Introduction
Chronotropic incompetence (CI), broadly defined as the inability of the heart to increase its rate commensurate with increased activity or demand, is common in patients with cardiovascular disease, produces exercise intolerance which impairs quality-of-life, and is an independent predictor of major adverse cardiovascular events and overall mortality. However, the importance of CI is under-appreciated and CI is often overlooked in clinical practice. This may be due partly due to multiple definitions, the confounding effects of aging, medications, and the need for formal exercise testing for definitive diagnosis. This review discusses the definition, mechanisms, diagnosis, and treatment of CI, with particular emphasis on its prominent role in HF. CI is common, can be diagnosed by objective, widely available, inexpensive methods, is potentially treatable, and its management can lead to significant improvements in exercise tolerance and quality-of-life.
Contribution of Heart Rate to Exercise Performance
The ability to perform physical work is an important determinant of quality-of-life1 and is enabled by an increase in oxygen uptake (VO2).2 During maximal aerobic exercise in healthy humans, VO2 increases approximately 4-fold.2 This is achieved by 2.2-fold increase in heart rate (HR), a 0.3-fold increase in stroke volume, and a 1.5-fold increase arteriovenous oxygen difference.2 Thus, the increase in HR is the strongest contributor to the ability to perform sustained aerobic exercise.3 It is therefore not surprising that CI can be the primary cause or a significant contributor to severe, symptomatic exercise intolerance.
Heart Rate Control
Heart rate at any moment in time reflects the dynamic balance between the sympathetic and parasympathetic divisions of the autonomic nervous system. Although the intrinsic rate of depolarization of the SA node is a 100 min, resting HR in humans is generally much lower (60-80 b/min) due to the predominating influence of the parasympathetic nervous system efferent Vagus nerve. Increased resting HR levels, due to increased sympathetic and/or decreased parasympathetic “tone”, have been associated with increased cardiovascular death, ischemic heart disease, and sudden cardiac death in both asymptomatic men and women.4;5 Furthermore, a resting HR ≥ 70b/min has been associated with increased mortality in a patients with stable coronary artery disease and left ventricular (LV) dysfunction.6;7
An intact HR response is vital for tightly matching a subject's cardiac output to their metabolic demands during exertion.4 Failure to achieve maximal HR, inadequate submaximal HR, or HR instability during exertion are all examples of impaired chronotropic response. These conditions are relatively common in patients with sick sinus syndrome, atrioventricular block, coronary artery disease, and heart failure (HF).4
Immediately after the termination of exertion, sympathetic withdrawal and increased parasympathic tone to the SA node combine to cause a rapid decline in HR. A delayed recovery of HR after exertion has been associated with increased all-cause mortality risk in a variety of asymptomatic and diseased populations,8 even after adjusting for severity of cardiovascular disease, LV function, and exercise capacity.9 While there are a number of methods of evaluating HR recovery, the most widely used threshold for increased risk of all-cause mortality has been a decrease in HR from peak exercise to 1 minute of passive supine recovery of < 12 b/min (or < 18 b/min if recovery was “active” i.e. unloaded cycling or slow walking) and/or a decrease in HR from peak exercise to 2 minutes of recovery of < 42 b/min.10
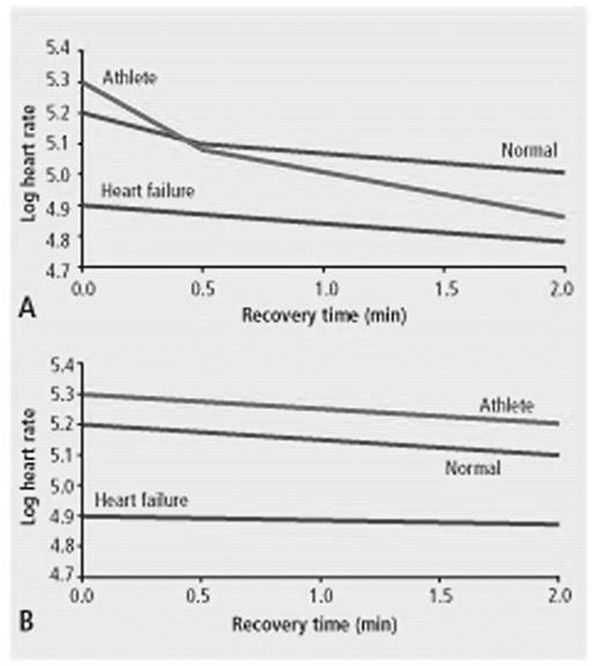
In contrast, highly trained athletes often display a rapid and profound drop in HR of ≥30-50 beat during the 1st minute of recovery from strenuous exertion.11 The rate and magnitude of HR recovery after exertion appears to be directly related to level of parasympathetic tone. The association between early HR recovery and parasympathetic nervous system function was elegantly demonstrated in a study of three groups of subjects – athletes, normal subjects, and patients with HF. Among athletes and normal subjects, there was a biexponential pattern of HR during early recovery, with a steep non-linear decrease during the first 30 seconds followed by a more shallow decline (Figure 1A). When the same subjects were given atropine and exercise testing was repeated, the initial steep decrease in HR observed among athletes and normal subjects disappeared (Figure 1B).11
Figure 1.
Influence of parasympathetic tone on heart rate recovery. A. Absolute heart rates (after log transformation) during the first 3 minutes ater exercise in 3 groups of subjects. Among athlets and normal subjects, there is a biexponential relationship which is absent in heart failure patients. B. After atropine, the initial steep slope is absent. From Lauer MS, Cleve Clin J of Med. 2009;76:S18-S22.8
In the the Framingham Offspring study, Nearly 3,000 healthy men and women were followed for an average of 15 years. Individuals in the top quintile of HR recovery at 1-minute after exercise had the lowest risk of coronary heart disease and cardiovascular disease (hazard ratios of 0.54 and 0.61, respectively) compared to those in the lower 4 quintiles of HR recovery.12 The MRFIT study also demonstrated that a delayed HR recovery (<50 beats after 3 minutes) was an independent predictor of all-cause death in asymptomatic men.13 In a long-term, 23 year follow-up study of asymptomatic working men who underwent exercise stress testing,14 factors independently associated with increased risk of fatal myocardial infarction, were a resting HR more than 75 beats/min, an increase in HR from rest to peak exercise of less than 89 beats/min and a decrease in HR of less than 25 beats after the cessation of exercise. In conclusion, the autonomic imbalance of sympathetic and parasympathetic activity, observable through HR responses at rest as well as during and after exercise, is strongly associated with increased risk of adverse cardiovascular outcomes and sudden death.8
Effect of age and gender on the HR response to exercise
There is no change in resting HR with adult aging. However, in healthy humans, there is a marked age-related decrease in maximum heart rate in response to exercise that is inexorable, highly predictable, and occurs in other mammalian species as well as humans.3;15;16 The age-related decline in maximal HR is the most substantial age-related change in cardiac function, both in magnitude and consequence.3;17;18 It is primarily responsible for the age-related decline in peak aerobic exercise capacity.3;18 Starting from early adulthood, maximal heart rate declines with age at a rate of ~0.7 beats min−1 year−1 in healthy sedentary, recreationally active and endurance exercise-trained adults.19. Though its mechanism(s) are not fully understood, dual-blockade studies show that intrinsic HR declines by 5-6 beats per minute for each decade of age such that resting HR in an 80 year old is not much slower than the intrinsic HR.15 This indicates that at rest there is minimal parasympathetic tone. In support of this, the increase in HR after atropine in an older person is less than half that in the young.17 There are also significant alterations in the sympathetic influence on heart rate response to exercise in aging, with increased circulating catecholamines and reduced responsiveness.17 Doses of isoproterenol that increase HR by 25 b/min in young healthy men produce an increase of only 10 b/min in older persons.17
The normal, age-related decline in maximal HR during exercise is not significantly modified by vigorous exercise training, suggesting that it is not due to the age-related decline in physical activity level.15 Also, it does not appear to be due to inadequate sympathetic stimulation, since both as serum norepinephrine and epinephrine are increased rather than decreased at rest in healthy elderly. Further, with exertion or stress, catecholamines increase even more than in young persons under the same stress conditions.
The traditional equation to predict maximal HR (220 b/min –age), was developed based on studies primarily in middle-aged men, some of whom had known coronary artery disease and were taking beta-blockers.19;20 This equation has been associated with tremendous inter-subject variability with a standard deviation of ± 11b/min21 that equation increases to ±40 b/min in patients with coronary heart disease receiving beta blockers.22 Consequently an alternative formula from Tanaka et al (208 − 0.7 × age) is becoming more accepted for determining age-predicted maximal HR (APMHR) even though it may still under-predict APMHR in older adults (Figure 2).21
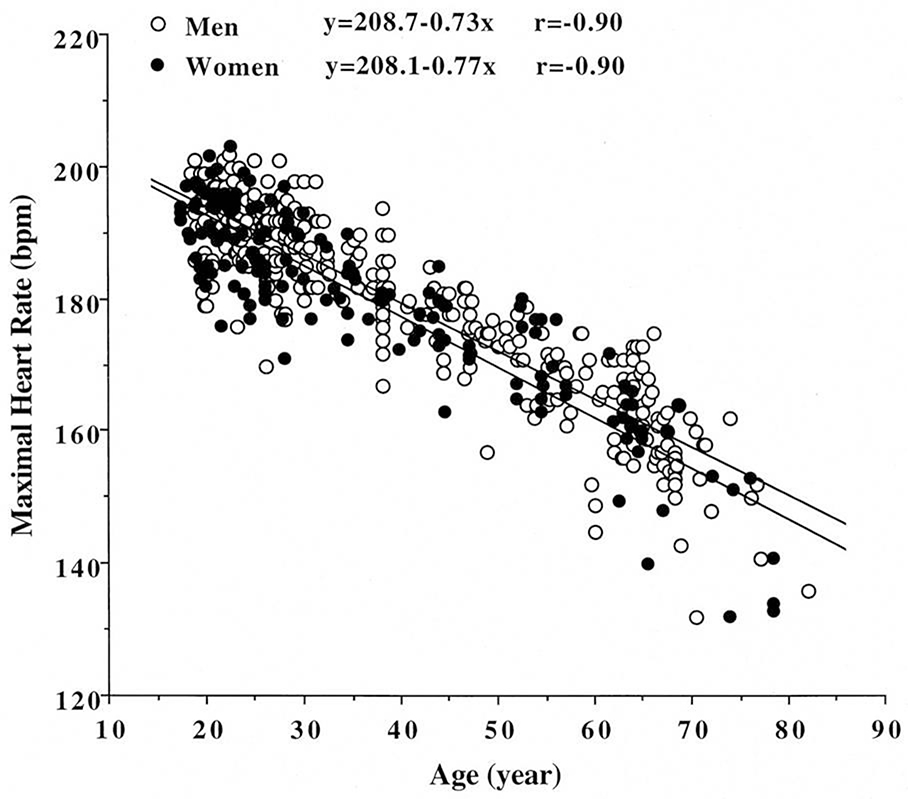
Figure 2.
Relationship between age and maximal heart rate in over 5,000 asymptomatic women, with 95% confidence limts. From these data, a new prediction equation was proposed: peak heart rate = 206 − 0.88 (age). From Tanaka H, JACC 2001;37:153-156. 21
Several earlier studies suggested that gender affected the heart rate trajectory during exercise and recovery, and that the traditional equation (220-age) overestimates maximal heart rate in younger women but underestimates in older women.19;21 A meta-analysis indicated that maximal HR was unaffected by gender.21 A recent, large prospective study in over 5,000 asymptomatic women showed that the traditional equation significantly overestimates maximal HR and thus proposed a new equation where; maximal HR=206−0.88(age)19.
Brawner et al.22 demonstrated that the 220 – age equation is not valid in patients with coronary heart disease (CHD) taking beta-adrenergic blockade (βB) therapy and developed the equation; [164-0.7(age)] for this population.
All of aforementioned studies improve on the estimations of maximal heart rate versus the traditional 220-age approach, but still produce substantial standard error of estimates (10-22 beats/min). Given the inherent variability in maximal heart, regression equations using a single predictor variable, such as age, are unlikely to be 100% accurate, and increasing the number of predictor variables adds little improvement and reduces practicality for clinical use. Thus, for estimating predicted maximal heart rate, we suggest selecting an equation that was generated in a population that most closely matches the population of interest. In this regard, the equation of Tanaka et al19 is recommended for apparently healthy persons, and the equation of Brawner et al22 is recommended for those with known or suspected cardiovascular disease. While these also are imperfect, they are superior to the traditional “220-age” equation and are practical.
Definition, Criteria, and Measurement of CI
A barrier to progress in studies of CI and its clinical management has been a lack of consistent methodology for determining CI. The lack of standardized criteria likely accounts for the wide range in reported prevalence of CI (9-89%) in the literature.23-26 In an evaluation of more than 1,500 CI patients referred for pacemaker implantation, the use of 5 different definitions of CI resulted in a prevalence of CI of 34-87%.27 CI has been most commonly diagnosed when HR fails to reach an arbitrary percentage (either 85%, 80% or less commonly, 70%) of the age-predicated maximal HR (usually based on 220-age equation described earlier) obtained during an incremental dynamic exercise test.28-30 CI has also been determined from change in HR from rest to peak exercise during an exercise test, commonly referred to as the HR reserve. Since the proportion of actual HR achieved during exercise depends in part on the resting HR level, the chronotropic response to exercise can also be assessed as the fraction of HR achieved at maximal effort. Thus, adjusted HR reserve, determined from the change in HR from rest to peak exercise divided by the difference of the resting HR and the age-predicated maximal HR has been commonly used.31 The majority of studies in the literature have used failure to obtain ≥ 80% of the HR reserve, obtained during a graded exercise test, as the primary criteria for CI.
However, before concluding that a patient has CI, it is important to consider their level of effort and reasons for terminating the exercise test. Patients should be encouraged to continue on the exercise modality until a true symptom-limited (exchaustive) maximal levels are achieved. Symptoms and subjective ratings of perceived exertion (RPE) can provide an estimate of exertion level,and is an acceptable method. Respiratory exchange ratio (RER, i.e. volume of carbon dioxide produced/volume of oxygen consumed) obtained from expired respiratory gas analysis at peak exertion during the exercise test, is the most definitive, objective, clinically available measure of physiologic level of effort during exercise. RERis reliable, and while requires expired gas analysis equipment, current generation equipment is automated and is moderate in cost. RER is a continuous variable, ranging from <0.85 at quiet rest to > 1.20 during intense, exhaustive exercise. Higher RER values indicate increasing confidence of maximal effort. It is generally accepted that an RER < 1.05 at peak exercise suggests submaximal effort or that the test was terminated prematurely and should lead to caution in diagnosing CI.
Wilkoff et al32 utilized the expired gas analysis technique to more objectively evaulate CI using the relationship between HR and VO2 during exercise. In this approach, the metabolic-chronotropic relationship (MCR) (also known as the chronotropic index) is calculated from the ratio of the HR reserve to the metabolic reserve during submaximal exercise. The advantage of using the MCR is that it adjusts for age, physical fitness, and functional capacity and it appears to be unaffected by the exercise testing mode or protocol. In normal adults, the percentage of HR reserve achieved during exercise equals the percentage of metabolic reserve achieved. This physiologic concept allows for a single HR achieved at any point during an exercise study (HR stage) to be determined as consistent or inconsistent with normal chronotropic function. This is accomplished by using the following formula, where METs = VO2 in ml . kg−1 . min−1 / 3.5:
. The Wilkoff model predicts the MCR slope of the normal sinus response to be 1.0 with a 95% confidence interval between .8 and 1.3.32 An MCR slope or any single MCR value (from one stage) of ≤ 0.80 is considered indicative of CI.
Consequently, the information that should be recorded for each patient during an exercise test to evaluate CI includes: age; resting HR (HR rest); age-predicted maximal HR (APMHR) = 220 b/min − (patient's age); age-predicted HR reserve (APHRR) = (APMHR) − (HR rest ); observed maximal HR during exercise test (HRmax); oxygen consumption (VO2 = ml . kg−1 . min−1) at each stage and at peak effort; and RER. For example, in a 60 year old subjects who only achieved an RER of 0.96 at peak exertion (i.e. submaximal effort!), entering following data from a submaximal stage (25 watts) of exercise (HRrest = 67; HRpeak= 100, HR@25w= 97, MET@25w= 3.3, METpeak= 3.7. into Wilkoff equation would result in a CI index = .66 (actual HR stage of 97/estimated HR stage of 147) which is well below the CI cut-off of ≤ .80. The Wilkoff approach32 can be combined with other methods to determine the presence of CI in challenging situations: 1) if despite reaching a peak exercise RER of greater than 1.05 (suggesting adequate effort), the patients fails to achieve a HRmax ≥ 80-85% of APHRR (or 80-85% HR reserve); 2) if respiratory exchange ratio does not reach 1.05 (suggesting submaximal effort) an MCR relationship of < 0.80 can be used.
While a variety of exercise testing protocols (Bruce, RAMP, etc) and modes of testing can be employed, a specific CI exercise testing protocol has been employed in some laboratories and evaluates the MCR relationship from two stages on a treadmill protocol (stage 1 = 1.3 mph & 0.5% grade and stage 2 = 3.0 mph & 1.5% grade). The process of data collection and analysis described above is subsequently used to determine the adequacy of the chronotropic responses.32
Savonen et al33;34 have proposed methods attempting to separate the effects of parasympathetic withdrawal versus sympathetic stimulation on the HR response to exercise. This is based the physiological observations that found that the HR increase below 100 b/min is predominantly controlled by gradual withdrawal of parasympathetic tone, whereas from 100 b/min to maximum, the HR increase is predominantly the result of increasing sympathetic nervous system activity. Savonen et al have termed this a “delineational” approach. Their work indicates that in men with and without coronary heart disease, an increase in HR from 40-100% of maximal work capacity in the exercise test predicts mortality and acute myocardial infarction better than the peak HR or HR reserve approaches. Similarly, another study35 demonstrated that a blunted HR increase from rest to 33% of maximal work capacity was not as strong of a predictor of death as a low HR reserve in patients referred for exercise testing. While provocative, these innovative approaches for assessing chronotropic response to exercise will require further validation before clinical application.
Effect of Medications and other Confounding Influences on CI
A number of commonly used cardiovascular medications including, beta blockers, digitalis, certain calcium channel blockers, amiodarone, and others can confound the determination of CI.36;37 Beta-blockers may result in pharmacological induced CI and obscure identification of an underlying intrinsic abnormality in neural balance.37 In one study38 a suitable threshold for CI among HF patients using beta blockers was found to be ≤ 62% of APHRR. Using this lower HR threshold, CI was able to be reliably identified and was an independent predictor of death.38 These modified criteria have been utilized to design clinical trials.39 Care should be taken before applying the modified threshold criteria to ensure that the patient is on a non-trivial dose and is compliant with the medication.
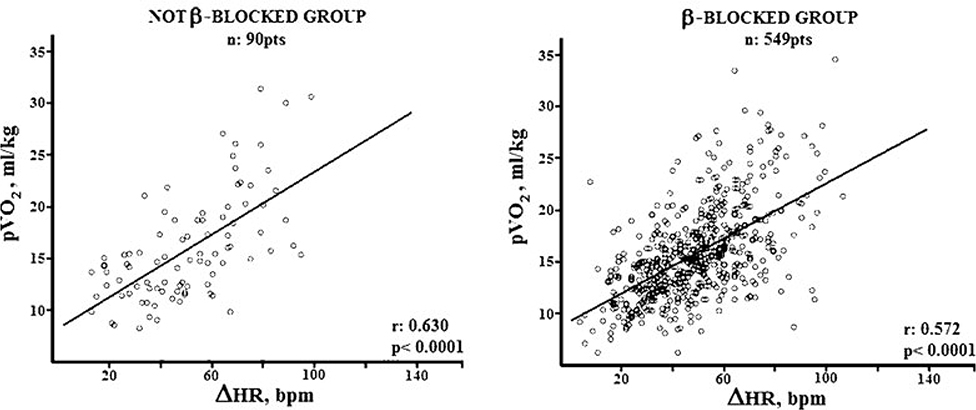
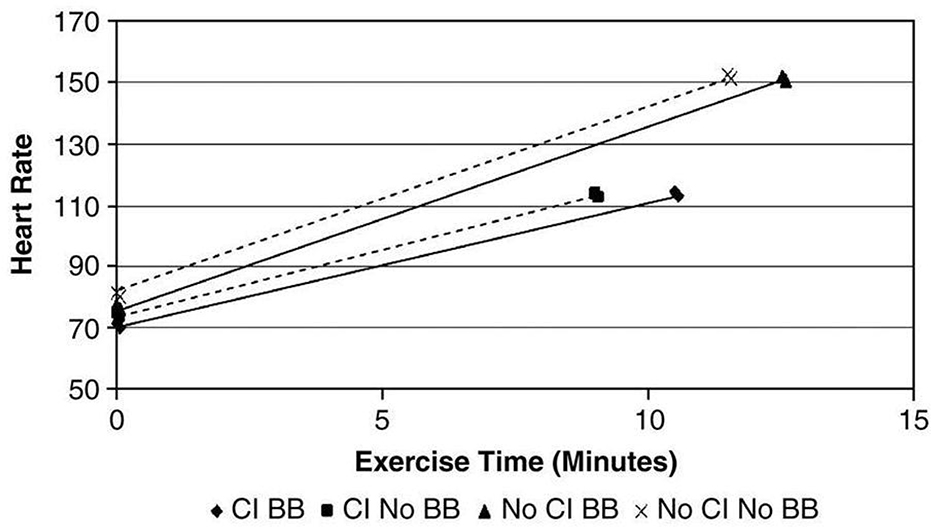
The use of separate CI criteria for patients taking beta blocker medications has been challenged by other studies that failed to demonstrate that any effect on beta blockers, including at high dose, on the occurrence of CI.40 Figure 3 shows the similar relationship between HR reserve and VO2 peak in HF patients that are either taking or not taking beta blockers. Similarly, Jorde and colleagues41 examined the relationship between exercise time and HR during treadmill exercise testing in HF patients. As seen in Figure 4, the HR slope is abnormal is abnormal in HF patients with CI, yet beta blockers have no impact on this relationship in these patients.42 While a still an evolving concept, chronic treatment of HF patients with beta blockers may paradoxically improve chronotropic response by decreasing sympathetic tone and/or by increasing beta receptor activity.43
Figure 3.
There is a significant relationship between change in heart rate during exercise and VO2peak in patients with HFrEF, but there is no significant difference in this relationship between those patients taking beta-blockers verson not taking them. From Magri et al; Cardiovascular Therapeutics 2010; In press. 40
Figure 4.
In patients with HF, beta blockers do not significantly impact the relationship between heart rate and exercise time, regardless of whether CI is present. From Jorde et al; European J of Heart Failure 2008;96-101.41
Chronic atrial fibrillation confounds the assessment of CI and criteria for its diagnosis have not been established. Exercise testing can be used to assess adequacy of response following pacemaker insertion for CI. Intrinsic HR response can be assessed in patients with existing pacemakers by reprogramming or suspending the device with a magnet, taking care to ensure the patient is not completely pacemaker dependent beforehand.
Relationship between Chronotropic Incompetence and Mortality
The relationship between CI and increased cardiac and all-cause mortality was first reported more over 30 years ago by Hinkle et al. 44 He described a group of men who were unable to reach an expected HR on a standard exercise protocol and who subsequently experienced increased frequency of cardiac events during 7 year follow-up. These investigators initially termed this inadequate HR response a “sustained relative bradycardia”. Subsequently, Rubinstein et al45 and Eckberg et al46 described a relationship between this phenomenon and autonomic dyfunction. Ellestad et al47 confirmed the finding increased risk of cardiac events during long-term follow-up,and showed that the risk of cardiac events associated with an abnormal HR response during exercise was greater than that associated with ischemic ST segment depression. He suggested the term “chronotropic incompetence” to describe this abnormal HR response during exercise.
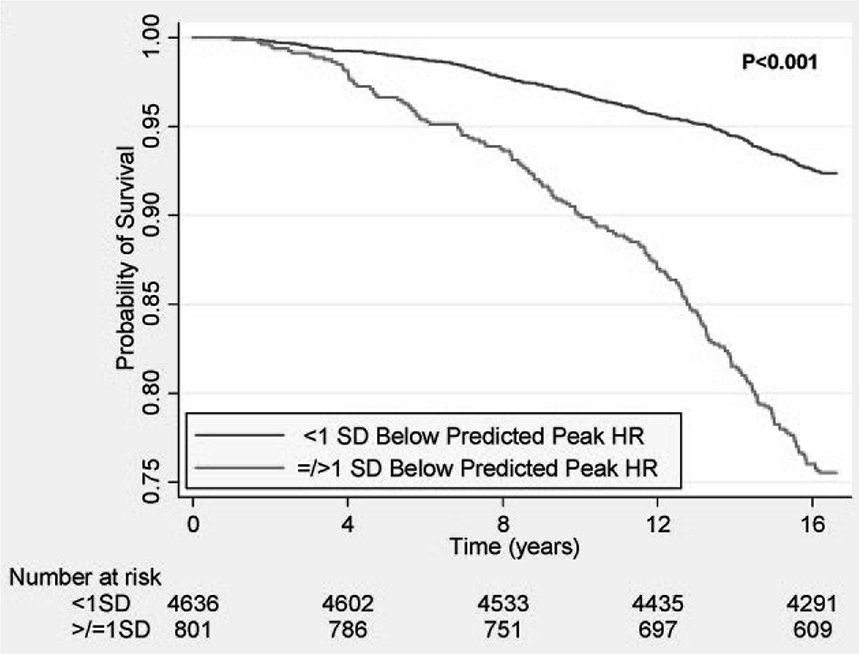
Subsequently, a number of studies, expanded on these findings and reported that an attenuated HR response to exercise is predictive of increased mortality and coronary heart disease risk, independent of a variety of other confounding factors, including age, gender, physical fitness, traditional cardiovascular risk factors, and ST-segment changes during exercise.19;28;48;49 In over 5,000 asymptomatic women, those with peak exercise HR > 1 SD below the predicted mean had markedly increased mortality during long-term follow-up (Figure 5).19 An attenuated HR response was found to be predictive of myocardial perfusion defects.28 A combination of CI and a myocardial perfusion defect during exercise stress testing carried a particularly high risk group of patients as potential candidates for heightened treatment.28 The prognostic value of an impaired HR response to exercise appears to persist even after considering the adverse effects of coronary artery disease and/or LV dysfunction.29
Figure 5.
Markedly reduced survival during long-term follow-up among asymptomatic women with peak HR ≥ 1 SD below the average. From Gulati et al, Circulation 2010;122:130-137.19
In another study30 of 3,221 patients who underwent treadmill exercise echocardiography with a median follow-up of 3.2 years, failure to achieve 85% of maximal predicted HR was associated with increased mortality and cardiac death even after adjusting for LV function and exercise-induced myocardial ischemia. Azarbal et al50 showed that a low % HR reserve was a superior predictor compared to an inability to achieve 85% of APMHR as the later method identified 2.2 times more individuals at increased risk of cardiac death. An attenuated HR response to exercise also predicts major adverse cardiac events among persons with known or suspected cardiovascular disease.51 Furthermore, in HF patients not taking beta blockers, the presence of CI appears to increase mortality risk.52
Thus, the HR profiles both during and after exercise are strong predictors of sudden death in asymptomatic and selected clinical populations, including those with coronary artery disease or HF. Collectively these findings provide the rationale for increased screening for inappropriate/inadequate HR responses during exercise testing and recovery to assist with more effective risk stratification and prognosis.
Mechanisms of Exercise Intolerance in HF
In contrast to most other forms of heart disease, the incidence of HF, a debilitating disorder, is increasing with 500,000 new cases in the U.S. per year and a 175% increase in the number of hospital discharges for HF over the past 20 years.53 It has been shown that a majority of persons with HF living in the community have a preserved LV EF.54-56 A hallmark characteristic of chronic HF, either HFrEF or HfpEF, is a markedly reduced capacity for physical exertion, with a subsequent reduction in VO2 peak that is 15-40% below that age-matched controls.57 Work from our group and other has shown that patients with HFpEF have similar reductions in exercise tolerance measured as peak exercise oxygen consumption (VO2 peak), have reduced submaximal exercise measures, ventilatory anaerobic threshold, 6 minute walk distance, quality-of-life, and markers of prognosis including VE/VCO2 slope as those with HF with reduced EF (HFrEF).58-61 These findings have been replicated by Smart, et al and others.62
According to the Fick equation, an appropriate increase in VO2 peak during exertion is dependent on both an increase in cardiac output as well as concomitant widening of the arterial-venous oxygen content difference.63;64 The latter is related to abnormalities of skeletal muscle and vascular function that limit exercise intolerance associated in HF.57;63;65 In addition, patients with HF often achieve less than 50% of the maximal cardiac output achieved by healthy individuals at peak exercise.57 The impairment in cardiac output response of HF patients correlates significantly with reductions in VO2 peak.66 The reduced cardiac output response of HF is often attributed to an attenuated stroke volume, subsequent to either systolic and/or diastolic LV dysfunction. Stroke volume, already diminished at rest in the HF patient subsequent to systolic and/or diastolic abnormalities, rises only modestly to a peak of 50-65 ml versus ≥ 100 ml in healthy subjects.67 Consequently, HF patients must rely to a greater extent on increases in HR to augment cardiac output to compensate for their inadequate stroke volume during physical exertion. Whereas maximal HR during exercise may be only mildly reduced at peak exertion, HR reserve (i.e. degree of HR augmentation above resting levels) is often blunted more substantially in HF patients due to the sympathetically-driven elevation in resting HRs.67
Contribution of Impaired HR Response to Exercise Intolerance in HF
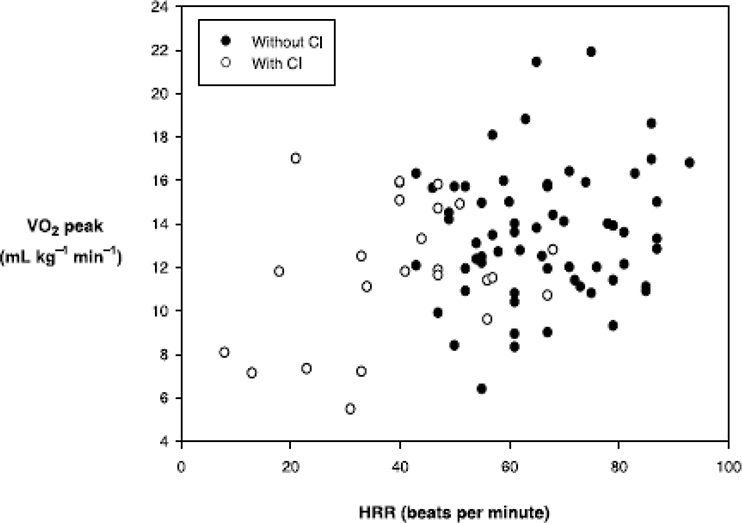
As previously described, the Fick equation dictates that an increase in cardiac output during exertion is dependent on an increase in stroke volume and/or HR. In HFrEF and HFpEF patients, the primary limiting factor during exertion is generally assumed to be an inability to increase the stroke volume commensurate with degree of effort. Yet given the potential impact of HR responsiveness on cardiac output and subsequently VO2 peak, it is surprising there has not been more interest on CI in a patient population where exercise intolerance is so problematic. We68 recently demonstrated that in a group of 102 elderly patients with either HFrEF or HFpEF, that HR reserve (the difference between resting and peak HR achieved on bicycle exercise test) was significantly correlated (r= .40) with VO2 peak (Figure 6). Moreover, these findings indicated that the increase in HR during exercise accounts for an appreciable portion (i.e. 15%) of the observed differences in VO2 peak in these older HF patients. This means that in a patient population with an average VO2 peak of 14 ml . kg−1 . min−1, abnormal HR accounts for about 2 ml.kg−1.min−1 (±16%) in VO2peak, and therefore has significant functional and prognostic ramifications.
Figure 6.
Relationship of heart rate reserve (HRR) to peak exercise oxygen consumption (VO2 peak) in older patients with HFrEF and HFpEF with (open circles) and without (closed circles) CI. There is a significant correlation between HRR and VO2 peak in those with (R=.39 p=0.04) and without CI (R=.41 p =0.01 ). From Brubaker, et al, Journal of Cardiopulmonary Rehabilitation 2006; 26:86-89. 68
Similarly, Witte et al37 found using < 80% of either APMHR or HR reserve, the average VO2 peak was significantly lower ( − 2.6 ml . kg−1 . min−1= 14% and − 4.6 ml . kg−1 . min−1= 25%, respectively) in HFrEF patient with CI verses those without CI. Furthermore, Witte et al37 reported a correlation between VO2 peak and delta HR (peak exercise HR – rest HR) of .56 and .60 for beta-blocked and non-beta blocked HF patients further supporting the significance and impact of inadequate HR increase during exertion in this population.
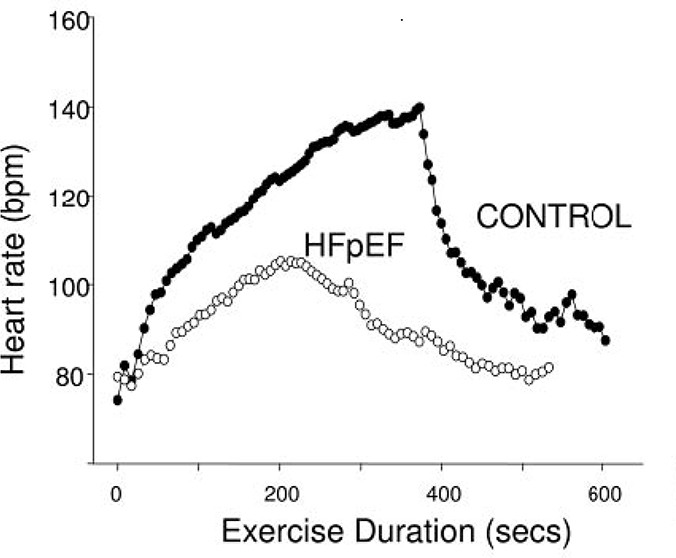
Borlaug et al69 evaluated parameters of exercise tolerance in HFpEF patients versus a control group without HF but matched on age, gender, important co-morbidities, and LV hypertrophy (controls). At peak exertion, the HEpEF patients had significant reductions in VO2 peak (9.0 ± 3.4 vs 14.4 ± 3.4 ml . kg−1 . min−1, respectively) and HR (87 ± 20 vs. 115 ± 22 beats/min, respectively) compared to controls. Exercise capacity, expressed as VO2 peak, correlated directly with cardiac output but was primary determined by HR and afterload responses during exercise. In contrast, change in end-diastolic volume and stroke volume were not correlated with exercise capacity. Furthermore, HFpEF patients had a slower HR rise, lower peak exercise HR, and impaired HR recovery, indicating abnormal autonomic function in these patients (Figure 7).69
Figure 7.
Heart rate profiles during and after cycle ergometry in patients with HFpEF compared to age and gender matched subjects who possessed similar co-morbidities to the HFpEF group including left ventricular hypertrophy (Control). These data demonstrate the delayed and attenuated heart rate response often seen in chronotropically impaired heart failure patients. From Borlaug et al, Circulation 2006; 114:2138-2147. 69
Prevalence of CI in HF
The reported prevalence of CI within the HF population has varied considerably with a range of 25-70%. This substantial variability is likely influenced by the criteria employed to determine CI as well as differing patient characteristics (age, disease severity, type/dose medications). In one of the earliest papers to evaluate the prevalence of CI in HF, Clark and Coates,70 using <80% of APMHR as the criteria, found that approximately 28% of stable, non beta-blocked systolic HFrEF patients (mean age = 59) demonstrated CI. In contrast, Roche et al,71 using achievement of ≤ 80% of APHRR as predetermined criteria, determined that 14 of 21 (66%) of stable, non beta-blocked, HFrEF patients (mean age 54±10 yrs) demonstrated CI. Furthermore, Roche et al71 found no significant difference in age, HR, peak oxygen update or LV ejection fraction between patient with and without CI. In a slightly older group of HFrEF patients, Witte et al37 found that 103 of 237 (43%) HF patients met the criteria of < 80% of APMHR whereas 170 of 237 (72%) met the criteria of < 80% of APHRR. Witte et al did indicate that patients taking beta blockers were more likely to have CI than those not taking beta blockers when < 80 % APMHR was used (49 vs. 32%, respectively) or < 80% APHRR was used (75 vs. 64%, respectively). In contrast, the criteria of ≤ 62% APHRR is used for HF patients on beta blocker therapy, a significantly smaller percentage (22%) of patients were identified with CI.38
We evaluated the prevalence of CI in older (≥60yrs) HFrEF and HFpEF as well as in age-matched healthy subjects using ≤80% of APMHR and the Wilkoff approach.68 While CI was uncommon in healthy older adults (just 2 out of 28 subjects = 7%), the prevalence of CI was relatively similar between older HFrEF ( 12 of 46 = 26%) and HFpEF (11 of 56 = 19%). A more recent unpublished analysis of 207 older HFpEF patients, tested in our lab indicated that 28% of these patients met the CI criteria as described earlier. Phan et al42 has also observed abnormal HR responses to exercise in HFpEF patients versus age-matched hypertensive and healthy control subjects. Using the criteria of <80% APMHR, Phan et al. observed a similar prevalence of CI among HFpEF of 35. Like other studies employing multiple criteria, the prevalence of CI increased to 63% of HfpEF patients when 80% of HR reserve criteria was employed as the definition of CI.42 Consequently , in addition to the central and peripheral pathophysiological derangements observed in HF patients, a significant portion (one-third or more depending on criteria employed) of both HFrEF and HFpEF populations also have to significant CI contributing to their exercise intolerance.
Mechanisms of CI in HF
Studies in the 1980s by Bristow et al72 and Colucci et al73 were the first to associate CI in HF with down-regulation of beta receptors and desensitization in the presence of increased circulating catecholamine levels. Bristow and colleagues72 found a 50% or more reduction in beta-adrenergic receptor density in the LV myocardium of failing hearts explanted during transplant surgery. Colucci et al demonstrated that norepinephrine infusion results in a reduced HR response in HF patients versus healthy subjects.73 During maximal isoproterenol stimulation, Bristow et al observed a 45% reduction compared to normal in adenylate cylase elaboration and up to 73% reduction in muscle contraction. These findings suggest that in HF patients, a decrease in beta-receptor density leads to a diminished sensitivity of the beta-adrenergic pathway and decrease in beta-agonist stimulated muscle contractility.72 Samejima et al74 demonstrated that the ratio of change in HR to change in log of norepinephrine (delta HR/delta log NE), an index of sinoatrial node sympathetic responsiveness, decreased progressively with the severity of HF. Furthermore, the delta HR/log delta NE ratio during exercise was significantly correlated with anaerobic threshold, VO2 peak, and VE/VCO2 slope.74 An electrophysiology study75 of symptomatic HFrEF patients and age-matched normal subjects undergoing radiofrequency ablation for AV tachycardia or AV nodal tachycardia demonstrated that when compared to non-HF subjects, HF patients with no prior atrial arrhythmias have significant sinus node remodeling characterized by (1) anatomical and structural changes along the crista terminalis, (2) prolonged sinus node recovery and sinoatrial conduction , and (3) caudal localization of the sinus node complex with circuitous propagation of the sinus impulse. This reduction in sinus node reserve appears to be responsible, at least in part, for the bradycardia and possibly the CI seen commonly in HF.75
Management of CI in HF
Exercise training. In addition to many other health benefits, endurance exercise training in healthy individuals results in favorable changes in chronotropic function such as decreased resting and submaximal exercise HRs, as well as a more rapid decline in post-exercise HRs. Most of these HR adaptations appear to be related to alteration in the balance of sympathetic and parasympathetic influence of the autonomic nervous system. Moreover, endurance exercise training generally improves exercise tolerance in HFrEF patients through a variety of potential central and peripheral mechanisms. The specific effects of exercise training on autonomic dysfunction and neurohormal activation in chronic HF include with increased baroreflex sensitivity and HR variability and reduced sympathetic outflow, plasma levels of catecholamines, angiotensin II, vasopressin, and brain natriuretic peptides at rest76.77 Consequently, it appears that exercise training modifies the abnormal afferent stimuli from the failing heart that tend to increase sympathetic outflow, leading to autonomic derangement and neurohumoral activation.76 Moreover, Hasking et al78 found that plasma norepinephrine concentrations sampled during supine rest were increased in patients with asymptomatic LV dysfunction and increase further with the progression to overt HF; at the later stages of overt HF, total body spillover was on average double that of control subjects and norepinephrine clearance was reduced by a third. While beneficial, the specific mechanism responsible for modification of the neurohumoral activation and autonomic derangement in HF patients during exercise training is yet to clarified.
Several exercise training studies79-81 have demonstrated that peak exercise HR increases 5-7% and contributes to the increase in cardiac output and VO2 peak usually observed in HF patients with exercise training. In a meta-analysis of 35 randomized studies of exercise training HF patients82 indicated that peak HR increased by an average of 4 b/min or 2.5% of the pre-training level. Keteyian et al81 demonstrated that after 24 weeks of endurance exercise training, peak exercise HR increased by 7 % (approximately 9 beats/min) yet remained unchanged in a non-exercise control group. Furthermore, the training-induced increase in peak HR accounted for 50% of the increase in VO2 peak (+ 2 ml . kg−1 . min−1 = 14%) in the exercise training group. While alterations in beta-adrenergic receptor sensitivity may explain these findings, other mechanisms responsible for or contributing to the improved chronotropic response in HF patients cannot be excluded. Further information is needed regarding the impact of exercise training on the chronotropic response of HFrEF and HFpEF patients.
Rate adaptive pacing. There is a linear relationship between HR and VO2 during exercise in a variety of patient populations, including HF,83 where a 2-6 beat/min increase in HR is associated with a 1 ml . kg−1 . min−1 increase in VO2 during exercise. Consequently, rate-adaptive pacing has been shown to enhance functional capacity in patients with an inadequate chronotropic response84 and those meeting formal definitions of CI.32;85 Despite the potential to improve HR, cardiac output, and subsequently VO2 during exertion in HF patients with chronotropic impairment, rate-adaptive pacing in this population has received minimal attention.86;87 Furthermore, it may be counterintuitive for some clinicians to believe certain HF patients may benefit from a pacemaker particularly in the absence of bradycardiac/heart block.
The potential benefit of rate-adaptive pacing, in conjunction with cardiac resynchronization therapy, on exercise performance in HFrEF patients has been assessed by Tse et al.88 Twenty HFrEF patients with CI (defined as achieving < 85% APMHR and < 80% APHRR) with an implanted cardiac resynchronization device (> 6 months) underwent treadmill exercise testing with measurement of VO2. During the exercise testing, the cardiac resynchronization device was programmed to: 1) DDD mode with fixed AVI (DDD-off); 2) DDD mode with AVI algorithm on (DDD-ON); and 3) DDDR mode. None of the 20 patients in the study achieved > 85% APMR and 11 (55%) failed to reach > 70% APMHR, a level indicative of severe CI. In the overall group, rate-adaptive pacing during cardiac resynchronization therapy increased peak exercise HR and exercise time but did not have an incremental benefit on peak exercise VO2 peak.. However, in the HF patients with more severe CI (those achieving < 70% APMHR), rate-adaptation significantly increased peak HR, exercise time, and VO2peak. Further, in the majority (82%) of these patients, the improvement in chronotropic response with rate-adaptive pacing was associated with ~ 20% increase in VO2peak.
It should be noted that for the majority of patients with less severe CI (those achieving 70-85% APMHR) there was little or no benefit, and one-third of the patients experienced a reduction in exercise capacity with rate-adaptive pacing.88 While it appears that rate-adaptive pacing has potential benefit in carefully selected patients with HFrEF, advances in this area are hindered by lack of standardized, accepted definitions and selection criteria. Furthermore, at this time it is unclear if CI is causal or simply a marker of advanced disease and if treating this with a pacemaker would improve functional status in HFrEF patients. Clearly, this issue requires further investigation in the future.
Even less is known regarding pacing in patients with HFpEF. The current RESET trial is designed to evaluate the effect of rate-adaptive pacing in HFpEF patients with overt CI.39 The rationale for this intervention is based on the observations that ~ 30 % of this population have CI and that impairment in chronotropic function contributes significantly to their objectively measured exercise intolerance.42;68;69. The outcomes of this randomized, controlled trial has the potential to help determine if rate-responsive pacing is an effective approach for improving exercise functional in this patient population.
Conclusions and Suggested Approach to Assessment and Management
Chronotropic incompetence is common, an important cause of exercise intolerance, and an independent predictor of major adverse cardiovascular events and mortality. It is present in up to 1/3 of patients with HF and contributes to their prominent exertional symptoms and reduced quality-of-life. Although the underlying mechanisms for CI in HF and other disorders are incompletely understood, available data suggest roles for reduced beta-receptor density and/or sensitivity secondary to increased sympathetic drive.89
The diagnosis of CI should take into account the confounding effects of aging, physical condition, and medications, but can be achieved objectively with widely available exercise testing methods and standardized definitions. A three step approach to assessment is suggested. First, perform a progressive, exhaustive, symptom-limited exercise test. If practical, this should include automated expired gas analysis using a standard, commercially available system, for assessment of RER, which objectively verifies level of effort, and peak VO2. Then, utilize a formula for peak heart rate that is relevant to the patient's profile. In general this will be the Tanaka formula for apparently healthy persons21 and the Brawner formula for those with cardiovascular disease or on beta-blockers.22 If the patient fails to achieve 80% of their APMHR on this test despite good/maximal effort (judged with RPE, symptoms, and RER levels), then the Wilkoff Chronotropic Index should be calculated. If CI is found to be present, a search for potentially reversible causes is warranted.
In HF, beta-adrenergic blockade may have less detrimental effect on exercise capacity than previously thought, and may even paradoxically improve exercise performance. Beta blockers and other negative inotropes do not appear to have as a major impact on HR response to exercise in the HF patients and thus separate CI criteria for these patients does not appear necessary. Furthermore, it appear that beta blockers may not substantially increase the prevalence of CI in HF patients. The potential of more novel beta-blockers to reduce the prevalence of CI in HF patients is unclear.
While exercise training and rate-adaptive pacing have been shown to improve chronotropic responses and exercise capacity in HF, clearly more research is needed to fully evaluate the impact of these therapies on key clinical outcomes.
CI is a common, easily diagnosed, and potentially treatable cause of exercise intolerance and merits more attention by clinicians when they encounter patients with symptoms of effort intolerance.
Acknowledgement
We gratefully acknowledge Rickie Henderson, MD, for critical review of the manuscript and Belinda Youngdahl for administrative assistance.
Both authors participated in drafting of the manuscript, reviewed and edited the manuscript for critical intellectual content, and approved the final version for submission. The sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Funding Sources
Supported in part by: National Institutes of Health grants R37AG18915 and P30AG21332.
Footnotes
The authors wish to declare the following potential conflicts of interests or financial disclosures
Dr. Brubaker: Boston Scientific (> $10K)
Dr. Kitzman: Synvista (> $10K), Bristol-Meyers-Squibb (> $10K), Novartis (> $10K), Boston Scientific (> $10K), Relypsa (> $10K), Forest Laboratories (< 10K).
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitzman DW. Exercise Intolerance. Prog Cardiovasc Dis. 2005;47:367–379. doi: 10.1016/j.pcad.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RD, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 3.Higginbotham MB, Morris KG, Williams RS. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. 1986;57:1374–1379. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 4.Orso F, Baldasseroni S, Maggioni A. Heart rate in coronary syndromes and heart failure. Prog Cardiovasc Dis. 2009;52:38–45. doi: 10.1016/j.pcad.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB. New perspectives on cardiovascular risk factors. Am Heart J. 1987;114:213–219. doi: 10.1016/0002-8703(87)90964-1. [DOI] [PubMed] [Google Scholar]
- 6.Tardif JC. Heart rate as a treatable cardiovascular risk factor. Br Med Bull. 2009;90:71–84. doi: 10.1093/bmb/ldp016. [DOI] [PubMed] [Google Scholar]
- 7.Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R, BEAUTIFUL investigators Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 8.Lauer MS. Autonomic function and prognosis. Cleve Clin J of Med. 2009;76:S18–S22. doi: 10.3949/ccjm.76.s2.04. [DOI] [PubMed] [Google Scholar]
- 9.Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–838. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 10.Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 12.Morshedi-Meibodi A, Larson MG, Levy D, O'Donnell CJ, Vasan RS. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study) Am J Cardiol. 2002;90:848–852. doi: 10.1016/s0002-9149(02)02706-6. [DOI] [PubMed] [Google Scholar]
- 13.Adabag AS, Grandits GA, Prineas RJ, Crow RS, Bloomfield HE, Neaton JD, MRFIT Research Group Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am J Cardiol. 2008;101:1437–1443. doi: 10.1016/j.amjcard.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jouven X, Empana JP, Schwartz PJ. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman DW, Taffet G. Effects of aging on cardiovascular structure and function. In: Halter JB, Ouslander JG, Tinetti ME, Studenski SA, High KP, Asthana S, editors. Hazzard's Geriatric Medicine and Gerontology. McGraw Hill; New York: 2009. [Google Scholar]
- 16.Haidet GC. Dynamic exercise in senescent beagles: oxygen consumption and hemodynamic responses. Am J Physiol. 1989;257:H1428–H1437. doi: 10.1152/ajpheart.1989.257.5.H1428. [DOI] [PubMed] [Google Scholar]
- 17.Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol Heart Circ Physiol. 1990;258:H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB. Effect of aging, sex, and physical training on cardiovacular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 19.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women. The St. James Women Take Heart Project. Ciculation. 2010;122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 20.Astrand PO. Physical performance as a function of age. JAMA. 1968;205:729–733. doi: 10.1001/jama.205.11.729. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 22.Brawner CA, Ehrman JK, Schairer JR, Cao JJ, Keteyian SJ. Predicting maximum heart rate among patients with coronary heart disease receiving beta-adrenergic blockade therapy. Am Heart J. 2004;148:910–914. doi: 10.1016/j.ahj.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Corbelli R, Masterson M, Milkoff BL. Chronotropic response to exercise in patients with atrial fibrillation. Pacing Clin Electrophysiol. 1990;13:179–187. doi: 10.1111/j.1540-8159.1990.tb05068.x. [DOI] [PubMed] [Google Scholar]
- 24.Coyne JC, Rohrbaugh MJ, Shoham V, Sonnega JS, Nicklas JM, Cranford JA. Prognostic importance of marital quality for survival of congestive heart failure. Am J Cardiol. 2001;88:526–529. doi: 10.1016/s0002-9149(01)01731-3. [DOI] [PubMed] [Google Scholar]
- 25.Gwinn N, Leman R, Kratz J, White JK, Gillette P. Chronotropic incompetence: a common and progressive finding in pacemaker patients. Am Heart J. 1992;123:1216–1219. doi: 10.1016/s0002-8703(10)80001-8. [DOI] [PubMed] [Google Scholar]
- 26.Lamas GA, Knight JD, Sweeney M, Mianulli M, Jorapur V, Khalighi K, Cook JR, Silverman R, Rosenthal L, Clapp-Channing N, Lee KL, Mark DB. Impact of rate-modulated pacing on quality of life and exercise capacity--evidence from the Advanced Elements of Pacing Randomized Controlled Trial (ADEPT) Heart Rhythm. 2007;4:1125–1132. doi: 10.1016/j.hrthm.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Coman J, Freedman R, Koplan BA, Reeves R, Santucci P, Stolen KQ, Kraus SM, Meyer TE, LIFE Study Results A blended sensor restores chronotropic response more favorable than an accelerometer alone in pacemaker patients: the LIFE study results. 2008;31(11):1442. doi: 10.1111/j.1540-8159.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 28.Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality [see comments] JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 29.Dresing TJ, Blackstone EH, Pashkow FJ. Usefulness of impaired chronotropic response to exercise as a predictor of mortality, independent of the severity of coronary artery disease. Am J Cardiol. 2000;86:602–609. doi: 10.1016/s0002-9149(00)01036-5. [DOI] [PubMed] [Google Scholar]
- 30.Elhendy A, van Domburg RT, Bax JJ, Nierop PR, Geleijnse ML, Ibrahim MM, Roelandt JR. The functional significance of chronotropic incompetence during dobutamine stress test. Heart. 1999;81:398–403. doi: 10.1136/hrt.81.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okin PM, Lauer MS, Kligfield P. Chronotropic response to exercise. Improved performance of ST-depression criteria after adjustment for heart rate reserve. Circulation. 1996;94:3226–3231. doi: 10.1161/01.cir.94.12.3226. [DOI] [PubMed] [Google Scholar]
- 32.Wilkoff BL, Miller RE. Exercise testing for chronotropic assessment. Cardiology Clinics. 1992;10:705–717. [PubMed] [Google Scholar]
- 33.Savonen KP, Kiviniemi V, Laukkanen JA, Lakka TA, Rauramaa TH, Salonen JT, Rauramaa R. Chronotropic incompetence and mortality in middle-aged men with known or suspected coronary heart disease. Eur Heart J. 2008;29:1896–1902. doi: 10.1093/eurheartj/ehn269. [DOI] [PubMed] [Google Scholar]
- 34.Savonen KP, Lakka TA, Laukkanen JA, Rauramaa TH, Salonen JT, Rauramaa R. Usefulness of chronotropic incompetence in response to exercise as a predictor of myocardial infarction in middle-aged men without cardiovascular disease. Am J Cardiol. 2008;101:992–998. doi: 10.1016/j.amjcard.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Leeper NJ, Dewey FE, Ashley EA, Sandri M, Tan SY, Hadley D, Myers J, Froelicher V. Prognostic value of heart rate increase at onset of exercise testing. Circulation. 2007;115:468–474. doi: 10.1161/CIRCULATIONAHA.106.666388. [DOI] [PubMed] [Google Scholar]
- 36.Gauri AJ, Raxwal V, Roux L. Effects of chronotropic incompetence and beta blocker use on the treadmill test in men. Am Heart J. 2001;142:136–141. doi: 10.1067/mhj.2001.115788. [DOI] [PubMed] [Google Scholar]
- 37.Witte KK, Cleland J, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockers. Heart. 2006;92:481–486. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan ME, Pothier CE, Lauer MS. Chronotropic incompetence asa predictor of death among patients with normal electrocardiograms taking beta blockers. Am J Cardiol. 2005;96:1328–1333. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 39.Kass DA, Kitzman DW, Alvarez GE. The Restoration of Chronotropic CompEtence in Heart Failure PatientS with Normal Ejection FracTion (RESET) Study: Rationale and Design. J Card Fail. 2010;16:17–24. doi: 10.1016/j.cardfail.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magri D, Palermo P, Cauti FM, Contini M, Farina S, Cattadori G, Apostolo A, Salvioni E, Magini A, Vignati C, Vignati C, Alimento M, Sciomer S, Bussotti M, Agostoni P. Chronotropic incompetence and functional capacity in chronic heart failure: no role of beta-blockers and beta-blocker dose. Cardiovasc Ther. 2010 doi: 10.1111/j.1755-5922.2010.00184.x. Online Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 41.Jorde UP, Vittorio T, Kasper ME, Arezzi E, Colombo PC, Goldsmith RI, Ahuja K, Tseng CH, Haas F, Hirsh DS. Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: time to pace? Eur J Heart Fail. 2008;10:96–101. doi: 10.1016/j.ejheart.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Phan T, Shivu G, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 43.Vittorio T, Lanier G, Zolty R, Sarswat N, Tseng CH, Colombo PC, Jorde UP. Association between endothelial function and chronotropic incompetence in subjects with chronic heart failure receiving optimal medical therapy. Echocardiography. 2010;27:294–299. doi: 10.1111/j.1540-8175.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 44.Hinkle LE, Carver ST, Plakum A. Slow heart rates and increased risk of death cardiac death in middle aged men. Arch Intern Med. 1972;129:58–8. [PubMed] [Google Scholar]
- 45.Creager MA, Massie BM, Faxon DP, Friedman SD, Kramer BL, Weiner DA, Ryan TJ, Topic N, Melidossian CD. Acute and long-term effects of enalapril on the cardiovascular response to exercise and exercise tolerance in patients with congestive heart failure. J Am Coll Cardiol. 1985;6:163–173. doi: 10.1016/s0735-1097(85)80269-2. [DOI] [PubMed] [Google Scholar]
- 46.Eckberg DL, Brabinsky M, Braumwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- 47.Ellestad MH, Wan MK. Predictive implications of stress testing. Follow-up of 2700 subjects after maximum treadmill stress testing. Circulation. 1975;51:363–369. doi: 10.1161/01.cir.51.2.363. [DOI] [PubMed] [Google Scholar]
- 48.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise: prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 49.Ellestad MH. Chronotropic incompetence: The implications of heart rate response to exercise (compensatory parasympathetic hyperactivity?) Circulation. 1996;93:1485–1487. doi: 10.1161/01.cir.93.8.1485. [DOI] [PubMed] [Google Scholar]
- 50.Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol. 2004;44:423–430. doi: 10.1016/j.jacc.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 51.Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114:2070–2082. doi: 10.1161/CIRCULATIONAHA.105.561944. [DOI] [PubMed] [Google Scholar]
- 52.Myers J, Tan SY, Abella J, Aleti V, Froelicher V. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. 2007;14(2):221. doi: 10.1097/HJR.0b013e328088cb92. [DOI] [PubMed] [Google Scholar]
- 53.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Hong YL, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics - 2006 update - A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:E85–E151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 54.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 55.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 56.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study*. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan MJ, Hawthorne M. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;28:1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 58.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 59.Moore B, Brubaker PH, Stewart KP, Kitzman DW. VE/VCO2 Slope in Older Heart Failure Patients With Normal Versus Reduced Ejection Fraction Compared With Age-Matched Healthy Controls. J Card Fail. 2007;13:259–262. doi: 10.1016/j.cardfail.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Brubaker PH, Marburger CT, Morgan TM, Fray B, Kitzman DW. Exercise responses of elderly patients with diastolic versus systolic heart failure. Med Sci Sports Exerc. 2003;35:1477–1485. doi: 10.1249/01.MSS.0000084416.71232.EA. [DOI] [PubMed] [Google Scholar]
- 61.Maldonado-Martin S, Brubaker PH, Kaminsky LA, Moore JB, Stewart KP, Kitzman DW. The relationship of a 6-min walk to VO(2 peak) and VT in older heart failure patients. Med Sci Sports Exerc. 2006;38:1047–1053. doi: 10.1249/01.mss.0000222830.41735.14. [DOI] [PubMed] [Google Scholar]
- 62.Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: Effects on cardiac function, functional capacity, and quality of life. American Heart Journal. 2007;153:530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Williams AG, Rayson MP, Jubb M, World M, Woods DR, Hayward M, Martin J, Humphries SE, Montgomery HE. The ACE gene and muscle performance. Nature. 2000;403:614. doi: 10.1038/35001141. [DOI] [PubMed] [Google Scholar]
- 64.De Cort SS, Innes JA, Barstow T, Guz A. Cardiac output, oxygen consumption and arteriovenous oxygen difference following a sudden rise in exercise level in human. J Physiol. 1991;441:501–512. doi: 10.1113/jphysiol.1991.sp018764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myers J, Froelicher V. Hemodynamic determinants of exercise capacity in chronic heart failure. Ann Intern Med. 1991;115:377–386. doi: 10.7326/0003-4819-115-5-377. [DOI] [PubMed] [Google Scholar]
- 66.Higginbotham MB, Morris KG, Conn E, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:52–60. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 67.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 68.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 70.Clark AL, Coats AJ. Chronotropic incompetence in chronic heart failure. Int J Cardiol. 1995;49:225–231. doi: 10.1016/0167-5273(95)02316-o. [DOI] [PubMed] [Google Scholar]
- 71.Roche F, Pichot V, Da Costa A. Chronotropic Incompetence response to exercise in congestive heart failure, relationship with autonomic status. Clinical Physiology. 2001;3:335–342. doi: 10.1046/j.1365-2281.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 72.Bristow MR, Hershberger RE, Port JD. Beta-adrenergic pathways in non-failing and failing human ventricular myocardium. Circulation. 1990;82:12–25. [PubMed] [Google Scholar]
- 73.Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, Gauthier DF, Hartley LH. Impaired chronotropic response to exercise in patients wtih congestive heart failure. Circulation. 1989;80:314–323. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 74.Samejima H, Omiya K, Uno M. Relationship between impaired chronotropic response, cardiac output during exercise, exercise tolerance in patients with chronic heart failure. Jpn Heart J. 2003;44:515–525. doi: 10.1536/jhj.44.515. [DOI] [PubMed] [Google Scholar]
- 75.Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897–903. doi: 10.1161/01.CIR.0000139336.69955.AB. [DOI] [PubMed] [Google Scholar]
- 76.Gademan MG, Swenne CA, Verwey HF, van der Laarse A, Maan AC, van de Vooren H, van Pelt J, van Exel HF, Lucas C, Cleuren GV, Somer S, Schalij MJ, van der Wall EE. Effect of exercise training on autonomic derangement and neurohumoral activation in chronic heart failure. J Card Fail. 2007;13:294–303. doi: 10.1016/j.cardfail.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 77.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA. Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION): Design and rationale. American Heart Journal. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Grossman GB, Rohde LE, Clausell N. Evidence for increased peripheral production of tumor necrosis factor-alpha in advanced congestive heart failure. Am J Cardiol. 2001;88:578–581. doi: 10.1016/s0002-9149(01)01746-5. [DOI] [PubMed] [Google Scholar]
- 79.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA. 2000;283:3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 80.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects of functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 81.Kavanaugh T, Myers MG, Baigrie R. Quality of life and cardiorespiratory function in chronic heart failure: effects of 12 months of aerobic training. Heart. 1996;76:42–49. doi: 10.1136/hrt.76.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 83.Keteyian SJ, Brawner CA, Schairer JR. Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J. 1999;138:233–240. doi: 10.1016/s0002-8703(99)70106-7. [DOI] [PubMed] [Google Scholar]
- 84.McElroy PA, Janicki JS, Weber KT. Physiologic correlates of the heart rate response to upright isotonic exercise; relevance to rate-responsive pacemakers. J Am Coll Cardiol. 1988;11:94–99. doi: 10.1016/0735-1097(88)90172-6. [DOI] [PubMed] [Google Scholar]
- 85.Kappenberger L, Herpers L. Rate responsive dual chamber pacing. Pacing Clin Electrophysiol. 1986;9:987–991. doi: 10.1111/j.1540-8159.1986.tb06658.x. [DOI] [PubMed] [Google Scholar]
- 86.Buckingham TA. Effects of ventricular function on exercise hemodynamics of variable rate pacing. J Am Coll Cardiol. 1998;11:1269–1277. doi: 10.1016/0735-1097(88)90291-4. [DOI] [PubMed] [Google Scholar]
- 87.Freedman RA. Assessment of pacemaker chronotropic response: implementation of the wilkoff mathematical model. Pacing Clin Electrophysiol. 2001;24:1748–1754. doi: 10.1046/j.1460-9592.2001.01748.x. [DOI] [PubMed] [Google Scholar]
- 88.Tse HF, Siu CW, Lee KLF, Fan K, Chan HW, Tang MO, Tsang V, Lee SWL, Lau CP. The Incremental Benefit of Rate-Adaptive Pacing on Exercise Performance During Cardiac Resynchronization Therapy. Journal of the American College of Cardiology. 2005;46:2292–2297. doi: 10.1016/j.jacc.2005.02.097. [DOI] [PubMed] [Google Scholar]
- 89.Kawasaki T, Kalmoto S, Sakatani T, Miki S, Kamitani T, Kuribayashi T, Matsubara H, Sugihara II Chronotropic incompetence and autonomic dysfunction in patients without structural heart disease. Eurospace. 2010;12:561–566. doi: 10.1093/europace/eup433. [DOI] [PubMed] [Google Scholar]