Abstract
Study Objectives:
Investigate the efficacy of a novel nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA).
Design:
A prospective, multicenter, sham-controlled, parallel-group, randomized, double-blind clinical trial.
Setting:
19 sites including both academic and private sleep disorder centers
Patients:
Obstructive sleep apnea with a pre-study AHI ≥ 10/hour
Interventions:
Treatment with a nasal EPAP device (N = 127) or similar appearing sham device (N = 123) for 3 months. Polysomnography (PSG) was performed on 2 non-consecutive nights (random order: device-on, device-off) at week 1 and after 3 months of treatment. Analysis of an intention to treat group (ITT) (patients completing week 1 PSGs) (EPAP N = 119, sham N = 110) was performed.
Measurements and Results:
At week 1, the median AHI value (device-on versus device-off) was significantly lower with EPAP (5.0 versus 13.8 events/h, P < 0.0001) but not sham (11.6 versus 11.1 events/h, P = NS); the decrease in the AHI (median) was greater (−52.7% vs. −7.3%, P < 0.0001) for the ITT group. At month 3, the percentage decrease in the AHI was 42.7% (EPAP) and 10.1% (sham), P < 0.0001. Over 3 months of EPAP treatment the Epworth Sleepiness Scale decreased (9.9 ± 4.7 to 7.2 ± 4.2, P < 0.0001), and the median percentage of reported nights used (entire night) was 88.2%.
Conclusions:
The nasal EPAP device significantly reduced the AHI and improved subjective daytime sleepiness compared to the sham treatment in patients with mild to severe OSA with excellent adherence.
Clinical Trial Information:
Registrations: ClinicalTrials.gov. Trial name: Randomized Study of Provent Versus Sham Device to Treat Obstructive Sleep Apnea (AERO). URL: http://www.clinicaltrials.gov/ct2/show/NCT00772044?term=Ventus&rank=1. Registration Number: NCT00772044.
Citation:
Berry RB; Kryger MH; Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. SLEEP 2011;34(4):479-485.
Keywords: Obstructive sleep apnea, expiratory positive airway pressure, CPAP
INTRODUCTION
Obstructive sleep apnea (OSA) is a very common disorder1 often resulting in adverse cardiovascular consequences, daytime sleepiness, and disturbed nocturnal sleep of the patient and bed partner.2,3 Although effective and safe treatment options including continuous positive airway pressure (CPAP),4–11 oral appliances,12,13 and upper airway surgery14 exist, none are ideal. Given the high prevalence of OSA, new effective treatment options would be welcomed.
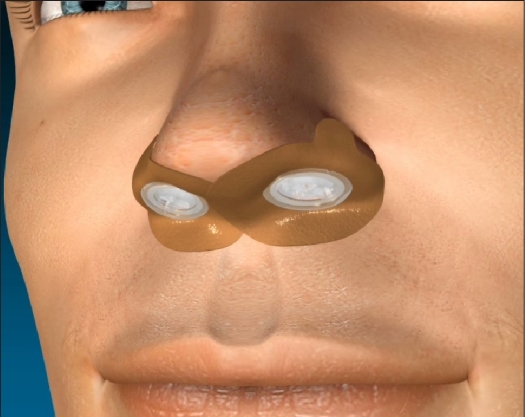
A novel expiratory positive airway pressure (EPAP) nasal device has been developed to provide a new therapeutic option for OSA (Provent Therapy, Ventus Medical Inc., Belmont, CA). A single use EPAP device containing a mechanical valve with very low inspiratory resistance but high expiratory resistance is applied to each nostril with adhesive to provide a seal (Figure 1). The high expiratory resistance results in positive pressure throughout exhalation, which splints open the upper airway, making it more resistant to collapse on subsequent inspiration.15
Figure 1.
Nasal EPAP device. Single use valves are inserted into each nostril and sealed with adhesive.
A small pilot study16 and a subsequent larger prospective multi-center trial17 found the nasal EPAP device to significantly reduce the AHI in groups of patients with varying severity of sleep apnea. The goal of the current study was to determine the effectiveness of the EPAP device and adherence to treatment compared to a sham device over a 3-month period in a larger group of patients with OSA.
METHODS
Study Design
The study was a prospective, multi-center, parallel group, sham-controlled, randomized, double-blinded clinical trial. Nineteen sites participated in the study (study investigators listed in the Acknowledgment). The study was registered on clinicaltrials.gov (NCT00772044).The local institutional review board (or authorized national institutional review board) at each site approved the study.
Patient Recruitment, Randomization, and Study Initiation
Patients were recruited from the sleep clinic of participating investigators. All consecutively seen patients with newly diagnosed OSA or previously diagnosed but untreated OSA who met inclusion and exclusion criteria were considered for enrollment (Table 1, Supplement). Inclusion criteria were pre-study AHI ≥ 10/h and age ≥ 18 years. Patients with severe nocturnal arterial oxygen desaturation, previous upper airway surgery, nasal occlusion, or previous treatment with CPAP or an oral appliance were excluded. After signing an informed consent, patients underwent a baseline clinic evaluation that included the Epworth Sleepiness Scale (ESS, a subjective measure of propensity to fall asleep in common situations)18 and a medical evaluation by the study physician.
Once the subject completed the baseline assessment, the randomization assignment was determined from sealed opaque envelopes provided by a third-party data management group, Advance Research Associates. The envelopes were mailed directly from their offices to the study site. In the envelopes, a sheet of paper contained the treatment group assignment, order of device-on vs device-off PSG, and PSG ID number.
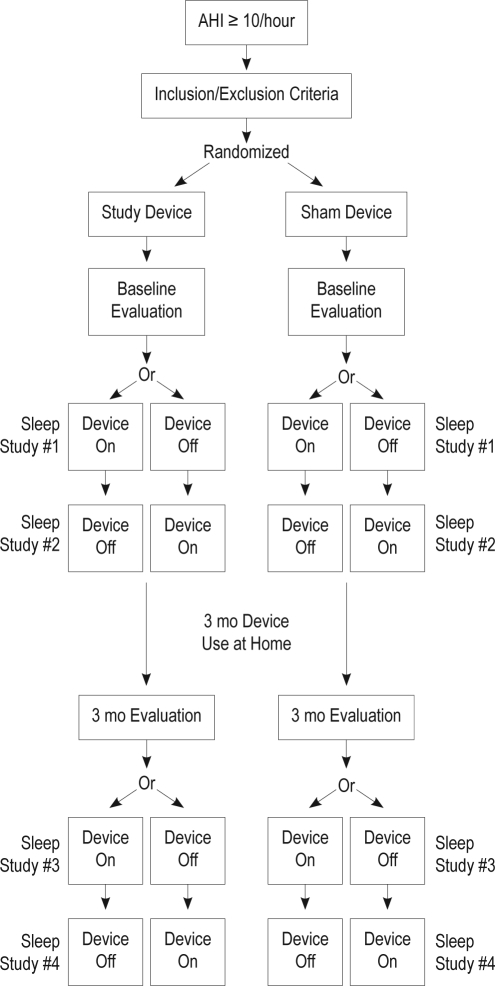
After randomization to either the nasal EPAP or sham device study arm, patients were trained on the use of the assigned device and began using the device nightly for the 3-month study duration (Figure 2). They were also asked to complete a daily diary entry each morning after awakening, documenting if the device was in place in the morning.
Figure 2.
Study design
Active and Sham Devices
The EPAP nasal device consists of a single-use valve inserted into each nostril and held in place by adhesive. The valve has minimal inspiratory resistance but an expiratory resistance of 80 cm H2O/L/sec at a flow rate of 100 mL/sec. The sham device was similar in appearance but with an expiratory resistance of < 1 cm H2O/L/sec. The adhesive substrate, similar to that found in adhesive bandages, was applied to the outer edges of the nares, resulting in a leak-free seal between the valve and the nose.
Treatment Initiation and Evaluations
During treatment week 1, after ≥ 3 nights of device use, patients underwent 2 sleep studies on non-consecutive nights (one device-on, one device-off, in randomly assigned order). After 3 months of treatment, patients underwent another clinic evaluation by the study physician. Patients again completed the ESS questionnaire and were asked about adverse effects or any change in health status. Following the 3-month clinic evaluation, another 2 sleep studies were performed on non-consecutive nights (device-on, device-off, randomly assigned).
Polysomnography
Attended polysomnography (PSG) was performed using standard techniques including monitoring of EEG derivations (frontal, central, and occipital), right and left electrooculographic derivations, a chin electromyographic (EMG) derivation, nasal pressure, an oral thermal sensor, chest and abdominal effort belts, a body position monitor, a left leg EMG derivation, a single ECG channel, and pulse oximetry. On device-on nights a specially designed nasal cannula (Ventus Medical Inc., Belmont, CA) was used; this securely attached to the nasal EPAP device or sham device (device-on nights) for measurement of nasal airflow. The polysomnographic data were analyzed by a central scoring center. Sleep was manually staged in 30-sec epochs, and arousals and respiratory events were scored using the recommended criteria published in the American Academy of Sleep Medicine Scoring Manual.19 Hypopneas were defined as reductions in airflow of 30% or more from baseline with a duration > 10 sec associated with a drop in arterial oxygen saturation (SpO2) ≥ 4%. The arterial oxygen desaturation index (ODI) was the number of desatu-rations ≥ 3% per hour of total sleep time (TST).
Statistical Analysis
Data analysis was completed by Advance Research Associates (Mountain View, CA). Analysis was performed on the intention to treat (ITT) and the modified intention to treat groups (mITT). The ITT group included all patients in whom data were available. The mITT group included patients who used the study device post randomization, did not experience major pro- tocol violations, completed the week 1 PSGs, and had an AHI ≥ 5/h on the week 1 device-off PSG night. The mITT analysis was performed to assess device efficacy in patients who actually had sleep apnea.
The primary and secondary endpoints were established a priori. The primary endpoint of the study was comparison of the difference in the AHI values between device-on and device-off nights between the EPAP and sham devices during PSG at week 1. Secondary endpoints were device-on versus device-off comparisons of the AHI at month 3 and the change in ESS between baseline and month 3.
Treatment group differences (EPAP versus sham) in the primary endpoint were statistically evaluated using the analysis of covariance (ANCOVA) on the ranked values for change in AHI. The covariate was the change in percent time supine from the device-off night to the device-on night. Treatment group differences for all other endpoints were statistically evaluated using analysis of variance (ANOVA) if tests of normality were met and the Kruskal-Wallis test when tests of normality failed, both stratified by study center. Within-group differences, for example AHI device-off versus AHI device-on for the EPAP group, were compared using the Wilcoxon rank sum method (nonparametric distributions). Daily compliance rates between sham and EPAP groups were compared by χ2 analysis. A P < 0.05 was considered statistically significant. Results are presented as means ± standard deviation or median (25th, 75th percentile).
RESULTS
Baseline Characteristics
Two hundred fifty (250) patients were randomized (127 nasal EPAP, 123 sham device). A total of 229 completed week 1 sleep studies (119 EPAP, 110 sham). This group was the ITT group. Of these, 173 had an AHI ≥ 5/h on the week 1 device-off night and comprised the mITT group (92 EPAP, 81 sham). The characteristics of the ITT and mITT groups are shown in Table 1. The patients in the EPAP and sham groups were well matched.
Table 1.
Patient demographics and dropout rates
| EPAP | Sham | P Value | EPAP | Sham | P Value | |
|---|---|---|---|---|---|---|
| ITT | ITT | EPAP versus Sham | mITT | mITT | EPAP versus Sham | |
| Sample size | 119 | 110 | 92 | 81 | ||
| Age | 47.7 ± 13.4 | 46.8 ± 12.0 | NS | 49.0 ± 13.1 | 47.3 ± 12.3 | NS |
| Gender | 71.4% male | 65.5% male | NS | 72.8% male | 66.7% male | NS |
| BMI (kg/m2) | 32.6 ± 7.0 | 33.8 ± 6.5 | NS | 32.8 ± 6.7 | 34.6 ± 6.6 | NS |
| Median baseline AHI (week 1 device-off PSG) | 13.8 (5.3, 22.6) | 11.1 (4.8, 21.8) | NS | 16.7 (9.5, 26.3) | 15.1 (10.3, 24.1) | NS |
| Dropouts by Month 3 | 19 (16.0%) | 15 (13.6%) | NS | 15 (16.3%) | 14 (17.3%) | NS |
ITT, intention to treat group, all subjects randomized for which data were available; mITT, modified intention to treat – patients finishing both week 1 PSG studies with an AHI ≥ 5 on device-off night of week 1.
A total of 195 patients in the ITT group (100 EPAP, 95 sham) completed the 3-month study. The percentage of dropouts in the EPAP and sham groups did not differ (P = NS). A total of 144 patients in the mITT group completed the 3-month study (77 EPAP, 67 sham). The percentage of dropouts in the EPAP and sham groups and did not differ (P = NS). CONSORT diagrams20 detailing patient flow for both the ITT and mITT groups are provided (Figures 1 and 2, Supplement). The ITT group dropouts (EPAP, sham) at month 3 were due to patients lost to follow-up (2, 5), patient non-compliance with the protocol unrelated to device use (4, 2), adverse events or device acclimation (11, 3), preference for an alternative treatment (0, 2), and other (0, 3). A detailed explanation for dropouts is provided (Table 3, Supplement).
AHI—Week 1 and Month 3
At week 1, the median AHI during device-off nights in both the EPAP and sham groups (ITT and mITT analysis) was in the mild to moderate range (Tables 2 and 3). In the EPAP group, there was a (median) percent reduction in the AHI of 52.7% (ITT) and 55.1% (mITT) device-on versus device-off night (P < 0.001). The sham device did not significantly reduce AHI,and the reduction in AHI with the EPAP device was significantly greater than the reduction with the sham device (in both ITT and mITT analysis) (P < 0.001). In a group of 17 patients with severe sleep apnea (AHI > 30, device-off nasal EPAP group), the median (25, 75 percentile values) AHI decreased from 48.2 (39.4, 50.2) to 18.9 (5.6, 28.0)/h (P < 0.001), showing that the nasal EPAP device also significantly reduced the AHI in patients with severe OSA.
Table 2.
Week 1 and month 3 AHI results (ITT group)
| P Value | |||||||
|---|---|---|---|---|---|---|---|
| Device-off | Device-on | Median of % change | Device-off | Device-on | Median of % change | EPAP vs Sham (% change) | |
|
EPAP Week 1 (N = 119) |
Sham Week 1 (N = 110) |
||||||
| AHI | 13.8 (5.3, 22.6) | 5.0* (1.7, 11.6) | −52.7 (−80.9, 1.2) | 11.1 (4.8, 21.8) | 11.6 (4.0, 21.0) | −7.3 (−48.5, 46.0) | < 0.0001 |
|
EPAP Month 3 (N = 100) |
Sham Month 3 (N = 95) |
||||||
| AHI | 14.4 (5.5, 21.4) | 5.6* (2.1, 12.5) | −42.7 (−80.2, 0.1) | 10.2 (3.4, 19.3) | 8.3 (4.2, 20.6) | −10.1 (−47.9, 88.5) | < 0.0001 |
EPAP Device-on vs Device-off
P < 0.0001.
Values are medians (25, 75 quartiles).
Table 3.
Week 1 and month 3 results (mITT group)
| P Value | |||||||
|---|---|---|---|---|---|---|---|
| Device-off | Device-on | Median of % change | Device-off | Device-on | Median of % change | EPAP vs Sham (% change) | |
|
EPAP Week 1 (N = 92) |
Sham Week 1 (N = 81) |
||||||
| AHI | 16.7 (9.5, 26.3) | 7.1* (2.2, 17.1) | −55.1 (−83.3, −21.3) | 15.1 (10.3, 24.1) | 13.6 (8.6, 25.8) | −13.8 (−50.5, 30.4) | < 0.001 |
|
EPAP Month 3 (N = 77) |
Sham Month 3 (N = 67) |
||||||
| AHI | 16.7 (9.7, 26.0) | 8.1* (3.8, 17.6) | −42.8 (−78.5, −10.8) | 14.5 (8.4, 23.5) | 13.3 (5.9, 25.0) | −12.3 (−42.7, 77.9) | < 0.001 |
EPAP Device-on vs Device-off
P < 0.001.
Values are medians (25, 75 quartiles).
TST, total sleep time.
Oxygenation—Week 1 and Month 3
Analysis of oxygenation was performed in the mITT group (Table 4). The ODI and %TST with SpO2 < 90% at week 1 were both significantly lower on EPAP device-on nights than device-off nights. The reductions in the ODI and %TST with SpO2 < 90% with the sham device (device-on vs. device-off)were not statistically significant. In addition, the reductions in the ODI and %TST with SpO2 < 90% with the EPAP device were significantly greater than corresponding reduction with the sham device. At month 3, the AHI, ODI, and %TST with SpO2 < 90% showed similar results, with significant decreases during device-on compared to device-off nights with the nasal EPAP device. In addition, the percentage decreases were all significantly greater for nasal EPAP than the sham device.
Table 4.
Oxygenation data week 1 and month 3 (mITT group)
| P Value | |||||||
|---|---|---|---|---|---|---|---|
| Device-off | Device-on | Median of % change | Device-off | Device-on | Median of % change | EPAP vs Sham (% change) | |
|
EPAP Week 1 (N = 92) |
Sham Week 1 (N = 81) |
||||||
| ODI | 13.7 (7.8, 23.6) | 7.3* (3.5, 13.8) | −43.2 (−66.1, −2.2) | 14.6 (8.7, 22.3) | 12.2 (6.5, 21.9) | −15.5 (−40.9, 19.7) | < 0.001 |
| %TST SpO2 < 90% | 1.5 (0.2, 5.1) | 0.6** (0.0, 1.7) | −65.6 (−95.1, 0.0) | 2.2 (0.2, 6.2) | 1.0 (0.2, 4.7) | −40.0 (−74.2, 64.9) | 0.004 |
|
EPAP Month 3 (N = 77) |
Sham Month 3 (N = 67) |
||||||
| ODI | 12.6 (7.1, 23.8) | 8.6* (3.7, 13.5) | −35.2 (−64.1, 2.8) | 13.3 (7.5, 23.1) | 12.7 (6.4, 21.2) | −16.0 (−39.8, 29.6) | 0.025 |
| %TST SpO2 < 90% | 1.3 (0.2, 5.0) | 0.7** (0.0, 3.5) | −64.1 (−91.7, −26.5) | 1.8 (0.3, 5.1) | 1.8 (0.1, 6.2) | 0.0 (−60.0, 66.0) | 0.002 |
EPAP Device-on vs Device-off
P < 0.001,
P = 0.004.
Values are medians (25, 75 quartiles).
TST, total sleep time; ODI, oxygen desaturation index; SpO2, arterial oxygen saturation.
Sleep Architecture (mITT Analysis)
The TST, sleep stage durations (% of TST), and arousal index for device-off and device-on in both EPAP and sham treatment groups are shown for week 1 in Table 5. At week 1, the amount of stage N1 and the arousal index were slightly but significantly reduced by EPAP (device-on versus device-off) compared with sham treatment. However, at month 3, stage N1 and the arousal index were not significantly reduced by EPAP compared to sham treatment (Table 2, Supplement).
Table 5.
Sleep architecture and effects of supine position and REM sleep (mITT analysis) week 1 results.
| EPAP |
Sham |
P Value | |||
|---|---|---|---|---|---|
| Device-off | Device-on | Device-off | Device-on | EPAP vs Sham (absolute change) | |
| N = 92 |
N = 81 |
||||
| TST | 364.0 ± 56.7 | 352.4 ± 65.1 | 357.6 ± 73.4 | 344.4 ± 66.2 | 0.87 |
| Wake After Sleep Onset | 57.6 ± 42.4 | 54.0 ± 36.8 | 57.3 ± 43.3 | 58.8 ± 37.8 | 0.43 |
| Stage N1 | 16.9 ± 10.6 | 14.5 ± 8.6** | 17.6 ± 12.6 | 19.0 ± 13.1 | 0.034 |
| Stage N2 | 60.4 ± 10.1 | 62.7 ± 8.8** | 59.7 ± 10.1 | 59.9 ± 11.6 | 0.22 |
| Stage N3 | 5.4 ± 6.5 | 5.7 ± 6.6 | 5.8 ± 6.3 | 4.7 ± 5.0 | 0.071 |
| Stage REM | 17.3 ± 5.8 | 17.1 ± 6.4 | 17.0 ± 6.0 | 16.4 ± 6.1 | 0.88 |
| Arousal Index | 19.9 (13.5, 28.8) | 15.3* (11.7, 22.5) | 20.2 (13.9, 26.2) | 19.2 (15.0, 26.8) | < 0.001 |
| N = 85 |
N = 72 |
||||
| AHI NREM1 | 14.9 (7.7, 24.0 ) | 6.0* (1.7, 12.6) | 10.8 (5.4, 22.0) | 11.5 (5.0, 24.0) | < 0.001 |
| AHI REM1 | 26.5 (10.3, 45.1) | 8.7* (2.5, 27.9) | 31.6 (9.7, 46.9) | 22.4 (7.6, 43.2) | 0.003 |
| N = 57 |
N = 57 |
||||
| AHI Supine1 | 29.2 (18.2, 46.5) | 12.7* (3.3, 36.6) | 24.8 (14.7, 42.7) | 25.9 (12.8, 47.2) | 0.036 |
| AHI Non-Supine1 | 6.0 (2.4, 11.3) | 2.5** (0.7, 6.3) | 6.6 (1.9, 15.2) | 4.8 (2.1, 16.2) | 0.093 |
Values are median (25, 75 quartile) or, mean ± standard deviation.
Device-off versus Device-on P < 0.01.
Device-off versus Device-on P < 0.05.
Patients with > 20 minutes in each position and state.
Impact of Position and Sleep Stage (mITT Analysis)
The effects of body position and sleep stage are shown in Table 5. The EPAP device significantly reduced the AHI (device-on versus device-off) during both NREM and REM sleep and in the supine position during week 1 (Table 5). At month 3, the AHI during NREM and REM sleep as well as in the supine position were significantly lower (device-on compared to device-off) for the nasal EPAP device, but the reduction compared to sham device was only significant for AHI REM and AHI supine (Table 2, Supplement).
Treatment Success
For the mITT analysis, we defined treatment success on EPAP as a ≥ 50% reduction in the AHI or an AHI reduced to < 10/h (if week 1 device-off AHI was ≥ 10/h) comparing device-on to device-off nights and found that at week 1, success was achieved in 62.0% (EPAP) and 27.2% (sham) of patients (P < 0.001). At month 3, success was achieved in 50.7% (EPAP) and 22.4% (sham) of patients (P = 0.001).
Impact on Subjective Sleepiness
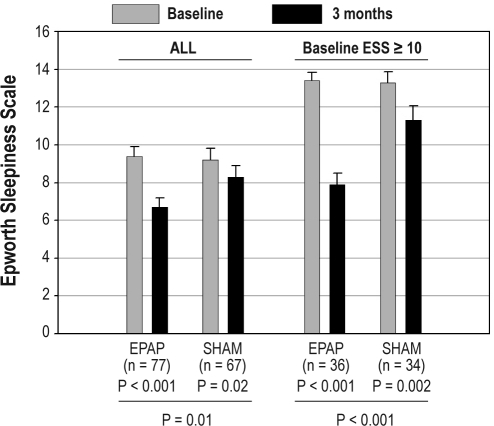
Changes in the ESS (subjective sleepiness) between baseline and month 3 follow-up were analyzed using both an ITT and mITT analysis. For ITT analysis, the ESS changed from 9.9 ± 4.7 to 7.2 ± 4.2 (P < 0.0001) in the EPAP group and from 9.6 ± 4.9 to 8.3 ± 5.1 in the sham group (P = 0.001). However, the change was significantly greater in the EPAP than sham group (P = 0.04). In the mITT analysis, significant reductions in the ESS (less subjective sleepiness) were found for both the EPAP and sham groups between baseline and the 3-month follow-up (Figure 3). The reduction in ESS was significantly larger with EPAP treatment than with sham treatment. Additional mITT analysis of the change in ESS was performed for those with a baseline ESS ≥ 10 (increased sleepiness). In this group, the mean ESS score dropped into the normal range with EPAP treatment (Figure 3).
Figure 3.
Epworth Sleepiness Scale at baseline and after 3 months of treatment for the mITT analysis. There was a significant decrease in both the EPAP device and sham device groups. However, the decrease was significantly greater for the EPAP device. Similar results were noted for the group with an Epworth Sleepiness Scale ≥10 at baseline. The error bars are standard error of the mean.
Adherence (ITT Analysis)
The adherence to treatment was high in both the EPAP and sham groups (ITT analysis). The median percentage (25, 75 percentile) of reported nights the device was worn for the entire night for the EPAP device and sham device were respectively 88.2% (67.5, 96.4) versus 92.3% (84.0, 97.5), P = 0.02. The median percentages of nights that the diary was completed were above 97% in both treatment groups.
Adverse Events (ITT Analysis)
There were no serious device-related adverse events in either the EPAP or the sham treated groups. Device-related adverse events were reported by 45% (53/119) of patients in the EPAP group and 34% (37/110) of patients in the sham group (P = 0.36). Device-related adverse events resulting in study discontinuation occurred in 7% (8/119) of patients in the EPAP group. Detailed device-related adverse event summary tables are provided (Tables 4 and 5, Supplement).
DISCUSSION
The major finding of the study was that the EPAP device significantly decreased the AHI compared to device-off nights on the week 1 sleep study, and that the difference was significantly greater than with the sham device (52.7% versus 7.3%, ITT analysis). At repeat testing at month 3, 51% of the EPAP device users had a 50% or greater reduction in the AHI (or reduction to < 10/h) on device-on compared to device-off nights. The week 1 PSG also found that the EPAP device improved oxygenation as exhibited by a small but significant decrease in the ODI and %TST with SpO2 less than 90%. Subjective sleepiness (ESS) also improved after 3 months of nasal EPAP use. The side effects of EPAP treatment were mild, and a significant proportion of patients completed the 3-month study. Median device use (adherence), as reported by patient diary, was excellent, with the nasal EPAP device worn all night for approximately 88% of nights.
The effectiveness of nasal EPAP in this study compares favorably to other treatment modalities. Although CPAP often reduces the AHI to less than 5/h, inadequate adherence to treatment often reduces effectiveness.8,9 For example, if CPAP reduces the AHI from 40 to 10/h but is only used for one-half of the total sleep time, the effective AHI is 25/h. In one crossover study comparing oral appliances and CPAP, the mean AHI dropped from baseline of 21.3/h to 4.8/h with CPAP and to 14.0/h with an oral appliance.13 In a meta-analysis of the effectiveness of surgery for OSA, procedures less complex than maxillary-mandibular advancement (usually reserved for severe OSA) reduced the AHI to less than 10/h in 31% of patients.14 At month 3, the nasal EPAP device achieved a 51% treatment success rate, defined as a 50% or greater reduction in the AHI or a reduction to less than 10/h. This result compares favorably with treatment with oral appliances, upper airway surgery, and even with CPAP when one computes an “effective AHI.”
Our study has a number of limitations. There were a large number of exclusion criteria, including patients with severe arterial oxygen desaturation, upper airway surgery, or those that had tried CPAP. The top four exclusions were prior CPAP use, other serious uncontrolled medical conditions, other sleep disorders, and medications affecting neurocognitive function. The exclusions were designed so that 3 months of sham treatment would not impose a significant health risk and that treatment naïve patients would be studied. Because of our exclusions, the results of the study may not generalize to less selected populations that may contain CPAP failures, prior upper airway surgery, or severe arterial oxygen desaturation. Our modified intention to treat group included patients with an AHI ≥ 5/h. The reason a substantial number of patients failed to have an AHI ≥ 5/h on device-off nights is not clear. There can be night-to-night variability in patients with milder OSA. The pre-study AHI was determined with less standardized scoring from sleep centers at the research sites. In any case, the mITT definition was applied to both nasal EPAP device and sham groups, and PSGs were scored by a central lab with scorers blinded to treatment assignment and time point. Therefore, the fact that patient numbers in the mITT group used for analysis were lower after randomization should not have influenced our results. In addition, using an intention to treat analysis, the EPAP device significantly reduced both the AHI and ESS. The reduction in the AHI and ESS were significantly greater with EPAP than with sham.
Another limitation of our study was that adherence determination depended on patient report rather than an objective measure. However, adherence was similar on both the sham and active device.
Although the nasal EPAP device met predetermined efficacy criteria in roughly 50% of patients, no baseline predictors of treatment success were identified by post hoc analysis. In the absence of further clinical trials documenting even longer term efficacy, it is reasonable to consider nasal EPAP for patients who have failed treatment with CPAP and who do not have life-threatening drops in the arterial oxygen saturation. Confirmation of efficacy by a home sleep study or full sleep study and follow-up with the physician to assure adequate adherence would be consistent with recommendations for other treatments for OSA.2
In summary, a randomized, double-blind, sham-controlled study documented that the nasal EPAP device effectively reduced the AHI and improved oxygenation at both week 1 and month 3 in a substantial percentage of patients with mild to severe OSA with minimal side effects. There was significant improvement in subjective sleepiness compared to the sham device group, and self-reported adherence was > 88% with device treatment. The results of the study suggest that nasal EPAP is an effective treatment alternative for a substantial percentage of OSA patients.
DISCLOSURE STATEMENT
This study was funded by Ventus Medical, Inc. Dr. Berry has received research support from Respironics, ResMed, and Ventus Medical. Dr. Kryger has received research support from Respironics, Ventus Medical, and Cephalon. Dr. Massie has received research support from Ventus Medical.
ACKNOWLEDGMENTS
Study Investigators: James Andry, Sleep Therapy and Research Center, San Antonio, Texas; Safwan Badr, Wayne State University, Detroit, Michigan; David Baratz, Pulmonary Associates (2 sites), Phoenix and Glendale, Arizona; Richard Berry, University of Florida, Gainesville, Florida; Michael Biber, Neurocare Inc., Center for Research, Newton, Massachusetts; Richard Bogan, Sleep Med of South Carolina, Columbia, South Carolina; Sean Caples, Mayo Clinic, Rochester, Minnesota; Mark Goetting, Sleep Health, Portage, Michigan; Douglas Kirsch, Sleep Health Centers, LLC, Brighton, Massachusetts; Meir Kryger, Gaylord Sleep Medicine, Wallingford, Connecticut; Clete Kushida, Stanford University, Palo Alto, California; D. Alan Lankford, Sleep Disorders Center of Georgia, Atlanta, Georgia; Clifford Massie, Chicago Sleep Group, Suburban Lung Associates, Elk Grove Village, Illinois; Mark Reploeg, The Corvallis Clinic, Corvallis, Oregon; Leon Rosenthal, Sleep Medicine Associates of Texas, Dallas, Texas; Paula Schweitzer, Sleep Medicine & Research Center, St. Luke's Hospital, Chesterfield, Missouri; Richard Simon, Kathryn Severyns Dement Sleep Disorders Center, Walla Walla, Washington; David Winslow, Kentucky Research Group, Louisville, Kentucky
ABBREVIATIONS
- AHI
Apnea hypopnea index
- AE
Adverse event
- BMI
Body mass index
- CONSORT
Consolidated standards of reporting trials
- CPAP
Continuous positive airway pressure
- EPAP
Expiratory positive airway pressure
- ESS
Epworth Sleepiness Scale
- ODI
Oxygen desaturation index
- OSA
Obstructive sleep apnea
- MedDRA
Medical Dictionary for Regulatory Activities terminology
- mITT
Modified intention to treat
Table S1.
Inclusion and exclusion criteria
| Inclusion Criteria |
| 1. Age ≥ 18 years |
| 2. Diagnosis of OSA |
| 3. AHI ≥ 10 on diagnostic PSG performed within last 3 months |
| 4. Investigator believes that patient can benefit from OSA treatment |
| 5. Patient understands and is willing and able to comply with study requirements |
| Exclusion Criteria |
| 1. Use of any device that interferes with nasal or oral breathing |
| 2. Persistent blockage of one or both nostrils which prevents airflow in one or both nostrils |
| 3. Any chronic sores or lesions on the inside or outside of the nose |
| 4. Chronic use of nasal decongestants other than nasal steroids |
| 5. Oxygen saturation < 75% for > 10% of the diagnostic PSG |
| 6. Oxygen saturation < 75% for > 25% of the first 4 hours of the diagnostic PSG |
| 7. Prior or near-miss motor vehicle accident due to sleepiness in the past 12 months |
| 8. Current use of hypnotics, anxiolytics, sedating antidepressants, anticonvulsants, sedating antihistamines, stimulants, or other medications likely to affect neurocognitive function and/or alertness |
| 9. History of allergic reaction to acrylic-based adhesives (such as those found in BAND-AIDS) |
| 10. Current acute upper respiratory (including nasal, sinus, or middle ear) inflammation or infection or perforation of the tympanic membrane* |
| 11. History of frequent and/or poorly treated severe nasal allergies or sinusitis which may interfere with the ability to use Provent |
| 12. Narcolepsy, idiopathic hypersomnolence, chronic insomnia, restless legs syndrome, REM sleep behavior disorder, or any other diagnosed or suspected sleep disorder other than OSA that could affect sleepiness scales or the likelihood of apneas/hypopneas during a PSG |
| 13. Current use of diurnal or nocturnal supplemental oxygen |
| 14. History of CPAP use in the home for the treatment of OSA. Temporary use of CPAP in a laboratory setting does not exclude the patient from participating. |
| 15. History of use of oral appliances in the home for the treatment of OSA |
| 16. History of prior surgery for OSA (e.g., somnoplasty, uvulopalatopharyngoplasty, laser-assisted uvulopalatoplasty, mandibular advancement, Pillar procedure). Patients may participate if prior surgery was limited to the nose, sinuses, and/or turbinates, etc. |
| 17. Currently working night or rotating shifts |
| 18. Consumption of > 10 caffeinated beverages per day (approximately 1000 mg per day) |
| 19. History of severe cardiovascular disease, including New York Heart Association Class III or IV heart failure, coronary artery disease with angina, or myocardial infarction in the past 6 months; stroke in the past 6 months |
| 20. History of cardiac rhythm disturbance (defined as a 5-beat run of sustained ventricular tachycardia or bradycardia if < 30 beats per min for a 10-second run or previously undiagnosed and untreated atrial fibrillation or Mobitz II or third-degree heart block) |
| 21. Uncontrolled hypertension, defined as SBP > 180 or DBP > 105 mm Hg |
| 22. Uncontrolled hypotension, defined as SBP < 80 or DBP < 55 mm Hg |
| 23. History of severe respiratory disorders (including respiratory muscle weakness, bullous lung disease, bypassed upper airway, pneumothorax, pneumomediastinum, etc.) or unstable respiratory disease (e.g., asthma or chronic obstructive pulmonary disease with exacerbation in the last 3 months) |
| 24. Any other serious, uncontrolled medical condition that may impair follow-up or put the patient at undue risk |
| 25. Females of child bearing age who are pregnant or intending to become pregnant. Proof of non-pregnancy with a urine or blood test is not required. |
| 26. Consumes on average more than 3 drinks of alcohol per day |
| 27. Chronic neurologic disorders affecting neurocognitive abilities or daily function |
| 28. Cancer, unless in remission for more than 1 year. A patient with a history of a small basal cell carcinoma (without metastasis) that was excised with wide margins may be included at the discretion of the Investigator. |
| 29. Current psychiatric illness likely to impair ability to participate in study without undue risk |
| 30. Smokers whose habit interferes with the overnight PSG |
| 31. Any known illicit drug usage |
Subject may be reconsidered for participation after the acute episode resolves.
mITT consort diagram of patient flow.
ITT consort diagram of patient flow
Table S2.
Sleep architecture and effects of supine position and REM sleep—month 3 results (mITT analysis)
| P Value | |||||
|---|---|---|---|---|---|
| EPAP |
Sham |
EPAP vs Sham (absolute change) | |||
| Device-off | Device-on | Device-off | Device-on | ||
| N = 77 |
N = 67 |
||||
| TST | 363.3 ± 65.3 | 347.4 ± 69.0** | 347.7 ± 76.9 | 346.2 ± 72.7 | 0.33 |
| Wake After Sleep Onset | 48.6 ± 30.2 | 59.6 ± 37.5** | 57.6 ± 45.5 | 56.9 ± 44.8 | 0.18 |
| Stage N1 | 18.6 ± 12.2 | 14.9 ± 9.3* | 16.2 ± 9.9 | 16.7 ± 12.7 | 0.045 |
| Stage N2 | 59.9 ± 11.2 | 61.6 ± 10.3 | 60.7 ± 10.5 | 61.8 ± 11.9 | 0.66 |
| Stage N3 | 4.3 ± 5.7 | 5.5 ± 6.8 | 5.6 ± 6.5 | 5.3 ± 5.9 | 0.16 |
| Stage REM | 17.2 ± 6.5 | 17.9 ± 6.5 | 17.4 ± 6.2 | 16.3 ± 5.7 | 0.091 |
| Arousal Index | 17.2 (10.0, 23.8) | 17.3 (11.5, 25.4) | 16.5 (10.3, 23.9) | 15.6 (12.8, 23.1) | 0.58 |
| N = 66 |
N = 55 |
||||
| AHI NREM1 | 13.8 (6.3, 20.4) | 5.3* (2.4, 14.6) | 9.9 (6.2, 21.2) | 8.8 (4.6, 23.5) | 0.16 |
| AHI REM1 | 25.3 (13.1, 51.9) | 11.7* (4.3, 31.8) | 20.9 (8.4, 44.7) | 20.2 (8.2, 45.7) | 0.033 |
| N = 39 |
N = 38 |
||||
| AHI Supine1 | 26.2 (14.9, 48.0) | 12.3* (3.7, 28.8) | 21.8 (14.3, 39.4) | 21.2 (13.5, 44.1) | 0.015 |
| AHI Non-Supine1 | 8.1 (1.9, 13.3) | 2.8 (1.2, 7.2) | 4.9 (2.2, 9.8) | 4.3 (1.7, 8.5) | 0.32 |
Device-off versus Device-on P < 0.01.
Device-off versus Device-on P < 0.05.
Patients with > 20 minutes in each position and state.
Table S3.
Patient drop-out reasons (ITT group at month 3)
| EPAP | Sham | |
|---|---|---|
| N = 19/119 (16.0%) | N = 15/110 (13.6%) | |
| Lost to follow-up | 2 | 5 |
| Device-related adverse events | ||
| Dry mouth/throat | 3 | |
| Breathing discomfort | 2 | |
| Nasal itching | 1 | |
| Sleep maintenance insomnia | 1 | |
| Vertigo | 1 | |
| Oxygen desaturation | 1 | |
| Adverse events not device related | ||
| Tonsil infection | 1 | |
| Transient ischemic attack | 1 | |
| Severe degenerative disc disease and neuropathic pain | 1 | |
| Unable to acclimate to device | 1 | 1 |
| Patient non-compliant with protocol | ||
| Due to study assessment requirements | 2 | |
| Unwilling to do last 2 PSGs | 1 | |
| Missed month 3 office visit | 1 | 1 |
| Patient using CPAP | 1 | |
| Site protocol deviation | ||
| Violation of study entry criteria | 2 | |
| Patient prefers alternative treatment | 2 | |
| Other | ||
| Patient not allowed on hospital property | 1 | |
| Patient personal emergency | 1 | |
| Site withdrawal from study (staff lay-offs) | 1 |
EPAP vs sham dropout rate P = 0.62.
Table S4.
Device-related adverse event summary (ITT analysis)
| EPAP | Sham | ||
|---|---|---|---|
| N = 119 | N = 110 | P value | |
| Patients reporting device-related AEs | 53 (44.5%) | 37 (33.6%) | 0.36 |
| # Device-related AEs reported | 106 | 63 | |
| # Serious device-related AEs reported | 0 (0.0%) | 0 (0.0%) |
Table S5.
Device-related adverse events*—number of patients reporting AEs (ITT analysis) (Patients may report more than 1 AE)
| EPAP | Sham | |
|---|---|---|
| N = 119 | N = 110 | |
| Respiratory, thoracic and mediastinal disorders | 25 (21.0%) | 22 (20.0%) |
| Nasal congestion | 10 (8.4%) | 5 (4.5%) |
| Nasal discomfort | 9 (7.6%) | 13 (11.8%) |
| Cough | 2 (1.7%) | 0 (0.0%) |
| Nasal dryness | 1 (0.8%) | 0 (0.0%) |
| Oropharyngeal pain | 1 (0.8%) | 0 (0.0%) |
| Rhinorrhea (discharge of nasal mucus) | 1 (0.8%) | 1 (0.9%) |
| Sinusitis | 1 (0.8%) | 1 (0.9%) |
| Snoring | 1 (0.8%) | 0 (0.0%) |
| Suffocation feeling | 1 (0.8%) | 0 (0.0%) |
| Dyspnea (shortness of breath) | 0 (0.0%) | 2 (1.8%) |
| Epistaxis (nosebleed) | 0 (0.0%) | 2 (1.8%) |
| Productive cough | 0 (0.0%) | 1 (0.9%) |
| Sinus congestion | 0 (0.0%) | 1 (0.9%) |
| Upper respiratory infection | 0 (0.0%) | 1 (0.9%) |
| Nervous system disorders | 16 (13.4%) | 10 (9.1%) |
| Insomnia | 5 (4.2%) | 1 (0.9%) |
| Headache | 5 (4.2%) | 5 (4.5%) |
| Initial insomnia | 4 (3.4%) | 2 (1.8%) |
| Poor quality sleep | 2 (1.7%) | 0 (0.0%) |
| Drooling | 2 (1.7%) | 0 (0.0%) |
| Somnolence (sleepiness) | 1 (0.8%) | 3 (2.7%) |
| Vertigo | 1 (0.8%) | 0 (0.0%) |
| Abnormal dreams | 0 (0.0%) | 2 (1.8%) |
| Dizziness | 0 (0.0%) | 1 (0.9%) |
| Gastrointestinal disorders | 18 (15.1%) | 3 (2.7%) |
| Dry mouth | 13 (10.9%) | 3 (2.7%) |
| Dry throat | 4 (3.4%) | 0 (0.0%) |
| Chapped lips | 1 (0.8%) | 0 (0.0%) |
| Dysgeusia (dysfunction of sense of taste) | 1 (0.8%) | 0 (0.0%) |
| Dry lips | 1 (0.8%) | 0 (0.0%) |
| Injury, poisoning, and procedural complications | 18 (15.1%) | 7 (6.4%) |
| Device interaction (exhalation difficulty, discomfort with device) | 17 (14.3%) | 4 (3.6%) |
| Medical device site reaction (moisture in nose behind device) | 2 (1.7%) | 0 (0.0%) |
| Instillation site pain (nostril soreness) | 0 (0.0%) | 3 (2.7%) |
| Psychiatric disorders | 2 (1.7%) | 3 (2.7%) |
| Anxiety | 2 (1.7%) | 3 (2.7%) |
| Eye disorders | 1 (0.8%) | 0 (0.0%) |
| Lacrimation increased (tear production) | 1 (0.8%) | 0 (0.0%) |
| Investigations | 1 (0.8%) | 1 (0.9%) |
| Blood pressure increased | 1 (0.8%) | 0 (0.0%) |
| Oxygen saturation decreased | 0 (0.0%) | 1 (0.9%) |
| Skin and subcutaneous tissue disorders | 1 (0.8%) | 0 (0.0%) |
| Dermatitis | 1 (0.8%) | 0 (0.0%) |
| Vascular disorders | 1 (0.8%) | 0 (0.0%) |
| Worsening hypertension | 1 (0.8%) | 0 (0.0%) |
| Cardiac disorders | 0 (0.0%) | 1 (0.9%) |
| Worsening of coronary artery disease | 0 (0.0%) | 1 (0.9%) |
Device-related adverse events were categorized using MedDRA coding. MedDRA, the Medical Dictionary for Regulatory Activities terminology, is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA).
REFERENCES
- 1.Young T, Palta M, Dempsey J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Epstein LJ, Kristo D, Strollo P, et al. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Augusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomized prospective parallel trial. Lancet. 1999;353:2100–5. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 5.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 6.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–72. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 7.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 8.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;15:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008;15:365–9. doi: 10.1155/2008/534372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulgrew AT, Lawati NA, Ayas NT, et al. Residual sleep apnea on polysomnography after 3 months of CPAP therapy: clinical implications, predictors and patterns. Sleep Med. 2010;11:119–25. doi: 10.1016/j.sleep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep-related breathing disorder. Eur Respir J. 2000;16:921–7. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 12.Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11:1–22. doi: 10.1007/s11325-006-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 14.Elshaug AG, Moss JR, Southcott AM, Hiller JE. Redefining success in airway surgery for obstructive sleep apnea: a meta-analysis and synthesis of the evidence. Sleep. 2007;30:461–7. doi: 10.1093/sleep/30.4.461. [DOI] [PubMed] [Google Scholar]
- 15.Mahadevia AK, Onal E, Lopata M. Effects of expiratory positive airway pressure on sleep-induced respiratory abnormalities in patients with hypersomnia-sleep apnea syndrome. Am Rev Respir Dis. 1983;128:708–11. doi: 10.1164/arrd.1983.128.4.708. [DOI] [PubMed] [Google Scholar]
- 16.Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2008;4:426–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med. 2009;5:532–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Inclusion and exclusion criteria
| Inclusion Criteria |
| 1. Age ≥ 18 years |
| 2. Diagnosis of OSA |
| 3. AHI ≥ 10 on diagnostic PSG performed within last 3 months |
| 4. Investigator believes that patient can benefit from OSA treatment |
| 5. Patient understands and is willing and able to comply with study requirements |
| Exclusion Criteria |
| 1. Use of any device that interferes with nasal or oral breathing |
| 2. Persistent blockage of one or both nostrils which prevents airflow in one or both nostrils |
| 3. Any chronic sores or lesions on the inside or outside of the nose |
| 4. Chronic use of nasal decongestants other than nasal steroids |
| 5. Oxygen saturation < 75% for > 10% of the diagnostic PSG |
| 6. Oxygen saturation < 75% for > 25% of the first 4 hours of the diagnostic PSG |
| 7. Prior or near-miss motor vehicle accident due to sleepiness in the past 12 months |
| 8. Current use of hypnotics, anxiolytics, sedating antidepressants, anticonvulsants, sedating antihistamines, stimulants, or other medications likely to affect neurocognitive function and/or alertness |
| 9. History of allergic reaction to acrylic-based adhesives (such as those found in BAND-AIDS) |
| 10. Current acute upper respiratory (including nasal, sinus, or middle ear) inflammation or infection or perforation of the tympanic membrane* |
| 11. History of frequent and/or poorly treated severe nasal allergies or sinusitis which may interfere with the ability to use Provent |
| 12. Narcolepsy, idiopathic hypersomnolence, chronic insomnia, restless legs syndrome, REM sleep behavior disorder, or any other diagnosed or suspected sleep disorder other than OSA that could affect sleepiness scales or the likelihood of apneas/hypopneas during a PSG |
| 13. Current use of diurnal or nocturnal supplemental oxygen |
| 14. History of CPAP use in the home for the treatment of OSA. Temporary use of CPAP in a laboratory setting does not exclude the patient from participating. |
| 15. History of use of oral appliances in the home for the treatment of OSA |
| 16. History of prior surgery for OSA (e.g., somnoplasty, uvulopalatopharyngoplasty, laser-assisted uvulopalatoplasty, mandibular advancement, Pillar procedure). Patients may participate if prior surgery was limited to the nose, sinuses, and/or turbinates, etc. |
| 17. Currently working night or rotating shifts |
| 18. Consumption of > 10 caffeinated beverages per day (approximately 1000 mg per day) |
| 19. History of severe cardiovascular disease, including New York Heart Association Class III or IV heart failure, coronary artery disease with angina, or myocardial infarction in the past 6 months; stroke in the past 6 months |
| 20. History of cardiac rhythm disturbance (defined as a 5-beat run of sustained ventricular tachycardia or bradycardia if < 30 beats per min for a 10-second run or previously undiagnosed and untreated atrial fibrillation or Mobitz II or third-degree heart block) |
| 21. Uncontrolled hypertension, defined as SBP > 180 or DBP > 105 mm Hg |
| 22. Uncontrolled hypotension, defined as SBP < 80 or DBP < 55 mm Hg |
| 23. History of severe respiratory disorders (including respiratory muscle weakness, bullous lung disease, bypassed upper airway, pneumothorax, pneumomediastinum, etc.) or unstable respiratory disease (e.g., asthma or chronic obstructive pulmonary disease with exacerbation in the last 3 months) |
| 24. Any other serious, uncontrolled medical condition that may impair follow-up or put the patient at undue risk |
| 25. Females of child bearing age who are pregnant or intending to become pregnant. Proof of non-pregnancy with a urine or blood test is not required. |
| 26. Consumes on average more than 3 drinks of alcohol per day |
| 27. Chronic neurologic disorders affecting neurocognitive abilities or daily function |
| 28. Cancer, unless in remission for more than 1 year. A patient with a history of a small basal cell carcinoma (without metastasis) that was excised with wide margins may be included at the discretion of the Investigator. |
| 29. Current psychiatric illness likely to impair ability to participate in study without undue risk |
| 30. Smokers whose habit interferes with the overnight PSG |
| 31. Any known illicit drug usage |
Subject may be reconsidered for participation after the acute episode resolves.
mITT consort diagram of patient flow.
ITT consort diagram of patient flow
Table S2.
Sleep architecture and effects of supine position and REM sleep—month 3 results (mITT analysis)
| P Value | |||||
|---|---|---|---|---|---|
| EPAP |
Sham |
EPAP vs Sham (absolute change) | |||
| Device-off | Device-on | Device-off | Device-on | ||
| N = 77 |
N = 67 |
||||
| TST | 363.3 ± 65.3 | 347.4 ± 69.0** | 347.7 ± 76.9 | 346.2 ± 72.7 | 0.33 |
| Wake After Sleep Onset | 48.6 ± 30.2 | 59.6 ± 37.5** | 57.6 ± 45.5 | 56.9 ± 44.8 | 0.18 |
| Stage N1 | 18.6 ± 12.2 | 14.9 ± 9.3* | 16.2 ± 9.9 | 16.7 ± 12.7 | 0.045 |
| Stage N2 | 59.9 ± 11.2 | 61.6 ± 10.3 | 60.7 ± 10.5 | 61.8 ± 11.9 | 0.66 |
| Stage N3 | 4.3 ± 5.7 | 5.5 ± 6.8 | 5.6 ± 6.5 | 5.3 ± 5.9 | 0.16 |
| Stage REM | 17.2 ± 6.5 | 17.9 ± 6.5 | 17.4 ± 6.2 | 16.3 ± 5.7 | 0.091 |
| Arousal Index | 17.2 (10.0, 23.8) | 17.3 (11.5, 25.4) | 16.5 (10.3, 23.9) | 15.6 (12.8, 23.1) | 0.58 |
| N = 66 |
N = 55 |
||||
| AHI NREM1 | 13.8 (6.3, 20.4) | 5.3* (2.4, 14.6) | 9.9 (6.2, 21.2) | 8.8 (4.6, 23.5) | 0.16 |
| AHI REM1 | 25.3 (13.1, 51.9) | 11.7* (4.3, 31.8) | 20.9 (8.4, 44.7) | 20.2 (8.2, 45.7) | 0.033 |
| N = 39 |
N = 38 |
||||
| AHI Supine1 | 26.2 (14.9, 48.0) | 12.3* (3.7, 28.8) | 21.8 (14.3, 39.4) | 21.2 (13.5, 44.1) | 0.015 |
| AHI Non-Supine1 | 8.1 (1.9, 13.3) | 2.8 (1.2, 7.2) | 4.9 (2.2, 9.8) | 4.3 (1.7, 8.5) | 0.32 |
Device-off versus Device-on P < 0.01.
Device-off versus Device-on P < 0.05.
Patients with > 20 minutes in each position and state.
Table S3.
Patient drop-out reasons (ITT group at month 3)
| EPAP | Sham | |
|---|---|---|
| N = 19/119 (16.0%) | N = 15/110 (13.6%) | |
| Lost to follow-up | 2 | 5 |
| Device-related adverse events | ||
| Dry mouth/throat | 3 | |
| Breathing discomfort | 2 | |
| Nasal itching | 1 | |
| Sleep maintenance insomnia | 1 | |
| Vertigo | 1 | |
| Oxygen desaturation | 1 | |
| Adverse events not device related | ||
| Tonsil infection | 1 | |
| Transient ischemic attack | 1 | |
| Severe degenerative disc disease and neuropathic pain | 1 | |
| Unable to acclimate to device | 1 | 1 |
| Patient non-compliant with protocol | ||
| Due to study assessment requirements | 2 | |
| Unwilling to do last 2 PSGs | 1 | |
| Missed month 3 office visit | 1 | 1 |
| Patient using CPAP | 1 | |
| Site protocol deviation | ||
| Violation of study entry criteria | 2 | |
| Patient prefers alternative treatment | 2 | |
| Other | ||
| Patient not allowed on hospital property | 1 | |
| Patient personal emergency | 1 | |
| Site withdrawal from study (staff lay-offs) | 1 |
EPAP vs sham dropout rate P = 0.62.
Table S4.
Device-related adverse event summary (ITT analysis)
| EPAP | Sham | ||
|---|---|---|---|
| N = 119 | N = 110 | P value | |
| Patients reporting device-related AEs | 53 (44.5%) | 37 (33.6%) | 0.36 |
| # Device-related AEs reported | 106 | 63 | |
| # Serious device-related AEs reported | 0 (0.0%) | 0 (0.0%) |
Table S5.
Device-related adverse events*—number of patients reporting AEs (ITT analysis) (Patients may report more than 1 AE)
| EPAP | Sham | |
|---|---|---|
| N = 119 | N = 110 | |
| Respiratory, thoracic and mediastinal disorders | 25 (21.0%) | 22 (20.0%) |
| Nasal congestion | 10 (8.4%) | 5 (4.5%) |
| Nasal discomfort | 9 (7.6%) | 13 (11.8%) |
| Cough | 2 (1.7%) | 0 (0.0%) |
| Nasal dryness | 1 (0.8%) | 0 (0.0%) |
| Oropharyngeal pain | 1 (0.8%) | 0 (0.0%) |
| Rhinorrhea (discharge of nasal mucus) | 1 (0.8%) | 1 (0.9%) |
| Sinusitis | 1 (0.8%) | 1 (0.9%) |
| Snoring | 1 (0.8%) | 0 (0.0%) |
| Suffocation feeling | 1 (0.8%) | 0 (0.0%) |
| Dyspnea (shortness of breath) | 0 (0.0%) | 2 (1.8%) |
| Epistaxis (nosebleed) | 0 (0.0%) | 2 (1.8%) |
| Productive cough | 0 (0.0%) | 1 (0.9%) |
| Sinus congestion | 0 (0.0%) | 1 (0.9%) |
| Upper respiratory infection | 0 (0.0%) | 1 (0.9%) |
| Nervous system disorders | 16 (13.4%) | 10 (9.1%) |
| Insomnia | 5 (4.2%) | 1 (0.9%) |
| Headache | 5 (4.2%) | 5 (4.5%) |
| Initial insomnia | 4 (3.4%) | 2 (1.8%) |
| Poor quality sleep | 2 (1.7%) | 0 (0.0%) |
| Drooling | 2 (1.7%) | 0 (0.0%) |
| Somnolence (sleepiness) | 1 (0.8%) | 3 (2.7%) |
| Vertigo | 1 (0.8%) | 0 (0.0%) |
| Abnormal dreams | 0 (0.0%) | 2 (1.8%) |
| Dizziness | 0 (0.0%) | 1 (0.9%) |
| Gastrointestinal disorders | 18 (15.1%) | 3 (2.7%) |
| Dry mouth | 13 (10.9%) | 3 (2.7%) |
| Dry throat | 4 (3.4%) | 0 (0.0%) |
| Chapped lips | 1 (0.8%) | 0 (0.0%) |
| Dysgeusia (dysfunction of sense of taste) | 1 (0.8%) | 0 (0.0%) |
| Dry lips | 1 (0.8%) | 0 (0.0%) |
| Injury, poisoning, and procedural complications | 18 (15.1%) | 7 (6.4%) |
| Device interaction (exhalation difficulty, discomfort with device) | 17 (14.3%) | 4 (3.6%) |
| Medical device site reaction (moisture in nose behind device) | 2 (1.7%) | 0 (0.0%) |
| Instillation site pain (nostril soreness) | 0 (0.0%) | 3 (2.7%) |
| Psychiatric disorders | 2 (1.7%) | 3 (2.7%) |
| Anxiety | 2 (1.7%) | 3 (2.7%) |
| Eye disorders | 1 (0.8%) | 0 (0.0%) |
| Lacrimation increased (tear production) | 1 (0.8%) | 0 (0.0%) |
| Investigations | 1 (0.8%) | 1 (0.9%) |
| Blood pressure increased | 1 (0.8%) | 0 (0.0%) |
| Oxygen saturation decreased | 0 (0.0%) | 1 (0.9%) |
| Skin and subcutaneous tissue disorders | 1 (0.8%) | 0 (0.0%) |
| Dermatitis | 1 (0.8%) | 0 (0.0%) |
| Vascular disorders | 1 (0.8%) | 0 (0.0%) |
| Worsening hypertension | 1 (0.8%) | 0 (0.0%) |
| Cardiac disorders | 0 (0.0%) | 1 (0.9%) |
| Worsening of coronary artery disease | 0 (0.0%) | 1 (0.9%) |
Device-related adverse events were categorized using MedDRA coding. MedDRA, the Medical Dictionary for Regulatory Activities terminology, is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA).