Abstract
Study Objectives:
Oral appliances are increasingly being used for treatment of obstructive sleep apnea (OSA). Mandibular advancement splint (MAS) mechanically protrudes the mandible, while the tongue stabilizing device (TSD) protrudes and holds the tongue using suction. Although both appliances can significantly improve or ameliorate OSA, their comparative effects on upper airway structure have not been investigated.
Design:
Cohort study.
Setting:
Sleep Investigation Unit.
Patients:
39 patients undergoing oral appliance treatment for OSA.
Interventions:
OSA patients underwent magnetic resonance imaging (MRI) of the upper airway during wakefulness at baseline and with MAS and TSD in randomized order. Treatment efficacy was determined by polysomnography in a subset of 18 patients.
Measurements and Results:
Upper airway lumen and surrounding soft tissue structures were segmented using image analysis software. Upper airway dimensions and soft tissue centroid movements were determined. Both appliances altered upper airway geometry, associated with movement of the parapharyngeal fat pads away from the airway. TSD increased velopharyngeal lateral diameter to a greater extent (+0.35 ± 0.07 vs. +0.18 ± 0.05 cm; P < 0.001) and also increased antero-posterior diameter with anterior displacement of the tongue (0.68 ± 0.04 cm; P < 0.001) and soft palate (0.12 ± 0.03 cm; P < 0.001). MAS resulted in significant anterior displacement of the tongue base muscles (0.35 ± 0.04 cm). TSD responders (AHI reduction ≥ 50%) increased velopharyngeal volume more than non-responders (+2.65 ± 0.9 vs. –0.44 ± 0.8 cm3; P < 0.05). Airway structures did not differ between MAS responders and non-responders.
Conclusions:
These results indicate that the patterns and magnitude of changes in upper airway structure differ between appliances. Further studies are warranted to evaluate the clinical relevance of these changes, and whether they can be used to predict treatment outcome.
Citation:
Sutherland K; Deane SA; Chan ASL; Schwab RJ; Ng AT; Darendeliler MA; Cistulli PA. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. SLEEP 2011;34(4):469-477.
Keywords: Magnetic resonance imaging, mandibular advancement splints, obstructive sleep apnoea, tongue stabilising device, upper airway
INTRODUCTION
Oral appliances offer an effective alternative to continuous positive airway pressure (CPAP) in the treatment of obstructive sleep apnea (OSA).1 Oral appliances can be categorized into two design types; the mandibular advancement splint (MAS) and the tongue stabilizing device (TSD). MAS devices attach to the dental arches and mechanically protrude the mandible, whereas TSDs consist of a preformed bulb, which holds and protrudes the tongue using suction.
MAS are the most common type of oral appliance, and mounting evidence supports their use for the treatment of OSA.2 The clinical practice parameters of the American Academy of Sleep Medicine currently recommend the use of MAS for the treatment of mild to moderate OSA, and for severe OSA when patients refuse or are unable to tolerate CPAP.3
TSD are used less commonly, and investigations into their efficacy remain limited.4–8 Tongue protrusion using these devices has been shown to lead to improvements in OSA, with demonstrated reductions in AHI, arousal frequency, and oxygen desaturation,4,6–8 as well as improvement in daytime sleepiness.6,9 TSD has been proposed as a treatment option for patients with dental contraindications (hypodontia, edentulism, periodontal disease), which preclude the use of MAS.
The aim of oral appliance therapy is to improve upper airway patency, thereby preventing pharyngeal collapse during sleep. Various imaging techniques have demonstrated increased upper airway dimensions with mandible or tongue protrusion.2,10–12 However, understanding of the exact mechanisms by which oral appliances improve OSA remains limited. Magnetic resonance imaging (MRI) is the modality of choice for 3-dimensional analyses of the upper airway and surrounding soft tissue structures.13,14 We have recently used MRI in the largest and most detailed assessment to date of the effects of MAS on upper airway structure.15 However, there are few studies examining the effects of TSD on upper airway structure and no data about the commercially available TSD.
The different modes of action of these appliances (mandible versus tongue protrusion) are likely to differentially influence upper airway caliber and shape and surrounding soft tissues. There have been no direct comparisons of the anatomical effects of these oral appliances. Assessment of upper airway structures with MAS and TSD could provide insights into their respective sites and mechanisms of action. This study aimed to assess and compare the effects of two oral appliances on upper airway structure.
METHODS
Subjects
Patients with OSA were prospectively recruited from a sleep disorders clinic in a university teaching hospital for treatment with a customized MAS. The study was approved by the institutional ethics committee, and written informed consent was obtained from all patients. Inclusion criteria required a minimum of 2 OSA symptoms (snoring, witnessed apneas, fragmented sleep, and daytime sleepiness) plus confirmation of OSA by polysomnography (apnea-hypopnea index [AHI] ≥ 10 events/h). Patients were excluded if they had periodontal disease, insufficient number of teeth, or an exaggerated gag reflex (contraindications to MAS use).
Oral Appliances
The MAS provided to patients was a custom-made 2-piece appliance (SomnoDent MAS; SomnoMed Ltd, Crows Nest, Australia) with previously published design features and efficacy.16–21 To enable wear during MRI, the standard adjustable screw mechanism of this appliance was replaced with a modifiable acrylic coupling mechanism.15 The TSD was a preformed silicon appliance (Aveo-TSD, Innovative Health Technologies, New Zealand).6 The flanges of the TSD are placed outside the lips while the tongue is inserted into the bulb. The bulb is squeezed and released to generate suction until the tongue is retained without excessive discomfort. There was no method to standardize the suction pressure of the TSD; however, each patient adjusted the appliance to their own comfort level.
Magnetic Resonance Imaging of the Upper Airway
Magnetic resonance imaging was performed with a Philips INTERA 1.5T MRI scanner (Philips Electronics, Netherlands) while patients were awake, supine, and positioned with the Frankfort plane perpendicular to horizontal as previously described.15 Scans of each patient were acquired without and with each of the appliances in a randomized order. Contiguous T1-weighted spin-echo images were acquired through the mid-sagittal plane (1.25 mm thickness, 50 slices, 272 × 512 matrix) and axial plane (3 mm thickness, 50 slices, 224 × 12 matrix).
Anatomic Definitions, Measurements and Analysis
MRI data were assessed by the segmentation of upper airway lumen and soft tissue structures and identification of cephalometric landmarks. MR images were processed with image analysis software (Amira 4.1; Visage Imaging Inc., Carlsbad, CA) using previously described and validated techniques.15,22–24 Upper airway regions were defined as follows: velopharynx (hard palate to tip of uvula), oropharynx (tip of uvula to tip of epiglottis), and hypopharynx (tip of epiglottis to vocal cords) (Figure 1A). The airway lumen was segmented on axial image slices using a region-growing tool of the software, which marks a boundary around a seed point encapsulating pixels below a patient-specific threshold intensity for air (black).25 Airway volumetric information, as well as cross-sectional area (CSA) and anteroposterior (A-P) and lateral (L) diameters were obtained. Airway shape was calculated by the A-P:L ratio where a ratio of 1.0 represents a circle, while values < or > 1.0 indicate an elliptical shape oriented with the long axis in the coronal or sagittal plane, respectively.26
Figure 1.
Image analysis. (A) Mid-sagittal MRI demonstrating upper airway regions. (B) Representative axial MRI illustrating segmentation of upper airway structures; airway lumen (blue), soft palate (purple), tongue (red), parapharyngeal fat pads (yellow), and lateral pharyngeal walls (green). (C) Volumetric reconstructions of upper airway soft tissues showing the principle of calculation of magnitude and direction (x, y, z) of centroid movement. (D) Cephalometric linear and angular measurements of maxilla, mandible and hyoid position and anterior facial height. S, sella; N, nasion; ANS, anterior nasal spine; PNS, posterior nasal spine; A, A point; B, B point; Gn, gnathion; H, hyoid; C3, C3 vertebra.
Parapharyngeal fat pads, soft palate, tongue (genioglossus and base of tongue muscles) and lateral pharyngeal wall (retropalatal and retroglossal) were segmented on axial slices (Figure 1B), and reconstructions were used to obtain volumes and assess tissue movements. To assess movement x, y, z coordinates of the centroid (a point analogous to the centre of mass of an object) of each tissue structure were obtained, and the magnitude and direction of centroid movement with mandibular and tongue advancement determined (Figure 1C).
Cephalometric landmarks were identified on mid-sagittal images. Sella-nasion-A (SNA) angle, sella-nasion-B (SNB) angle, A-nasion-B (ANB) angle, basion-sella-nasion (BaSN) angle, and anterior nasal spine to gnathion distance (ANS-Gn, to measure lower anterior facial height) were obtained. Hyoid to C3 vertebra (H-C3), hyoid to posterior nasal spine (H-PNS), and hyoid to gnathion (H-Gn) distances were used to evaluate hyoid position (Figure 1D).
Polysomnography
All patients underwent polysomnography to determine treatment outcome with MAS. A subset of 18 patients additionally underwent polysomnography with TSD as part of a randomized controlled trial comparing the efficacy of the 2 oral appliances.6 Findings pertaining to TSD and MAS treatment response are described in this 18-patient subgroup. Polysomnography was scored in accordance with standard criteria.15,27–29
Treatment Outcome
Treatment outcome was based on definitions previously described.16,18,20 In keeping with our previous imaging study,15 “responders” were defined by ≥ 50% AHI reduction and “non-responders” by < 50% AHI reduction.
Statistical Analysis
Statistical analyses were performed using statistical software package SPSS (version 16.0 for Windows; SPSS, Inc., Chicago, IL). Descriptive statistics for patient clinical characteristics and MRI parameters are presented as mean ± standard deviation (SD) and means ± standard error of the mean (SEM), respectively. Continuous variables between conditions (baseline, MAS, TSD) were compared using repeated-measures ANOVA. If the F-statistic indicated a significant difference, paired t-tests comparing the 3 conditions were performed with a Bonferroni adjusted α level of 0.05/3 (0.017) (Holm procedure) for each comparison. In cases of non-normally distributed data, a non-parametric test was used (Wilcoxon rank-sum). Differences between treatment responders and non-responders were assessed by Student's t-test. Relationships between anthropometric, polysomnographic and airway measurements were assessed with Pearson correlation coefficient, or Spearman correlation coefficient if data were not normally distributed. Statistical significance was accepted at P < 0.05.
RESULTS
Clinical Characteristics
Thirty-nine OSA patients underwent upper airway imaging with both the MAS and TSD. The clinical characteristics of these patients are shown in Table 1. Characteristics of the subgroup of 18 patients who completed overnight polysomnography with TSD did not significantly differ from those who did not.
Table 1.
Patient characteristics
| n = 39 | n = 18 | |
|---|---|---|
| Gender (% male) | 64 | 70 |
| Age (years) | 50 ± 10.7 | 47.7 ± 11.3 |
| BMI (kg/m2) | 29.2 ± 5.5 | 27.7 ± 5.1 |
| Neck circumference (cm) | 39.3 ± 4.2 | 39.3 ± 4.2 |
| Baseline AHI (events/h) | 26.9 ± 17.1 | 26.8 ± 18.1 |
| Baseline AHI range (events/h) | 10.3 – 75.7 | 10.3 – 75.7 |
| AHI with MAS (events/h) | 12.0 ± 12.6 | 12.0 ± 9.6 |
| AHI with TSD (events/h) | N/A | 11.0 ± 9.1 |
| Mandibular advancement (% of maximum) | 75.4 ± 14.1 | 75.6 ± 12.6 |
Characteristics are shown for the patient sample as a whole (n = 39) and for the subset of these patients who had efficacy data for both oral appliances (n = 18). Mean values ± standard deviation.
TSD, tongue stabilizing device; BMI, body mass index; AHI, apnea hypopnea index.
Comparison of Effects of MAS and TSD on Upper Airway Structure
Airway variables
Airway volume changes with MAS and TSD are shown in Table 2. Volumetric reconstructions of the upper airway from a single patient are shown in Figure 2. There was a small but significant decrease in upper airway length from baseline (8.9 ± 0.2 cm) with both MAS (8.6 ± 0.2 cm; P < 0.001) and TSD (8.7 ± 0.2 cm; P < 0.02). The effects of MAS and TSD on airway CSA are shown in Figure 3. Both oral appliances increased mean CSA of the total upper airway, but TSD did so to a significantly greater extent. There were regional differences in the effects of both appliances (Figure 3). Lateral and A-P dimensions as well as A-P:L ratio are shown in Figure 4. Both appliances had the greatest effect on velopharyngeal lateral diameter. However, TSD had a larger effect on lateral diameter and additionally increased A-P diameter. Despite this TSD and MAS similarly influenced velopharyngeal shape to be more elliptical with a lateral long axis. This is represented schematically in Figure 4B.
Table 2.
Upper airway volume at baseline and with MAS and TSD (n = 39)
| Baseline | MAS | TSD | |
|---|---|---|---|
| Total airway (cm3) | 13.8 ± 1.0 | 14.3 ± 1.1 | 17.14 ± 1.6*# |
| Velopharynx (cm3) | 5.1 ± 0.4 | 5.4 ± 0.5 | 6.4 ± 0.7*# |
| Oropharynx (cm3) | 2.9 ± 0.3 | 3.1 ± 0.3 | 3.3 ± 0.3 |
| Hypopharynx (cm3) | 5.7 ± 0.5 | 5.8 ± 0.5 | 6.4 ± 0.6 |
Mean values ± standard error of mean.
P < 0.05 vs. baseline;
P < 0.05 MAS vs. TSD.
MAS, mandibular advancement splint; TSD, tongue stabilizing device.
Figure 2.
Volumetric reconstructions of the upper airway at baseline and with MAS and TSD in a single OSA patient. Corresponding mid-sagittal magnetic resonance images are shown.
Figure 3.
Upper airway cross-sectional area at baseline and with MAS and TSD. (A) Total upper airway. (B) Velopharynx. (C) Oropharynx. (D) Hypopharynx. *P < 0.05 vs. baseline, #P < 0.05 MAS vs. TSD.
Figure 4.
Upper airway lateral (L) and antero-posterior (AP) diameters at baseline and with MAS and TSD. The A-P:L ratio measure of shape is also shown. (A) Velopharynx. (B) Schematic representation of L and A-P diameter and shape changes in the velopharynx with MAS and TSD. (C) Oropharynx. (D) Hypopharynx. *P < 0.05 vs. baseline, #P < 0.05 MAS vs. TSD.
TSD changes in oropharyngeal A-P diameter (r = −0.32, P < 0.05), oropharyngeal minimum CSA (r = −0.36, P < 0.05), and hypopharyngeal volume (r = −0.33, P < 0.05) were inversely related to BMI. Similarly, changes in A-P diameter (r = −0.455, P < 0.01), lateral diameter (r = −0.331, P < 0.05), and minimum CSA (r = −0.468, P < 0.01) showed inverse relationships with neck circumference. There were no relationships between MAS airway changes and BMI or neck circumference.
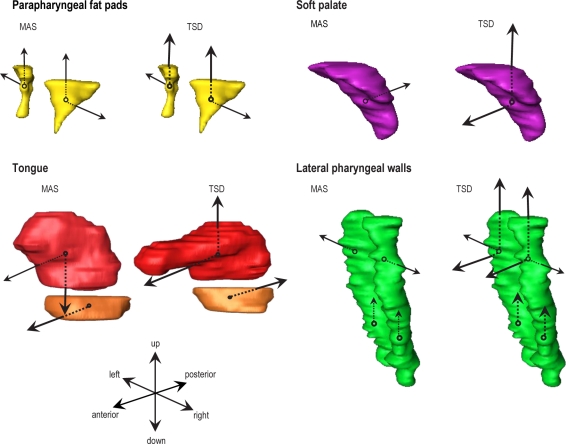
Soft tissue centroid movements
Overall centroid movements were relatively small; however, different patterns were observed. Both appliances resulted in lateral movement of the parapharyngeal fat pads away from the airway (MAS 0.28 ± 0.03 cm, TSD 0.23 ± 0.03 cm). Superior displacement of the fat pads was significantly greater with TSD than MAS (0.44 ± 0.05 vs. 0.12 ± 0.03 cm; P < 0.001). MAS resulted in slight posterior displacement of the soft palate (0.06 ± 0.03 cm), but TSD produced anterior (0.12 ± 0.03 cm) and superior (0.19 ± 0.04) movement.
TSD moved the tongue further forward than MAS (0.68 ± 0.04 vs. 0.06 ± 0.04 cm; P < 0.001). The tongue also moved superiorly with TSD (0.11 ± 0.05 cm) but inferiorly with MAS (0.11 ± 0.06 cm; P < 0.01). Muscles at the base of the tongue shifted forward with MAS (0.35 ± 0.04 cm; P < 0.001).
Velopharyngeal lateral pharyngeal walls moved laterally with MAS (0.14 ± 0.02 cm) and TSD (0.17 ± 0.02 cm). Anterior (0.14 ± 0.02 cm; P < 0.001) and superior (0.2 ± 0.05 cm; P < 0.001) movement also occurred with TSD. The oropharyngeal pharyngeal walls moved more superiorly with TSD (0.32 ± 0.05 vs. 0.11 ± 0.05 cm; P < 0.001). The relative movements of upper airway soft tissue centroids are illustrated in Figure 5.
Figure 5.
Schematic representation of soft tissue centroid movements with MAS and TSD. Larger and bolder arrows represent significantly greater centroid movements with each appliance.
Cephalometric analyses
Cephalometric measurements are shown in Table 3. SNB angle showed an increase with MAS and decrease with TSD. TSD increased lower face height (ANS-Gn) to a greater extent than MAS. Both appliances decreased H-PNS distance, but TSD additionally decreased H-C3 and H-Gn distances.
Table 3.
Cephalometric measurements at baseline and with MAS and TSD (n = 39)
| Baseline | MAS | TSD | |
|---|---|---|---|
| SNA (°) | 82.9 ± 0.8 | 83.4 ± 0.7 | 83.3 ± 0.8 |
| SNB (°) | 79.8 ± 0.8 | 82.0 ± 0.8* | 76.2 ± 0.8*# |
| ANB (°) | 3.0 ± 0.8 | 1.4 ± 0.7* | 7.1 ± 0.7*# |
| ANS-Gn (cm) | 6.9 ± 0.1 | 7.6 ± 0.1* | 8.7 ± 0.1*# |
| H-C3 (cm) | 3.8 ± 0.1 | 3.8 ± 0.1 | 3.7 ± 0.1*# |
| H-PNS (cm) | 7.4 ± 0.1 | 7.1 ± 0.1* | 7.2 ± 0.1* |
| H-Gn (cm) | 4.6 ± 0.1 | 4.7 ± 0.1 | 4.2 ± 0.1*# |
Mean values ± standard error of mean.
P < 0.01 vs. baseline;
P < 0.01 MAS vs. TSD.
SNA, sella-nasion-A point; SNB, sella-nasion-B point; ANB, A point-nasion-B point; ANS-Gn, anterior nasal spine to gnathion distance; H-C3, hyoid to C3 vertebra distance; H-PNS, hyoid to posterior nasal spine distance; H-Gn, hyoid to gnathion distance.
Upper Airway Structure and Treatment Outcome
In the subset of 18 patients with treatment outcome data, 10 patients were TSD responders and 8 non-responders. Responders and non-responders did not differ in terms of age (46.2 ± 11.7 vs. 49.5 ± 11.3 years), BMI (26.3 ± 4.8 vs. 30.5 ± 5.4 kg/ m 2), or neck circumference (38.7 ± 4.8 vs. 41.9 ± 4.1 cm), but baseline AHI did differ (34.8 ± 21.1 vs. 16.9 ± 4.5 events/h; P < 0.05). There were no differences between responders and non-responders in baseline upper airway structure (data not shown). However differences were observed in changes in velopharyngeal measurements. Responders showed a greater increase in A-P diameter (+0.2 ± 0.08 vs. –0.08 ± 0.06 cm; P < 0.02), minimum CSA (+0.44 ± 0.2 vs. –0.12 ± 0.1 cm2; P < 0.05), mean CSA (+0.75 ± 0.2 vs. +0.02 ± 0.2 cm2; P < 0.01), and volume (+2.65 ± 0.9 vs. –0.44 ± 0.8 cm3; P < 0.05).
Of these 18 patients, 12 were MAS responders and 6 non-responders. There were no differences in age (47.5 ± 11.8 vs. 48.1 ± 11.5 years), BMI (27.0 ± 4.4 vs. 30.5 ± 6.7 kg/m2), neck circumference (40.0 ± 3.9 vs. 41.3 ± 5.5 cm), or baseline AHI (30.6 ± 20.7 vs. 19.2 ± 8.2 events/h) between MAS response groups. Baseline or changes in upper airway structure did not differ between MAS responders and non-responders. There was no linear relationship between changes in AHI and airway volume with either appliance (Figure 6). Cephalometric measurements or changes and soft tissue centroid movements did not differ between treatment responders and non-responders for either appliance. In particular, it did not appear that differences in the degree of mouth opening, as assessed by the cephalo-metric measure ANS-Gn, induced by the 2 appliances had an impact on treatment outcome.
Figure 6.
Changes in total airway volume and AHI with oral appliance treatment. (A) MAS. (B) TSD. There was no significant relationship between airway size and treatment efficacy with either appliance. N = 18.
A cross-tabulation of these 18 patients by MAS and TSD response are shown in Table 4. Of these patients, 77.7% had the same category of response (responder or non-responder) with either appliance. There was no correlation between total airway or velopharyngeal volume changes between the two appliances (r = 0.01, P = 0.96). However, changes in oropharyngeal volume appear to show some consistency between the two appliances (r = 0.71, P = 0.001).
Table 4.
Cross-tabulation showing treatment response with MAS and TSD (n = 18)
| MAS outcome |
TSD Total | ||
|---|---|---|---|
| TSD outcome | Responder | Non-responder | |
| Responder | 9 | 1 | 10 |
| Non-responder | 3 | 5 | 8 |
| MAS Total | 12 | 6 | 18 |
DISCUSSION
This is the first study to compare the effects of two oral appliances, MAS and TSD, on upper airway structure using MRI. Clinically MAS are more widely used and investigated, while TSD have received less attention, and their role in OSA treatment remains uncertain. Our results indicate that both MAS and TSD increase upper airway dimensions but that there are differences in their effects on upper airway structure. Overall TSD have a greater effect on upper airway size than MAS.
Previous imaging studies of tongue protrusion are limited. A nasopharyngoscopic investigation of voluntary tongue and mandible protrusion30 reported a greater increase in velopharyngeal and oropharyngeal CSA with tongue compared to mandible protrusion, which is in accordance with the findings of the current study using oral appliances. The greater effects of TSD on upper airway structure may be a result of this appliance mechanically producing more anterior movement of the tongue. This action of protruding the tongue outside the oral cavity may cause greater displacement of other tissues. The level of mandibular advancement achieved with MAS averaged 6 millimeters, and therefore effects on upper airway structures may be more subtle.
This comparative imaging study suggests both appliances primarily improve upper airway caliber in the velopharynx. The velopharynx is the most common site of primary pharyngeal collapse in OSA.31,32 Increased velopharyngeal lateral diameter appears to be the main effect of MAS on the upper airway.12,15 This is likely a consequence of stretching soft tissue connections between the tongue, soft palate, and lateral pharyngeal walls through the palatopharyngeal and palatoglossal arches.10,33 Although TSD increases airway A-P diameter via forward displacement of the tongue, traction on these intra-pharyngeal connections via the tongue base may additionally increase the lateral dimension. The primarily lateral airway increase with both appliances and subsequent shape change to an ellipse in this orientation may favor reduced collapsibility. These data are consistent with respiratory-related changes in the airway that occur primarily in the lateral rather than A-P direction, suggesting more compliant lateral walls.25,34
A significant reduction in upper airway length was noted with both appliances. However, as mean length change was less than image slice thickness, this airway length change requires further investigation. Nonetheless, pharyngeal length has been shown to increase in OSA patients when supine,35 and reduction in pharyngeal length with oral appliances may represent a potential mechanism of action. Superior movement of the hyoid bone towards the posterior nasal spine was also associated with both appliances. An inferiorly positioned hyoid bone is a cephalometric variable commonly associated with OSA.36,37 It is unclear whether the inferiorly positioned hyoid is a cause or result of OSA; however, it appears that oral appliances have a corrective effect on hyoid position.
We have previously shown differences in the effects of MAS related to treatment outcome with improved airway caliber only evident in responders (although in the patient subset analyzed in this study these differences were not significant).15,29 In this study we were able to demonstrate significant increases in velopharyngeal airway dimensions with TSD in treatment responders only, suggesting efficacy is related to improved airway caliber. However, there is no direct linear relationship between changes in airway structure and improvement in total AHI with either appliance. This is not completely surprising and may relate to issues with night-to-night variability in AHI and sleep positions, and the relationship between upper airway size and OSA severity itself is unlikely to be linear.
In this sample, ∼77% of patients showed an equivalent treatment response with both appliances suggesting that some patients may characteristically respond to either form of oral appliance. We did identify a moderate correlation between the changes in oropharyngeal volume produced with both appliances. Previous studies have found that site of pharyngeal collapse is a significant determinant of treatment outcome, with patients who have primary oropharyngeal collapse more likely to respond to treatment.38,39 In the current study, oropharyngeal improvements were similar with both appliances and may explain why TSD (which improved velopharyngeal dimensions significantly more than MAS) was not more efficacious. Nevertheless, although structural characteristics are likely to be relevant, neuromuscular factors may additionally be important. Indeed a normalizing effect of TSD on the time lag between peak inspiratory genioglossus muscle activity and maximum inspiratory effort during apneic events has been demonstrated40 and is dependent on active retention of the tongue in the bulb.
The “holy grail” in terms of oral appliance treatment is the ability to predict which patients will respond to treatment. In our previous MRI study with MAS there were no baseline upper airway structures that allowed discrimination between responders and non-responders. Similarly, this study revealed no baseline characteristics related to TSD treatment response. However, there appears to be some relationship between BMI and neck circumference and the effect of TSD on upper airway size, with smaller body measures indicative of a greater improvement in measures of airway caliber. Larger body measures may be indicative of excess soft tissue surrounding the upper airway, which cannot be easily displaced to effectively increase upper airway size with TSD.
We have previously demonstrated that MAS and TSD similarly improve AHI.6 However MAS is effective in a greater proportion of patients and is additionally associated with greater symptomatic improvement, compliance, and patient preference. This may relate to issues with wearing the TSD, such as discomfort and involuntary removal during sleep, which result in reduced usage time. Therefore although TSD appears to have more favorable effects on upper airway caliber than MAS, practical issues may circumvent these positive effects. However, for patients who are able to tolerate TSD or are not suitable for MAS treatment, this study demonstrates highly favorable effects of this device on upper airway structure. Although TSD are not commonly used in clinical practice, a role for this type of appliance has previously been proposed for patients in whom MAS is contraindicated, such as those with insufficient teeth to retain the appliance or those with periodontal disease. Patients in this study had to be suitable candidates for MAS therapy, and therefore all patients had enough teeth to use MAS. How airway structural changes and efficacy with TSD are affected by issues such as edentulism would need to be addressed by future studies.
There are some limitations to this study. The MAS used was modified by removing the screw mechanism in order to permit imaging, and this appears to have resulted in lesser degrees of mandibular advancement than reported in our earlier efficacy studies.16,17,19 Hence this may have attenuated the airway changes observed with MAS in the current study. Image acquisition with MRI occurs over many minutes, and therefore the data represent averages across respiratory cycles. Imaging was performed during wakefulness, and the effects of MAS and TSD on upper airway structure may not be identical during sleep. However, imaging during sleep is technically challenging, and valuable insights can be gained using awake imaging as a surrogate.23 TSD application was not standardized; therefore, degree of tongue protrusion may not have been consistent between the sleep study and image acquisition. However, this only affects results related to treatment outcome. Moreover, the use of TSD in this manner reflects the intended use by the manufacturer as the appliance is commercially available. Relating our supine imaging findings to treatment outcome based on total AHI may not be completely applicable. Airway shape, in both OSA patients and healthy controls, changes from an ellipse while supine to more circular in the lateral recumbent position.41 What effect posture has on airway dimensions with oral appliances has not been investigated. It is possible that effects on airway dimensions and efficacy may differ with body position; however, both appliances reduce supine and non-supine AHI.6 Although we employed a novel method to examine soft tissue movements by measuring displacement of the structure's centroid, this is likely to be an oversimplification, which neglects morphological changes that require more complex analysis methods. Nonetheless this method was able to detect differences in soft tissue movements between MAS and TSD, giving insight into the differential structural effects of these oral appliances.
In conclusion both MAS and TSD increase upper airway dimensions and move surrounding soft tissues; however, the magnitude and pattern of changes differ between appliances. Further research evaluating whether these changes or the site of change within the airway can be used to predict treatment outcome is warranted.
DISCLOSURE STATEMENT
The oral appliances used in this study were provided at no cost by SomnoMed Ltd. Australia (mandibular advancement splints) and Innovative Health Technologies Ltd., New Zealand (tongue stabilizing devices). These companies had no other role in the study. Dr. Cistulli contributed to the development of the mandibular advancement splint used in this study. He has consulted for and has been on the advisory board of SomnoMed. He is a consultant for ExploraMed and has financial interest in the company. His department has received research support from ResMed and SomnoMed. He is a board member of the ResMed Foundation, a nonprofit, charitable organization. Dr. Schwab has participated in speaking engagements for Nuvigil. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Research was undertaken at Sydney Dental Hospital, St George Hospital and Royal North Shore Hospital, Sydney, Australia. The authors are grateful for the assistance of Dr. Biao Zeng, Dr. Jin Qian, Dr. Belinda Liu, and the sleep laboratory staff. The authors are grateful to Innovative Health Technologies, New Zealand for providing the tongue stabilizing devices and SomnoMed Ltd. Australia for providing the mandibular advancement splints.
REFERENCES
- 1.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693–9. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 3.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 4.Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14:546–52. doi: 10.1093/sleep/14.6.546. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright RD, Samelson CF. The effects of a nonsurgical treatment for obstructive sleep apnea. The tongue-retaining device. JAMA. 1982;248:705–9. [PubMed] [Google Scholar]
- 6.Deane SA, Cistulli PA, Ng AT, Zeng B, Petocz P, Darendeliler MA. Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial. Sleep. 2009;32:648–53. doi: 10.1093/sleep/32.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higurashi N, Kikuchi M, Miyazaki S, Itasaka Y. Effectiveness of a tongue-retaining device. Psychiatry Clin Neurosci. 2002;56:331–2. doi: 10.1046/j.1440-1819.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- 8.Kingshott RN, Jones DR, Taylor DR, Robertson CJ. The efficacy of a novel tongue-stabilizing device on polysomnographic variables in sleep-disordered breathing: a pilot study. Sleep Breath. 2002;6:69–76. doi: 10.1007/s11325-002-0069-1. [DOI] [PubMed] [Google Scholar]
- 9.Dort L, Brant R. A randomized, controlled, crossover study of a noncustomized tongue retaining device for sleep disordered breathing. Sleep Breath. 2008;12:369–73. doi: 10.1007/s11325-008-0187-5. [DOI] [PubMed] [Google Scholar]
- 10.Isono S, Tanaka A, Sho Y, Konno A, Nishino T. Advancement of the mandible improves velopharyngeal airway patency. J Appl Physiol. 1995;79:2132–8. doi: 10.1152/jappl.1995.79.6.2132. [DOI] [PubMed] [Google Scholar]
- 11.Johal A, Battagel JM. An investigation into the changes in airway dimension and the efficacy of mandibular advancement appliances in subjects with obstructive sleep apnoea. Br J Orthod. 1999;26:205–10. doi: 10.1093/ortho/26.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54:972–7. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed MM, Schwab RJ. Upper airway imaging in obstructive sleep apnea. Curr Opin Pulm Med. 2006;12:397–401. doi: 10.1097/01.mcp.0000245706.77064.51. [DOI] [PubMed] [Google Scholar]
- 14.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 15.Chan ASL, Sutherland K, Schwab RJ, et al. the effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65:726–31. doi: 10.1136/thx.2009.131094. [DOI] [PubMed] [Google Scholar]
- 16.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:743–8. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 17.Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934–41. doi: 10.1093/sleep/27.5.934. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 19.Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:860–4. doi: 10.1164/rccm.200204-342OC. [DOI] [PubMed] [Google Scholar]
- 20.Zeng B, Ng AT, Darendeliler MA, Petocz P, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:726–30. doi: 10.1164/rccm.200608-1205OC. [DOI] [PubMed] [Google Scholar]
- 21.Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31:543–7. doi: 10.1093/sleep/31.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwab RJ, Pasirstein M, Kaplan L, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–63. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 24.Welch KC, Foster GD, Ritter CT, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–42. [PubMed] [Google Scholar]
- 25.Schwab RJ, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med. 1996;154:1106–16. doi: 10.1164/ajrccm.154.4.8887615. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JH, Leigh MS, Paduch A, et al. Evaluation of pharyngeal shape and size using anatomical optical coherence tomography in individuals with and without obstructive sleep apnoea. J Sleep Res. 2008;17:230–8. doi: 10.1111/j.1365-2869.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 27.EEG arousals: Scoring rules and examples: A preliminary report from the sleep disorders atlas task force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. Los Angeles. [DOI] [PubMed] [Google Scholar]
- 29.Chan AS, Lee RW, Srinivasan VK, Darendeliler MA, Grunstein RR, Cistulli PA. Nasopharyngoscopic evaluation of oral appliance therapy for obstructive sleep apnoea. Eur Respir J. 2010;35:836–46. doi: 10.1183/09031936.00077409. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson KA, Love LL, Ryan CF. Effect of mandibular and tongue protrusion on upper airway size during wakefulness. Am J Respir Crit Care Med. 1997;155:1748–54. doi: 10.1164/ajrccm.155.5.9154887. [DOI] [PubMed] [Google Scholar]
- 31.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 32.Morrison DL, Launois SH, Isono S, Feroah TR, Whitelaw WA, Remmers JE. Pharyngeal narrowing and closing pressures in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;148:606–11. doi: 10.1164/ajrccm/148.3.606. [DOI] [PubMed] [Google Scholar]
- 33.Isono S, Tanaka A, Tagaito Y, Sho Y, Nishino T. Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology. 1997;87:1055–62. doi: 10.1097/00000542-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 35.Pae EK, Lowe AA, Fleetham JA. A role of pharyngeal length in obstructive sleep apnea patients. Am J Orthod Dentofacial Orthop. 1997;111:12–7. doi: 10.1016/s0889-5406(97)70296-8. [DOI] [PubMed] [Google Scholar]
- 36.Hui DS, Ko FW, Chu AS, et al. Cephalometric assessment of craniofacial morphology in Chinese patients with obstructive sleep apnoea. Respir Med. 2003;97:640–6. doi: 10.1053/rmed.2003.1494. [DOI] [PubMed] [Google Scholar]
- 37.Johal A, Patel SI, Battagel JM. The relationship between craniofacial anatomy and obstructive sleep apnoea: a case-controlled study. J Sleep Res. 2007;16:319–26. doi: 10.1111/j.1365-2869.2007.00599.x. [DOI] [PubMed] [Google Scholar]
- 38.Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep. 2006;29:666–71. [PubMed] [Google Scholar]
- 39.Sanner BM, Heise M, Knoben B, et al. MRI of the pharynx and treatment efficacy of a mandibular advancement device in obstructive sleep apnoea syndrome. Eur Respir J. 2002;20:143–50. doi: 10.1183/09031936.02.00268902. [DOI] [PubMed] [Google Scholar]
- 40.Ono T, Lowe AA, Ferguson KA, Fleetham JA. A tongue retaining device and sleep-state genioglossus muscle activity in patients with obstructive sleep apnea. Angle Orthod. 1996;66:273–80. doi: 10.1043/0003-3219(1996)066<0273:ATRDAS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 41.Walsh JH, Leigh MS, Paduch A, et al. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. Sleep. 2008;31:1543–9. doi: 10.1093/sleep/31.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]