Abstract
Study Objective:
Studies in adults and children have shown that African American race is a risk factor for the obstructive sleep apnea syndrome (OSAS). Therefore, we hypothesized that non-obese, non-snoring African American children would have a more collapsible upper airway during sleep than age-, gender-, and size-matched Caucasians.
Design:
Upper airway dynamic function was measured during sleep in normal African American and Caucasian children.
Setting:
Sleep laboratory.
Patients or Participants:
56 normal children between the ages of 8-18 years.
Interventions:
Pressure-flow relationships were measured during NREM sleep. Nasal pressure was decreased to subatmospheric levels, using previously described techniques that resulted in an activated and relatively hypotonic upper airway.
Measurements and Results:
The activated and hypotonic critical pressures (Pcrit) were -25 (-25, -3) (median, range) and -19 (-25, -3) for African Americans, and -25 (-25, -4) and -25 (-25.0, -4) cm H2O, respectively, for Caucasians. The slopes of the pressure-flow response (SPF) under activated and hypotonic conditions for African Americans were 10 (-9, 46) and 13 (-20, 46), and for Caucasians 9 (-9, 64) and 8 (-5, 54) mL/s/cm H2O, respectively. There were no significant differences between groups for Pcrit or SPF under either activated or hypotonic conditions.
Conclusion:
Upper airway collapsibility was similar in asymptomatic, non-obese African American and Caucasian children. Differences in upper airway characteristics and neuromotor function cannot explain the increased prevalence of OSAS in African American children.
Citation:
Pinto S; Huang J; Tapia I; Karamessinis L; Pepe M; Gallagher PR; Bradford R; Nixon T; Lee NY; Marcus CL. Effects of race on upper airway dynamic function during sleep in children. SLEEP 2011;34(4):495-501.
Keywords: Pcrit, obstructive sleep apnea, children, African American, Caucasian
INTRODUCTION
The obstructive sleep apnea syndrome (OSAS) occurs in approximately 2% to 3% of children1 and is a common cause of morbidities such as failure to thrive, neurocognitive abnormalities,2 cor pulmonale, and systemic hypertension.3 Several risk factors are known to be associated with OSAS, such as obesity, race, age, and gender.1
Epidemiological data collected in adults and children have shown that race is an independent risk factor for OSAS, with African Americans being more likely to have OSAS compared to Caucasians.4–7 Studies done in a large sample of 399 children found that African American children were 3.5 times more likely to have OSAS than their Caucasian peers,7 and that this increased prevalence of OSAS remained when data were adjusted for obesity and asthma.
The reason for this propensity for OSAS in African Americans is unknown. It may be due to different upper airway anatomy or neuromotor control, genetic factors, and environmental or cultural factors. OSAS appears to be the result of a combination of anatomic and neuromotor abnormalities.8 In children, anatomic factors resulting in a narrower upper airway, such as adenotonsillar hypertrophy, obesity, and craniofacial anomalies, partly explain the pathophysiology of OSAS.9 However, not all children with enlarged tonsils and adenoids develop OSAS and children with OSAS do not obstruct while awake. In addition, OSAS persists in a proportion of children after anatomic factors are corrected, e.g., following adenotonsillectomy.10,11 These facts indicate the importance of the neuromotor component in maintaining upper airway patency.
Most studies evaluating the epidemiology and pathophysiology of racial differences in OSAS have relied on polysomnograms, questionnaires or radiological evaluations, and have focused on anatomic factors. However, to our knowledge there are no studies evaluating upper airway dynamic responses during sleep in different racial groups in order to determine any potential physiological differences behind the different prevalence of OSAS in African American versus Caucasian children.
Upper airway dynamic studies during sleep have shown that normal children have an upper airway that is resistant to collapse, and that upper airway collapsibility increases with age.12 The understanding of normal upper airway dynamics has led to extensive research in pediatric and adult OSAS.12–17 We hypothesized that non-obese, non-snoring African American children would have different upper airway dynamic function andq a more collapsible upper airway during sleep compared to age and gender-matched Caucasians.
METHODS
A retrospective analysis of data from previous research studies was performed. All subjects had been enrolled as controls and had been studied by the senior author (CLM) using the same techniques.14 Following screening to ensure no symptoms of OSAS or other medical conditions, subjects underwent a baseline polysomnogram to ensure normalcy. They then underwent a repeat sleep study on a separate night during which upper airway dynamic function was assessed using activated and hypotonic techniques.
Study Group
Normal, healthy subjects aged 5 to 18 years were recruited from the general community by means of advertisements. The lower age limit was chosen to exclude children too young to cooperate with wearing a nasal mask. Subjects with a history of nightly snoring, upper airway surgery, lower respiratory tract disease other than mild asthma, obesity (body mass index [BMI] > 95th percentile for age, race, and sex)18 or with medical conditions requiring daily medications were excluded. Subjects with an apnea hypopnea index > 1.5/h on baseline polysomnography were excluded. Caucasian and African American subjects were matched for age, gender and BMI. The Children's Hospital of Phila delphia Institutional Review Board for human studies approved the protocol. Informed consent was obtained from 18-year-old subjects and parents or guardians of subjects younger than 18, and assent from those subjects younger than 18 years.
Baseline Polysomnography
The following parameters were recorded on a Rembrandt polysomnography system (Embla, Broomfield, CO): electroencephalogram (EEG)(C3/M2, C4/M1, O1/M2, O2/M1), left and right electrooculograms, submental electromyogram (EMG), tibial EMG, electrocardiogram, oro nasal airflow with a 3-pronged thermistor (Pro-Tech Services, Inc., Mukilteo, WA), nasal pressure with a pressure transducer (Pro-Tech Services, Inc.), rib cage and abdominal wall motion using respiratory inductance plethysmography (Viasys Health care, Yorba Linda, CA), end-tidal PCO2 (Novametrix Medical Systems, Inc., Wall-ingford, CT) and arterial oxygen saturation (SpO2) with pulse waveform (Masimo, Irvine, CA). Subjects were also recorded on digital video. Studies were scored using standard pediatric criteria.19
Upper Airway Dynamic Measurements During Sleep
Routine polysomnographic parameters were measured as described previously. In addition, the subject wore a continuous positive airway pressure mask (CPAP)(Respironics, Murrysville, PA) attached to a heated pneumotachometer (Hans Rudolph, Inc., Kansas City, MO) and transducer (Validyne Engineering Corp., Northridge, CA). Nasal pressure (PN) was measured at the mask, using a differential pressure transducer referenced to atmosphere. Selected signals, including PN and flow, were acquired on a Power Lab system (ADInstruments,Colorado Springs, CO) and simultaneously displayed on Rembrandt. PN was altered in either a positive or subatmospheric direction, using a device provided by Respironics. A toggle switch allowed the patient to be switched rapidly between positive and negative pressure, ranging from –25 to +25 cm H2O. Measurements were performed during non-REM sleep, as subjects were least likely to arouse during that stage.20
Activated technique
Subjects slept while receiving a level of CPAP sufficient to abolish inspiratory airflow limitation. Inspiratory airflow limitation was considered to occur when airflow failed to increase despite increasing respiratory effort, as demonstrated by the characteristic flow waveform consisting of increasing inspira-tory flow followed by a mid-inspiratory plateau.21 Once inspiratory flow limitation occurred, PN was lowered in 2 cm H2O step-like decrements every 5 breaths until flow approached zero or an arousal occurred. This slow, stepwise protocol allowed for recruitment of upper airway reflexes, and resulted in a neuromuscularly activated airway.12,22
Hypotonic technique
Subjects slept while receiving a level of CPAP sufficient to abolish inspiratory airflow limitation (the holding pressure). PNwas decreased abruptly by 2 cm H2O for 5 breaths, following which it was rapidly returned to the holding pressure. PN was dropped repeatedly to incrementally lower levels, with a return each time to the holding pressure, until either flow approached zero or arousal occurred. Previous studies have shown that it takes several breaths at subatmospheric pressure before the upper airway reflexes are activated12,23,24; thus analyzing only the first 3 breaths provides data on a relatively hypotonic upper airway.
Data Analysis
For the activated runs, the average mid-inspiratory flow was taken from the lowest two consecutive breaths at each level of pressure. For hypotonic runs, data were taken from the first 3 breaths after the pressure drop. Pressure-flow curves were constructed by plotting maximal inspiratory airflow (VImax) against PN. PN vs VImax curves were fitted by least squares linear regression, and the X-axis intercept (Pcrit; VImax = 0) was determined. As many pediatric subjects are able to maintain airflow even at markedly subatmospheric pressures, the X-intercept cannot always be determined without extreme extrapolation. Therefore, as in previous studies, we arbitrarily assigned a threshold value of -25 cm H2O (the lowest PN deliverable by our equipment) to Pcrit data that were extrapolated to < -25 cm H2O.12,17 The slope of the pressure-flow curve (SPF) was also used to characterize the upper airway response.12,14
Statistical Analysis
Histograms and Kolmogorov-Smirnov tests of normal distribution were examined. Depending on normal versus skewed distribution of the data, either t-tests for independent samples or Mann-Whitney tests were used to examine differences in parameters between the 2 groups. Hodges-Lehmann estimates of population median differences and 95% confidence intervals (CIs) for the differences between population medians25 were calculated. Wilcoxon matched-pairs signed-rank tests were used to compare activated versus hypotonic versions of Pcrit and SPF.
An observed effect size index was calculated based on a generalization of the Mann-Whitney U statistic. This effect size measure θ1 = P (Y > X) has been labeled a probabilistic index that provides an intuitive and simple effect size estimate for nonparametric outcomes.26 The formula is [2(U/mn)-1], where U/mn is the Mann-Whitney U statistic divided by the product of the 2 sample sizes.27 The resulting probabilistic index is an indication of the probability of observing a higher outcome value for a randomly chosen African American subject relative to a randomly chosen Caucasian subject.
RESULTS
Fifty-six subjects were studied: 28 were African American and 28 were Caucasian. Demographic and polysomnographic data are shown in Table 1. Typical examples of the dynamic upper airway responses are shown in Figures 1 and 2. The Pcrit and SPF parameters of the groups are shown in Table 2 and Figures 3 and 4. Overall, the activated Pcrit was more subatmospheric than the hypotonic Pcrit (P = 0.004) and the activated slope of the pressure-flow curve had a strong tendency to be flatter than the hypotonic slope (P = 0.055). No statistically significant differences were found between the race groups for any parameter. In addition, we observed that for all the Pcrit and SPF outcomes, the 95% confidence intervals of the median differences between the groups included zero, indicating again that there was no statistical significant differences between the race groups.
Table 1.
Demographic and polysomnographic data
| African American | Caucasian | |
|---|---|---|
| N | 28 | 28 |
| Age (yrs) | 13 ± 3 | 13 ± 3 |
| Females (N, %) | 16 (57%) | 16 (57%) |
| BMI (kg/m2) | 20.7 ± 2.9 | 21.3 ± 2.9 |
| AHI (N/h) | 0.4 ± 0.6 | 0.3 ± 0.5 |
BMI, body mass index; AHI, apnea hypopnea index.
Data shown as mean ± SD. There were no statistical differences between groups.
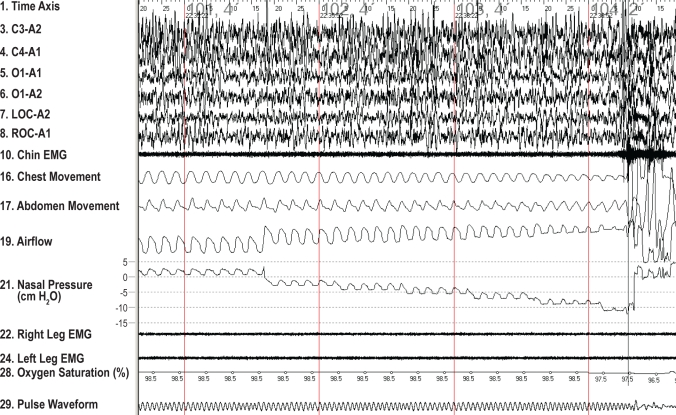
Figure 1.
Typical example of the activated pressure-flow relationship in a 16-year-old Caucasian male. The subject aroused at a nasal pressure of -10 cm H2O.
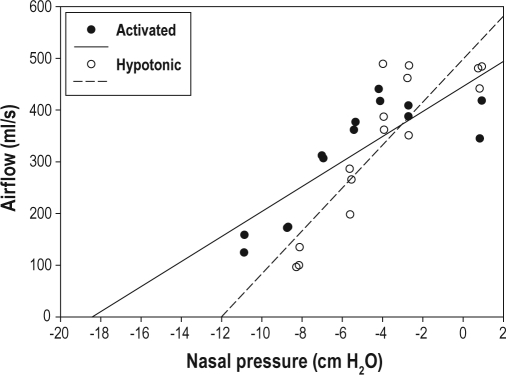
Figure 2.
Typical example of the pressure-flow relationship using both activated (solid circles) and hypotonic (hollow circles) techniques, obtained from the same subject as Figure 1. The hypotonic technique resulted in a more collapsible upper airway, with a steeper slope and a higher critical closing pressure.
Table 2.
Dynamic upper airway responses
| Outcome | African-American Median (range) | Caucasian Median (range) | M-W P-value | Probabilistic Index* | Observed Median Difference | Population Median Difference | 95% CI of the Population Difference |
|---|---|---|---|---|---|---|---|
| SPF (activated) | 9.6 (-8.8, 46.1) | 9.0 (-8.6, 64.3) | 1.00 | 0.00 | 0.6 | 0.0 | -5.0, 5.1 |
| Pcrit (activated) | -25.0 (-25.0, -2.8) | -25.0 (-25.0, -3.8) | 0.89 | 0.02 | 0.0 | 0.0 | 0.0, 1.1 |
| SPF (hypotonic) | 12.8 (-20.4, 46.4) | 8.1 (-5.0, 53.8) | 0.39 | 0.14 | 4.7 | 3.4 | -5.4, 9.5 |
| Pcrit (hypotonic) | -18.8 (-25.0, -2.8) | -24.8 (-25.0, -3.6) | 0.41 | 0.13 | 6.0 | 0.0 | -0.5, 5.5 |
SPF, slope of the pressure-flow response (ml/s/cm H2O); Pcrit, critical closing pressure (cm H2O); M-W, Mann-Whitney P-value; CI, 95% confidence interval.
Probabilistic Index = [2(U/mn) -1], where U/mn is the Mann-Whitney Ustatistic divided by the product of the 2 sample sizes
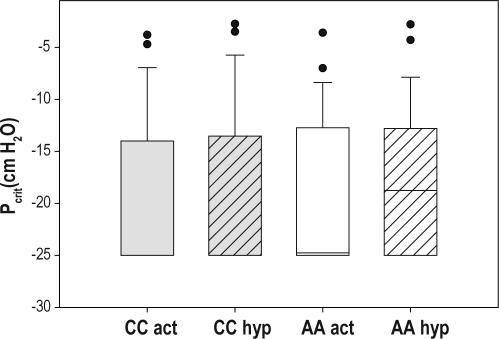
Figure 3.
Critical closing pressure under activated and hypotonic conditions. A boxplot of the critical closing pressures (Pcrit) under activated and hypotonic conditions for the different races is shown. The box represents the interquartile range that contains 50% of the val ues. The line across the box indicates the median. The whiskers extend from the box to the highest and lowest values, excluding outliers (O), which are defined as cases with values between 1.5 and 3 box lengths from either end of the box. No significant differences were observed between African Americans and Caucasians. CC, Caucasian; AA, African American; act, activated technique; hyp, hypotonic technique.
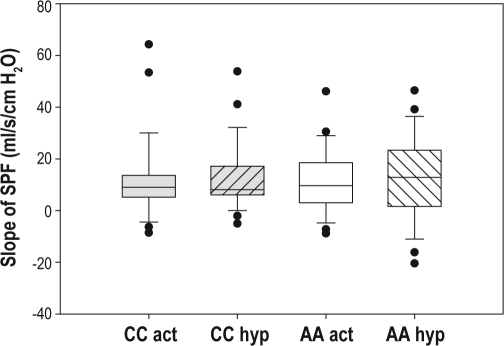
Figure 4.
Slope of the pressure-flow response under activated and hypotonic conditions. A boxplot of the slopes of the pressure-flow response (SPF) under activated and hypotonic conditions for the different races is shown. The box represents the interquartile range that contains 50% of the values. The line across the box indicates the median. The whiskers extend from the box to the highest and lowest values, excluding outliers (O), which are defined as cases with values between 1.5 and 3 box lengths from either end of the box. No significant differences were observed between African Americans and Caucasians. CC, Caucasian; AA, African American; act, activated technique; hyp, hypotonic technique.
The failure to reject the null hypothesis of no differences inevitably raises questions regarding adequate power and sample size, and might also invoke the distinction between statistical versus clinical significance. A sample size of 28 in each group had 80% power to detect a probability of 0.72 or higher that an observation in the Caucasian group was less than an observation in the African American group using the Mann-Whitney rank sum test with a 0.05 two-sided significance level. Probabilistic indices also indicated a low probability of observing higher Pcrit or SPF values for any randomly chosen African American subject relative to any randomly chosen Caucasian subject.
DISCUSSION
This study has shown that upper airway collapsibility and upper airway dynamic function during sleep is similar in non-obese, non-snoring African American and Caucasian children after controlling for gender, age, and size. This indicates that African American and Caucasian children do not have different upper airway dynamic responses to subatmospheric pressure challenges, and that the increased prevalence of OSAS in African American children is not related to upper airway physiological differences.
Prevalence of OSAS in African Americans and Caucasians
Several studies have shown an increased prevalence of OSAS in African Americans. Studies done in children by Red-line and colleagues found that African American children were 3.5 as more likely to have OSAS as Caucasian children.1 This study included children from 2-18 years of age and related family members. In a following study, Rosen et al. reported increased susceptibility of African American children to OSAS.28 This study only included children from 8-11 years of age, and children who were recruited were not family members, thus eliminating the confounding factors of familial similarities and pubertal changes in upper airway collapsibility.29 Studies in adults have also reported increased OSAS in African Americans compared to Caucasians.30,31 In one study, the increased prevalence of OSAS in African Americans was noted predominantly in younger subjects < 25 years of age.31 However, most studies of racial differences in the prevalence of OSAS have relied on polysomnograms or questionnaires. There may be sub-clinical differences, especially in young, non-obese subjects, that may be detected by physiological measurements, even in the presence of a normal polysomnogram. These physiological differences may help explain the clinical differences seen in a portion of the population.
Measurement of Upper Airway Dynamic Responses During Sleep
Measurement of upper airway dynamic function during sleep has proved to be a useful tool in understanding upper airway physiology and the propensity for obstructive apnea, even when this tool is applied to asymptomatic populations. For example, it has been shown that normal, non-obese and non-snoring children have a less collapsible upper airway than asymptomatic adults, and that upper airway collapsibility increases during puberty, even in subjects without any obstructive apneas.12,14,29 The finding of subclinical abnormalities in upper airway function during sleep would suggest that subjects are at increased risk for developing OSAS in the future if they acquired additional risk factors such as increasing age, obesity, or alcohol ingestion.
The Starling resistor model of the upper airway describes the major determinants of airflow in terms of the mechanical properties of collapsible tubes.13 According to this model, under conditions of flow limitation, maximal inspiratory flow is determined by the pressure changes upstream (nasal) to a collapsible locus of the upper airway and is independent of the downstream (tracheal) pressure generated by the diaphragm. The critical closing pressure (Pcrit) occurs at the X-intercept of the pressure-flow response curve, i.e., the pressure at which there is zero flow due to upper airway closure. In normal children, however, Pcrit is often undeterminable due to the flat slope of the pressure-flow curve. The slope of the pressure-flow curve represents the conductance of the upstream pressure-flow relationship, and is used to characterize upper airway function in children.12,14,29 The original Starling resistor model did not take into account the fact that the upper airway is affected not only by mechanical forces, but also by activation of the upper airway muscles. Positive nasal pressure suppresses upper airway tone,32,33 whereas subatmospheric nasal pressure results in increased upper airway tone.22 Thus, sequentially lowering PN to subatmospheric levels results in an activated upper airway. However, in children the effect of subatmospheric pressure takes approximately 3 breaths before reflexes are activated.12,23,24 Therefore, maintaining positive nasal pressure and then acutely dropping to negative pressure for 1-3 breaths results in a relatively hypotonic airway. This hypotonic technique primarily tests upper airway structure, whereas the activated technique tests the combination of upper airway structure and neuromotor function.12,14,17,23
Past studies have shown that children and adults with OSAS have positive or slightly subatmospheric Pcrit values.16,17 Normal children and adolescents have Pcrit values in the subatmospheric range and a flat slope of the pressure-flow response, similar to the range seen in the current study.12,29 In young children with active upper airway reflexes, the activated Pcrit is more subatmospheric than the hypotonic Pcrit, and the activated SPF is flatter than the hypotonic SPF. These differences between the activated and hypotonic upper airway responses decrease during adolescence and into adulthood. This is consistent with the results of the current study (more subatmospheric Pcrit and tendency towards a flatter SPF in the activated condition), which included adolescents.
In the current study, there were no significant differences in upper airway dynamic responses between African American and Caucasian children under either the activated or the hypotonic conditions, indicating that both structural factors and neuromotor factors were similar between the races.
Structural Factors Affecting Upper Airway Collapsibility
The upper airway consists of muscle, soft tissue, cartilage, and bony components. The interplay between these structural components and the neural pathways determine the functioning of the upper airway. At birth, the face is about 40% of adult size, increasing to 65% at 3 years of age and completing growth by puberty.34 Thus, most facial growth was near completion in the subjects in this study. This suggests that skeletal factors do not play a major role in the differing prevalence of OSAS among the races, despite cephalometric studies showing differences between African American and Caucasian skeletal structure.35 Other studies have attributed differences in the prevalence of OSAS amongst the races to other soft tissue causes, such as tongue mass.35,36 Another possibility is that intrinsic airway properties (i.e., stiffness of the actual airway tissues) differ between the races. One fact supporting the latter theory is that pulmonary function differs between races, and this can only partly be explained by known factors such as anthropometric and nutritional differences.37 The lower lung volumes in African American children would be expected to result in decreased tracheal traction and hence increased upper airway collapsibility.38 Nevertheless, the current study showed no difference in the hypotonic Pcrit between races, suggesting that none of the above structural factors played a major role in the differing prevalence of OSAS between the races.
Neuromuscular Factors Affecting Upper Airway Collapsibility
The drive to the upper airway muscles is affected by the overall central nervous system ventilatory drive.39–41 Previously, it has been shown that the occlusion pressure in 100 milliseconds (P0.1) during sleep correlates with the SPF, indi cating that ventilatory drive affects upper airway collapsibili ty.14 To our knowledge, no studies have evaluated differences in ventilatory control in African Americans compared to Caucasians. Nevertheless, the lack of difference in activated upper airway dynamics between the races in the current study suggests that differences in upper airway neuromotor control cannot explain the differing prevalence of OSAS between the races. It is possible that other central nervous system factors affecting OSAS, such as the arousal threshold, differ between the races.
Environmental, Cultural, and Socioeconomic Factors
The Cleveland Children's Sleep And Health Study established an association between OSAS and residence in severely disadvantaged or distressed neighborhoods.42 After controlling for obesity, ethnicity, prematurity, socioeconomic status, and health characteristics like asthma, enlarged tonsils, and maternal smoking, children residing in a distressed neighborhood had over three times the odds of OSAS. The etiology of this risk is unclear. It is possible that factors such as high allergen or irritant exposure may lead to inflammation of the upper airway, thus narrowing the airway due to edema, or altering the upper airway mucosa and hence decreasing the upper airway afferent reflex responses.43,44 Other factors such as increased environmental noise and crowded sleeping conditions may lead to sleep fragmentation and sleep deprivation, which may exacerbate OSAS. In addition, racial differences in subjective sleep measures have been found, with African American children reporting less sleep than Caucasians.45
Limitations
This study focused on normal children in order to determine whether intrinsic, race-related differences in upper airway dynamics could explain the difference in OSAS prevalence between African Americans and Caucasians. Further physiologic studies of children with OSAS are now needed to determine if there are racial differences in upper airway collapsibility in children with obesity or adenotonsillar hypertrophy. For example, it is possible that obese children of different races have different patterns of fat distribution, which may affect upper airway patency. Future studies involving larger sample of children, and including children from additional races and ethnicities such as Hispanic and Asian children are needed.
CONCLUSIONS
Children of African American race have similar upper airway dynamic function during sleep compared to children of Caucasian race. As these upper airway dynamic studies comprise both structural and neuromuscular factors, the higher prevalence of OSAS based on epidemiological studies in African American children compared to Caucasians may not be explained on the basis of structural or neuromotor differences between the races, but may be due to environmental or socioeconomic factors. Identifying the factors contributing to the racial distribution of OSAS in children may help identify susceptible children in order to formulate screening strategies and thereby target susceptible populations in order to reduce the overall morbidity of OSAS in children.
DISCLOSURE STATEMENT
Research equipment for this study was provided by Respironics. Respironics supplied no other support. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all of the children and their families for their participation in this study. We thank the sleep technologists who assisted with this study for their dedication and professionalism.
Dr. Marcus was supported by NIH grants RO1 HL58585 and UL1 RR-024134.
REFERENCES
- 1.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 2.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 5.Chervin RD, Clarke DF, Huffman JL, et al. School performance, race, and other correlates of sleep-disordered breathing in children. Sleep Med. 2003;4:21–7. doi: 10.1016/s1389-9457(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery-Downs HE, Jones VF, Molfese VJ, Gozal D. Snoring in preschoolers: associations with sleepiness, ethnicity, and learning. Clin Pediatr (Phila) 2003;42:719–26. doi: 10.1177/000992280304200808. [DOI] [PubMed] [Google Scholar]
- 7.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 8.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 9.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C, Huang YS, Glamann C, Li K, Chan A. Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey. Otolaryngol Head Neck Surg. 2007;136:169–75. doi: 10.1016/j.otohns.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–8. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 12.Marcus CL, Fernandes do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 13.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626–33. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 15.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–40. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 17.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57:99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 18.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr. 1998;132:211–22. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- 19.The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Karamessinis LR, Pepe ME, et al. Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep. 2009;32:1173–81. doi: 10.1093/sleep/32.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapo-port DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–80. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 22.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–53. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1051–7. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 24.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–9. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges J, Lehmann E. Estimates of location based on rank tests. Ann Mathematical Stat. 1963;34:598–611. [Google Scholar]
- 26.Acion L, Peterson J, Temple S, Arndt S. Probabilistic index: an intuitive non-parametric approach to measuring the size of treatment effects. Stat Med. 2006;25:591–602. doi: 10.1002/sim.2256. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Kianifard F. A nonparametric procedure associated with a clinically meaningful efficacy measure. Biostatistics. 2000;1:293–8. doi: 10.1093/biostatistics/1.3.293. [DOI] [PubMed] [Google Scholar]
- 28.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:682–7. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 29.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman M, Bliznikas D, Klein M, Duggal P, Somenek M, Joseph NJ. Comparison of the incidences of obstructive sleep apnea-hypopnea syndrome in African-Americans versus Caucasian-Americans. Otolaryngol Head Neck Surg. 2006;134:545–50. doi: 10.1016/j.otohns.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 32.Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis. 1986;134:555–8. doi: 10.1164/arrd.1986.134.3.555. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport DM, Garay SM, Goldring RM. Nasal CPAP in obstructive sleep apnea: mechanisms of action. Bull Eur Physiopathol Respir. 1983;19:616–20. [PubMed] [Google Scholar]
- 34.Katz ES, D'Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:253–62. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 36.Lee JJ, Ramirez SG, Will MJ. Gender and racial variations in cephalometric analysis. Otolaryngol Head Neck Surg. 1997;117:326–9. doi: 10.1016/S0194-5998(97)70121-9. [DOI] [PubMed] [Google Scholar]
- 37.Harik-Khan RI, Muller DC, Wise RA. Racial difference in lung function in African-American and White children: effect of anthropometric, socioeconomic, nutritional, and environmental factors. Am J Epidemiol. 2004;160:893–900. doi: 10.1093/aje/kwh297. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–95. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz AR, Thut DC, Brower RG, et al. Modulation of maximal inspiratory airflow by neuromuscular activity: effect of CO2. J Appl Physiol. 1993;74:1597–605. doi: 10.1152/jappl.1993.74.4.1597. [DOI] [PubMed] [Google Scholar]
- 40.Weiner D, Mitra J, Salamone J, Cherniack NS. Effect of chemical stimuli on nerves supplying upper airway muscles. J Appl Physiol. 1982;52:530–6. doi: 10.1152/jappl.1982.52.3.530. [DOI] [PubMed] [Google Scholar]
- 41.Shea SA, Akahoshi T, Edwards JK, White DP. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Respir Crit Care Med. 2000;162:559–65. doi: 10.1164/ajrccm.162.2.9908111. [DOI] [PubMed] [Google Scholar]
- 42.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–7. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 43.Huang J, Colrain IM, Melendres MC, et al. Cortical processing of respiratory afferent stimuli during sleep in children with the obstructive sleep apnea syndrome. Sleep. 2008;31:403–10. doi: 10.1093/sleep/31.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tapia IE, Bandla P, Traylor J, Karamessinis L, Huang J, Marcus CL. Upper airway sensory function in children with obstructive sleep apnea syndrome. Sleep. 2010;33:968–72. doi: 10.1093/sleep/33.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaughlin C V, Beal KJ, Montgomery-Downs HE, Faye J V, O'Brien LM, Gozal D. Cultural influences on the bedtime behaviors of young children. Sleep Med. 2005;6:319–24. doi: 10.1016/j.sleep.2005.02.001. [DOI] [PubMed] [Google Scholar]