Abstract
Background:
Endometrial cancer incidence is increasing in industrialised countries. High body mass index (BMI, kg m−2) is associated with higher risk for disease. We wanted to investigate if BMI is related to clinico-pathological characteristics, hormone receptor status in primary tumour, and disease outcome in endometrial cancer.
Patients and methods:
In total, 1129 women primarily treated for endometrial carcinoma at Haukeland University Hospital during 1981–2009 were studied. Body mass index was available for 949 patients and related to comprehensive clinical and histopathological data, hormone receptor status in tumour, treatment, and follow-up.
Results:
High BMI was significantly associated with low International Federation of Gynaecology and Obstetrics (FIGO) stage, endometrioid histology, low/intermediate grade, and high level of progesterone receptor (PR) mRNA by qPCR (n=150; P=0.02) and protein expression by immunohistochemistry (n=433; P=0.003). In contrast, oestrogen receptor (ERα) status was not associated with BMI. Overweight/obese women had significantly better disease-specific survival (DSS) than normal/underweight women in univariate analysis (P=0.035). In multivariate analysis of DSS adjusting for age, FIGO stage, histological subtype, and grade, BMI showed no independent prognostic impact.
Conclusion:
High BMI was significantly associated with markers of non-aggressive disease and positive PR status in a large population-based study of endometrial carcinoma. Women with high BMI had significantly better prognosis in univariate analysis of DSS, an effect that disappeared in multivariate analysis adjusting for established prognostic markers. The role of PR in endometrial carcinogenesis needs to be further studied.
Keywords: body mass index, endometrial carcinoma, prognosis, progesterone receptor
Endometrial cancer is the most common gynaecological malignancy in industrialised countries (Parkin et al, 2005), and the incidence has been increasing over the last decades (Cancer Registry of Norway, 2009). Obesity is a known risk factor for disease development with a higher risk with increasing body mass index (BMI, kg m−2) (Schouten et al, 2004; Bjorge et al, 2007). It has recently been shown that morbidly obese women (BMI⩾40) have a six-fold increase in risk of disease development (Lindemann et al, 2008). This is presumably related to unopposed oestrogen exposure. After menopause, the ovaries and adrenal glands continue to produce androstenedione, which is converted to oestrone in adipose tissue by the aromatase enzyme. This weaker oestrogen may stimulate chronic endometrial proliferation and cancer development after menopause (Kaaks et al, 2002). Tumours arising in such hyper-oestrogenic environment are typically type I endometrial carcinomas, characterized by endometrioid histology, low grade, hormone receptor-positive status, and good prognosis. In contrast, tumours of type II are typically not oestrogen driven, of non-endometrioid histology, high grade, with loss of hormone receptors and poor prognosis (Bokhman, 1983; Amant et al, 2005). However, the prognostic value of the distinction between type I and type II endometrial cancer is limited, as up to 20% of type I endometrial cancers recur and 50% of type II cancers do not (Engelsen et al, 2009). Diagnostic accuracy and reproducibility of histological subtyping is a challenge. Therefore, there is need for new prognostic markers. Even though it is well established that obesity gives higher risk for endometrial cancer, studies relating BMI to clinical and histopathological markers and survival are scarce, and partly contradictive (Anderson et al, 1996; Duska et al, 2001; von Gruenigen et al, 2006; Temkin et al, 2007; Munstedt et al, 2008; Jeong et al, 2010). In particular, no previous studies have identified molecular markers for hormone receptor status in the tumour tissue related to BMI.
On this background, we have investigated the relationship between BMI and a large panel of clinical and histopathological data, hormone receptor status in primary tumours, and disease outcome in a large population-based endometrial carcinoma series.
Patients And Methods
Patient series
The patient series include 1129 women primarily treated for endometrial carcinoma at Haukeland University Hospital during the period 1981 through 2009. This is the referral hospital for Hordaland county, with ∼475 000 inhabitants, representing about 10% of the Norwegian population (SSB, 2010). The endometrial cancer incidence rate and prognosis in this area are similar to data for the total population (Cancer Registry of Norway, 2009).
Information concerning height, weight, age, menopausal status, International Federation of Gynaecology and Obstetrics (FIGO) stage, histological subtype and grade, treatment, and follow-up was collected by review of the medical records and through correspondence with the primary physicians. In all, 91% of the women underwent hysterectomy with bilateral salpingo-oophorectomy as primary treatment and were classified according to the FIGO 1988 criteria (Mikuta, 1993). If surgical treatment was contraindicated, the staging was based on the available information from curettage results, clinical examination, chest X-ray, and abdomino-pelvic CT.
Follow-up time was defined as the time interval between date of primary diagnosis and date of death or last follow-up. The median follow-up time was 4.9 years (range 0.01–23.2). In all, 223 patients (20%) died from endometrial carcinoma during the follow-up period, while 207 (18%) died from other causes. These data were cross-checked with information from the Cancer Registry of Norway and the Register of Statistics Norway. Last follow-up was 20 December 2009.
Body mass index was calculated as weight (kg) divided by squared height (m2), both measured at the time of diagnosis. These data were available for 949 patients (84%). For the statistical analyses on BMI we used the quartiles for the data set as cut points, as well as the established WHO classification system; BMI under 18.5 (underweight), between 18.5 and 24.9 (normal), between 25 and 29.9 (overweight), and >30 (obese). Height and weight of outliners (BMI<15 and BMI>50, n=7) was double-checked. All analyses were also performed excluding these; this did not affect any of the conclusions.
Immunohistochemistry
Formalin-fixed paraffin-embedded tumour specimens were mounted in tissue microarrays (TMAs) as previously described (Hoos et al, 2001; Stefansson et al, 2004). Briefly, TMA was constructed by identifying the area of highest tumour grade on HE-stained slides, followed by punching out three tissue cylinders from the selected areas of the donor block and mounting these into a recipient paraffin block using a custom-made precision instrument (Beecher Instruments, Silver Spring, MD, USA). Immunohistochemical staining for receptor status was assessed for oestrogen- and progesterone receptors (ERα and PR) and available for 437 and 433 patients for ERα and PR, respectively (38% of study population). The method for immunohistochemical staining was as previously described, using the lower quartile to define receptor loss (Engelsen et al, 2008b).
qPCR analysis
From a subset of 150 patients (13%), fresh frozen tumour tissue was collected prospectively and was available for mRNA analysis in parallel with the immunohistochemical staining. Total RNA was extracted using the RNeasy kit (Qiagen, Hilden, Germany), with quality control and method for data processing as previously reported (Engelsen et al, 2008a; Salvesen et al, 2009). mRNA expression levels in tumours for ERα and PR were investigated by qPCR using the TaqMan Low Density Array technique (Engelsen et al, 2008a).
Statistical methods
Body mass index in WHO categories was applied to assess the distribution of various clinico-pathological variables, using the Pearson's χ2-test. Hormone receptor status in primary tumour in relation to BMI was assessed by the Mann–Whitney U-test. Univariate survival analyses for disease-specific survival (DSS) and overall survival (OS) were performed using the Kaplan–Meier method (log-rank test). The Cox proportional hazard regression analysis was applied to evaluate the prognostic impact of BMI adjusted for the established prognostic markers in endometrial carcinoma. We compared the distribution of clinico-pathological variables and prognosis for patients with available data for BMI to patients where these data were missing (16%). Women lacking BMI data were older, with median age 69.3 years compared with 65.2 years for the group where BMI was registered, P=0.004 (Mann–Whitney U-test). No other significant differences were identified. The statistical software PASWStatistics18.0 was used for data analyses (SPSS Inc., Chicago, IL, USA).
The study was approved by the IRB (NSD 15501, REK III nr 052.01).
Results
High BMI associates with clinico-pathological markers for non-aggressive disease
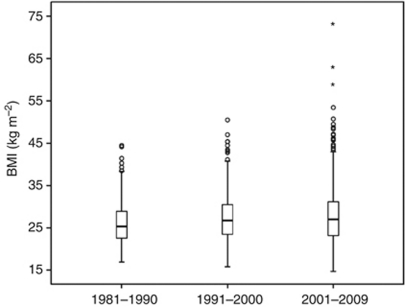
The median BMI at diagnosis was 26.4 (range 14.7–73.0), with significantly increasing BMI throughout the study period, P=0.002 (Figure 1). There was a significant association between BMI and patient age at diagnosis, FIGO stage, and histological subtype, as shown in Table 1. The proportion of patients with BMI<25 was larger in the lower and upper age quartiles compared with BMI⩾25, whereas there was a tendency for the patients of the middle age quartiles to be overweight or obese. The proportion of normal/lean patients was larger for FIGO stages III and IV compared with FIGO stages I and II. High BMI was also associated with endometrioid histology. There was no significant association between BMI and menopausal status nor BMI and grade. Also, there was no significant difference in number of performed lymphadenectomies related to BMI (P=0.99), but a tendency to more adjuvant therapy given to patients with BMI<25 (P=0.06).
Figure 1.
Distribution of BMI for endometrial carcinoma patients treated in one defined region in Norway (Hordaland county) in the periods 1981–1990, 1991–2000, and 2001–2009. Median BMI and range increase significantly from 25.3 (16.9–44.5) to 26.7 (15.8–50.5) and 26.9 (14.7–73.0) for the time periods studied, P=0.002 (Kruskal–Wallis test).  =minor outliers and
=minor outliers and  =major outliers.
=major outliers.
Table 1. Distribution of clinico-pathological factors in 949 patients with endometrial carcinoma according to body mass index (BMI).
| Variable | Total no. of patients | Median BMI | Lean (%) | Normal (%) | Overweight (%) | Obese (%) | P-valuea |
|---|---|---|---|---|---|---|---|
| Age, quartiles b | 949 | 0.002 | |||||
| 1 (age 26–58) | 25.6 | 5 (2) | 109 (45) | 66 (27) | 65 (27) | ||
| 2 (age 58–66) | 27.1 | 8 (3) | 76 (32) | 82 (34) | 73 (31) | ||
| 3 (age 66–74) | 27.3 | 2 (1) | 76 (31) | 91 (37) | 76 (31) | ||
| 4 (age 74–95) | 25.1 | 8 (4) | 97 (44) | 70 (32) | 45 (21) | ||
| Menopause c | 949 | 0.116 | |||||
| Pre/peri | 26.1 | 1 (1) | 54 (44) | 31 (25) | 38 (31) | ||
| Post | 26.4 | 22 (3) | 304 (37) | 278 (34) | 221 (27) | ||
| FIGO stage | 949 | <0.0001 | |||||
| I | 26.6 | 10 (2) | 246 (36) | 224 (33) | 197 (29) | ||
| II | 27.3 | 3 (3) | 30 (29) | 45 (44) | 26 (25) | ||
| III | 24.4 | 7 (6) | 55 (49) | 30 (27) | 21 (19) | ||
| IV | 24.0 | 3 (6) | 27 (49) | 10 (18) | 15 (27) | ||
| Histological subtype | 949 | ||||||
| Endometrioid | 26.6 | 16 (2) | 297 (37) | 269 (33) | 229 (28) | 0.030 | |
| Non-endometrioid | 25.1 | 7 (5) | 61 (44) | 40 (29) | 30 (22) | ||
| Grade d | 905 | 0.174 | |||||
| 1 or 2 | 26.7 | 14 (2) | 242 (36) | 224 (34) | 188 (28) | ||
| 3 | 25.7 | 9 (4) | 99 (42) | 71 (30) | 58 (25) | ||
| PR | 433 | 0.003e | |||||
| Positive | 26.9 | ||||||
| Negative | 25.5 | ||||||
| ER | 437 | 0.08e | |||||
| Positive | 26.7 | ||||||
| Negative | 25.5 |
Abbreviation: FIGO=International Federation of Gynaecology and Obstetrics.
χ2-test when no other specified.
Truncated to closest integer.
Menopausal status was determined based on the information from the patient records.
Data missing for 44 patients.
Mann–Whitney U-test.
High BMI associates with positive PR status in tumour
When investigating biomarkers for receptor status in tumours related to BMI we found that patients with PR-negative tumours (by IHC) had lower median BMI compared with the patients who had PR-positive tumours, median 25.5 vs 26.9, respectively (P=0.003, Mann–Whitney U-test). We did not find any significant correlation between BMI and ERα status in tumours (P=0.08) (Table 1). To further validate this finding, we examined a subset of 150 fresh frozen patient samples for mRNA expression levels for hormone receptors by qPCR. This confirmed a significantly higher mRNA expression level for PR in patients with BMI>25 compared with patients with lower BMI (P=0.02, Mann–Whitney U-test). For ERα, no such association with BMI was observed for mRNA expression levels (P=0.21). Loss of ERα and PR (by IHC) was associated with postmenopausal status (P=0.01 and P=0.006, respectively, Pearson's χ2-test).
BMI and prognosis
Univariate analysis
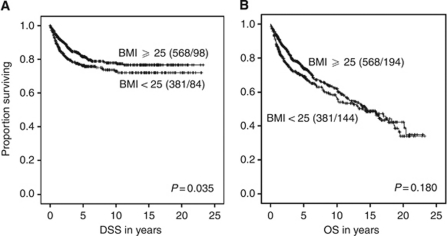
The established clinico-pathological variables showed, as expected, a highly significant impact on DSS, as listed in Table 2. There was a trend towards better prognosis for patients with higher BMI in univariate analysis (Table 2). Patients being overweight/obese vs normal/underweight as defined by the WHO had better DSS, with a 5-year survival of 82% for women with BMI⩾25 compared with 76% for BMI<25 (P=0.035; Figure 2A; Table 2). For OS, we found that patients with BMI<25 had a 5-year survival of 69% compared with 74% for patients with BMI⩾25 (P=0.18; Figure 2B). In the OS analysis, we also see a pattern of diminishing survival difference between the two BMI groups >10 years after diagnosis. This may relate to the higher risk of developing other diseases for overweight women, being more important than the risk for cancer-related deaths >10 years after diagnosis.
Table 2. Univariate survival analysis (Kaplan–Meier estimates) according to clinico-pathological factors and BMI in 1129 endometrial carcinoma patients.
| Variable | No. of patients (no. of deaths)a | 5-year survival | P (log-rank) |
|---|---|---|---|
| Age, quartiles b | <0.0001 | ||
| 1 (age 27–58) | 282 (17) | 94.5 | |
| 2 (age 58–66) | 282 (44) | 84.4 | |
| 3 (age 66–74) | 283 (73) | 73.8 | |
| 4 (age 74–94) | 282 (89) | 63.8 | |
| Sum | 1129 | ||
| Menopausal status | <0.0001 | ||
| Pre/peri | 145 (13) | 93.9 | |
| Post | 983 (87) | 77.1 | |
| Sumc | 1128 | ||
| FIGO stage | <0.0001 | ||
| I | 812 (79) | 90.8 | |
| II | 119 (27) | 74.2 | |
| III | 132 (68) | 39.4 | |
| IV | 65 (48) | 16.3 | |
| Sumd | 1128 | ||
| Histological subtype | <0.0001 | ||
| Endometrioid | 966 (146) | 84.4 | |
| Non-endometrioid | 163 (77) | 46.8 | |
| Sum | 1129 | ||
| Grade | <0.0001 | ||
| 1 | 345 (26) | 92.0 | |
| 2 | 454 (81) | 82.6 | |
| 3 | 283 (105) | 56.9 | |
| Sume | 1082 | ||
| BMI WHO | 0.066f | ||
| Underweight (<18.5) | 23 (7) | 63.3 | |
| Normal (18.5–24.9) | 358 (77) | 77.0 | |
| Overweight (25–29.9) | 309 (51) | 81.9 | |
| Obese (⩾30) | 259 (47) | 81.1 | |
| Sumg | 949 | ||
| BMI quartiles | 0.096f | ||
| 1 (14.7–23.1) | 237 (54) | 75.3 | |
| 2 (23.1–26.3) | 240 (46) | 79.1 | |
| 3 (26.3–30.5) | 236 (39) | 81.3 | |
| 4 (30.5–73.0) | 236 (43) | 81.4 | |
| Sum | 949 | ||
| BMI 2 groups h | 0.035f | ||
| <25 | 381 (84) | 76.3 | |
| ⩾25 | 568 (98) | 81.6 | |
| Sum | 949 | ||
Abbreviations: BMI=body mass index; FIGO=International Federation of Gynaecology and Obstetrics; WHO=World Health Organization.
Number of patients varies due to missing data.
Truncated to closest integer.
Data for menopausal status missing for one patient.
Data for FIGO stage missing for one patient.
Data for grade missing for 67 patients.
P-value with linear trend test.
Data for BMI missing for 180 patients.
Endometrioid carcinomas only: 5-year survival: BMI<25=81.2%, BMI⩾25=85.6% (P=0.134).
Figure 2.
Univariate survival plot by Kaplan–Meier for estimation of DSS (A) and OS (B) in patients with endometrial carcinoma related to BMI. The total number of patients in each group is followed by number of deaths, given in parentheses; P-value based on the Mantel–Cox test.
Multivariate analysis
The survival effect of BMI observed in univariate analysis for DSS disappeared when adjustment was made for age at diagnosis (continuous variable), FIGO stage, histological subtype, and grade in the Cox multivariate regression analysis as listed in Table 3. Adjusted HR for BMI<25 vs ⩾25 was 0.93 (CI 0.68–1.27, P=0.65). When BMI was applied as a continuous variable in the same Cox model, we found a similar insignificant HR for BMI of 1.01 (CI 0.98–1.04), and pattern for the other variables with independent impact for FIGO stage and age only. In contrast, when using OS as end point in the Cox model, we found that BMI had independent impact on prognosis when introduced as a continuous variable with an HR 1.02 (CI 1.00–1.04, P=0.035). FIGO stage and age were also independent predictors of prognosis (P<0.0001 for both), while histology was of borderline significance (P=0.053) and grade was non-significant (P=0.166).
Table 3. Survival analysis of 905 endometrial carcinoma patients based on the Cox proportional hazards model.
| Variable | No. of patients (%) | Unadjusted HRa | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
|---|---|---|---|---|---|---|---|
| FIGO stage | <0.0001 | <0.0001 | |||||
| I | 646 (71) | 1.00 | 1.00 | ||||
| II | 96 (11) | 3.25 | 1.98–5.31 | 2.83 | 1.72–4.65 | ||
| III | 111 (12) | 9.75 | 6.73–14.12 | 8.13 | 5.52–11.97 | ||
| IV | 52 (6) | 32.60 | 21.10–50.35 | 24.41 | 14.80–40.26 | ||
| Histological subtype | <0.0001 | 0.08 | |||||
| Endometrioid | 777 (86) | 1.00 | 1.00 | ||||
| Non-endometrioid | 128 (14) | 4.76 | 3.44–6.57 | 1.49 | 0.95–2.32 | ||
| Grade | <0.0001 | 0.11 | |||||
| 1 or 2 | 668 (74) | 1.00 | 1.00 | ||||
| 3 | 237 (26) | 3.84 | 2.83–5.19 | 1.41 | 0.93–2.13 | ||
| Age b | 904 (100) | 1.06 | 1.04–1.07 | <0.0001 | 1.05 | 1.03–1.06 | <0.0001 |
| BMI c | 0.04 | 0.65 | |||||
| <25 | 364 (40) | 1.38 | 1.02–1.86 | 0.93 | 0.68–1.27 | ||
| ⩾25 | 541 (60) | 1.00 | 1.00 | ||||
Abbreviations: BMI=body mass index; CI=confidence interval; FIGO=International Federation of Gynaecology and Obstetrics; HR=hazard ratio.
Analyses based on patients with complete information for all variables (n=905).
Age at primary operation, continuous variable with HR given per year.
When including patients with endometrioid histology only: adjusted HR for BMI was 1.07, 95% CI 0.73–1.55, P=0.7.
Discussion
To our knowledge, this is the most comprehensive study of clinico-pathological variables to date. It is also the largest study to date exploring the relationship between BMI and a large panel of markers for tumour phenotype in endometrial carcinoma. The large sample size with careful characterisation of FIGO stage, histological subtype, and grade confers more accuracy to the estimates for the independent prognostic impact of BMI compared with smaller previous studies. Also, the fact that the patient series studied was derived from a well-defined geographic region in Norway, previously shown to be representative for the total Norwegian population (Salvesen et al, 1999), suggests that the findings may be representative for a Caucasian patient population in general.
We found a positive association between high BMI and favourable DSS in univariate analysis but not in multivariate analysis. However, in multivariate analysis of OS, we found an independent unfavourable prognostic impact of increasing BMI. Previous studies exploring the effect of BMI on survival have reported conflicting results, which may be due to sample sizes, choice of cut point for BMI, outcome variables applied, and the panel of clinico-pathological markers adjusted for in the multivariate analyses. Like the present study, several have reported a trend towards better survival in the overweight compared with the more slender women (Anderson et al, 1996; Temkin et al, 2007; Munstedt et al, 2008). Others have concluded with no difference (Jeong et al, 2010) and even poorer survival for women with higher BMI (von Gruenigen et al, 2006). Disease-specific survival applied in the present study is more likely to be accurate in detecting deaths directly related to the disease studied. Previous studies, mostly applying OS, may have underestimated the positive biological impact of obesity, as obese women have increased risk of dying from intercurrent disease (Anderson et al, 1996; Temkin et al, 2007). Our findings that OS is less favourable for obese women when adjusted for the standard clinico-pathological risk factors may support this.
A limit of our study is that BMI is measured at the time of diagnosis. This may lead to a bias, as aggressive cancers often are associated with weight loss, cachexia, and anorexia (Keller 1993). Hence, we may have underestimated the weight of patients presenting with high stage cancers.
The rise in endometrial carcinoma incidence has been associated with an epidemic of obesity and physical inactivity (Amant et al, 2005). Unopposed oestrogen exposure leads to endometrial hyperplasia, and increased risk of atypical hyperplasia and type I endometrial cancer (Shang, 2006). The significance of progesterone in controlling oestrogen-driven proliferation is underlined by its efficacy in preventing endometrial cancer (Kim and Chapman-Davis, 2010). Still, the molecular basis and cross talk between hormone receptor pathways are poorly understood (Kim and Chapman-Davis, 2010). In previous smaller immunohistochemical studies (Duska et al, 2001; Gates et al, 2006), no significant relationship between hormone receptor status and BMI was identified (n=41 and n=165, respectively). We found that BMI was significantly linked to alterations in PR but not ERα status in tumours, confirmed by two different techniques estimating mRNA and protein levels for PR and ERα. The biological function of PR may be altered by genetic variations. Interestingly, recent studies have identified a single-nucleotide polymorphism in the gene coding for the PR, which has been associated with increased risk for endometrial carcinoma (Xu et al, 2009; O’Mara et al, 2010). This support the complexity in the hormone receptor interactions related to carcinogenesis and tumour development in endometrial cancer, and further studies of these interactions are needed.
Acknowledgments
We acknowledge Helse Vest, The University of Bergen, The Norwegian Cancer Society (Harald Andersens legat), The Research Council of Norway. We thank Britt Edvardsen, Ingjerd Bergo, Erlend S Njølstad, Pål-Christian S Njølstad, Gerd Lillian Hallseth, and Bendik Nordanger for technical assistance.
References
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I (2005) Endometrial cancer. Lancet 366: 491–505 [DOI] [PubMed] [Google Scholar]
- Anderson B, Connor JP, Andrews JI, Davis CS, Buller RE, Sorosky JI, Benda JA (1996) Obesity and prognosis in endometrial cancer. Am J Obstet Gynecol 174: 1171–1179 [DOI] [PubMed] [Google Scholar]
- Bjorge T, Engeland A, Tretli S, Weiderpass E (2007) Body size in relation to cancer of the uterine corpus in 1 million Norwegian women. Int J Cancer 120: 378–383 [DOI] [PubMed] [Google Scholar]
- Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15: 10–17 [DOI] [PubMed] [Google Scholar]
- Cancer Registry of Norway (2009) Cancer in Norway 2008-Cancer Incidence, Mortality, Survival and Prevalence in Norway. Cancer Registry of Norway: Oslo [Google Scholar]
- Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF (2001) Endometrial cancer in women 40 years old or younger. Gynecol Oncol 83: 388–393 [DOI] [PubMed] [Google Scholar]
- Engelsen IB, Akslen LA, Salvesen HB (2009) Biologic markers in endometrial cancer treatment. APMIS 117: 693–707 [DOI] [PubMed] [Google Scholar]
- Engelsen IB, Mannelqvist M, Stefansson IM, Carter SL, Beroukhim R, Oyan AM, Otte AP, Kalland KH, Akslen LA, Salvesen HB (2008a) Low BMI-1 expression is associated with an activated BMI-1-driven signature, vascular invasion, and hormone receptor loss in endometrial carcinoma. Br J Cancer 98: 1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsen IB, Stefansson IM, Akslen LA, Salvesen HB (2008b) GATA3 expression in estrogen receptor alpha-negative endometrial carcinomas identifies aggressive tumors with high proliferation and poor patient survival. Am J Obstet Gynecol 199: 543.e1–7. [DOI] [PubMed] [Google Scholar]
- Gates EJ, Hirschfield L, Matthews RP, Yap OW (2006) Body mass index as a prognostic factor in endometrioid adenocarcinoma of the endometrium. J Natl Med Assoc 98: 1814–1822 [PMC free article] [PubMed] [Google Scholar]
- Hoos A, Urist MJ, Stojadinovic A, Mastorides S, Dudas ME, Leung DH, Kuo D, Brennan MF, Lewis JJ, Cordon-Cardo C (2001) Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 158: 1245–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong NH, Lee JM, Lee JK, Ki JW, Cho CH, Kim SM, Seo SS, Park CY, Kim KT, Lee J (2010) Role of body mass index as a risk and prognostic factor of endometrioid uterine cancer in Korean women. Gynecol Oncol 118: 24–28 [DOI] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Kurzer MS (2002) Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 11: 1531–1543 [PubMed] [Google Scholar]
- Keller U (1993) Pathophysiology of cancer cachexia. Support Care Cancer 1: 290–294 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Chapman-Davis E (2010) Role of progesterone in endometrial cancer. Semin Reprod Med 28: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann K, Vatten LJ, Ellstrom-Engh M, Eskild A (2008) Body mass, diabetes and smoking, and endometrial cancer risk: a follow-up study. Br J Cancer 98: 1582–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuta JJ (1993) International Federation of Gynecology and Obstetrics staging of endometrial cancer 1988. Cancer 71: 1460–1463 [DOI] [PubMed] [Google Scholar]
- Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE (2008) Influence of body mass index on prognosis in gynecological malignancies. Cancer Causes Control 19: 909–916 [DOI] [PubMed] [Google Scholar]
- O’Mara TA, Fahey P, Ferguson K, Marquart L, Lambrechts D, Despierre E, Vergote I, Amant F, Hall P, Liu J, Czene K, Rebbeck TR, Ahmed S, Dunning AM, Gregory CS, Shah M, Webb PM, Spurdle AB (2010) Progesterone receptor gene variants and risk of endometrial cancer. Carcinogenesis, e-pub ahead of print, doi:10.1093/carcin/bgq263 [DOI] [PMC free article] [PubMed]
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108 [DOI] [PubMed] [Google Scholar]
- Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB, Trovik J, Wik E, Greulich H, Bo TH, Jonassen I, Thomas RK, Zander T, Garrayway LA, Oyan AM, Sellers WR, Kalland KH, Meyerson M, Akslen LA, Beroukhim R (2009) Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA 106: 4834–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen HB, Iversen OE, Akslen LA (1999) Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J Clin Oncol 17: 1382–1390 [DOI] [PubMed] [Google Scholar]
- Schouten LJ, Goldbohm RA, Van Den Brandt PA (2004) Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst 96: 1635–1638 [DOI] [PubMed] [Google Scholar]
- Shang Y (2006) Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer 6: 360–368 [DOI] [PubMed] [Google Scholar]
- SSB (2010) Fylkesstatistikk Hordaland Fylkeskommune [Online]. Statistisk Sentralbyrå (SSB). Available: http://statistikk.ivest.no/hf/
- Stefansson IM, Salvesen HB, Akslen LA (2004) Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol 22: 1242–1252 [DOI] [PubMed] [Google Scholar]
- Temkin SM, Pezzullo JC, Hellmann M, Lee YC, Abulafia O (2007) Is body mass index an independent risk factor of survival among patients with endometrial cancer? Am J Clin Oncol 30: 8–14 [DOI] [PubMed] [Google Scholar]
- Von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR (2006) Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study. Cancer 107: 2786–2791 [DOI] [PubMed] [Google Scholar]
- Xu W, Long JR, Zheng W, Ruan Z, Cai Q, Cheng JR, Xiang YB, Shu XO (2009) Association of the progesterone receptor gene with endometrial cancer risk in a Chinese population. Cancer 115: 2693–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]