Abstract
Study Objectives:
We explored differences between individuals with DSM-IV-TR diagnoses of primary insomnia (PI) and insomnia related to a mental disorder (IMD) by using serial measurements of self-reported sleep variables (sleep onset latency, SOL; wake after sleep onset, WASO; total sleep time, TST; sleep efficiency, SE), and visual analogue scale ratings of 2 forms of bedtime arousal (cognitive and emotional). Furthermore, we sought to examine the relationship between sleep and arousal within each diagnostic subgroup.
Design:
Between-group and within-group comparisons.
Setting:
Duke and Rush University Medical Centers, USA.
Participants:
One hundred eighty-seven insomnia sufferers (126 women, average age 47.15 years) diagnosed by sleep specialists at 2 sleep centers as PI patients (n = 126) and IMD patients (n = 61).
Interventions:
N/A
Measurements and Results:
Multilevel models for sleep measures indicated that IMD displayed significantly more instability across nights in their TST (i.e., larger changes) than did PI patients. With respect to pre-sleep arousal, IMD patients exhibited higher mean levels of emotional arousal, as well as more instability on the nightly ratings of this measure. Within the PI group, correlational analyses revealed a moderate relationship between the 2 arousal variables and SOL (r values 0.29 and 0.26), whereas the corresponding correlations were negligible and statistically nonsignificant in the IMD group.
Conclusions:
We found a number of differences on nighttime variables between those diagnosed with primary insomnia and those diagnosed with insomnia related to a mental disorder. These differences imply different perpetuating mechanisms involved in their ongoing sleep difficulties. Additionally, they support the categorical distinctiveness and the concurrent validity of these insomnia subtypes.
Citation:
Sánchez-Ortuño MM; Carney CE; Edinger JD; Wyatt JK; Harris A. Moving beyond average values: assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. SLEEP 2011;34(4):531-539
Keywords: DSM-IV-TR, primary insomnia, insomnia related to a mental disorder, measurement, instability, arousal, concurrent validity
INTRODUCTION
Primary insomnia (PI) and insomnia related to a mental disorder (IMD), as defined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Text Revision (DSM-IV-TR), are the 2 most common insomnia diagnoses,1 but distinguishing among them may be difficult in clinical practice.2–5 The implied distinction, based on diagnostic criteria, suggests differential etiologic roles for negative conditioning, inadequate sleep hygiene, and comorbid symptoms as precipitating and/or perpetuating factors of the observed insomnia syndrome.1,6 Whereas both types of insomnia may have negative conditioning, poor sleep habits, and even comorbid psychiatric conditions, PI is thought to relate more strongly to conditioned nighttime arousal and poor sleep habits, and IMD is thought to relate more strongly to the emotional and cognitive arousal associated with the comorbid condition. Nonetheless, the literature evidencing distinctive features of the insomnia syndrome in these 2 diagnostic groups is scarce.
Research focused on demonstrating a categorical distinction between these 2 diagnoses has traditionally compared the prototypical subtype of PI,7 psychophysiological insomnia, with IMD. With this aim, a study published in 2005 by Kohn and Espie8 showed that there were no significant differences between the 2 groups on self-reported sleep. By contrast, they found that the IMD sufferers exhibited poorer sleep hygiene. Moreover, the levels of self-reported somatic arousal, cognitive arousal, and depressive symptomatology were significantly higher in individuals with a diagnosis of IMD relative to those with psychophysiological insomnia. When conducting a forward stepwise logistic-regression model to identify which variable or subset of variables might best discriminate between the 2 groups, they found that, rather than any insomnia specific variable, only depressive symptomatology discriminated IMD patients from PI patients. Based on these results, the authors suggested that psychophysiological insomnia and IMD may be on a continuum of insomnia severity, rather than being categorically distinct.
Nevertheless, the results of a previous study9 provide an alternate view of the distinction between these 2 diagnostic sub-types. In this study, both psychophysiological insomnia and IMD patients experienced cognitive and somatic arousal at bedtime to the same extent. However, within the group of psychophysiological insomnia, nocturnal sleep disturbance (e.g., sleep onset latency) was positively related to the level of pre-sleep arousal, whereas this relationship was not observed within the IMD group. By contrast, within the IMD group, pre-sleep arousal was related to daytime symptoms, such as anxiety and depression. These findings suggest that the mechanisms underlying the 2 disorders may be different. Within the IMD group there appears to be a linkage between sleep disturbance and the psychiatric disorder per se, whereas in the PI group sleep disturbance appears more directly related to a circumscribed sleep-focused arousal. Based on such findings, IMD and PI may be viewed as categorically distinctive entities.
However, some caveats should be noted in regard to the literature addressing differences between these diagnostic categories. First, the majority of studies in this area use sleep variables that are based on either a single night of sleep monitoring or an average across nights. This approach loses sight of an important component of sleep measures that has been often overlooked, the nightly intra-individual variability.10,11 A recently published study considering intra-individual variability in sleep duration and fragmentation12 shows that higher intra-individual variability is associated with higher psychological and physiological indices of stress, especially among those individuals scoring high in negative affect. Given this observation, nightly variability of sleep parameters may be important to consider when comparing PI with IMD. Admittedly, if the insomnia symptoms are related to the psychiatric symptoms in patients with IMD, one would expect an accrued instability in the sleep parameters that may mirror the temporal instability of affect characterizing anxiety or depression disorders.13–15
Another limitation of previous studies relates to the use of one-time point measures of sleep-related symptoms (e.g., pre-sleep arousal). This raises the important question of whether a measure taken at one point in time reflects the “habitual” state or is merely an isolated observation that may be typical of a longer time horizon. Indeed since, by definition, “state” refers to an acute and situationally driven episode,16 multiple assessments of the state would ultimately offer a more naturalistic and valuable approximation to the construct within a predetermined time window.
Finally, given the important and sometimes controversial role of pre-sleep arousal in insomnia,17,18 a more detailed delineation of its sub-dimensions or channels is warranted. Indeed, it would be interesting to examine if individuals with insomnia vary in their tendency to manifest arousal via different “channels,” such as mood-related activation or purely cognitive activation, for example. Hence, differences between PI patients and IMD patients regarding pre-sleep arousal could emerge not only in overall level of arousal, but also in mode or dimension of cognitive/emotional arousal. A more detailed study of pre-sleep arousal may provide important information regarding mechanisms contributing to disturbed sleep in both diagnostic groups and may suggest more precise therapeutic alternatives.
The present study was undertaken to overcome some of the limitations in the literature mentioned thus far in order to ascertain differences in PI and IMD patients. In the current investigation, we employed a number of strategies to improve upon previous studies including: (1) multiple nights of assessment; (2) a decomposition of pre-sleep arousal into 2 dimensions, such as active mind and anxiety; (3) an innovative measure of variability/instability that uses adjacent temporal changes15; and (4) a statistical procedure, multilevel modelling analysis, that adjusts for the number of observations each individual has available.19 We sought to examine differences between PI and IMD patients on self-reported sleep parameters and self-reported pre-sleep arousal. In addition, we aimed to investigate, within each of the 2 diagnostic groups, the relationship between these forms of pre-sleep arousal and sleep variables.
METHODS
Participants
Participants for this study were a subset of those participating in a larger investigation designed to examine the reliability and validity of the DSM-IV-TR1 and ICSD-26 insomnia diagnoses conducted at 2 sites, Duke University Medical Center, Durham, NC, and Rush Medical Center, Chicago, IL (MH067057). The recruitment of participants for this specific study took place from August 2004 to May 2008 through posted announcements and referrals by providers working at the collaborating study sites. Thus, we aimed to recruit not only research volunteers, but also treatment-seeking individuals.
Given the objectives of the parent study, the inclusion and exclusion criteria were designed to allow enrollment of a broad and diverse group of insomnia sufferers. To be included, study candidates had to: (1) meet Research Diagnostic Criteria for a general insomnia disorder,20 (2) be 18 years of age or older, and (3) be fluent in English. The exclusion criteria were designed to prevent enrollment of those insomnia sufferers who would be unable to fully participate in this study completely and safely. Thus, we excluded those who were: (1) suffering an unstable or life-threatening medical condition that would make it difficult to complete study procedures; (2) imminently suicidal; (3) at least moderately cognitively impaired as evidenced by a score < 24 on the Mini Mental Status Exam; or (4) medical or psychiatric inpatients at the time of volunteering for the study. Finally, we excluded study candidates who had previously been evaluated by any of the study clinicians.
Questionnaires
Once enrolled, participants were given several questionnaires to complete as part of their intake evaluation. Included among these were the 21-item Beck Depression Inventory (BDI-I),21 the 21-item Beck Anxiety Inventory (BAI),22 the Epworth Sleepiness Scale (ESS),23 the Fatigue Severity Scale (FSS),24 and the Pre-Sleep Arousal Scale, (PSAS).18 In addition, study participants completed a self-report sleep history questionnaire. This is a 10-page instrument containing questions about demographic information, current and past sleep complaints, medical and psychiatric history, and previous treatment history.
Sleep Diary Data and Pre-Sleep Arousal Visual Analogue Scale Ratings
All participants were asked to gather 2 weeks of sleep diary data. Variables derived from the diaries included bedtime, sleep onset latency (SOL), number of nocturnal awakenings, time awake after sleep onset and prior to final awakening (WASO), time of final awakening, time of rising out of bed, total time awake, time in bed (TIB: calculated as time from reported lights out to time of reported rising out of bed), total sleep time (TST: calculated by subtracting the final wake-up time, WASO, and SOL from TIB) and sleep efficiency (SE = total sleep time/time in bed * 100%). For the purposes of the current study, nightly estimates of SOL, WASO, TST, and SE were selected for our analyses.
Along with the sleep diaries, the subset of participants included in this embedded study were requested to complete daily a form with 5 questions related to their perceived level of arousal at bedtime the previous night. The questions were: (1) How physically tense did you feel last night? (2) How active was your mind last night? (3) How anxious did you feel last night? (4) How worried were you last night? and (5) How frustrated/angry did you feel last night? The participants were asked to answer to each question by means of a visual analogue scale (VAS). The VAS consisted of a 12-cm line on paper with verbal anchors labeling the ends, “Not at all” and “Extremely.” Respondents were asked to mark the location that best corresponded to their level of arousal the previous night. These ratings were converted into numeric values based on their location on the VAS line. Responses to each VAS ranged from 0 to 120.
Since it is expected arousal measures to be highly correlated, we elected to reduce the number of arousal measures included in this study in order to simplify the analyses. We explored our arousal measures by means of a factor analysis. This statistical technique allowed us to determine how the different dimensions of arousal cluster into characteristically similar factors. A principal-components factor analysis with oblique rotation was performed on the 5 arousal measures. Two factors best fitted the data, explaining 89.1% of the total variance of observations. Factor 1 grouped together physical tense, anxiety, worry, and frustration ratings, with anxiety and worry loading most highly on this factor, whereas the active mind rating loaded highly on the second factor. This finding was theoretically interpretable. Actually, it distinguishes a pure measure of cognitive arousal, “active mind,” from other arousal manifestations additionally incorporating somatic and emotional components, such as “anxiety,” as a classical example. Based on these observations, we selected active mind and anxiety as the arousal measures to be included herein. We computed the correlation between these 2 selected VAS ratings. Since we had repeated observations for each individual, we computed the weighted correlation coefficient, which takes into account the different number of observations on each individual.25 Consistent with expectation, this correlation was statistically significant, 0.72, P < 0.01. Nonetheless, even if this correlation coefficient is large, it does suggest some distinctiveness between the 2 types of arousal.
Polysomnography
Since insomnia patients show considerable night-to-night sleep variability, all participants underwent 2 consecutive nights of laboratory polysomnographic (PSG) monitoring instead of one. PSGs were scored using standard scoring criteria for sleep stages,26 apneas/hypopneas,27 and periodic limb movements (PLMS) and related arousals.28 PSG summary data included an apnea-hypopnea index, PLMS index, and a desatu-ration index (i.e., number of O2 declines ≥ 3% from baseline per hour of sleep), along with bedtime, SOL, number of nocturnal awakenings, WASO, time of final awakening, time of rising out of bed, TST, total time awake, TIB, and SE.
Diagnostic Interviews
Six clinicians with specialty training in sleep disorders medicine were identified to serve as study clinicians at each site. These 6 clinicians were paired to form 3 clinician dyads, according to the following procedure: they were first stratified by sleep medicine experience level (< 10 yr vs. 10 yr) and by professional training background (MD vs. PhD). They then were randomly assigned to dyads within strata so as to equate the pairs in terms of their experience and clinical specialty. Each dyad was randomly assigned to one of 3 different assessment methods: the first method included the use of the Duke Structured Interview for Sleep Disorders (DSISD).29 The DSISD includes questions that incorporate criteria for ascertaining sleep disorders within both the DSM-IV-TR and ICSD-2 sleep disorder nosologies. The second method included a standard clinical interview complemented by the patients' self-reported sleep history questionnaires and sleep diaries. Lastly, the third method added the PSG reports to a standard clinical interview, sleep history questionnaires, and sleep diaries. Immediately after the interview with each participant, the clinicians used Electronic Diagnostic Rating Forms to assign insomnia diagnoses. The rating forms consisted of 2 series of insomnia diagnoses presented on the screen of a specially programmed PDA hand-held computer. The first series of diagnostic choices consisted of 10 insomnia diagnoses taken from the DSM-IV-TR manual. The second series of diagnoses consisted of 34 choices selected from the ICSD-2 manual. Each diagnosis appeared on the PDA screen individually accompanied by a 100 pixel visual analogue scale (VAS) labeled “doesn't fit at all” at its left extreme and “fits extremely well” at its right extreme. Clinicians were instructed to consider each diagnosis separately and rate how well that diagnosis “fit” the study participant in question. Clinicians used the PDA stylus to move a sliding pointer to the location on the VAS to indicate the goodness of fit for each DSM-IV-TR and ICSD-2 insomnia diagnosis listed. These ratings were converted into numeric values based on their location on the 100 pixel VAS line.
Procedure
Participants fulfilling the inclusion criteria completed the self-report test battery and underwent the Structured Clinical Interview for DSM-IV, SCID,30 conducted by the study coordinator at the study site. Once the sleep history questionnaire, 2 weeks of sleep diary monitoring, VAS ratings, and the PSGs were completed, each participant was randomly assigned to one of the 6 possible orders of the 3 interview methods. That way, each participant was assessed, at different time periods, by the 3 different methods and, thus, by each one of the dyads.
A total of 223 individuals (150 from Duke University Medical Center and 73 from Rush Medical Center) in this embedded study underwent the procedures mentioned above and, thus, were assigned DSM-IV-TR diagnoses and ICSD-2 diagnoses by the 6 clinicians at each site. Just the DSM-IV-TR-based diagnoses were considered for the purposes of the present study. For each participant, diagnostic ratings provided by the 6 clinicians on each of the 10 DSM-IV-TR categories were combined into an average value. That way, each participant had 10 mean ratings (one for each of the 10 DSM-IV-TR sleep diagnostic categories that appeared in the PDA) with higher mean values connoting the better the fit of a diagnosis.
From the 223 individuals that were assessed, we selected those whose highest mean diagnostic rating was for the diagnosis “insomnia related to mental disorder” (n = 61) as well as those whose highest mean diagnostic rating was for the diagnosis “primary insomnia” (n = 126). Obtaining the highest mean rating in, for example, the category “primary insomnia” connoted that, combining the input of 6 different clinicians, the diagnosis of primary insomnia best fit the symptoms of this particular participant. These 187 individuals contributed 2600 valid nights of sleep diary and pre-sleep arousal VAS ratings: on average 13.9 (SD = 2.5) nights per participant.
All study procedures were reviewed and approved by the institutional review boards of the 2 collaborating study sites. All participants were required to provide written informed consent prior to enrolling the research and undergoing the study-related procedures. All participants received parking expenses plus a maximum $400.00 payment if they completed all study procedures.
Data Analyses
Three different sets of analyses were conducted in this study. With our first set of analyses we compared diagnostic groups (PI and IMD) in regard to sociodemographic variables and questionnaire measures taken at one-time point. Group differences in continuous measures were analyzed with t-tests and, when the distributions were not normal, with Mann-Whitney U tests. Between-group differences on categorical variables were assessed using χ2 analyses for contingency tables. These analyses were performed using SPSS, version 15.0.1 (Chicago, IL).
The goal of the second set of analyses was to explore diagnostic group differences in measures obtained at several time points, such as sleep diary variables and pre-sleep arousal VAS ratings. Prior to conducting analyses with the pre-sleep arousal VAS ratings, we explored their concurrent validity by computing their correlation with the PSAS scores. As can be seen in Table 1, the 4 correlations were statistically significant. As expected, “anxious” rating showed a slightly higher correlation with the PSAS Somatic scale score than did the “active mind” rating. For purely descriptive purposes, we calculated the mean values and standard deviation of each variable within each diagnostic group before conducting group comparisons. Since the number of measurement occasions (nights) of individuals were unequal (nights ranging from 3 to 18, mean = 13.9, SD = 2.5), each individual's mean was weighted by nights, following the procedure suggested by Bland and Kerry,31 to compute the overall mean for each diagnostic group. To explore between-group differences, a multilevel model (MLM) was then fitted for each sleep variable and each pre-sleep arousal measure, using the MIXED procedure19 of SPSS, version 15.0.1. MLMs were chosen for these specific analyses because they take into account the hierarchical structure present in data such as ours, wherein multiple observations (named level 1 variables) were nested within individuals, and individuals (named level 2 variables) were grouped into diagnostic categories (PI or IMD). Inasmuch as the difference in the gender composition of the 2 diagnostic groups approached statistical significance (P = 0.058), we added gender as a covariate in our MLMs. Subsequently, 2 binary variables, i.e., diagnostic group and gender, were included as fixed-effects predictors in the MLMs.
Table 1.
Correlations between arousal measures
| VAS – Active mind | VAS – Anxious | |
|---|---|---|
| PSAS – Somatic scale | 0.31** | 0.36** |
| PSAS – Cognitive scale | 0.48** | 0.48** |
P < 0.01 (two-tailed)
Since we had several consecutive observations of sleep parameters and pre-sleep arousal ratings for each individual, another parameter could be analyzed in our dataset: the night-to-night instability of these sleep and arousal measures. Following the procedure proposed by Jahng et al.15 we calculated, for each individual included in the dataset, the differences between consecutive measurements, and then squared each of those differences. For example, if a given individual had 10 nights of observations, there would be 9 successive differences in the variable of interest, e.g., [value of night 2 – value of night 1]^2, [value of night 3 – value of night 2]^2, and so forth. As can be deduced, these successive differences capture both variability and temporal dependency in a time series.32
To ascertain whether group differences existed on our indices of instability, we used generalized MLMs. We chose generalized MLMs due to the characteristics of the dependent variable: the square successive differences (SSDs). Although successive differences may be normally distributed, their squared terms are positively skewed. Thus, following the guidelines provided by Jahng et al. for the analysis of SSDs, we fitted generalized MLMs with the NLMIXED procedure15 in SAS, version 9.1 (SAS Institute, Cary, NC).
Finally, to test whether there was a relationship between measures of pre-sleep arousal and sleep variables, correlation coefficients were computed separately in each of the 2 diagnostic groups. Inasmuch as we had different numbers of measurements on each individual, we calculated weighted correlation coefficients following the procedure suggested by Bland and Altman.25 These correlational analyses were performed using the statistical package SPSS, version 15.0.1 (Chicago, IL),
All dependent variables were examined for normality of distribution. Logarithmic transformations (log to the base of ten) were applied to the skewed distributions of SOL and WASO. For SE and VAS data, arcsine square-root transformations were used. Since we conducted multiple hypothesis testing, to guard against false positives we used a family-wise α level. For each family of pairwise comparisons (questionnaire measures, sleep diary measures, and arousal measures) we divided the type I error probability, 0.05, by the number of comparisons being made. That way, statistical significance for the set of comparisons involving questionnaire measures was set at < 0.0084. Concerning diary-derived sleep measures, the α value associated to statistical significance was set at < 0.0125. Lastly, for the comparisons involving arousal ratings, statistical significance was set at < 0.025.
RESULTS
Sociodemographic and Clinical Characteristics of Patients
The study sample included 126 individuals with the diagnosis of PI and 61 individuals with the diagnosis of IMD. Descriptive data of sociodemographic and clinical characteristics of patients within each diagnostic group are summarized in Table 2.
Table 2.
Sociodemographic and clinical characteristics of patients
| Primary insomnia group (n = 126) | Insomnia related to a mental disorder group (n = 61) | P value | |
|---|---|---|---|
| Age, mean (SD), y | 48.07 (15.02) | 45.21 (13.44) | 0.212a |
| Education duration, mean (SD), y | 15.35 (3.14) | 14.77 (2.84) | 0.194b |
| Sex, No. (%), Female | 91 (72.2) | 35 (58.3) | 0.058 |
| Ethnic group, No. (%) | 0.298 | ||
| Caucasian | 80 (63.5) | 31 (51.7) | |
| African American | 39 (31.0) | 24 (40.0) | |
| Other | 7 (5.6) | 5 (8.3) | |
| Marital status, No. (%) | 0.001 | ||
| Single | 32 (25.4) | 27 (45.0) | |
| Married or live-in partner | 71 (56.3) | 16 (26.7) | |
| Separated, divorced, or widowed | 23 (18.3) | 17 (28.3) | |
| Beck Anxiety Inventory (BAI) | 9.02 (7.14) | 14.18 (10.07) | < 0.001b |
| Beck Depression Inventory (BDI) | 13.86 (9.46) | 19.38 (11.00) | 0.001a |
| Pre-sleep cognitive arousal (PSAS-COG) | 18.42 (7.56) | 21.64 (7.10) | 0.008a |
| Pre-sleep somatic arousal (PSAS-SOM) | 11.48 (3.96) | 13.91 (4.65) | < 0.001b |
| Fatigue Severity Scale (FSS) | 4.21 (1.41) | 4.54 (1.55) | 0.168a |
| Epworth Sleepiness Scale (ESS) | 8.57 (4.79) | 9.17 (5.25) | 0.444a |
| Sleep medication use, nights/month, mean (SD) | 7.38 (10.97) | 10.13 (12.54) | 0.148a |
| Medication use for anxiety or depression, No. (%) | 23 (18.5) | 27 (44.3) | < 0.001 |
P value from a t-test analysis.
P value from a Mann-Whitney analysis.
In addition to those, results from the structured interview for psychiatric disorders (SCID) indicated 49 of the 61 participants assigned IMD diagnoses met SCID criteria for at least one comorbid psychiatric disorder. Thirty-three individuals had a comorbid depressive disorder diagnosis, 8 had a bipolar disorder, 5 had an anxiety disorder, and 3 had a substance-dependence disorder. Of the remaining 12 individuals with an IMD diagnosis, 2 were taking psychiatric medication, 3 fulfilled criteria for a SCID lifetime diagnosis, and 4 self-reported an anxiety and/or depression disorder on the sleep history questionnaire.
Furthermore, data collected in the sleep history questionnaire also showed that the 3 most common self-reported health conditions within the PI group were fatigue (46%), weight control problems (38.9%), and problems related to the back, neck, or spine (29.4%). These health conditions were also the most frequently selected within the IMD group: fatigue (63.9%), weight control issues (41%), and back, neck, or spine problems (41%). Differences on self-reported mental health status appeared more salient: approximately three-quarters of the IMD group self-reported depression, and 57% self-reported anxiety. Less than one-fifth of the PI group declared the presence of an anxiety (18.3 %) or depression problem (16.7%).
Sleep Diary Measures and Pre-Sleep Arousal VAS Ratings
Mean values of self-reported sleep variables, i.e., SOL, WASO, TST, and SE, and pre-sleep arousal VAS scores, i.e., Active mind and Anxious, are displayed in Table 3. No significant between-group differences emerged when considering the sleep diary variables as the dependent variables in the MLMs (Table 4). In terms of pre-sleep arousal, and in agreement with the results obtained from the pre-sleep arousal questionnaire (PSAS), the IMD group scored significantly higher on the VAS measure reflecting emotional arousal, whereas the hypothesis that the IMD group exhibited higher cognitive arousal approached statistical significance.
Table 3.
Descriptive statistics of sleep diary and pre-sleep arousal ratings.
| Primary insomnia group (n = 126) | Insomnia associated to a mental disorder group (n = 61) | |
|---|---|---|
| Sleep diary measures | ||
| SOL (in minutes) | 42.69 (31.24) | 57.53 (38.71) |
| WASO (in minutes) | 75.22 (40.58) | 72.49 (42.20) |
| TST (in minutes) | 367.83 (62.40) | 364.77 (80.84) |
| SE (in %) | 75.83 (10.22) | 73.44 (12.70) |
| Pre-sleep arousal ratings (VAS) | ||
| Active mind | 47.58 (26.44) | 56.75 (25.18) |
| Anxious | 34.25 (24.24) | 45.24 (24.52) |
Values are mean (SD). SOL, sleep onset latency; WASO, wake after sleep onset; TST, total sleep time; SE, sleep efficiency; VAS, visual analogue scale
Table 4.
Estimates of fixed effects of multilevel model for sleep diary measures and pre-sleep arousal measures
| Dependent variable | Fixed effects | Coefficient | SE | t | P |
|---|---|---|---|---|---|
| Sleep diary measures | γ | ||||
| Sleep onset latency | Intercept | 1.48 | 0.04 | 32.16 | < 0.001 |
| Gender | -0.07 | 0.05 | -1.41 | 0.160 | |
| Group | 0.10 | 0.05 | 2.05 | 0.041 | |
| Wake after sleep onset | Intercept | 1.66 | 0.05 | 34.51 | < 0.001 |
| Gender | 0.02 | 0.05 | 0.49 | 0.624 | |
| Group | -0.04 | 0.05 | -0.77 | 0.444 | |
| Total sleep time | Intercept | 353.44 | 10.15 | 24.60 | < 0.001 |
| Gender | 19.08 | 11.09 | 1.72 | 0.087 | |
| Group | -0.84 | 11.08 | -0.07 | 0.940 | |
| Sleep efficiency | Intercept | 1.06 | 0.02 | 52.75 | < 0.001 |
| Gender | 0.02 | 0.02 | 0.80 | 0.424 | |
| Group | -0.02 | 0.02 | -1.02 | 0.307 | |
| Pre-sleep arousal ratings (VAS) | |||||
| Active mind | Intercept | 0.65 | 0.04 | 15.81 | < 0.001 |
| Gender | 0.01 | 0.04 | 0.34 | 0.731 | |
| Group | 0.10 | 0.04 | 2.23 | 0.027 | |
| Anxious | Intercept | 0.53 | 0.04 | 13.61 | < 0.001 |
| Gender | -0.02 | 0.04 | -0.45 | 0.650 | |
| Group | 0.10 | 0.04 | 2.41 | 0.017 |
Gender variable was coded as 0: Male and 1: Female. Group variable was coded as 0: Primary insomnia and 1: Insomnia related to a mental disorder.
VAS, visual analogue scale.
Instability in Sleep Diary Measures and Pre-Sleep Arousal VAS Ratings
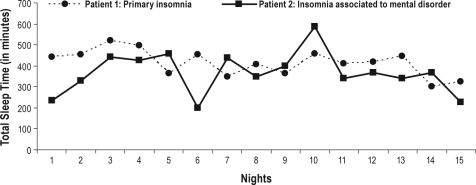
Results derived from the generalized MLMs for each one of the dependent variables are presented in Table 5. As can be seen, patients in the IMD group had more nightly intra-individual instability in TST than PI patients. Figure 1 illustrates such a result with a representation of the night-to-night self-reported TST of a PI patient and an IMD patient. This figure shows that the IMD patient exhibits more dramatic changes in TST across nights when compared with the PI patient. In contrast, no significant differences were found between the groups on SOL instability, WASO instability, or SE instability.
Table 5.
Estimates of fixed effects of generalized multilevel model for night-to-night instability (square successive differences, SSDs) of sleep diary and pre-sleep arousal measures
| Dependent variable | Fixed effects | Coefficient | SE | t | P |
|---|---|---|---|---|---|
| Sleep diary measures | γ | ||||
| Sleep onset latency – SSDs | Intercept | 6.90 | 0.23 | 30.42 | < 0.001 |
| Gender | -2.30 | 3.47 | -0.66 | 0.509 | |
| Group | 0.09 | 0.07 | 1.21 | 0.226 | |
| Wake after sleep onset – SSDs | Intercept | 8.01 | 0.14 | 57.33 | < 0.001 |
| Gender | -2.95 | 2.15 | -1.37 | 0.171 | |
| Group | 0.02 | 0.04 | 0.48 | 0.632 | |
| Total sleep time - SSDs | Intercept | 9.19 | 0.09 | 95.04 | < 0.001 |
| Gender | -1.28 | 1.46 | -0.88 | 0.381 | |
| Group | 0.14 | 0.03 | 4.53 | 0.000 | |
| Sleep efficiency – SSDs | Intercept | 5.29 | 0.12 | 42.01 | < 0.001 |
| Gender | -1.64 | 1.95 | -0.84 | 0.400 | |
| Group | 0.07 | 0.04 | 1.81 | 0.072 | |
| Pre-sleep arousal ratings (VAS) | |||||
| Active mind – SSDs | Intercept | 6.28 | 0.16 | 38.07 | < 0.001 |
| Gender | -0.96 | 2.48 | -0.39 | 0.697 | |
| Group | 0.09 | 0.05 | 1.69 | 0.093 | |
| Anxious – SSDs | Intercept | 5.77 | 0.22 | 26.66 | < 0.001 |
| Gender | -1.83 | 3.20 | -0.57 | 0.567 | |
| Group | 0.16 | 0.07 | 2.35 | 0.019 |
Gender variable was coded as 0: Male and 1: Female. Group variable was coded as 0: Primary insomnia and 1: Insomnia related to a mental disorder.
VAS, visual analogue scale.
Figure 1.
Chart of self-reported total sleep time across nights for two age- and gender-matched patients
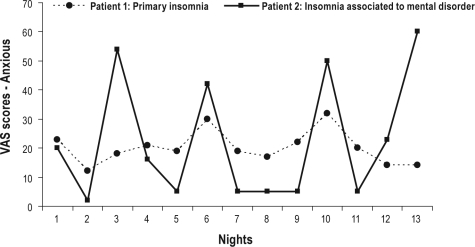
The results of the generalized MLM used to examine instability in the level of cognitive arousal (i.e., Active mind) did not reveal any statistically significant group effect. By contrast, the IMD group showed greater instability than did the PI group with regard to pre-sleep anxious mood ratings. A graphical example of these findings can be seen in Figure 2. It depicts the night-to-night scores on anxiety of a PI patient and an IMD patient. The amplitude of the fluctuations from night-to-night scores is wider for the IMD patient, thus indicating a greater instability in this emotional arousal measure.
Figure 2.
Chart of visual analogue scale ratings (Anxious) across nights for two age- and gender-matched patients
Correlations Between Sleep Diary Measures and Pre-Sleep Arousal VAS Scores
Weighted correlation coefficients between sleep measures and pre-sleep arousal ratings in both diagnostic groups are shown in Table 6. Overall, the association between pre-sleep arousal and sleep seemed stronger in the PI group than in the IMD group. In the PI group, the correlation coefficients be-tween SOL and the 2 pre-sleep arousal measures were statistically significant and in the moderate range in magnitude (r values 0.26 and 0.29), thus indicating that PI patients with high values of pre-sleep arousal also tend to have high values of SOL. With respect to the rest of sleep measures, SE and TST were also correlated with pre-sleep arousal. However, the relationship between pre-sleep arousal and TST was in the opposite direction. That is, the positive, although relatively small in absolute magnitude, correlation coefficient, 0.18, suggested that higher pre-sleep cognitive arousal was related to longer TST. The corresponding correlations were all low and statistically nonsignificant in the IMD group.
Table 6.
Weighted Pearson correlation coefficients of sleep diary measures and pre-sleep arousal ratings
| Sleep diary measures | Primary insomnia group (n = 126) |
Insomnia related to a mental disorder group (n = 61) |
||||||
|---|---|---|---|---|---|---|---|---|
| SOL | WASO | TST | SE | SOL | WASO | TST | SE | |
| Pre-sleep arousal VAS ratings | ||||||||
| Active mind | 0.29*** | 0.04 | 0.18* | −0.11 | 0.04 | 0.07 | −0.21 | −0.10 |
| Anxious | 0.26** | 0.12 | −0.02 | −0.19* | −0.02 | 0.04 | −0.21 | −0.08 |
SOL, sleep onset latency; WASO, wake after sleep onset; TST, total sleep time; SE, sleep efficiency; VAS, visual analogue scale.
P < 0.05;
P < 0.01;
P < 0.001 (2-tailed)
DISCUSSION
Compared to a group of IMD sufferers, PI sufferers exhibited lower night-to-night instability in their TSTs, as measured by sleep diary. Likewise, ratings of pre-sleep emotional arousal also showed a lower instability across nights in the PI group. In this group, self-reported levels of pre-sleep arousal were positively correlated with SOL and negatively correlated with SE, whereas in the IMD group there were virtually no statistically significant associations between pre-sleep arousal and the sleep diary-derived measures. These findings highlighted a number of notable differences between the 2 diagnostic subtypes that may imply differing perpetuating mechanisms involved in their ongoing sleep difficulties and, therefore, support their categorical distinctiveness.
Regarding measures collected at one point in time, we found that depression and anxiety symptoms, as well as self-reported somatic and cognitive pre-sleep arousal, were higher within the IMD group. These between-group differences on self-reported pre-sleep arousal were further reinforced by our nightly assessment of various forms of bedtime arousal. Overall, these findings are in agreement with previous research distinguishing PI from IMD.2,8 However, our findings are not in accord with the idea that a continuum may exist across “primary” and “secondary” insomnia, and that differences between those diagnoses may be more “quantitative” than categorical, as stated elsewhere.2,8 Actually, our results may reveal an alternative hypothesis: that the mechanisms underlying the sleep disruption within each group are different.
Under normal conditions, short and fragmented sleep on one night leads to a longer and more solid sleep the next night as a result of enhanced sleep debt and subsequent elevated sleepiness.33 Therefore, the more unstable night-to-night TST within the IMD group could indicate the existence of a quicker and more efficient mechanism of sleep recovery. In stark contrast, the lower variability exhibited by PI sufferers may suggest that the sleep recovery mechanism is less responsive in this group.
This hypothesis is compatible and builds upon earlier work addressing sleep variability in PI sufferers. The studies by Vallières et al.34 and Perlis et al.35 suggest that a high night-to-night variability in sleep variables is precluded in, at least, a subset of PI sufferers. An appealing explanation to these findings is that there may be a primary defect in the sleep homeostat of PI sufferers that simply requires more sustained sleep debt to produce normal sleep.35 This tenet, as well as our findings, fit well with accepted models about the development and maintenance of PI.36 One of the pivotal elements in those models is the concept of “plasticity of the sleep-wake system.” According to Espie,36 the stability in the sleep pattern would be displayed by small standard deviations, and plasticity would be demonstrated by regression from higher values of wakefulness to lower values of wakefulness. Therefore, the time taken to adjust from above to below some threshold value might be a useful measure of plasticity. Arguably, in the absence of between-group differences in mean TST, the more stable TST values within the PI group would suggest a more impaired plasticity of their sleep-wake system. This impaired plasticity would in turn explain why the repayment of sleep debt may not function efficiently in PI.
Likewise, the more stable level of pre-sleep arousal found within the PI group would be congruent with the idea that conditioned hyper-arousal or, arguably, a conditioned inhibition of de-arousal, is a hallmark in PI.36–38 That is, assuming that heightened arousal or inhibition of de-arousal is conditioned to external and situational cues (i.e., bedroom, bedtime routines), one would expect some stability of the arousal response when and if the situation (e.g., bedroom) remains the same. By contrast, a higher instability of emotional arousal from night to night suggests that different factors, other than the bedroom or bedtime routines, may trigger the bedtime arousal response within the IMD group.
The tenet that different mechanisms underlie the sleep disruption within the 2 diagnostic groups is further supported by our correlational findings. The association found between sleep and arousal measures indicates that the 2 diag-nostic groups may vary in how their overall level of arousal at bedtime relates to diminished self-reported sleep. Indeed, the statistically significant correlations found within the PI would imply that low levels of arousal tend to be linked to shorter SOLs. That was not the case for the IMD group, wherein low levels of bedtime arousal are not associated with better sleep. These findings are in agreement with the results reported by Broman and Hetta,9 and suggest a less straightforward relation-ship of arousal and sleep within the IMD group when compared to the PI group.
It could be argued, however, that the higher use of psychiatric medication within the IMD group may have distorted the relationship between arousal and sleep. So as to test this hypothesis, we selected individuals not taking any psychiatric medication and computed again the correlations coefficients between the 2 indexes of bedtime arousal and SOL in this subset of “non-treated” IMD patients (n = 34). As was the case for the entire IMD group, these correlations were all non-statistically significant.
In reviewing our findings, we keep in mind the issues highlighted by previous research concerning the difficulties in distinguishing PI from IMD and the influence of the clinician's background on diagnosis assignment.3–5 Nonetheless, the procedure used herein to assign insomnia diagnoses may have overcome some of these limitations. Our method for diagnosis assignment didn't allow us to determine whether the PI and IMD groups were composed exclusively of “pure” cases of these distinctive diagnostic subtypes. However, by combining the diagnostic impressions provided by 6 clinicians with different backgrounds, it would seem that participants included in each group had either a “predominant” PI disorder or a “predominant” IMD disorder. Furthermore, it should be noted that the clinicians' diagnostic assignments were independent from the scores on the self-report measures (none of the study clinicians had access to data derived from those measures). Hence, the between-group differences noted on our questionnaire measures of anxiety, depression and pre-sleep arousal do support the diagnostic validity of our clinically identified insomnia sub-types. Admittedly, the presence of impure subtypes may have obscured the categorical differences between the 2 groups compared and, therefore, the results presented may underestimate the differences that would be noted between “pure” samples of these distinctive subtypes. Indeed, to test this assumption, we selected within each diagnostic group just “pure” cases based on the SCID diagnoses. Therefore, for the “pure” PI group we selected those individuals not having a SCID diagnosis of another mental disorder, n = 70, and for the “pure” IMD group we retained those individuals with a current SCID diagnosis, n = 49. We then used a generalized MLM to ascertain whether group differences existed on the instability of TST adjusting for the effect of gender. The results obtained confirmed our previous finding: TST instability across nights was greater within the “pure” IMD group when compared to the “pure” PI group (γgroup = 0.72, SE = 0.15; t117 = 4.65, P < 0.001). Furthermore, when comparing the 2 “pure” groups on instability of arousal across nights, the IMD group showed more instable arousal ratings (Ps < 0.04). These results suggest that, even considering more “pure” insomnia subtypes, between-group differences still emerge.
It also should be noted that, even if one of the study's inclusion criteria was that participants had to meet RDC for a general insomnia disorder, the presenting insomnia symptoms could have been associated with another occult sleep disorder, such as sleep apnea or periodic limb movements. Since only a subset of the study clinicians had access to the PSG results, it is then possible that the clinicians lacking this information made inaccurate judgements about the presence vs. absence of diagnoses such as sleep apnea. However, inasmuch as all the clinicians' judgements were integrated into a mean score, these possible diagnostic errors may have been offset to some degree.
There may be further limitations to this study. These results were obtained using self-report measures. Further comparative work is required to replicate our findings using concomitant objective measures of sleep and psychophysiologic arousal. Another potential problem with the methodology used is that the individuals were instructed to complete the VAS ratings at the time they completed the sleep diaries. We would probably have obtained more accurate indices of arousal had we collected the arousal ratings by ecological momentary assessment at bedtime,39 rather than by relying on retrospective reports provided the following day.
In summary, taking advantage of the fine-grain picture provided by using multiple time points of assessment, our results indicate that the nightly fluctuations in self-reported sleep duration were greater in a group of IMD patients. This may suggest the presence of a quick homeostatic rebound effect in the IMD group, which may be precluded in PI. Likewise, the evidence of a more stable bedtime emotional arousal, coupled with a relationship between arousal levels and SOL within the PI group, would also support the contention about the role of a conditioned arousal response in the PI disorder. By contrast, the relative independence of the sleep measures from the level of bedtime arousal in the IMD group, as well as a higher variability in night-to-night emotional arousal, may indicate that there are different pathways to sleep disturbance in these insomnia subtypes.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Edinger has consulted for Philips-Respironics and Kingsdown and has received research support from Philips-Respironics and Helicor. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by a Pickwick Fellowship Award (Dr. Carney) from the National Sleep Foundation and by the National Institute of Mental Health Grant # R01, MH067057 (Dr. Edinger and Dr. Wyatt). M. Montserrat Sánchez-Ortuño was supported by a research fellowship award from Fundación Séneca, Murcia, Spain.
REFERENCES
- 1.American Psychiatric Association., American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Moul D, Nofzinger E, Pilkonis P, Houck P, Miewald J, Buysse D. Symptom reports in severe chronic insomnia. Sleep. 2002;25:553–63. [PubMed] [Google Scholar]
- 3.Nowell P, Buysse D, Reynolds CF, et al. Clinical factors contributing to the differential diagnosis of primary insomnia and insomnia related to mental disorders. Am J Psychiatry. 1997;154:1412–6. doi: 10.1176/ajp.154.10.1412. [DOI] [PubMed] [Google Scholar]
- 4.Buysse DJ, Reynolds CF, Hauri PJ, et al. Diagnostic concordance for DSM-IV sleep disorders - a report from the apa/nimh DSM-IV field trial. Am J Psychiatry. 1994;151:1351–60. doi: 10.1176/ajp.151.9.1351. [DOI] [PubMed] [Google Scholar]
- 5.Buysse D, Reynolds C, Kupfer D, et al. Clinical diagnoses in 216 insomnia patients using the International Classification of Sleep Disorders (ICSD), DSM-IV and ICD-10 categories: a report from the APA/NIMH DSM-IV Field Trial. Sleep. 1994;17:630–7. doi: 10.1093/sleep/17.7.630. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Sleep Medicine. The International Classification of Sleep Disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 7.Reynolds C, Kupfer D, Buysse D, Coble P, Yeager A. Subtyping DSMIII-R primary insomnia: a literature review by the DSM-IV Work Group on Sleep Disorders. Am J Psychiatry. 1991;148:432–8. doi: 10.1176/ajp.148.4.432. [DOI] [PubMed] [Google Scholar]
- 8.Kohn L, Espie CA. Sensitivity and specificity of measures of the insomnia experience: a comparative study of psychophysiologic insomnia, insomnia associated with mental disorder and good sleepers. Sleep. 2005;28:104–12. doi: 10.1093/sleep/28.1.104. [DOI] [PubMed] [Google Scholar]
- 9.Broman JE, Hetta J. Perceived pre-sleep arousal in patients with persistent psychophysiological and psychiatric insomnia. Nord J Psychiatry. 1994;48:203–7. [Google Scholar]
- 10.Tworoger S, Davis S, Vitiello M, Lentz M, McTiernan A. Factors associated with objective (actigraphic) and subjective sleep quality in young adult women. J Psychosom Res. 2005;59:11–9. doi: 10.1016/j.jpsychores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Knutson K, Rathouz P, Yan L, Liu K, Lauderdale D. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30:793–6. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mezick E, Matthews K, Hall M, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–54. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen R, Clark M, Baetz M. Mood swings in patients with anxiety disorders compared with normal controls. J Affect Disord. 2004;78:185–92. doi: 10.1016/S0165-0327(02)00304-X. [DOI] [PubMed] [Google Scholar]
- 14.Ebner-Priemer U, Eid M, Kleindienst N, Stabenow S, Trull T. Analytic strategies for understanding affective (in)stability and other dynamic processes in psychopathology. J Abnorm Psychol. 2009;118:195–202. doi: 10.1037/a0014868. [DOI] [PubMed] [Google Scholar]
- 15.Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: indices using successive difference and group comparison via multilevel modeling. Psychol Methods. 2008;13:354–75. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman M. Development of a situation-specific trait-state test for prediction and measurement of affective responses. J Consult Clin Psychol. 1977;45:513–23. doi: 10.1037//0022-006x.45.4.513. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet M. Hyperarousal as the basis for insomnia: effect size and significance. Sleep. 2005;28:1500–1. doi: 10.1093/sleep/28.12.1500. [DOI] [PubMed] [Google Scholar]
- 18.Nicassio P, Mendlowitz D, Fussell J, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23:263–71. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 19.Peugh JL, Enders CK. Using the SPSS mixed procedure to fit cross-sectional and longitudinal multilevel models. Educ Psychol Meas. 2005;65:811–35. [Google Scholar]
- 20.Edinger J, Bonnet M, Bootzin R, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory - 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 22.Beck AT, Brown G, Epstein N, Steer RA. An inventory for measuring clinical anxiety - psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness-the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Krupp LB, Larocca NG, Muirnash J, Steinberg AD. The Fatigue Severity scale - application to patients with multiple-sclerosis and systemic lupuserythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Calculating correlation-coefficients with repeated observations. 2. correlation between subjects. BMJ. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechtshaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems of sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 27.Phillipson EA, Remmers JE. American Thoracic Society Consensus Conference on Indications and Standards for Cardiopulmonary Sleep Studies. Am Rev Respir Dis. 1989;139:559–68. doi: 10.1164/ajrccm/139.2.559. [DOI] [PubMed] [Google Scholar]
- 28.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 29.Edinger JD, Wyatt JK, Olsen MK, et al. Reliability and validity of the Duke structured interview for sleep disorders for insomnia screening. Sleep. 2009;32:0810. [Google Scholar]
- 30.Spitzer R, Williams J, Gibbons M, First M. Instruction Manual for the Structured Clinical Interview for DSM-IV (SCID-IV). (SCID 1996 Revision) New York: Biometrics Research Department, New York Psychiatric Institute; 1996. [Google Scholar]
- 31.Bland JM, Kerry SM. Statistics notes - Weighted comparison of means. BMJ. 1998;316:129. doi: 10.1136/bmj.316.7125.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trull TJ, Solhan MB, Tragesser SL, et al. Affective instability: Measuring a core feature of borderline personality disorder with ecological momentary assessment. J Abnorm Psychol. 2008;117:647–61. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- 33.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. Sleep loss and sleepiness - current issues. Chest. 2008;134:653–60. doi: 10.1378/chest.08-1064. [DOI] [PubMed] [Google Scholar]
- 34.Vallieres A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–53. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 35.Perlis ML, Swinkels CM, Gehrman PR, Pigeon WR, Matteson-Rusby SE, Jungquist CR. The incidence and temporal patterning of insomnia: a pilot study. J Sleep Res. 2010;19:31–5. doi: 10.1111/j.1365-2869.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espie CA. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 37.Hauri P, Fisher J. Persistent psychophysiological (learned) insomnia. Sleep. 1986;9:38–53. doi: 10.1093/sleep/9.1.38. [DOI] [PubMed] [Google Scholar]
- 38.Espie CA, Broomfield NM, MacMahon KMA, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Solhan MB, Trull TJ, Jahng S, Wood PK. Clinical assessment of affective instability: comparing EMA indices, questionnaire reports, and retrospective recall. Psychol Assess. 2009;21:425–36. doi: 10.1037/a0016869. [DOI] [PMC free article] [PubMed] [Google Scholar]