitation was eliminated and neuromuscular activity was attenuated, as reported previously.7,13,44,45 A minimum holding pressure of 4 cm H2O was applied to prevent re-breathing for control subjects. During stable, stage N2 sleep, PN was reduced rapidly by 1-2 cm H2O to a level where airflow became flow-limited for 5 breaths and then returned to holding pressure for 1-2 min before continuing with subsequent pressure drops. PN was repeatedly lowered in 1-2 cm H2O increments until complete (zero flow) or near complete (< 50 mL/s) upper airway closure was observed. A minimum of 2 series of stepwise reductions in PN by 1–2 cm H2O that eventually encompassed zero airflow (PCRIT) was collected for each assessment of passive PCRIT.
Active upper airway critical collapsing pressure (active PCRIT)
Dynamic neuromuscular responses to upper airway obstruction were determined by assessment of the active PCRIT, as previously described.8,44 Briefly, PN was reduced stepwise from holding pressure by 1-2 cm H2O until constant airflow limitation was exhibited for 10 min during NREM sleep. PN was subsequently further reduced stepwise by 1-2 cm H2O for 10 min during NREM sleep until recurrent obstructive apneas were observed or until sleep was no longer maintained. At pressures lower than 4 cm H2O, a continuous airflow of between 8-10 L/min was added through the nasal mask to prevent re-breathing.
Passive PCRIT sources of variability
In a group (n = 10) of separate control subjects (Table 1A), within-night passive PCRIT measurements were obtained in the supine position while controlling head position (head comfortably fixed in the same position) during hypnotic-induced sleep (0.50 mg triazolam, Pfizer Inc, NY, NY).
Analyses
Polysomnography
All polysomnography studies were analyzed for sleep stage, arousals, and respiratory-related events according to the standard published criteria.46–49
Upper airway critical closing pressure (PCRIT) and up-stream resistance (RUS)
A passive and active pressure-flow curve were separately constructed using flow-limited breaths, as previously described.8 A spline analysis was performed to identify the sloped, flow-limited portion of the pressure-flow curve. Median regression was performed using data from the flow-limited portion of the pressure-flow curve to obtain the x-intercept (PCRIT), and 1/slope (upstream resistance; RUS) for measurements of passive and active PCRIT and RUS.7
Statistical Analysis
In a larger database of PCRIT measurements at our center, we examined our ability to successfully obtain a passive and active PCRIT measurement in previously recruited subjects. A successful PCRIT measurement was defined as a PCRIT value extrapolated no more than 3 cm H2O from the lowest nasal pressure level successfully applied during sleep. We then examined the reliability of passive and active PCRIT and RUS measurements using several approaches. First, since differences in within-night and between-night measurements and subject characteristics were normally distributed, statistical significance was assessed using paired t-tests. Second, the intraclass-correlation (ICC) between measurements, an assessment of the within-individual variation, was determined using the method of Deyo et al.50 An ICC of 1.0 would indicate an absence of within-individual variability for the measurement. Third, Bland-Altman plots of the difference in measurements vs. the average of the 2 measurements were examined for evidence of systematic bias, the presence of heteroscedasticity, and to identify the limits of agreement that bound the mean difference between PCRIT and RUS measurements (mean difference ± 2 SD). Linear regression analyses were performed to determine whether differences in passive and active PCRIT and RUS measurements were a function of time within-night and between-nights. To examine if there was any evidence of a systematic bias on the reproducibility of the passive or active PCRIT due to the median regression approach, we compared the fitted x-intercept to the lowest pressure level obtained in a subgroup of 21 individuals. Linear regression analyses were also performed to determine the strength of any cross-sectional associations between the repeated measurements of PCRIT and RUS with age or BMI, stratified by disease status. Between-group differences by sleep apnea status were assessed using unpaired t-tests. Statistical significance was defined a priori at a P ≤ 0.05 and data are presented as mean ± SD, unless otherwise specified. Statistical analyses were performed using STATA 10.0 (College Station, TX).
RESULTS
In a larger database of previously collected passive and active PCRIT measurements at our center, we examined our ability to obtain a PCRIT measurement. In 148 subjects in whom measurements of passive PCRIT were attempted, we obtained a successful passive PCRIT in 86%. For the active PCRIT, however, we were only able to obtain a valid measurement of active PCRIT in 48%.
Table 1 describes the subject characteristics for the groups in the current study for the within-night passive PCRIT and RUS measurement (Table 1A) analysis, and the between-night analyses for both passive (Table 1B) and active (Table 1C) PCRIT and RUS.
Within-Night Reliability of Passive PCRIT and RUS: Natural Sleep
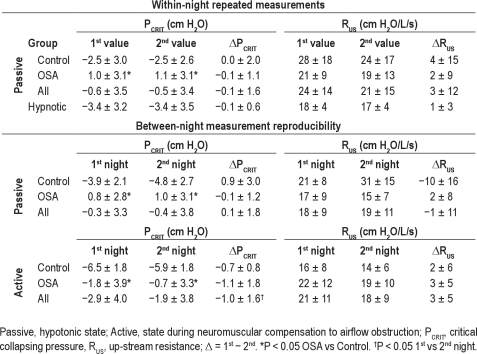
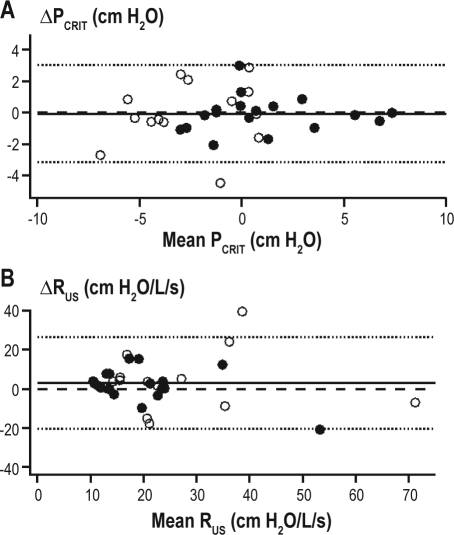
Thirty-two subjects (Table 1A) had within-night repeated measurements separated by an average of 134 ± 91 min (range: 4–304 min). The mean holding pressure for the first series of measurements was similar for the second series of measurements (8.6 ± 2.4 cm H2O vs. 8.6 ± 2.3 cm H2O, respectively; P = 0.75). For all subjects, there was no difference between the first and the second passive PCRIT (P = 0.93) or RUS (P = 0.40) measurements (Table 2). The ICC was 0.90 and 0.66 for comparisons of within-night passive PCRIT and RUS, respectively. The Bland-Altman analysis did not demonstrate a systematic bias between the first and second measurements of passive PCRIT, with a mean difference of −0.1 ± 1.6 cm H2O; P = 0.93 (see Figure 2A) and lower and upper limits of agreement of −3.2 and +3.0 cm H2O, respectively. Similarly, no significant group mean differences between the within-night repeated measurement of passive RUS (3.0 ± 3.6 cm H2O/L/s; P = 0.40; Figure 2B) were observed, with lower and upper limits of agreement of −20.5 and +26.5 cm H2O, respectively.
Table 2.
Repeatability and reproducibility of PCRIT and RUS
Figure 2.
Within-night passive PCRIT Bland-Altman plot. Bland-Altman plots displaying the difference of repeated measurements of (A) passive PCRIT and (B) passive RUS plotted against the average of the repeated measurements. The mean difference (−0.1 ± 1.6 cm H2O; solid line) was not different from zero (dashed line) and the limits of agreement (dotted lines) are represented as ± 2 standard deviations. The upper and lower limits of agreement were +3.0 and 3.2 cm H2O for passive PCRIT and +26.5 to −20.5 cm H2O/L/s for RUS. The intraclass correlation coefficient (ICC) for between-night measurements during sleep was 0.90 for passive PCRIT and 0.66 for RUS. The subject demographics of healthy control (open circles) and sleep apnea (closed circles) subjects are provided in Table 1A.
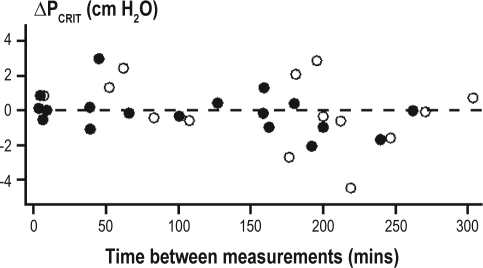
No association between the time interval (in minutes) and repeated measurements was observed for either the passive PCRIT (r2 = 0.09, P = 0.10; Figure 3) or RUS (r2 = 0.03, P = 0.37). When subjects were stratified based on the median time between repeated measurements (≤ 159 min [n = 17; mean = 63 minutes] and > 159 min [n = 15; mean = 216 min]), there was no change in the mean difference in passive PCRIT or RUS. The mean difference in both passive PCRIT and RUS measurements overtime was the same for OSA and control subjects.
Figure 3.
Difference between repeated passive PCRIT measurements vs. time between the measurements. Scatter-plot showing the difference between repeated passive PCRIT measurements (ΔPCRIT) and the time in minutes between the measurements. The time between measurements did not systematically alter the magnitude of ΔPCRIT (r2 = 0.09, P < 0.07). The subject demographics of healthy control (open circles) and sleep apneic (closed circles) subjects are provided in Table 1A.
Within-Night Reliability of Passive PCRIT and RUS: Hypnotic-Induced Sleep
During sleep with sedation, the average time between repeated passive PCRIT measurements was 78 ± 59 min (range: 5–189 min). PCRIT and RUS were not different between the first and second measurements (Table 2) with ICCs of 0.99 and 0.66, respectively. No systematic bias between PCRIT measurements were observed (mean difference −0.1 ± 0.6 cm H2O; P = 0.76) with upper and lower confidence intervals of +1.1 and −1.2 cm H2O (Figure 4). Similarly, no systematic differences in RUS measurements were observed (mean difference 3 ± 12 cm H2O/L/s; P = 0.44) with upper and lower confidence intervals of +6.5 and −5.0 cm H2O/L/s. No association between the time interval and repeated measurements of PCRIT (r2 = 0.21, P = 0.17) or RUS (r2 = 0.32, P = 0.086) were observed in all participants or when stratified by OSA status.
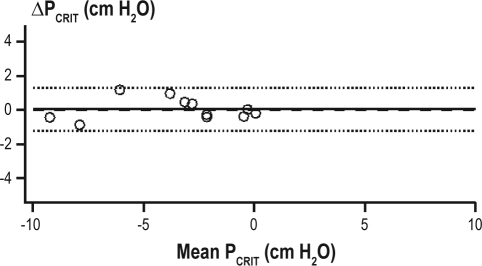
Figure 4.
Within-night passive PCRIT during hypnotic induced sleep. Bland-Altman plots displaying the difference of repeated measurements of passive PCRIT plotted against the average of the repeat measurements. The mean difference (−0.1 ± 0.6 cm H2O; solid line) was not different from zero (dashed line) and the limits of agreement (dotted lines) are represented as ± 2 standard deviations. The upper and lower limits of agreement were +1.1 and −1.2 cm H2O for passive PCRIT. The intraclass correlation coefficient (ICC) for between-night measurements during hypnotic-induced sleep was 0.99 for passive PCRIT and 0.66 for RUS. The subject demographics of the subjects, none of whom had OSA (open circles) are provided in Table 1A.
Between-Night Passive PCRIT and RUS Reliability
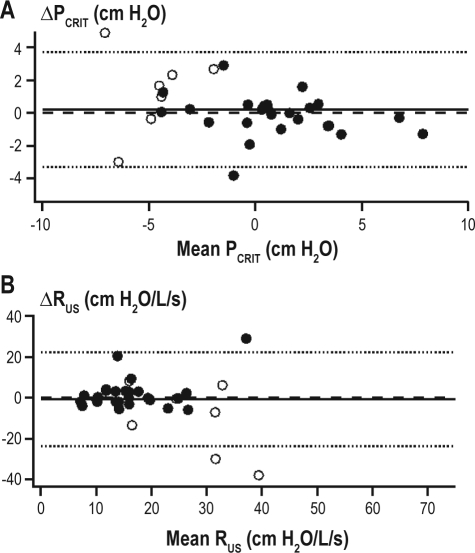
Twenty-five of the 33 subjects for the between-night passive PCRIT and RUS analysis were studied on consecutive nights. The remaining 9 subjects had their second study night a median of 3.5 months later (range: 2 weeks to 2 years) and there was no significant change in BMI with the time between the measurements in this group (P = 0.64). No significant difference in between-night passive PCRIT and RUS was observed between night 1 and night 2 (Table 2). The ICC was 0.87 and 0.41 for comparisons of between-night passive PCRIT and RUS, respectively. Bland-Altman plots did not demonstrate a systematic bias in the between-night passive PCRIT measurements (mean difference 0.1 ± 1.8 cm H2O; P = 0.73; Figure 5A) with lower and upper limits of agreement of −3.5 and +3.7 cm H2O, nor in the between-night passive RUS (mean difference −1 ± 11 cm H2O/L/s; P = 0.65; Figure 5B), with lower and upper limits of agreement of −23.1 and +21.3 cm H2O/L/s.
Figure 5.
Between-night passive PCRIT and RUS measurement reproducibility. Bland-Altman plots displaying the difference between night 1 and night 2 measurements of (A) passive PCRIT and (B) passive RUS plotted against the average of both measurements. The mean difference (0.1 ± 1.8 cm H2O; solid line) is not different from zero and the limits of agreement (dashed lines) are represented as ± 2 standard deviations. The upper and lower limits of agreement for between-night passive PCRIT were −3.3 and +3.5 cm H2O and for RUS were −23.5 and +20.7 cm H2O/L/s, respectively. The intraclass correlation coefficient (ICC) for between-night measurements was 0.87 for passive PCRIT and 0.41 for RUS. The subject demographics of healthy control (open circles) and sleep apneic (closed circles) subjects are provided in Table 1B.
The number of days between passive PCRIT measurements was not associated with the differences between the measurements for the entire group (r2 = 0.03, P = 0.30) or for the subgroup that had > 1 day between measurements (r2 = 0.001, P = 0.93). Similarly, for passive RUS measurements there was no association of time between measurements and the difference in passive RUS for the entire group (r2 = 0.01, P = 0.61) or for the subgroup with > 1 day between measurements (r2 = 0.06, P = 0.54). To examine the variability between repeated passive PCRIT measurements that may be due to the median regression approach, we examined the lowest observed pressure measurement compared to the calculated PCRIT. The difference between passive PCRIT and the lowest pressure level was the same on the first (−0.2 ± 0.6 cm H2O) and second (−0.5 ± 0.5 cm H2O) nights (P = 0.08).
Between-Night Active PCRIT and RUS Reliability
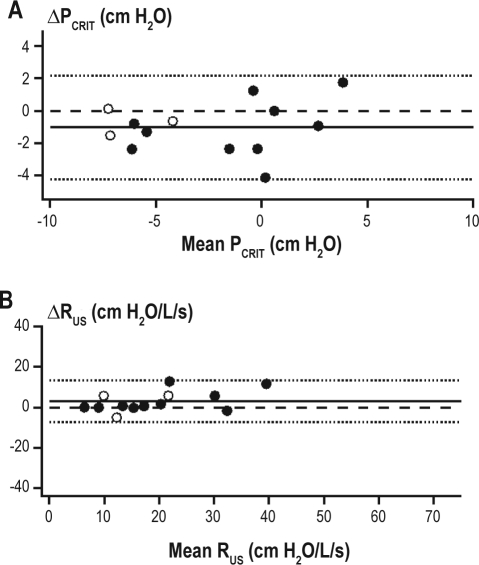
Active PCRIT measurements were performed on 18 of the subjects who had passive PCRIT measurements repeated between nights; however, adequate data were obtained from 13 of 18 subjects (70%). The active PCRIT was 1.0 ± 1.6 cm H2O lower (less collapsible) on night 1 than night 2 (P = 0.04; Table 2). Moreover, RUS exhibited a nonsignificant trend to be higher on night 1 compared to night 2 (P = 0.08; Table 2). Ten of 13 subjects had active PCRIT and RUS measurements on consecutive nights, and 3 had a second study night between 1 and 9 months (median 113 days). As participant characteristics may change over time and affect upper airway properties, a sub-analysis was performed of the 10 subjects in whom active PCRIT measurements were obtained on consecutive nights. There was no significant between-night active PCRIT (−0.8 ± 1.2 cm H2O; P = 0.07) or RUS (2 ± 5 cm H2O/L/s; P = 0.16) difference. For the entire group the ICC was 0.95 and 0.87 for comparisons of between-night active PCRIT and RUS, respectively. Bland Altman plots also demonstrated the small systematic difference between the first night and second night active PCRIT measurements of −1.0 ± 1.6 cm H2O (P = 0.04; Table 2) with the lower and upper limits of agreement for between-night active PCRIT at −4.2 and +2.2 cm H2O, respectively (Figure 6A). Similarly, a small systematic difference in active RUS was exhibited between nights that did not reach statistical significance (mean difference 3 ± 5 cm H2O/L/s; P = 0.06; Figure 6B) with lower and upper limits of agreement of −7.4 and +13.3 cm H2O/L/s, respectively. Similar to the passive PCRIT there was no difference between active PCRIT and the lowest pressure level on the first (−0.9 ± 0.3 cm H2O) compared to the second (−0.5 ± 0.4 cm H2O) night (P = 0.28).
Figure 6.
Between-night active PCRIT and RUS measurement reproducibility. Bland-Altman plots displaying the difference between night 1 and night 2 measurements of (A) active PCRIT and (B) active RUS plotted against the average of both measurements. The mean difference (solid line) shows a small systematic bias (−1.0 ± 1.6 cm H2O) and the limits of agreement (dashed lines) are represented as ± 2 standard deviations. The upper and lower limits of agreement for between-night passive PCRIT were –4.2 and +2.2 cm H2O and for RUS were -7.4 and +13.3 cm H2O/L/s, respectively. The intraclass correlation coefficient (ICC) for between-night measurements was 0.95 for passive PCRIT and 0.87 for RUS. The subject demographics of healthy control (open circles) and sleep apneic (closed circles) subjects are provided in Table 1C.
DISCUSSION
The findings in this study demonstrate that the passive PCRIT measurement is more readily obtained than the active PCRIT measurement. Furthermore, when passive and active PCRIT and RUS measurements were collected on multiple occasions, these measurements were reliable within the same night and between nights, using several approaches. First, comparison of repeated measures of PCRIT and RUS demonstrated negligible differences in group mean effects for passive measurements. Furthermore, no systematic differences in active measurements were observed when made on consecutive nights. Second, intra-class correlation between measurements for PCRIT and RUS were high. Third, examination of Bland-Altman plots demonstrated agreement between repeated passive PCRIT measurements both within and between nights and for active PCRIT measurements between nights. Specifically, no significant or minimal systematic bias in the mean difference between measurements, over a range of PCRIT or RUS measurements was observed, and relatively narrow limits of agreement were present. Furthermore, hypnotic-induced sleep and careful control of head and neck posture further reduced the variability of within-night repeated measurements of passive PCRIT.
Reliability of Passive PCRIT and RUS Measurements
The current study reports the reliability in repeated passive PCRIT and RUS measurements, a measure of the contribution of mechanical loads towards the development of upper airway obstruction. We observed negligible mean differences in repeated measurements of the passive PCRIT within- and between-nights and excellent ICC agreement (0.87–0.90), suggesting minimal within-individual variability in this measurement. Similarly, passive RUS demonstrated negligible mean differences in repeated measurements within and between nights, although the ICC agreement was more modest (0.41–0.66). The Bland-Altman analyses for within- and between-night passive PCRIT demonstrated similar limits of agreement, at ≈ ± 3 cm H2O and most likely represents the minimally significant change in passive PCRIT necessary to assess the effects of an intervention on upper airway mechanical loads. The use of a hypnotic to induce stable sleep and careful control of head, neck, and body posture further reduced the variability in the measurement of passive PCRIT. Under these carefully controlled conditions, the minimally significant change would be as little as ≈ ± 1 cm H2O. Others have demonstrated that measurement of a hypnotic PCRIT during hypnotic-induced sleep are strongly correlated when measurements are made during natural sleep.28 Our findings suggest that repeated measurements of passive PCRIT have validity within and between-nights and have sufficient reliability to assess potential significant effects of interventions on modifying upper airway mechanical properties.
Reliability of Active PCRIT and RUS Measurements
The current study also evaluated the reliability of repeated active PCRIT and RUS measurements, a global measure of upper airway collapsibility that assesses the contribution of mechanical loads and neuromuscular responses towards the development of upper airway obstruction. In subjects in whom active PCRIT measurements were performed on consecutive nights, there was no significant between-night difference. However, when all subjects with active measurements were considered (i.e., the addition of 3 individuals studied a median of 113 days apart), we observed a small but statistically significant increase in the mean active PCRIT by 1.0 cm H2O (i.e., more collapsible) during the second night compared to the first night. Nevertheless, excellent ICC agreement (0.95) was present for active PCRIT measurements. We speculate that the small systematic change in active PCRIT between nights may be due to changes in participant characteristics in the 3 individuals studied a median of 113 days apart. Other possibilities might include an improved tolerance of the nasal mask and sleep testing conditions resulting in improved sleep quality on the second night, thereby decreasing arousability and increasing airway collapsibility. This hypothesis, however, could not be tested in the context of the study design. Alternatively, the between-night difference could be due to a type I error, with a 5% chance that this systematic difference was a false positive finding. The active RUS demonstrated negligible mean differences in repeated measurements between-nights. In contrast to the passive RUS, the active RUS demonstrated a stronger ICC (0.87). We hypothesize that the higher ICC for the active RUS may in part be related to the availability of adequate active pressure-flow measurements primarily in a sample of sleep apnea subjects, with only a few control subjects included.
The Bland-Altman analyses for between-night active PCRIT demonstrated limits of agreement, at ≈ ± 3 cm H2O, similar to the passive PCRIT, and most likely represents the minimally significant change in active PCRIT necessary to assess the global effects (an integration of mechanical loads and neuromuscular responses) of an intervention on upper airway collapsibility. Quantitative differences in active PCRIT of approximately 4-5 cm H2O, generally distinguish between groups over the continuum of normal breathing (< −10 cm H2O), snoring (between −5 to −10 cm H2O), obstructive hypopneas (between −5 to 0 cm H2O), and obstructive apneas (> 0 cm H2O),8,9,12 with a threshold of approximately −5 cm H2O separating individuals with primarily snoring or normal breathing (no disease) from those with hypopneas and apneas (disease).8,12,43 The night-to-night variability in active PCRIT was greatest around a mean active PCRIT of 0 cm H2O, suggesting that the measurement is less reliable in predicting disease status in this range. However, with limits of agreement of ≈ ± 3 cm H2O, the active PCRIT would range from −3 cm H2O to +3 cm H2O, which is above the threshold of −5 cm H2O that is generally associated with the development of apneas and hypopneas.8,9,51 Therefore, repeated measurements of active PCRIT are reliable in predicting the severity of upper airway obstruction (i.e. apneas and hypopneas from snoring and normal breathing), and interventions which alter the active PCRIT beyond a ± 3 cm H2O threshold are very likely to correspond to significant changes in the severity of upper airway obstruction. The influence of sedation and control of head and neck posture on the variability of active PCRIT measurements was not determined in this study, however, we would hypothesize that the variability in the measurement would be further reduced, similar to what was observed with the passive PCRIT.
Sources of Variability in the Measurement of PCRIT
Several factors may explain the variability observed in the passive and active PCRIT. Sleep state has been shown to have small effects on the passive PCRIT, with an increase by 1 cm H2O in REM sleep compared to NREM sleep,13,30,35 and no significant difference between stage N2 or N3 sleep.35 In contrast, little is known about the effects of sleep state on the active PCRIT. Recent studies have reported a decrease in sleep apnea severity during stage N3 sleep when compared with stages N1 or N2, suggesting that the active PCRIT may be reduced during stage N3 sleep.52
Other sources that may contribute to variability of upper airway collapsibility measurements include changes in body posture13,53 as well as head, neck, and jaw position.31,38 Previous studies during sedation have shown that jaw opening as well as head and neck position can change the passive PCRIT by 4-8 cm H2O.17,18 Furthermore, sleep apnea severity can change as the night progresses, which would suggest alterations in the passive and/or active PCRIT during the night. In the current study, we found no significant differences in passive PCRIT over time within the same night, suggesting that such changes are rather small. We could not exclude significant within-night changes in the active PCRIT since this was not assessed.
We examined whether the analytic approach of calculating PCRIT using a median regression contributed to the between measurement variability. There was a trend for the calculated passive PCRIT to be between ∼0.2 to 0.5 cm H2O lower than the lowest observed pressure level and the active PCRIT to be between ∼0.5 and 0.9 cm H2O lower. The difference between PCRIT and the lowest pressure level is small and would therefore contribute to no greater than ∼0.4 cm H2O of the variability in between-night measurements.
Changes in lung volume could also represent another source of variability in repeated PCRIT measurements.54 We have recently shown that changing the trans-respiratory pressure by 10 cm H2O elevates end-expiratory lung volume by one liter and decreases passive PCRIT by 1-2 cm H2O.54 A PCRIT difference between measurements would translate into a 0.3 L change in lung volume thereby accounting for 0.3–0.6 cm H2O (10% to 20%) of the variability. These data imply that while changes in lung volume modify the upper airway collapsibility, only a relatively small portion of between measurement variability could be attributed to a change in lung volume.
Methodological Limitations
There are several additional limitations of the current study. First, the study was a retrospective analysis of sleep studies utilizing a convenience sample that included subjects who had a minimum of two passive and active PCRIT measurements, and may have resulted in an overestimation in the frequency of obtaining a valid PCRIT measurement. Indeed, analysis of our database of previously collected PCRIT measurements demonstrated that we obtained a passive PCRIT measurement in 86% of subjects and active PCRIT in 48%. Furthermore, review of the subjects that we were unable to obtain an active PCRIT measurement in the current study demonstrated that these subjects tended to be control subjects and/or female subjects.8,44 One explanation for this observation is that sleep disruption may occur at progressively lower nasal pressures and limit the ability to obtain an active PCRIT on successive occasions. Our estimates of successful PCRIT measurement may represent a minimum yield that could be improved with approaches to enhancing sleep continuity (e.g., use of a hypnotic).16 Second, in the current study we were not able to investigate the within-night variability of the active PCRIT, since the time taken to perform each measurement with stable sleep precluded our ability to obtain two series of active measurements in the same night. Third, although participants maintained a supine posture for measurements of PCRIT, head, neck, and jaw position were not rigorously controlled (see ‘Sources of Variability in the Measurement of PCRIT') and could account for some of the variability observed between measurements of passive and active PCRIT. The combination of hypnotic-induced sleep and control of body posture and head and neck position reduced the standard deviation for repeated measurements of passive PCRIT to almost one-third, suggesting that greater precision in PCRIT measurement can be attained by physical control of postural factors and control of sleep state. Our study, however, more likely simulates real world conditions with respect to minor variation in head and neck position while an individual sleeps; therefore, assessment of reliability of PCRIT measurements under these real world conditions are important in establishing the minimal significant difference in PCRIT measurements with interventions.
Implications and Conclusion
Data regarding the performance characteristics of passive and active PCRIT measurements are important for adequately powering cohort and treatment studies. For example, based on an effect size of 1.0 with a standard deviation (SD: 1.6), a sample of thirteen subjects would be required to obtain 90% power to detect a within-subject passive or active PCRIT difference of 1.6 cm H2O or RUS difference of 11 cm H2O/L/s. Furthermore, a 15 or 50% attrition rate should be incorporated into the study design for the passive or active PCRIT, respectively, to ensure adequate recruitment. Since the reliability is greater for repeated passive PCRIT measurements during hypnotic-induced sleep, only five subjects would provide equal or greater power to detect the same change in PCRIT or RUS. Agreement between repeated measurements of both PCRIT techniques indicates that these methods can reliably quantify mechanical and neuromuscular properties that modify airway collapsibility. The present findings provide critical information required to plan studies examining pathogenic mechanisms and predicting clinical responses to therapeutic interventions such as positional therapy, surgical interventions, oral appliance effects, and pharmacotherapy.
In conclusion, the agreement between repeated measurements of passive and active PCRIT and RUS during sleep suggests that upper airway collapsibility can be reliably used to characterize the relative contribution of mechanical and neuromuscular factors to upper airway collapse and could be used to characterize responses to interventions and determine appropriate sample sizes for clinical trial interventions. We anticipate that an approach utilizing measurements of upper airway collapsibility may complement the traditional approach to measurement of sleep apnea severity, the AHI, in defining factors and identifying therapies that predict successful therapeutic outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Kirkness has received donated CPAP equipment and research support from ResMed Science Center. Dr, Schwartz has received donated CPAP equipment and research support from ResMed Science Center and has consulted for Apnex, Cardiac Concepts and Sora Pharmaceuticals. Dr. Smith has consulted for Apnex. Dr. Patil has received donated CPAP equipment and research support from ResMed Science Center. Dr. Schneider has consulted for Fisher & Paykel and TNI Medical. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Adam Benjafield and Glenn Richards from ResMed Science Center for providing the pressure generators used to measure PCRIT. Support for this study was provided by NHLBI–HL50381, HL37379, and HL077137.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 4.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Kirkness JP, Schwartz AR, Schneider H, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol. 2008;104:1618–24. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 8.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–42. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 10.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 11.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 12.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–3. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 13.Boudewyns A, Punjabi N, Van de Heyning PH, et al. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–41. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 14.Fregosi RF, Quan SF, Morgan WL, et al. Pharyngeal critical pressure in children with mild sleep-disordered breathing. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.01444.2005. [DOI] [PubMed] [Google Scholar]
- 15.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–40. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino Y, Ayuse T, Kurata S, et al. The compensatory responses to upper airway obstruction in normal subjects under propofol anesthesia. Respir Physiol Neurobiol. 2009;166:24–31. doi: 10.1016/j.resp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda H, Ayuse T, Oi K. The effects of head and body positioning on upper airway collapsibility in normal subjects who received midazolam sedation. J Clin Anesth. 2006;18:185–93. doi: 10.1016/j.jclinane.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res. 2005;84:554–8. doi: 10.1177/154405910508400613. [DOI] [PubMed] [Google Scholar]
- 19.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–7. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Litman RS, McDonough JM, Marcus CL, Schwartz AR, Ward DS. Upper airway collapsibility in anesthetized children. Anesth Analg. 2006;102:750–4. doi: 10.1213/01.ane.0000197695.24281.df. [DOI] [PubMed] [Google Scholar]
- 21.Oliven A, O'Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–9. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- 22.Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol. 2010;108:445–51. doi: 10.1152/japplphysiol.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol. 2001;91:2248–54. doi: 10.1152/jappl.2001.91.5.2248. [DOI] [PubMed] [Google Scholar]
- 24.Series F, Cote C, Simoneau JA, St, Marc I. Upper airway collapsibility, and contractile and metabolic characteristics of musculus uvulae. FASEB J. 1996;10:897–904. doi: 10.1096/fasebj.10.8.8666167. [DOI] [PubMed] [Google Scholar]
- 25.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999;159:149–57. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 26.Isono S, Tanaka A, Tagaito Y, Ishikawa T, Nishino T. Influences of head positions and bite opening on collapsibility of the passive pharynx. J Appl Physiol. 2004;97:339–46. doi: 10.1152/japplphysiol.00907.2003. [DOI] [PubMed] [Google Scholar]
- 27.Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol. 2007;103:1379–85. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 28.Morrison DL, Launois SH, Isono S, Feroah TR, Whitelaw WA, Remmers JE. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;148:606–11. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 29.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57:520–7. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998;157:1051–7. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 31.Ayuse T, Inazawa T, Kurata S, et al. Mouth-opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J Dent Res. 2004;83:718–22. doi: 10.1177/154405910408300912. [DOI] [PubMed] [Google Scholar]
- 32.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 33.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153:255–9. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
- 34.Odeh M, Schnall R, Gavriely N, Oliven A. Dependency of upper airway patency on head position: the effect of muscle contraction. Respir Physiol. 1995;100:239–44. doi: 10.1016/0034-5687(94)00135-m. [DOI] [PubMed] [Google Scholar]
- 35.Penzel T, Moller M, Becker HF, Knaack L, Peter JH. Effect of sleep position and sleep stage on the collapsibility of the upper airways in patients with sleep apnea. Sleep. 2001;24:90–5. doi: 10.1093/sleep/24.1.90. [DOI] [PubMed] [Google Scholar]
- 36.Philip-Joet F, Marc I, Series F. Effects of genioglossal response to negative airway pressure on upper airway collapsibility during sleep. J Appl Physiol. 1996;80:1466–74. doi: 10.1152/jappl.1996.80.5.1466. [DOI] [PubMed] [Google Scholar]
- 37.Seelagy MM, Schwartz AR, Russ DB, King ED, Wise RA, Smith PL. Reflex modulation of airflow dynamics through the upper airway. J Appl Physiol. 1994;76:2692–700. doi: 10.1152/jappl.1994.76.6.2692. [DOI] [PubMed] [Google Scholar]
- 38.Walsh JH, Maddison KJ, Platt PR, Hillman DR, Eastwood PR. Influence of head extension, flexion, and rotation on collapsibility of the passive upper airway. Sleep. 2008;31:1440–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–9. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliven A, Odeh M, Geitini L, et al. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol. 2007;103:1662–8. doi: 10.1152/japplphysiol.00620.2007. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527–32. doi: 10.1164/ajrccm/145.3.527. [DOI] [PubMed] [Google Scholar]
- 42.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Surface tension of upper airway mucosal lining liquid in obstructive sleep apnea/ hypopnea syndrome. Sleep. 2005;28:457–63. doi: 10.1093/sleep/28.4.457. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 44.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105:197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz AR, Eisele DW, Smith PL. Pharyngeal airway obstruction in obstructive sleep apnea: pathophysiology and clinical implications. Otolaryngol Clin North Am. 1998;31:911–8. doi: 10.1016/s0030-6665(05)70098-0. [DOI] [PubMed] [Google Scholar]
- 46.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. US Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 47.Atlas Task Force. EEG Arousals: Scoring Rules and Examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 48.American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 49.Punjabi NM, O'hearn DJ, Neubauer DN, et al. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am J Respir Crit Care Med. 1999;159:1703–9. doi: 10.1164/ajrccm.159.6.9808095. [DOI] [PubMed] [Google Scholar]
- 50.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12:142S–58S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 51.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–88. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 52.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5:519–24. [PMC free article] [PubMed] [Google Scholar]
- 53.McEvoy RD, Sharp DJ, Thornton AT. The effects of posture on obstructive sleep apnea. Am Rev Respir Dis. 1986;133:662–6. doi: 10.1164/arrd.1986.133.4.662. [DOI] [PubMed] [Google Scholar]
- 54.Squier SB, Patil SP, Schneider H, Kirkness JP, Smith PL, Schwartz AR. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00080.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]