Abstract
Study Objectives:
Treating ovariectomized rats with physiological levels of estradiol and/or progesterone affects aspects of both baseline (24 h) sleep and recovery (18 h) sleep after 6 h of sleep deprivation. We have extended the analysis of these effects by examining several additional parameters of sleep architecture using the same data set as in our previous study (Deurveilher et al. SLEEP 2009;32(7):865-877).
Design:
Sleep in ovariectomized rats implanted with oil, 17 β-estradiol and/or progesterone capsules was recorded using EEG and EMG before, during, and after 6 h of sleep deprivation during the light phase of a 12/12 h light/dark cycle.
Measurements and Results:
During the baseline dark, but not light, phase, treatments with estradiol alone or combined with progesterone decreased the mean duration of non-rapid eye movement sleep (NREMS) episodes and the number of REMS episodes, while also increasing brief awakenings, consistent with the previously reported lower baseline NREMS and REMS amounts. Following sleep deprivation, the hormonal treatments caused a larger percentage increase from baseline in the mean durations of NREMS and REMS episodes, and a larger percentage decrease in brief awakenings, consistent with the previously reported larger increase in recovery REMS amount. There were no hormonal effects on NREMS and REMS EEG power values, other than on recovery NREMS delta power, as previously reported.
Conclusions:
Physiological levels of estradiol and/or progesterone in female rats modulate sleep architecture differently at baseline and after acute sleep loss, fragmenting baseline sleep while consolidating recovery sleep. These hormones also play a role in the diurnal pattern of NREMS maintenance.
Citation:
Deurveilher S; Rusak B; Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. SLEEP 2011;34(4):519-530.
Keywords: Ovariectomy, estradiol, progesterone, sleep architecture, sleep homeostasis, sleep deprivation
INTRODUCTION
Increasing evidence suggests that the ovarian hormones estradiol and progesterone not only control reproductive functions and behaviors but also influence a diverse range of physiological and psychological processes, including mood, sensory, and cognitive functions.1–3 Sleep is no exception: alterations of sleep patterns are associated with changing levels of endogenous estradiol and progesterone such as occur during the menstrual cycle, pregnancy, and menopause.4–6 Sleep patterns also change in response to hormonal treatments, such as oral contraceptive use in young women7,8 and hormone replacement therapy in menopausal women.9–11 While inadequate sleep is becoming increasingly common and can impair human health and performance,12–14 relatively little is known about whether and how endogenous or exogenous female sex hormones influence recovery from sleep loss.15,16
To determine how female sex hormones modulate spontaneous (baseline) sleep and sleep recovery after sleep loss, we previously used a rodent model of hormonal loss and replacement in a paradigm involving gentle handling to generate acute (6 h) sleep deprivation.17 We used subcutaneous steroid implants to produce stable, physiological levels of estradiol and progesterone in adult ovariectomized (OVX) rats in order to evaluate effects on baseline and recovery sleep in similar stable hormonal conditions. This approach is not possible in normally cycling females because of the changing estradiol and proges-terone levels across the estrous cycle.18 We reported that OVX rats treated with estradiol and/or progesterone spent less time in spontaneous non-rapid eye movement sleep (NREMS) and/or REMS during the dark phase, compared to untreated OVX rats. Following 6 h of sleep deprivation, the hormonally treated rats showed a more robust increase from baseline in REMS amount, but a less pronounced increase in NREMS EEG delta power (a measure of sleep intensity/drive), compared to hormonally untreated OVX rats.
These findings on baseline sleep were largely consistent with a number of previous studies which reported that subcutaneous injections of estradiol reduced spontaneous REMS amounts in OVX rats.19–22 Decreases in sleep, in particular REMS, have also been observed in intact female rats on the proestrus night (i.e., after spontaneous estradiol and progesterone peaks).23–25 However, in contrast to our previous results, a few studies have shown only minor changes during the estrous cycle in sleep recovery after 6 h of sleep deprivation in rats25 and little effect of endogenous or exogenous estradiol on sleep homeostasis in mice.26–28 Thus, the extent to which female sex hormones modulate sleep recovery after sleep deprivation in rodents remains unresolved.
In this report, we characterized the influence of female sex hormones on the homeostatic process of sleep recovery by analyzing several parameters of sleep architecture during spontaneous sleep and during recovery sleep in OVX rats treated with estradiol and/ or progesterone and in hormonally untreated male rats, using the data set from Deurveilher et al.17 The parameters analyzed in the present study included: the number and duration of NREMS and REMS episodes, the number of brief awakenings from sleep, and the EEG power spectra during NREMS and REMS.
MATERIALS AND METHODS
We used the same data base as in our previous report17 to perform additional analyses of sleep architecture. The details of surgery, experimental design, experimental procedures, and sleep/wake scoring were described previously.17 Briefly, adult Wistar rats of both sexes (Charles River Canada, St. Constant, QC, Canada; females: 211–264 g; males: 310–355 g at the time of surgery) were maintained on a 12/12 h light/dark cycle (lights on at 07:00) at 23 ± 1°C ambient temperature, with food and water available ad libitum. Under anesthesia (72/3.8/0.7 mg/kg ketamine/ xylazine/acepromazine, intraperitoneal), female rats were ovariectomized and implanted subcutaneously with Silastic capsules containing sesame oil (Oil group; n = 8); 10.5 μg of 17β-estradiol (Catalog No. E8875; Sigma-Aldrich, St Louis, MO) in sesame oil (low estradiol [LE] group; n = 8); 60 μg of 17 β-estradiol in sesame oil (high estradiol [HE] group; n = 8); 40 mg of crystalline progesterone (Catalog No. P0130; Sigma-Aldrich) [low progesterone (LP) group; n = 8]; or 10.5 μg of 17 β-estradiol and 40 mg of progesterone (low estradiol + progesterone [LEP] group; n = 8). The efficacy of the hormone manipulations was previously confirmed by measuring plasma estradiol levels, and uterus and body weights.17 ‘Low’ doses generated levels typical of diestrus, while ‘high’ doses generated levels typical of proestrus in cycling female rats.18 Males were left gonad-intact but implanted with oil-filled Silastic capsules. All animals were also implanted with supradural EEG and neck muscle EMG electrodes.
Two weeks after surgery and habituation to the recording apparatus, EEG and EMG were recorded for 24 h (baseline), followed by 6 h of sleep deprivation in the second half of the light phase, and an 18 h recovery period which encompassed the following dark phase and the first half of the light phase. Rats were sleep deprived by introducing novel objects into their cages, gently shuffling their bedding, cage tapping, and, when necessary, slowly moving their litter tray, whenever the rats showed behavioral or EEG signs of sleepiness. All experiments were performed in accordance with the guidelines of the Canadian Council on Animal Care and were approved by the Dalhousie University Committee on Laboratory Animals.
Behavioral states were scored automatically using SleepSign software (Kissei Comtec America, Irvine, CA) in 10-s epochs; the scoring was confirmed visually off-line. Each epoch was identified as wake (high frequency, low voltage EEG and high amplitude EMG), NREMS (low frequency, high voltage EEG and low amplitude EMG) or REMS (low amplitude EEG theta wave activity and EMG atonia).
Analyses of Sleep Architecture
The following parameters of sleep architecture were quantified for the 12 h baseline light phase, 12 h baseline dark phase, and 12 h recovery dark phase: the number and mean duration (s) of NREMS and REMS episodes (the minimum duration is a 10-s epoch). In addition, the durations of NREMS and REMS episodes were analyzed by plotting frequency histograms constructed for 9 consecutive bins of logarithmically increasing episode duration (10, 20-30, 40-70, 80-150, 160-310, 320-630, 640-1270, 1280-2550, and > 2560 s).29 As a measure of sleep fragmentation, we calculated the number of brief awakenings, which were defined as 10-s epochs of wakefulness bounded by NREMS or, rarely, REMS.
EEG Power Spectral Analysis
EEG power spectra for NREMS and REMS were computed in 2-s windows using fast Fourier transform (FFT; Hanning window) and tallied in 0.5 Hz bins in the following frequency ranges: delta, 0.5-4 Hz; theta, 4.5-8 Hz; sigma, 8.5-13 Hz; beta, 13.5-30 Hz; and gamma, 30.5-50 Hz.30 Five 2-s power spectra were averaged over a 10-s epoch of a scored behavioral state. Epochs containing EEG artifacts were visually identified and omitted from the spectral analysis. Both absolute and relative power values were used to evaluate EEG power during NREMS and REMS. Relative power values, expressed as a percentage of total EEG power in each sleep state, controlled for any differences in absolute EEG power among animals.
Statistical Analyses
All statistical analyses were conducted with Statview 5.0 (SAS Institute Inc., Cary, NC). Sleep-wake parameters were analyzed using one-way repeated measures ANOVA with “time interval” as the main factor for each group, or one-way factorial ANOVA with “group” as the main factor for each time interval. The EEG power density in NREMS and REMS was analyzed with one-way ANOVA with “group” as the main factor for each selected frequency bin or band. Post hoc Tukey-Kramer tests were used to analyze specific comparisons when main effects in ANOVA were significant. Values of P < 0.05 were considered statistically significant.
RESULTS
The results reported here were derived from 48 rats used in our previous study, including 40 OVX females, assigned to Oil, LE, HE, LP, and LEP groups (n = 8 per group), and 8 gonad-intact, hormonally untreated males (M group).17
Baseline Sleep
In our previous study, we reported that the amount of NREMS during the 24 h baseline period was significantly lower (by ∼10% or ∼1 h) in the HE and LEP groups compared to the Oil group, and this decrease tended to be more prominent during the 12 h dark phase.17 The amount of REMS during the 24 h baseline period was lower (by ∼20% or ∼30 min) in the LE, HE, and LP groups compared to the Oil group, and this decrease was significant during the dark phase. In the present study, we analyzed the number and mean duration of NREMS and REMS episodes, the number of brief awakenings from sleep (as a measure of sleep fragmentation), and EEG power spectra (0.5-50 Hz) during NREMS and REMS. The results are summarized in Table 1.
Table 1.
Summary of significant group differences in baseline sleep and recovery sleep after sleep deprivation
| Sleep Variables | Female Groups | Males vs. Females |
|---|---|---|
| BASELINE (24 h) | ||
| NREMS amount (min)17 | HE, LEP < Oil, LP | – |
| REMS amount (min)17 | LE, HE, LP < Oil | M > HE |
| NREMS episode number | – | M > LP, LEP |
| REMS episode number | – | M < Oil |
| NREMS episode duration (s) | – | M < LP |
| BASELINE (12 h light) | ||
| REMS amount (min)17 | LE, HE, LP < LEP | M > HE |
| NREMS episode number | – | M > LE, HE, LEP |
| Brief awakenings | – | M > LEP |
| BASELINE (12 h dark) | ||
| REMS amount (min)17 | HE, LP, LEP < Oil | M < Oil |
| REMS episode number | HE, LP, LEP < Oil | M < Oil |
| NREMS episode duration (s) | HE, LEP < Oil and LEP < LP | M < Oil, LP |
| Brief awakenings | LEP > Oil, LP | M > Oil |
| RECOVERY (12 h dark) | ||
| REMS amount (% 12 h baseline dark) | HE, LEP > Oil | – |
| REMS episode number | HE, LP, LEP < Oil | – |
| REMS episode duration (% 12 h baseline dark) | HE, LEP > Oil | – |
| NREMS episode number | – | M > LP, LEP |
| NREMS episode duration (s) | – | M < LP |
| NREMS episode duration (% 12 h baseline dark) | HE, LEP > Oil | – |
| Brief awakenings (% 12 h baseline dark) | LEP > Oil | – |
| NREMS delta power, first 2 h (% 24 h baseline)17 | HE, LP, LEP < Oil | – |
– indicates no significant group differences.
NREMS episodes
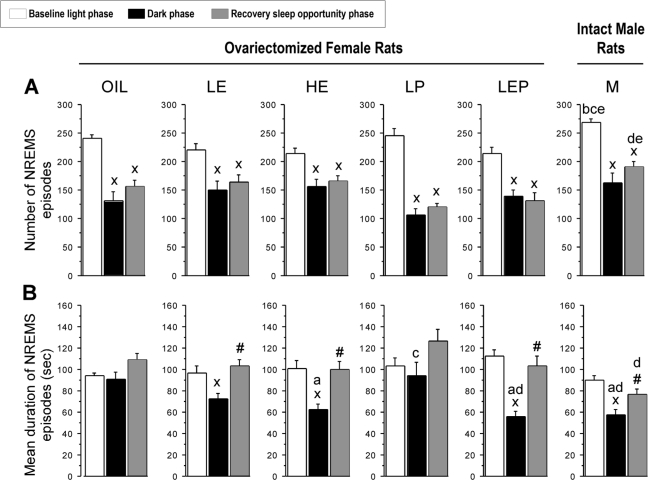
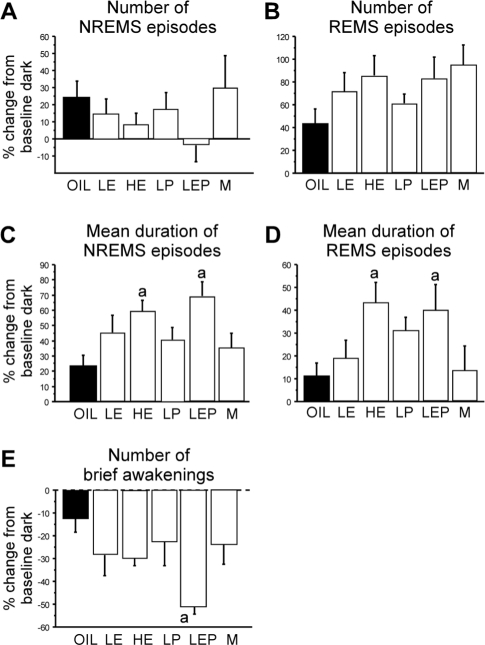
There were significant differences among groups in mean durations of NREMS episodes (Figure 1B, black bars). The mean duration of NREMS episodes during the dark phase was shorter in the HE, LEP, and M groups by 31 to 38% compared to the Oil and LP groups (Group: F5,42 = 5.36, P < 0.001; P < 0.05, HE, LEP, and M < Oil, and LEP and M < LP). The number of episodes did not differ among groups (Figure 1A, black bars), and the resulting total NREMS amounts, while somewhat lower for groups showing shorter episodes, also did not differ significantly among groups, as previously reported.17 The mean duration of NREMS episodes was significantly shorter in the dark than in the light phase in the LE, HE, LEP, and M groups (P < 0.05 vs. light phase), whereas it was similar between the light and dark phases in the Oil and LP groups (Figure 1B, white and black bars).
Figure 1.
The number (A) and mean duration (B) of non-rapid eye movement sleep (NREMS) episodes observed in ovariectomized (OVX) female and intact male (M) rats during the baseline 12 h light (white bars) and 12 h dark phases (black bars) and during 12 h of recovery sleep opportunity (dark phase; gray bars) following 6 h of sleep deprivation. OVX female rats had been treated for two weeks with oil, low estradiol (LE), high estradiol (HE), low progesterone (LP), or low estradiol plus low progesterone (LEP) doses. The number of NREMS episodes (A) differed significantly between the light and dark phases at baseline. During the recovery dark phase, the number of NREMS episodes (A) remained similar to that during the baseline dark phase. The mean duration of NREMS episodes (B) was shorter in the HE, LEP, and M groups than in the Oil and the LP groups during the dark phase, and it was shorter in the dark vs. the light phase in the LE, HE, LEP, and M groups only. During the recovery dark phase, the mean duration of NREMS episodes (B) was significantly increased compared to the corresponding baseline in the LE, HE, LEP, and M groups only. Data are shown as means + standard errors of the mean (SEM) for 8 animals per group. xdifferent from baseline light phase; #different from baseline dark phase; adifferent from Oil; bdifferent from LE; cdifferent from HE; ddifferent from LP; edifferent from LEP; all P < 0.05 (Tukey-Kramer post hoc comparisons).
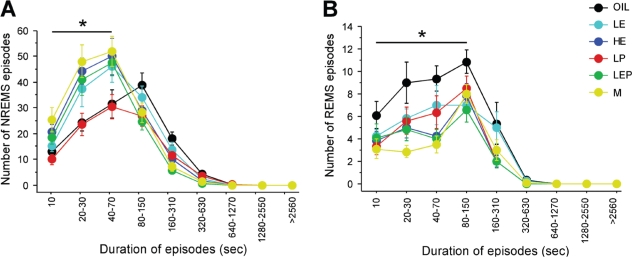
The pattern of NREMS was analyzed further by examining the number of NREMS episodes as a function of episode duration (Figure 3A). During the dark phase, the number of NREMS episodes lasting ≤ 70 s tended to be higher in the LE, HE, LEP, and M groups than in the Oil and LP groups, but this difference was only significant for the M group (Group: F5,42 = 3.81, P < 0.01; M > Oil and LP, P < 0.05). This increased number of shorter NREMS episodes is consistent with the shorter mean duration of NREMS episodes in the HE, LEP, and M groups (Figure 1B), as described above.
Figure 3.
The frequency distributions of NREMS (A) and REMS (B) episodes as a function of episode duration (s) during the baseline dark phase in the Oil, LE, HE, LP, LEP, and M groups. All episodes of NREMS and REMS were divided into 9 consecutive bins of increasing duration on a logarithmic scale. There were significant group differences in the number of NREMS episodes ≤ 70 s (F5,42 = 3.81; P < 0.01) and the number of REMS episodes ≤ 150 s (F5,42 = 4.71, P < 0.01). Specifically, the Oil and LP groups had fewer NREMS episodes ≤ 70 s than the M group (P < 0.05 vs. M), while the HE, LEP and M groups had fewer REMS episodes ≤ 150 s than the Oil group (P < 0.05 vs. Oil). Data are shown as means ± SEM of 8 animals per group. * indicates the combined bins for which the main factor of Group was significant (P < 0.05, ANOVA).
REMS episodes
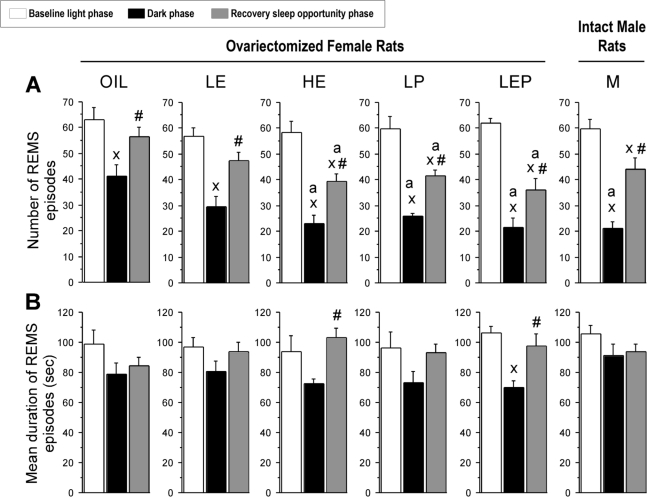
The lower amount of REMS in the HE, LP, LEP, and M groups compared to the Oil group in the dark phase (as previously reported17) was due to a decrease in the number, not the mean duration, of REMS episodes (Figure 2, black bars). The number of REMS episodes in the dark phase was significantly lower in the HE (−44%), LP (−37%), LEP (−46%), and M (−49%) groups compared to the Oil group (Group: F5,42 = 5.51, P < 0.001; P < 0.05 vs. Oil; Figure 2A, black bars). This reduction was reflected in a smaller number of REMS episodes ≤ 150 s in duration (Figure 3B). During the light phase, the number (Figure 2A, white bars), mean duration (Figure 2B, white bars), and duration distribution (data not shown) of REMS episodes did not differ among groups.
Figure 2.
The number (A) and mean duration (B) of rapid-eye movement sleep (REMS) episodes during baseline recordings for the 12 h light (white bars) and 12 h dark phases (black bars) and during 12 h of recovery sleep opportunity (dark phase; gray bars) following 6 h of sleep deprivation. See the legend of Figure 1 for further information regarding the hormonal treatment groups. The number of REMS episodes (A) in the dark phase was lower in the HE, LP, LEP, and M groups compared to the Oil group under both baseline and recovery conditions. The mean duration of REMS episodes (B) was significantly longer during the recovery dark phase relative to the baseline dark phase in the HE and LEP groups only. xdifferent from baseline light phase; #different from baseline dark phase; adifferent from Oil; all P < 0.05 (Tukey-Kramer post hoc comparisons).
Brief awakenings
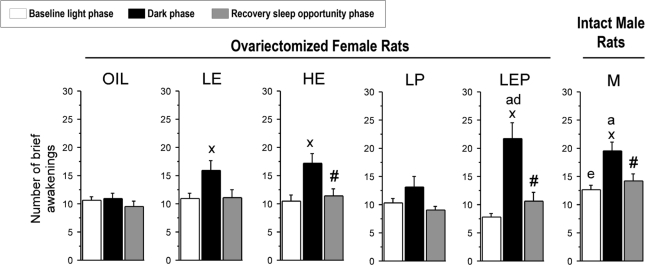
As a measure of sleep fragmentation, we examined the number of brief awakenings (episodes of 10 s wake; Figure 4) that interrupted sleep. To correct for differences in total sleep amount among the groups, the number of brief awakenings was expressed per hour of total sleep.29 Consistent with the finding of shorter durations of NREMS episodes in the dark versus the light phase, the number of brief awakenings was significantly higher during the dark phase in the LE, HE, LEP, and M groups (P < 0.05 vs. light phase), but not in the Oil and LP groups. The number of brief awakenings differed among groups during the dark phase (F5,42 = 4.75, P < 0.01): the LEP group had more brief awakenings than both the Oil and LP groups (+100% and +66%, respectively; P < 0.05 vs. LEP), and the M group had more brief awakenings than the Oil group (P < 0.05). During the light phase, the M group had more brief awakenings than the LEP group (Group: F5,42 = 3.50, P < 0.01; P < 0.05 vs. M), but there were no differences among the 5 female groups (consistent with results for mean duration of NREMS episodes).
Figure 4.
The number of brief awakenings from sleep during the baseline light (white bars) and dark (black bars) phases and during the recovery dark phase (gray bars) in the Oil, LE, HE, LP, LEP, and M group. A brief awakening was defined as a 10-s epoch of wakefulness bounded by NREMS (or rarely REMS) and expressed per hour of sleep time. At baseline, the number of brief awakenings was higher in the LEP group than in the Oil and LP groups during the dark phase, and it was higher during the dark than the light phase in the LE, HE, LEP, and M groups. During the recovery dark phase, the number of brief awakenings was significantly decreased compared to the corresponding baseline in the HE, LEP, and M groups. Data are shown as means + SEM of 8 animals per group. xdifferent from baseline light phase; #different from baseline dark phase; adifferent from Oil; ddifferent from LP; edifferent from LEP; all P < 0.05 (Tukey-Kramer post hoc comparisons).
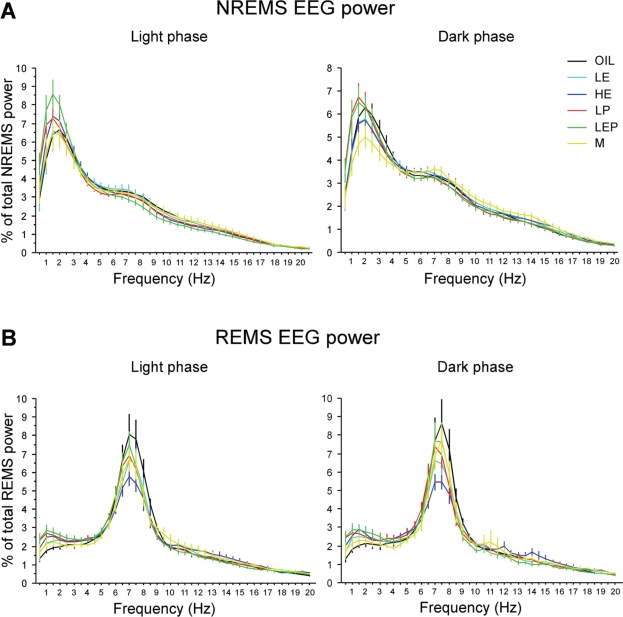
Sleep EEG power spectra
The NREMS EEG spectra typically showed a peak in the delta range (0.5-4 Hz; Figure 5A), whereas the REMS EEG spectra showed a peak in the theta range (4.5-8 Hz; Figure 5B). NREMS delta power (a measure of NREMS intensity) did not differ significantly among groups, and the results were similar for NREMS slow-wave energy, a measure which takes into account both NREMS intensity and NREMS amount (data not shown).25
Figure 5.
Power spectra of the EEG during NREMS (A) and REMS (B) in the baseline light (left) and dark (right) phases in the Oil, LE, HE, LP, LEP, and M groups. EEG power was normalized to the total power (0.5-50 Hz) for each 0.5 Hz bin in each animal. The power spectra during NREMS and REMS in the light and dark phases were similar among groups, except for a trend toward lower REMS theta power in the HE group. Data are shown as means ± SEM of 8 animals per group.
Despite an apparent ∼20% reduction in theta power during REMS in the HE group relative to the Oil group in both the light and dark phases (Figure 5B), spectral distribution, absolute and relative EEG spectral power did not differ significantly among groups. A recent report suggested that the magnitude of the effect of a synthetic estrogen on REMS EEG power varied across the dark phase.31 Thus, we further analyzed REMS EEG power in 4 h blocks across the dark phase. A trend toward lower theta power (∼20%) in the HE group compared to the Oil group was present in all three 4 h blocks, but there were no significant group differences in any block (data not shown).
In summary, hormonal treatment of OVX female rats affected spontaneous sleep parameters mostly during the dark (active) phase. Thus, during that phase, treatment with estradiol alone or combined with progesterone decreased both the mean duration of NREMS episodes and the number of REMS episodes, while treatments with progesterone alone decreased the number of REMS episodes; combined estradiol and progesterone treatment also increased the number of brief awakenings from sleep. Hormonal treatments did not significantly affect NREMS and REMS EEG power spectra, including delta and theta values, in either the light or the dark phase. Overall, males tended to be more similar to estradiol-treated OVX females than to Oil-treated OVX females in their baseline sleep architecture.
Recovery from Sleep Deprivation
To study homeostatic responses to sleep deprivation, all animals were kept awake by gentle interventions for 6 h during the second half of the light phase and then allowed to sleep during the subsequent 18 h (12 h in the dark and 6 h in the light phase). We reported previously that all 6 groups showed rebound increases in NREMS and REMS throughout the 18 h recovery period compared to the time-matched 18 h baseline period, but that the mag-nitude of the increase in REMS (but not NREMS) was smaller in the Oil group (23% increase) than in the other groups (up to 73% increase in the HE group).17 We also reported that the increase in NREMS EEG delta power (normalized to the 24 h baseline) during the first 2 h after sleep deprivation was larger in the Oil group (+179%) than in the other groups (up to +146% in the LEP group). Since rebound increases in NREMS delta power and in NREMS and REMS amounts occurred during the recovery dark phase, we focused on that 12 h period for analysis of changes in sleep architecture. The results are summarized in Table 1.
NREMS episodes
Increased amounts of NREMS in the recovery dark phase were due to an increase in the mean duration, not the number, of NREMS episodes in all groups, although this duration increase was not statistically significant in the Oil and LP groups (Figure 1, gray bars; Figure 6A, C). In the LE, HE, LEP, and M groups, the mean duration of NREMS episodes increased significantly during the recovery dark phase compared to the baseline dark phase (P < 0.05 vs. baseline for each comparison; Figure 1B, gray bars) and these levels were comparable to their light phase baseline NREMS durations (Figure 1B, white bars). The percentages of these increases in NREMS episode duration from the dark phase baseline levels in the HE and LEP groups (+60% and +69%, respectively) were significantly higher than those in the Oil group (+23%; Group: F5,41 = 3.51, P < 0.01; P < 0.05 vs. Oil; Figure 6C). During the recovery dark phase, the M group had shorter NREMS episodes than the LP group (Group: F5,41 = 3.99, P < 0.01; P < 0.05 vs. LP; Figure 1B), while there were no significant differences among the 5 female groups. The frequency distribution of NREMS episodes as a function of episode duration is shown in Supplementary Figure S1A.
Figure 6.
Changes during the recovery dark period relative to time-matched baselines in the numbers of NREMS (A) and REMS (B) episodes, the mean duration of NREMS (C) and REMS (D) episodes, and the numbers of brief awakenings (E) in the Oil (black bars), LE, HE, LP, LEP, and M (white bars) groups. The percentage increases in mean duration of both NREMS and REMS episodes were greater in the HE and LEP groups than in the Oil group. The percentage decrease in the number of brief awakenings was larger in the LEP group than in the Oil group. Data (means + SEM) are expressed as percentages of the corresponding baseline period; n = 8 per group. adifferent from Oil; all P < 0.05 (Tukey-Kramer post hoc comparisons).
Although the main contributor to the increase in NREMS amounts during recovery was an increase in average NREMS episode duration, the M group also had more NREMS episodes than the LP and LEP groups during the recovery dark phase (Group: F5,41 = 5.37, P < 0.001; P < 0.05 vs. M; Figure 1B), while there were no significant differences among the 5 female groups.
REMS episodes
The rebound increase in REMS amount in the recovery dark phase was mainly due to an increase in the number of REMS episodes in all groups, although a concomitant increase in their mean duration occurred in some groups (Figure 2, gray bars). In all groups, the number of REMS episodes was significantly higher during the recovery dark phase relative to the baseline dark phase (all P < 0.05 vs. baseline; Figure 2A, gray bars). Increases appeared to be related to levels of steroid hormones, ranging from the Oil group (+44%), to the LP (+61%), LE (+72%), LEP (+83%), HE (+86%), and M (+95%) groups, but these differences were not statistically significant (F5,41 = 1.42, NS; Figure 6B). During the recovery dark period, as was observed during the corresponding baseline, the number of REMS episodes was significantly lower in the HE, LP, and LEP groups compared to the Oil group (by 26% to 36%; Group: F5,41 = 4.21, P < 0.01; P < 0.05 vs. Oil; Figure 2A, gray bars). These reductions were reflected in the lower number of short REMS episodes (≤ 150 s; Supplementary Figure S1B).
The mean duration of REMS episodes was significantly increased during the recovery dark phase relative to the baseline dark phase, reaching the levels observed during the baseline light phase, in the HE and LEP groups only (+40 to 43%; both P < 0.05 vs. baseline; Figure 2B, gray bars). These increases were larger than those in the Oil group (Group: F5,41 = 2.54, P < 0.05; P < 0.05 vs. Oil; Figure 6D), and contributed to the more robust relative increases in REMS amount in the HE and LEP groups, reported in Deurveilher et al.17
Brief awakenings
As shown in Figure 4, consistent with the results for the mean duration of NREMS episodes, the number of brief awakenings (per hour of sleep time) was significantly lower during the recovery dark phase compared to the baseline dark phase in the HE (−33%), LEP (−51%), and M groups (−27%; P < 0.05 vs. baseline for each comparison) and was comparable to levels observed during the baseline light phase. This decrease in the LEP group was significantly larger than in the Oil group (−10%; Group: F5,41 = 3.37, P < 0.025; P < 0.05 vs. Oil; Figure 6E). There were no group differences in the absolute number of brief awakenings during the recovery dark phase.
Sleep EEG power spectra
During the 12 h recovery dark phase, NREMS EEG delta power (% of total power) was increased, while power in the other 4 EEG frequency bands—theta, sigma, beta, and gamma—was decreased compared to the corresponding baseline values (all P < 0.05), with no significant group differences (Supplementary Figure S2A). In addition, as observed during the corresponding baseline, the entire spectral distribution (0.5-50 Hz in 0.5 Hz bins) of NREMS EEG power did not differ significantly among groups (Supplementary Figure S2B). During the first 2 h of the recovery period, however, NREMS EEG delta power (% of 24 h baseline mean) was higher in the Oil group than in the other groups, as reported by Deurveilher et al.,17 while no group difference was observed in the other EEG frequency bands (data not shown).
During the 12 h recovery dark phase, in contrast to the EEG during NREMS, the EEG power in each of the 5 frequency bands (as a % of total power) during REMS did not differ from the respective baseline values in any treatment group (Supplementary Figure S2C). However, as also observed during the corresponding baseline, there was a trend for less theta power (−21%) in the HE group relative to the Oil group (Supplementary Figure S2D).
In summary, hormonal treatments decreased the absolute number of REMS episodes during both baseline and recov-ery sleep (in the dark phase). Increases in the duration of both NREMS and REMS episodes (and corresponding decreases in brief awakenings) after sleep deprivation were further enhanced by treatment with estradiol alone or combined with progesterone, while recovery NREMS delta power was specifically and transiently decreased by the same hormonal treatments.
DISCUSSION
The present results demonstrate that 2-week treatments with physiological levels of estradiol and/or progesterone modulate the sleep architecture of ovariectomized female rats under baseline conditions as well as during recovery from acute (6 h) sleep deprivation. At baseline, predominantly during the active (dark) phase, treatment with estradiol alone or combined with progesterone decreased the mean duration of NREMS episodes and the number of REMS episodes, and increased the number of brief awakenings; treatment with progesterone alone decreased the number of REMS episodes. During recovery sleep after sleep deprivation, estradiol and/or progesterone increased the duration of both NREMS and REMS episodes and decreased the number of brief awakenings relative to baseline levels. These changes account for the previously reported effects of these hormone treatments in reducing baseline amounts of REMS and NREMS, and causing more robust relative increases in recovery REMS amounts (REMS rebound).17 In general, a low dose of estradiol combined with progesterone was as effective as a high dose of estradiol alone in modifying baseline and recovery sleep architecture, suggesting a synergistic effect between the two hormones.
Hormonal Treatments Impair Baseline NREMS Maintenance and REMS Initiation in the Dark Phase
The effects of hormonal treatments during baseline sleep were remarkably dependent on daily phase. The Oil and LP groups showed NREMS episodes of similar average durations during the light and dark phases at baseline, while the LE, HE, and LEP groups showed significantly shorter NREMS episodes during the dark relative to the light phase (Figure 1B). Paralleling these changes were dramatic increases among hormonally treated rats in the number of brief awakenings in the dark phase, especially in the LEP group (Figure 4). In addition, the number of REMS episodes was reduced selectively in the dark phase, with no significant change in the duration of REMS episodes, in the HE, LEP, and LP groups compared to the Oil group.
The temporal gating of these hormonal effects to the dark phase may involve estradiol-induced alterations in the neuronal activity or timekeeping mechanisms of the suprachiasmatic nucleus (SCN), the site of the principal circadian clock in mammals.32 The SCN contains both subtypes of estrogen receptors (ER),33,34 and responds to estradiol administration with changes in neuronal firing35 and in the expression levels of various transcription factors,36 clock genes,37,38 and neuropeptides.39 Another possibility is that estradiol/progesterone modulates the diurnal rhythm of core body temperature or of other hormones, such as melatonin or cortisol,40–42 by acting at brain areas outside of the SCN or in the periphery, which may secondarily cause diurnal changes in sleep architecture.
Among previous studies of estradiol effects on sleep, only a few have examined sleep architecture in detail, and these have used various forms of estradiol and treatment strategies. Our results, using the natural 17 β-estradiol, are consistent with those in studies that used estradiol benzoate,19,21 but not with the results of one study31 using 17α-ethinyl estradiol (both are synthetic estrogens that are metabolized at a much slower rate than the natural 17 β-estradiol). In the latter study, when OVX rats were treated systemically with 4 daily injections of 17α-ethinyl estradiol, the duration but not the number of REMS episodes was decreased during the dark phase.31 Since this study did not report the number and duration of NREMS episodes, it is not possible to assess whether the increased durations of REMS episodes were a secondary result of changes in NREMS parameters. These differences may be related to the use of different strains of rats (Wistar [present study] vs. Sprague-Dawley31) and the length of the delay from the start of hormonal treatment until the EEG recordings began (2 weeks [present study] vs. 4 days31).
Another explanation for the different outcomes in these studies may be that different forms of estradiol have distinct effects on the mechanisms that govern the initiation and/or maintenance of REMS. The ER α and ER β nuclear receptors are differentially distributed in brain areas involved in REMS control, such as the pontine tegmentum and the preoptic region.33,43 Different forms of estradiol vary in their affinity for ER α and ER β,44 as well as in their regulatory effects on the expression of these ER subtypes.45–47 It is possible that different forms of estradiol differentially modulate ER subtypes and thereby influence different aspects of REMS regulation.
The LP group showed no significant changes in the number or duration of NREMS episodes, but showed a reduced number of REMS episodes (with no change in duration) during the dark phase, as compared to the Oil group. These results do not agree with previous studies using male rats, which showed that an acute injection of a high dose of progesterone (180 mg/kg, i.p.) at light onset caused fewer but longer episodes of NREMS and REMS during the light phase,48 while repeated injections of allopregnanolone (a metabolite of progesterone) for 5 days (15 mg/kg/day) at dark onset caused fewer but longer episodes of NREMS during the dark phase, with no changes in the number or duration of REMS episodes.49 These differences are likely due to the sex of the animals studied, including the presence of testosterone in males, as well as differences in dose, route of administration, and duration of drug treatment.
Our finding that treatments with estradiol alone or combined with progesterone influence NREMS mainly through changes in episode durations and in number of brief awakenings suggests that estradiol may modulate the mechanisms that regulate NREMS maintenance. Brief awakenings have been proposed to reflect the operation of mechanisms that govern NREMS maintenance, rather than being random disruptions of sleep.50 The ventrolateral preoptic nucleus is thought to be involved specifically in the maintenance, as opposed to the initiation, of NREMS51 and responds to estradiol administration with decreases in the expression of prostaglandin D synthase, which is responsible for the production of prostaglandin D2, a sleep promoting factor,24,52 and of adenosine A2A receptors, which bind adenosine, another endogenous hypnogen.53 Therefore, the disruption of NREMS maintenance found in the present study may be due to estradiol's action at the ventrolateral preoptic nucleus.
In contrast to their effect on the duration of NREMS episodes, treatments with estradiol and/or progesterone influence REMS mainly through changes in episode frequency. The reduction in the number of REMS episodes could be a secondary outcome related to the shorter average duration of NREMS episodes, because REMS occurrence is normally gated by a lengthy preceding NREMS episode. This interpretation would be reasonable for the HE and LEP groups, which do show significantly shorter NREMS episodes.
Alternatively, estradiol and progesterone may affect mechanisms enabling REMS initiation more directly. This is a more likely mechanism for progesterone acting alone, since the LP group did not show any change in the duration of NREMS episodes, but did show less frequent REMS episodes. During transitions from NREMS to REMS, the activity of wake-active orexinergic, serotonergic, and noradrenergic neurons is further decreased to allow for the reciprocal activation of REMS-active neurons in the brainstem, thus triggering REMS onset.54 Since estradiol treatments can increase the activity of these neurotransmitter systems by affecting synthetic enzymes,55,56 neurotrans mitter levels,57 and receptor expression,58 it is possible that estradiol and progesterone act directly on some of these neurons to inhibit transition to REMS, thus reducing REMS frequency.
Hormonal Treatments Had Little Effects on EEG Power during Baseline NREMS and REMS
Despite the decreased duration of NREMS episodes, as well as the decreased total NREMS amount17 in the HE and LEP groups, baseline NREMS EEG delta power (an index of the intensity or depth of NREMS) was not significantly affected by hormonal treatments during either the light or dark phase in these groups. It is possible that a small cumulative deficit of NREMS amount over a short term (i.e., the 2-week treatments) is not sufficient to affect NREMS delta power, although a longer-term deficit accumulation might be. In any case, the HE and LEP rats did not compensate for the reduced NREMS amount by sleeping more intensely (i.e., increased NREMS delta power) at baseline.
Similar to the NREMS EEG spectra, the REMS EEG spectra appeared to be unaffected by hormonal state, although REMS theta power tended to be smaller in the HE rats compared to the Oil rats during both the light and dark periods. Theta power in REMS has been proposed as a measure of REMS intensity and need.59 Our results are inconsistent with a previous study which showed a decrease in REMS theta power following treatment with 17 α-ethinyl estradiol in OVX rats.31 Since there was a trend toward decreased theta power in the present study, it is possible that our study lacked the statistical power to detect this effect. Alternatively, the decrease in REMS theta power observed previously31 may be associated with the decrease in the duration of REMS episodes that was also observed in that study, but not in the present study.
The present finding that the baseline sleep EEG was unaffected by hormonal treatment appears to be inconsistent with previously reported changes in the sleep EEG, notably, NREMS slow-wave activity (0.75-4 Hz) during the estrous cycle and particularly around proestrus in female (Sprague-Dawley) rats.25 These rat studies are also in disagreement with the finding in women of changes in EEG spindle activity (12-15 Hz), but not in slow-wave activity, during NREMS across the menstrual cycle.60–62 The reasons for these discrepancies are not clear, but it is possible that regulatory differences exist between species. It is also possible that the sleep EEG is more responsive to fluctuating levels of hormones than stable hormonal conditions (as achieved with hormone-filled Silastic implants).
Treatment with Estradiol Alone or Combined with Progesterone Facilitates Sleep Consolidation after Sleep Deprivation
After 6 h of sleep deprivation, the amount of NREMS was increased from baseline during the first 12 h (dark) recovery period in all groups (reported in Deurveilher et al.17). This increase tended to be enhanced by the hormonal treatments, and was associated with a larger increase from baseline in the duration of NREMS episodes in the HE and LEP groups, and a larger decrease in the number of brief awakenings in the LEP groups. These findings suggest that hormonal treatments directly facilitated consolidation of NREMS during recovery from sleep deprivation, and/or that the impact of sleep deprivation in promoting sleep may have overridden the effects of estradiol in promoting interruption of NREMS continuity in the dark phase.
Despite their larger increase in NREMS episode duration during recovery, the HE and LEP groups showed a smaller increase in recovery NREMS EEG delta power than the Oil group (reported in Deurveilher et al.17), suggesting the possibility that these rats are able to cope with, and are adapted to, shorter NREMS episodes in the dark at baseline because of altered NREMS homeostasis. This smaller increase in recovery NREMS delta power and increased recovery REMS in hormonally-treated rats may reflect the mutually inhibitory interaction between NREMS intensity and REMS propensity63 (see Deurveilher et al.17 for further discussion).
Similar to NREMS, the amount of REMS increased from baseline during recovery sleep in all groups. This increase was enhanced in the hormonally treated animals, in particular the HE and LEP groups, and may be related to their lower amount of REMS at baseline, as compared to the Oil group. This reduction in baseline REMS time may lead to a small cumulative loss in REMS time, causing a larger compensatory increase in REMS in the hormonally treated rats.
The increase in REMS amount in the HE and LEP groups was associated with an increase in both the number and mean duration of REMS episodes in the HE and LEP groups, and in the number of REMS episodes in the other female groups. As was the case at baseline, the absolute number of REMS episodes during recovery was lower in the HE, LP, and LEP groups compared to the Oil group, suggesting that the hormonal influence on the number of REMS episodes observed during baseline persisted during recovery from sleep deprivation.
The larger percentage increase from baseline in the mean duration of REMS episodes in the HE and LEP groups may be associated with the larger increase in the mean duration of NREMS episodes, and accounts for the reported higher percentage increase in REMS amount in these two groups.17
These findings suggest that the hormonal treatments help consolidate both NREMS and REMS during recovery from sleep deprivation, when sleep pressure is particularly high. It is possible that estradiol and progesterone influence the homeo-static mechanisms governing NREMS and REMS. Although we previously found significant effects of estradiol replacement on c-Fos levels (a marker of neuronal activation) in sleep/wake-promoting and limbic forebrain areas following 6 h of sleep deprivation,64 it is unknown whether estradiol modulation of c-Fos expression in those areas affects sleep homeostasis. In addition, the median preoptic nucleus has been shown to play a role in REMS homeostasis65 and contains ERs;33,66 estradiol may act on this nucleus to influence REMS homeostasis.
Males and Females Show Differences in Baseline and Recovery Sleep Architecture
The male group differed from the female groups in several aspects of sleep architecture under both baseline and recov-ery conditions (summarized in Table 1). During the baseline dark phase, when sleep pressure was low, the M group had more fragmented NREMS, as indicated by shorter episodes of NREMS and more brief awakenings, and spent less time in REMS, mainly due to fewer episodes, compared to the Oil group, but there were no significant differences between the M group and the hormonally treated female groups. During sleep deprivation (which occurred in the light phase), however, male rats tended to require more interventions to stay awake than any of the female groups.17 Finally, during the recovery dark phase, when sleep pressure was high, the M group had more NREMS episodes than the LP and LEP groups but shorter NREMS episodes than the LP group, with no significant differences from the Oil or HE group.
These results suggest that certain parameters of sleep architecture may be differentially regulated in male and female rats. Alternatively, it is possible that these differences result from the hormonal manipulations applied in the females, rather than representing a sex difference. This possibility is supported by the fact that, although there were differences between the male group and select female groups for certain sleep parameters, the male group did not differ from all female groups for any sleep parameter. It should also be noted that low levels of estradiol are present in male rats.67 Further studies using castrated males treated with E will be necessary to address these questions.
CONCLUSIONS
Our results show that physiological levels of estradiol and progesterone replacement in young adult OVX rats selectively modulate baseline sleep architecture during the daily dark phase, particularly the mean duration of NREMS episodes, and the numbers of REMS episodes and brief awakenings. It is possible that circulating estradiol and progesterone levels influence the mechanisms that regulate NREMS consolidation and its diurnal organization, as well as those that enable REMS initiation. Hormonally treated rats responded more strongly to acutely lost sleep than untreated rats, as indicated by larger compensatory changes in the mean duration of NREMS and REMS episodes and in the number of brief awakenings during recovery from sleep deprivation (but not in NREMS EEG delta power).17 These results suggest that estradiol and progesterone may regulate baseline sleep architecture by affecting both the circadian and homeostatic regulation of sleep. The use of young adult OVX rats as a model to study the effects of hormonal manipulations on sleep regulation may be relevant to young adult women undergoing surgically-induced menopause. However, older rats may better model the effects of hormone deficiency and replacement on sleep during human menopause, and we are currently investigating this possibility.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Rusak is a research consultant for Institut de Recherches Internationales Servier. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Ms. Elizabeth Cumyn and Ms. Nabilah Chowdhury for their technical assistance, Ms. Michelle Black for technical and editorial assistance, and Mr. Paul Black for creating software to support the data analysis. Supported by CIHR (MOP-67085).
ABBREVIATIONS
- HE
high estradiol
- LE
low estradiol
- LP
low progesterone
- LEP
low estradiol + low progesterone
- M
males
- NREMS
non-rapid eye movement sleep
- OVX
ovariectomized REMS, rapid eye movement sleep
- SCN
suprachiasmatic nucleus
- SEM
standard error of the mean
- ZT
zeitgeber time
The frequency distributions of NREMS (A) and REMS (B) episodes as a function of episode duration (s) during the recovery dark phase in the Oil, LE, HE, LP, LEP, and M groups. All episodes of NREMS and REMS were sorted into nine consecutive bins of increasing duration on a logarithmic scale. There were significant group differences in the number of NREMS episodes ≤ 70 s (F5,41 = 8.08; P < 0.0001) and the number of REMS episodes ≤ 150 s (F5,41 = 3.68; P < 0.01). Thus, the LE and HE groups had more NREMS episodes ≤ 70 s than the LP group (P < 0.05 vs. LP), while the M group had more NREMS episodes than the Oil, LP, and LEP groups (P < 0.05 vs. M). The HE and LEP groups had a lower number of REMS episodes ≤ 150 s than the Oil group (P < 0.05 vs. Oil). Data are shown as means ± SEM of 8 animals per group.* indicates the combined bins for which the factor Group was significant (P < 0.05, ANOVA).
The EEG power values in 5 frequency bands in NREMS (A) and REMS (C) during the baseline (black bars) and recovery (gray bars) dark phases, and spectral distributions of NREMS (B) and REMS (D) EEG power during the recovery dark phase in the Oil, LE, HE, LP, LEP, and M groups. EEG spectral power was normalized to the total power (0.5-50 Hz) in each animal, and the mean values were plotted in 0.5 Hz bins. As there were no significant group differences, the data shown in A and C are collapsed across the 6 groups. During recovery NREMS, irrespective of groups, delta power was increased, while theta, sigma, beta, and gamma power were decreased, compared to the respective baseline values. During recovery REMS, EEG power in all frequency bands was similar to corresponding baseline values. The spectral distribution of relative power of the EEG recorded during NREMS (B) and REMS (D) in the recovery dark phase was similar among groups. Data are shown as means ± SEM of 8 animals per group. #different from baseline, P < 0.05, paired t-test (A,C).
REFERENCES
- 1.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 2.Farage MA, Osborn TW, MacLean AB. Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Arch Gynecol Obstet. 2008;278:299–307. doi: 10.1007/s00404-008-0708-2. [DOI] [PubMed] [Google Scholar]
- 3.Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- 4.Dzaja A, Arber S, Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Shechter A, Boivin DB. Sleep, Hormones, and Circadian Rhythms throughout the Menstrual Cycle in Healthy Women and Women with Premenstrual Dysphoric Disorder. Int J Endocrinol. 2010;2010:1–17. doi: 10.1155/2010/259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–22. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Burdick RS, Hoffmann R, Armitage R. Short note: oral contraceptives and sleep in depressed and healthy women. Sleep. 2002;25:347–9. [PubMed] [Google Scholar]
- 8.Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442:729–37. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- 9.Polo-Kantola P, Erkkola R, Helenius H, Irjala K, Polo O. When does estrogen replacement therapy improve sleep quality? Am J Obstet Gynecol. 1998;178:1002–9. doi: 10.1016/s0002-9378(98)70539-3. [DOI] [PubMed] [Google Scholar]
- 10.Hachul H, Bittencourt LR, Andersen ML, Haidar MA, Baracat EC, Tufik S. Effects of hormone therapy with estrogen and/or progesterone on sleep pattern in postmenopausal women. Int J Gynaecol Obstet. 2008;103:207–12. doi: 10.1016/j.ijgo.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Antonijevic IA, Stalla GK, Steiger A. Modulation of the sleep electroencephalogram by estrogen replacement in postmenopausal women. Am J Obstet Gynecol. 2000;182:277–82. doi: 10.1016/s0002-9378(00)70211-0. [DOI] [PubMed] [Google Scholar]
- 12.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 13.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kalleinen N, Polo O, Himanen SL, Joutsen A, Urrila AS, Polo-Kantola P. Sleep deprivation and hormone therapy in postmenopausal women. Sleep Med. 2006;7:436–47. doi: 10.1016/j.sleep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Armitage R, Smith C, Thompson S, Hoffman R. Sex differences in slow-wave activity in response to sleep deprivation. Sleep Res Online. 2001;4:33–41. [Google Scholar]
- 17.Deurveilher S, Rusak B, Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep. 2009;32:865–77. [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–26. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 19.Barbe L, Faure JM, Bensch C, Dufy B, Vincent JD. The influence of ovarian steroids on the distribution of sleep elements in white female rats [in French] J Physiol (Paris) 1970;62(Suppl 2):240–1. [PubMed] [Google Scholar]
- 20.Branchey M, Branchey L, Nadler RD. Effects of estrogen and progesterone on sleep patterns of female rats. Physiol Behav. 1971;6:743–6. doi: 10.1016/0031-9384(71)90267-8. [DOI] [PubMed] [Google Scholar]
- 21.Matsushima M, Takeichi M. Effects of intraventricular implantation of crystalline estradiol benzoate on the sleep-wakefulness circadian rhythm of ovariectomized rats. Jpn J Psychiatry Neurol. 1990;44:111–21. doi: 10.1111/j.1440-1819.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 22.Pawlyk AC, Alfinito PD, Johnston GH, Deecher DC. Subchronic 17alpha-ethinyl estradiol differentially affects subtypes of sleep and wakefulness in ovariectomized rats. Horm Behav. 2008;53:217–24. doi: 10.1016/j.yhbeh.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7:173–81. doi: 10.1016/0006-8993(68)90095-4. [DOI] [PubMed] [Google Scholar]
- 24.Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–92. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwierin B, Borbely AA, Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811:96–104. doi: 10.1016/s0006-8993(98)00991-3. [DOI] [PubMed] [Google Scholar]
- 26.Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–23. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 27.Paul KN, Losee-Olson S, Pinckney L, Turek FW. The ability of stress to alter sleep in mice is sensitive to reproductive hormones. Brain Res. 2009;1305:74–85. doi: 10.1016/j.brainres.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyazovskiy VV, Kopp C, Wigger E, Jones ME, Simpson ER, Tobler I. Sleep and rest regulation in young and old oestrogen-deficient female mice. J Neuroendocrinol. 2006;18:567–76. doi: 10.1111/j.1365-2826.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 29.Tobler I, Deboer T, Fischer M. Sleep and sleep regulation in normal and prion protein-deficient mice. J Neurosci. 1997;17:1869–79. doi: 10.1523/JNEUROSCI.17-05-01869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawlyk AC, Alfinito PD, Deecher DC. Effect of 17alpha-ethinyl estradiol on active phase rapid eye movement sleep microarchitecture. Eur J Pharmacol. 2008;591:315–8. doi: 10.1016/j.ejphar.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 32.Moore RY, Leak RH. Suprachiasmatic nucleus. In: Takahashi JS, Turek FW, Moore RY, editors. Circadian Clocks. Handbook of Behavioral Neuro-biology. Vol. 141. New York: Kluwer Academic/Plenum Publishers; 2001. p. 79. [Google Scholar]
- 33.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol. 2008;20:1270–7. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 35.Fatehi M, Fatehi-Hassanabad Z. Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat. Neuropsychopharmacology. 2008;33:1354–64. doi: 10.1038/sj.npp.1301523. [DOI] [PubMed] [Google Scholar]
- 36.Abizaid A, Mezei G, Horvath TL. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res. 2004;1010:35–44. doi: 10.1016/j.brainres.2004.01.089. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura TJ, Moriya T, Inoue S, et al. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82:622–30. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura TJ, Shinohara K, Funabashi T, Kimura F. Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats. Neurosci Res. 2001;41:251–5. doi: 10.1016/s0168-0102(01)00285-1. [DOI] [PubMed] [Google Scholar]
- 39.Mahoney MM, Ramanathan C, Hagenauer MH, Thompson RC, Smale L, Lee T. Daily rhythms and sex differences in vasoactive intestinal poly-peptide, VIPR2 receptor and arginine vasopressin mRNA in the suprachiasmatic nucleus of a diurnal rodent, Arvicanthis niloticus. Eur J Neurosci. 2009;30:1537–43. doi: 10.1111/j.1460-9568.2009.06936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Cauter E, Golstein J, Vanhaelst L, Leclercq R. Effects of oral contraceptive therapy on the circadian patterns of cortisol and thyrotropin (TSH) Eur J Clin Invest. 1975;5:115–21. doi: 10.1111/j.1365-2362.1975.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 41.Brun J, Claustrat B, David M. Urinary melatonin, LH, oestradiol, progesterone excretion during the menstrual cycle or in women taking oral contraceptives. Acta Endocrinol (Copenh) 1987;116:145–9. doi: 10.1530/acta.0.1160145. [DOI] [PubMed] [Google Scholar]
- 42.Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103:185–94. doi: 10.1016/s0166-4328(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 43.Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468:347–60. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- 44.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- 45.Jin M, Jin F, Zhang L, Chen Z, Huang H. Two estrogen replacement therapies differentially regulate expression of estrogen receptors alpha and beta in the hippocampus and cortex of ovariectomized rat. Brain Res Mol Brain Res. 2005;142:107–14. doi: 10.1016/j.molbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–80. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 47.Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17beta-estradiol and the phytoestrogen, coumestrol. Brain Res Mol Brain Res. 1999;67:165–71. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 48.Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Physiol. 1996;271:E763, 72. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- 49.Damianisch K, Rupprecht R, Lancel M. The influence of subchronic administration of the neurosteroid allopregnanolone on sleep in the rat. Neuropsychopharmacology. 2001;25:576–84. doi: 10.1016/S0893-133X(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 50.Lo CC, Chou T, Penzel T, et al. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci U S A. 2004;101:17545–8. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mong JA, Devidze N, Frail DE, et al. Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: Evidence from high-density oligonucleotide arrays and in situ hybridization. Proc Natl Acad Sci U S A. 2003;100:318–23. doi: 10.1073/pnas.262663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribeiro AC, Pfaff DW, Devidze N. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci. 2009;29:795–801. doi: 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 54.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–95. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- 57.Alfinito PD, Chen X, Mastroeni R, Pawlyk AC, Deecher DC. Estradiol increases catecholamine levels in the hypothalamus of ovariectomized rats during the dark-phase. Eur J Pharmacol. 2009;616:334–9. doi: 10.1016/j.ejphar.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 58.Silveyra P, Cataldi NI, Lux-Lantos V, Libertun C. Gonadal steroids modulated hypocretin/orexin type-1 receptor expression in a brain region, sex and daytime specific manner. Regul Pept. 2009;158:121–6. doi: 10.1016/j.regpep.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–82. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- 60.Ishizuka Y, Pollak CP, Shirakawa S, et al. Sleep spindle frequency changes during the menstrual cycle. J Sleep Res. 1994;3:26–9. doi: 10.1111/j.1365-2869.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 61.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–35. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 62.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–91. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Endo T, Schwierin B, Borbely AA, Tobler I. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 64.Deurveilher S, Cumyn E, Peers T, Rusak B, Semba K. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in fore-brain arousal regions of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1328–40. doi: 10.1152/ajpregu.90576.2008. [DOI] [PubMed] [Google Scholar]
- 65.Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006;26:3037–44. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simerly RB, Carr AM, Zee MC, Lorang D. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 67.Hawkins RA, Freedman B, Marshall A, Killen E. Oestradiol-17 beta and prolactin levels in rat peripheral plasma. Br J Cancer. 1975;32:179–85. doi: 10.1038/bjc.1975.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The frequency distributions of NREMS (A) and REMS (B) episodes as a function of episode duration (s) during the recovery dark phase in the Oil, LE, HE, LP, LEP, and M groups. All episodes of NREMS and REMS were sorted into nine consecutive bins of increasing duration on a logarithmic scale. There were significant group differences in the number of NREMS episodes ≤ 70 s (F5,41 = 8.08; P < 0.0001) and the number of REMS episodes ≤ 150 s (F5,41 = 3.68; P < 0.01). Thus, the LE and HE groups had more NREMS episodes ≤ 70 s than the LP group (P < 0.05 vs. LP), while the M group had more NREMS episodes than the Oil, LP, and LEP groups (P < 0.05 vs. M). The HE and LEP groups had a lower number of REMS episodes ≤ 150 s than the Oil group (P < 0.05 vs. Oil). Data are shown as means ± SEM of 8 animals per group.* indicates the combined bins for which the factor Group was significant (P < 0.05, ANOVA).
The EEG power values in 5 frequency bands in NREMS (A) and REMS (C) during the baseline (black bars) and recovery (gray bars) dark phases, and spectral distributions of NREMS (B) and REMS (D) EEG power during the recovery dark phase in the Oil, LE, HE, LP, LEP, and M groups. EEG spectral power was normalized to the total power (0.5-50 Hz) in each animal, and the mean values were plotted in 0.5 Hz bins. As there were no significant group differences, the data shown in A and C are collapsed across the 6 groups. During recovery NREMS, irrespective of groups, delta power was increased, while theta, sigma, beta, and gamma power were decreased, compared to the respective baseline values. During recovery REMS, EEG power in all frequency bands was similar to corresponding baseline values. The spectral distribution of relative power of the EEG recorded during NREMS (B) and REMS (D) in the recovery dark phase was similar among groups. Data are shown as means ± SEM of 8 animals per group. #different from baseline, P < 0.05, paired t-test (A,C).