Abstract
IL-6 has received significant attention for its regulatory role in muscle wasting during cachexia. This review will examine the role of circulating IL-6 for decreasing muscle mass during cancer and emphasize some of the indirect actions of IL-6 that may cause muscle wasting.
Keywords: Colon cancer, wasting, inflammation, atrogin-1, exercise
INTRODUCTION
The whole body wasting that occurs with disease has been acknowledged clinically since the time of the ancient Greeks. An unintentional 10% loss of body weight over a 12-month period that is directly associated with an underlying disease is termed cachexia (29). Cachexia involves the loss of both muscle and adipose tissue, and can be associated with many disease states, such as certain cancers, congestive heart failure, kidney failure, and HIV/AIDS (36). Over 80% of patients with gastric or pancreatic cancers exhibit weight loss, and the degree of weight loss with cancer has been associated with shorter survival times. A generalized inflammatory state is also characteristic of cachexia. The inflammatory cytokines that have been implicated in wasting diseases are interleukin-6 (IL-6), tumor necrosis factor–α (TNF-α), interleukin-1β (IL-1β), and interferon-γ (IFN-γ). Although many inflammatory mediators may play a role in muscle wasting during cachexia, IL-6 has emerged as a critical factor related to the maintenance of body mass during disease. While IL-6 has been implicated in many different disease states, this review's primary focus is on the role of IL-6 during cancer cachexia.
Beyond inflammation and disease, elevated circulating IL-6 levels have been clearly associated with an acute bout of exercise. Contracting skeletal muscle is an established source of IL-6 synthesis and release during exercise. The physiological implications related to exercise-induced increases in circulating and skeletal muscle IL-6 have been recently reviewed and demonstrates the important regulatory role of IL-6 on metabolism and immune function during and after exercise (32). An interesting and somewhat paradoxical question is how a single signaling molecule could be involved in diverse processes related to the metabolic response to exercise, inflammation, muscle regeneration, and also be an important regulator of cancer-induced muscle wasting. One important consideration related to this question is the length of time the circulating IL-6 is actually elevated; a chronic response versus a somewhat brief increase that returns to baseline. With exercise, circulating IL-6 can be elevated for several hours to several days, while cancer patients can have circulating IL-6 levels elevated chronically for weeks. Since systemic IL-6 levels normally peak immediately following an acute bout of exercise and return to baseline levels by 2 days post-exercise (31), our definition of chronic IL-6 is anything longer than this normal, physiological response of systemic IL-6 seen during exercise. A second important consideration when examining the potential effects of IL-6 relates to the source of the circulating IL-6, and the potential interactions between IL-6 and its soluble or cell surface receptor. The cellular source and level of receptor expression could impact the type of tissues responding to IL-6, and directly or indirectly regulate muscle homeostasis. While muscle is an important source of circulating IL-6 with exercise, other sources of IL-6 such as immune cells responding to infection, or a tumor with cancer, are likely sources of IL-6 with disease. Lastly, a chronic elevation of circulating IL-6 is often associated with an underlying disease, and specific aspects of different disease states could serve to alter the systemic and tissue level response to elevated IL-6.
This review will analyze the effect of chronically elevated IL-6 on the regulation of skeletal muscle mass, and the potential for exercise to influence this response. Although expression methodologies that increase either circulating IL-6 or IL-6 directly in muscle have provided tremendous insight into skeletal muscle regulation, the scope of this review is to examine the relationship between cancer, chronically elevated IL-6, and skeletal muscle mass. Acknowledging that there is considerable work remaining to ultimately determine direct and indirect effects of chronic IL-6 levels on skeletal muscle mass regulation, the central hypothesis of this review is that low, chronic levels of circulating IL-6 have a greater impact on the acceleration of disease progression, rather than directly regulating skeletal muscle protein turnover. The impact of the cellular and tissue level sources of IL-6, dosage of IL-6, and potential direct and indirect actions of IL-6 on muscle will be discussed.
IL-6 AND IL-6 SIGNALING
IL-6 signaling cascade
IL-6 is a member of a family of multi-functional cytokines that includes IL-11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CT-1), among others (22). IL-6 is commonly described as a pleiotropic cytokine, which is both produced by a variety of cell types, targets a variety of cell types, and has the capacity to induce several different intracellular signaling pathways. Circulating IL-6 levels are normally very low or undetectable and are dramatically increased in response to inflammatory conditions, often related to the acute phase immune response to infection. Through ligand-receptor interactions, IL-6 can induce intracellular signaling in a wide range of cell types including; smooth muscle cells, hepatocytes, and hematopoietic stem cells. The diversity of IL-6 regulatory targets relates directly to interaction with both the membrane bound and soluble IL-6 receptors. The membrane associated IL-6 receptor on target cells forms a heterodimer with the cell surface gp130 receptor, and this complex activates intracellular signaling pathways. The gp130 receptor is known as the signal transducing receptor, and is expressed on the plasma membrane of most cell types.
The regulation of intracellular signaling initiated by IL-6 has been well described for a number of cell types (22). Briefly, IL-6 binding to the IL-6 receptor and the subsequent heterodimer formation with two gp130 receptors serves to activate two janus kinase (JAK) family members by trans-phosphorylation. The activated JAKs can then phosphorylate signal transducers and activators of transcription (STAT) family proteins. This signaling pathway is commonly referred to as JAK/STAT signaling, and allows activated STAT proteins to dimerize and translocate to the nucleus to activate gene transcription related to processes associated with inflammation, angiogenesis, cell survival, cell proliferation, and cell transformation related to cancer. IL-6-induced STAT signaling also initiates negative feedback regulation through the increased transcription of suppressor of cytokine signaling (SOCS) proteins.
IL-6 binding to the soluble IL-6 receptor allows for gp130 receptor complex formation in a greater range of target cells, since the gp130 receptor is expressed in most cell types. This type of IL-6 signaling is termed “trans-signaling”, and is a biological regulatory event where the soluble receptor serves as a signaling agonist, rather than antagonist (22). Circulating soluble IL-6 receptor expression is increased in patients with colon cancer (39). Alterations in soluble IL-6 receptor expression with cachexia associated diseases could be important for the systemic amplification of IL-6 signaling in a variety of tissues.
IL-6 AND CANCER CACHEXIA
Human studies
Many cancers that induce cachexia also have elevated systemic IL-6 levels. IL-6's relationship to cancer cachexia has been well documented (36). Although many cytokines are involved in inflammatory processes and have the potential to be involved in cachexia, circulating IL-6 is well described as either elevated or a predictor of weight loss in human cancer cachexia (23, 33). IL-6 has been found in larger quantities in weight-losing patients with cancer than in patients with the same disease, but without weight loss (33). Iwase et al. (2004) reported that IL-6 was the only cytokine measured that was elevated in all 28 cachectic patients included in their study, and IL-6 levels increased in patients as they approached death. Circulating TNF-α was elevated in only 1 of 28 cachectic patients.
Rodent studies
Mouse models of cancer have provided valuable insight for understanding the effect of IL-6 on skeletal muscle wasting. IL-6 is an important regulator of muscle mass loss in several mouse models used to study cancer cachexia. C-26 colon adenocarcinoma implantation in the mouse flank, a commonly used cachexia model, typically results in an approximate 25–50% reduction in gastrocnemius muscle mass by 3 weeks post-inoculation (35). Mice implanted with the murine C-26 adenocarcinoma have increased circulating IL-6, which coincides with muscle wasting (35). In fact, certain clones of the C-26 that do not cause cachexia coincidently also do not produce IL-6 (18). Administration of specific IL-6 neutralizing antibodies or IL-6 inhibitors has decreased muscle wasting in tumor-bearing mice (35, 40). While this model is advantageous, mice implanted with the C-26 adenocarcinoma typically do not live longer than a month once inoculated with the tumor. Additionally, the tumor size also can comprise up to 10% of the animal's body weight, which usually does not occur in human cancer or cachexia. This non-physiological tumor to body mass ratio could place excessive systemic inflammatory and metabolic challenges on the animal.
ApcMin/+mice
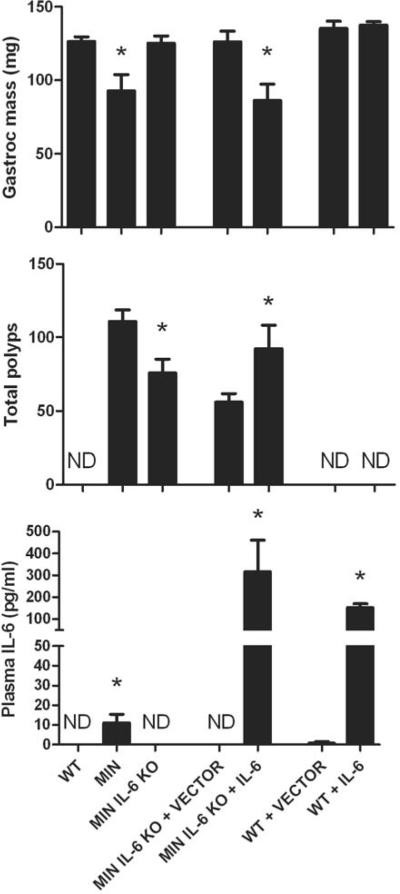
Our lab has used the ApcMin/+ mouse, which is widely used to study intestinal and colon polyp formation and growth, to study the impact of exercise and diet on intestinal tumor growth, as well as the regulation and progression of cancer cachexia (5–8, 26, 27). This mouse has a mutated adenomatous polyposis coli (Apc) gene, and spontaneously develops a large number of intestinal and colonic polyps by 4 weeks of age. During our original investigation into this mouse model, we found that body mass was 21% less and gastrocnemius muscle mass was 45% less than wild-type mice at 6 months of age (26). These mice also demonstrated chronic inflammation as they had splenomegaly and elevated IL-6 levels in the plasma, without any induction of circulating TNF-α levels. Our investigation then proceeded to examine if the induction of circulating IL-6 was necessary for the development and progression of cachexia in the ApcMin/+ mouse (7) (See Figure 1). First, we found that when 6-month-old ApcMin/+ mice were stratified by the degree of catechetic symptoms, mice exhibiting the greatest skeletal muscle mass loss also had the highest circulating IL-6 levels. We further addressed this question through the manipulation of circulating IL-6 levels by gene knockout or systemic over-expression methodologies. ApcMin/+ / IL-6−/− mice failed to develop cachexia and had skeletal muscle masses that were greater than cachectic ApcMin/+ mice, and similar to age-matched wild-type mice. This lack of cachexia was present in the ApcMin/+ / IL-6−/− mice, even though they carried a significant intestinal tumor burden. When IL-6 was replaced systemically in ApcMin/+ / IL-6−/− mice through the use of an IL-6 over-expression vector electroporated into the quadriceps muscle, gastrocnemius muscle mass and fat pad mass were significantly reduced. IL-6 over-expression at physiological levels (~180 pg/ml or ~18-fold greater) also accelerated cachexia in pre-cachectic ApcMin/+ mice. Both skeletal muscle wet masses and fiber cross-sectional areas were affected by the different manipulations to systemic IL-6 levels (7, 8). It is also worthy to note that IL-6 mRNA was not induced in the gastrocnemius muscle of the cachectic ApcMin/+ mice (8), suggesting another source of IL-6 that was causing the wasting. These data demonstrate that ApcMin/+ mice undergo an IL-6-dependent cachexia that is not related to local IL-6 production.
Figure 1. The dependence of IL-6 on muscle wasting and tumor formation.
Wild-type (WT), ApcMin/+ (MIN), ApcMin/+ / IL-6−/− mice (MIN IL-6 KO) were analyzed at 6 months of age for gastrocnemius muscle mass, total intestinal polyp number, and circulating IL-6. IL-6 or an empty vector was over-expressed in the circulation in ApcMin/+ / IL-6−/− mice (MIN IL-6 KO + VECTOR and MIN IL-6 KO + IL-6) and WT mice (WT + VECTOR and WT + IL-6). Treatment was done for 10 weeks and muscle mass, polyp counts, and circulating IL-6 were also analyzed at 6 months of age. ND=not detected. Figure 1 was created originally by the author and has not been previously published.
Source of IL-6 during cancer
While there may be many different tissues and cell types could be responsible for the increase in circulating IL-6 during some types of cancer, there is evidence that the tumors are an important source. It has been shown that when tumors are removed from cachectic rodents, body mass can return to normal and circulating IL-6 levels significantly decrease (35). Further work has demonstrated that clones of the C-26 adenocarcinoma that produced IL-6 cause cachexia, while those that do not produce IL-6 also did not have any effects on body mass (18). When IL-6-producing human melanoma or prostate cancer cells are implanted into mice to produce cachexia, administration of an IL-6 neutralizing antibody to human IL-6 prevented body mass loss (40), suggesting that the IL-6 that was being blocked was IL-6 that was being produced by the tumor. While IL-6 has been postulated to be a tumor growth factor, the functional role of tumor-produced IL-6 in mediating skeletal muscle mass has not been elucidated.
CHRONICALLY ELEVATED IL-6 WITHOUT UNDERLYING DISEASE
Many researchers have turned to non-disease models to delineate the effects of chronically elevated IL-6 on skeletal muscle. Chronic administration of IL-6 to otherwise healthy mice or rats through transgenic over-expression (12), systemic administration (19, 24), in vivo electroporation (17), or infused directly into skeletal muscle (10, 21), can decrease muscle mass, muscle protein content, or slow the rate of muscle growth. However, these effects appear related to the level of circulating IL-6, the type of administration, and the age of the rodent.
Human studies
The human response to altered circulating IL-6 levels at acute time points after administration has been examined. For example, Bente Pedersen's group has administered recombinant human IL-6 in healthy men at a dose of 140 pg/ml for 3 hours (37). They found a 50% decrease in muscle protein turnover, with protein synthesis suppressed more than protein breakdown. They also found that the release of amino acids from skeletal muscle increased 2-fold, while the concentration of amino acids in the blood decreased. Thus far, there have not been any studies conducted in humans looking solely at the long-term consequences of chronic, systemic IL-6 on skeletal muscle and adipose tissue mass.
Transgenic IL-6 mice
The overall mouse growth rate of transgenic mice over-expressing IL-6 is reduced 50–70% when compared to wild-type mice (12). The circulating IL-6 levels in these transgenic mice were (5,000–15,000 pg/ml), which are very high when compared to levels seen in many wasting disease conditions (100 pg/ml) in humans (23). When examining transgenic over-expression of IL-6, it must be recognized that IL-6 will be expressed in many, if not most tissues. This auto/paracrine regulation may not be present in most disease states, where the source of IL-6 is localized to an area of chronic infection. When interpreting these studies, it is important to consider there is both high circulating and tissue expression of IL-6.
Direct application of IL-6 to skeletal muscle
IL-6 infusion has been applied directly to skeletal muscle using mini-osmotic pumps (10, 21). Rats are implanted with these pumps and IL-6 is released over a period of 14 days, while circulating IL-6 levels remain the same or decrease compared to sham-operated, saline-infused rats. The dose used was designed to mimic plasma IL-6 concentrations after strenuous exercise (10–70 pg/ml). This model is advantageous for 2 reasons. First, the authors can delineate direct effects of IL-6 on skeletal muscle by administering it directly to the muscle without the complications of indirect effects of other tissues. Second, the dose they use is quite low compared to IL-6 transgenic mice and other models of high systemic IL-6, and nicely compliments IL-6 levels seen during cancer cachexia. The authors did report that the tibialis anterior muscles had 9% less protein content and 17% less myofibrillar protein content. However, changes in protein content were made between the IL-6-infused muscle and the contralateral, non-infused side, and it is not clear if muscle masses were altered with this type of treatment.
Systemic administration
The results of injecting rodents with IL-6 have provided conflicting results. One study injected human recombinant IL-6 into rats and measured protein breakdown 12 hours later (19). At a dose of 115 μg/kg body mass, IL-6 did not stimulate protein breakdown as measured by tyrosine and 3-methylhistine release in vitro. On the other hand, 2 injections of IL-6 at 125 μg/kg body mass, each separated by 3 hours, was enough to induce increases of 15–40% in tyrosine and 3-methylhistine release, respectively. The interesting observation is that this higher dose of IL-6 was also sufficient to induce fever in these rats, where fever was not induced with the lower dose. More recent work has administered recombinant human IL-6 to rats through continuous infusion through mini-osmotic pumps implanted subcutaneously for 7 days at a dose of 50, 100, or 250 μg/kg body mass/day (24). Only the high dose of IL-6 (250 μg/kg body mass) resulted in smaller muscle masses by approximately 15%. Serum measurements of IL-6 reached 12,000 pg/ml in the high-dose group and 765 pg/ml in the moderate dose group. These studies would suggest that only supraphysiological doses of IL-6 are capable of inducing atrophy in the absence of an underlying disease.
In vivo electroporation of IL-6 in mice
While some investigators have shown that IL-6 also is sufficient to induce cachexia, our lab has demonstrated that IL-6 does not affect overall body mass, lean tissue mass, or fat mass in mice without a tumor burden (Figure 1). Low level systemic IL-6 over-expression (152 pg/ml) for up to 10 weeks using in vivo electroporation had no effect on body mass, epididymal fat pad mass, or gastrocnemius muscle mass from wild-type mice (7).
A similar IL-6 plasmid and electroporation procedure has been used that involved injection of the tibialis cranealis, gastrocnemius, and quadriceps muscles of both limbs (17). As a result of the number of muscle electroporated the levels of serum IL-6 in this study were very high, approximately 800 pg/ml between days 7–15, and declined to 200 pg/ml 30 days after the electroporation procedure. Nevertheless, this procedure caused a 20% reduction in body mass after 7 days and remained suppressed during the 30-day experiment. IL-6 over-expression also caused an approximate 75% decrease in adipose tissue mass and a 9% reduction in quadriceps skeletal muscle mass. We have also reported that the muscle producing the IL-6 following electroporation (quadriceps) has a 16% reduction in quadriceps mass (8). This is likely related to the high dose of IL-6 being in this quadriceps muscle. An important consideration relates to the extremely high doses of circulating IL-6 with these methodologies, when compared to the levels seen in cachectic cancer. It remains to be determined if supraphysiological levels of IL-6 regulate wasting mechanisms similarly to the low-level chronic inflammation seen in many cachectic conditions. Of the research conducted thus far, low chronic levels of circulating IL-6 without underlying disease do not appear to be sufficient to induce body mass or skeletal muscle mass loss.
MUSCLE-SPECIFIC CHANGES DURING IL-6-DEPENDENT CACHEXIA
Most theoretical models describing muscle mass regulation clearly define the important balance between anabolic and catabolic stimuli for resultant growth or wasting. There is an unequivocal agreement in the literature that muscle mass loss with cachexia involves the regulation and induction of protein degradation, protein synthesis, and apoptosis (36). There has been a keen interest in better understanding the stimuli inducing wasting, the regulation of these mechanisms, and the type of muscle wasting that contributes to extreme loss of muscle mass. The identification of processes critical for muscle mass maintenance at different stages of cachexia, and how these processes are regulated by different types of underlying disease need better defining for the successful development of anti-cachectic therapeutics.
Glycolytic vs. oxidative muscle
Human skeletal muscle is a mix of oxidative and glycolytic muscle fibers. Besides differing in metabolic and physiological properties, fiber type also impacts sensitivity to atrophic stimuli. Atrophy induced by muscle disuse (i.e. bed-rest, spaceflight) affects these muscle phenotypes differentially; slow-oxidative muscle fibers initially undergo greater atrophy (11). Unlike disuse, the generally accepted consensus is that glycolytic fibers are more susceptible to wasting diseases. Additionally, the skeletal muscle mitochondria population is not homogenous, which may affect the ability of fast fibers to effectively deal with oxidative stress (4). With C-26 tumor implantation, there is significant wasting of the mouse fast-type gastrocnemius and tibialis anterior muscles, with minimal change in slow soleus muscle mass (2). Although these dramatic differences in muscle mass are not apparent in the ApcMin/+ mouse, we have reported a greater atrophy in the gastrocnemius compared to the soleus muscle (26). When quantifying muscle fiber cross-sectional area (CSA) of the gastrocnemius muscle, type IIb fiber CSA was 32% less in cachectic ApcMin/+ mice compared to age-matched wild-type mice, but the mean type IIa fiber CSA was unchanged (8). We have also found that the ApcMin/+ mouse soleus muscle undergoes regeneration and/or necrosis during cachexia, that is not present in the gastrocnemius muscle (26). When IL-6 was over-expressed in pre-cachectic ApcMin/+ mice to accelerate cachexia, only type IIb fibers demonstrated significant atrophy. Due to these differences related to muscle phenotype, it appears fiber type, possibly related to oxidative capacity, can have a significant role in regulatory processes related to cachexia. Further work is needed to establish if IL-6 preferentially target fast-type muscles to waste, while simultaneously altering regeneration and necrosis in oxidative muscle fibers.
Cell signaling
The lysosomal system involving the cathepsins, the calcium-activated system involving the calpains, and the ubiquitin system are well described processes for protein degradation in cells (36). Atrogin-1 and muscle ring finger-1 (MuRF-1) are 2 muscle specific E3 ligases that are induced in atrophying and wasting skeletal muscle. There have been conflicting reports as to whether MuRF-1 and Atrogin-1 are regulated by IL-6. IL-6 administration applied directly to the muscle for 14 days has been shown to not alter MuRF-1 (21). However, Atrogin-1 mRNA levels have been shown to both increase and not change with muscular treatment of IL-6 (10, 21).
Our lab has also measured Atrogin-1 and MuRF-1 mRNA and protein levels in the gastrocnemius using the ApcMin/+ mouse and our different IL-6 treatments (8). Atrogin-1, but not MuRF-1, was induced at both the gene and protein level as ApcMin/+ mice aged from 3 to 6 months of age, going from a pre-cachectic to a cachectic state. Atrogin-1 mRNA and protein levels were also elevated in ApcMin/+ mice when we over-expressed IL-6 in the circulation. However, there was a smaller increase in Atrogin-1 mRNA levels in wild-type, non-cachectic mice following IL-6 over-expression, which did not translate into increased Atrogin-1 protein. Further work will need to examine the regulatory importance of Atrogin-1 sensitivity to IL-6, and if this relates to the pathology of wasting muscle in IL-6-dependent cachexia.
INDIRECT EFFECTS OF IL-6
When examining systemic over-expression or disease-induced increases in IL-6, it is difficult, if not impossible, to delineate the direct effects on skeletal muscle versus indirect effects related to other systemic changes. Since many cell types in muscle have the capacity to respond to IL-6-initiated signaling, it is interesting to further determine which cell types are initiating the biological responses to the directly applied IL-6 stimulus. Additionally, understanding the integration of IL-6 signaling between endothelial cells, immune cells, fibroblast cells, satellite cells, and myofibers to regulate muscle plasticity and size remains an important question. Physiologically, increased circulating IL-6 due to underlying disease will almost certainly influence muscle mass through a combination of direct and indirect effects, and include a variety of cell types for the disruption of muscle homeostasis. The systemic alterations that accompany many diseases associated with cachexia are likely important for understanding the integrated signaling events that leads to muscle wasting. These integrative events may also provide the rationale for muscle wasting specifically related to the regulation of muscle loss with different underlying diseases. Since physiological concentrations of circulating IL-6 are insufficient to induce skeletal muscle wasting in wild-type mice, one must point to indirect effects of IL-6 to mediate its cachectic effect during cancer.
Tumor growth and formation
IL-6 may be working indirectly to affect the polyp burden since IL-6 is a potent tumor growth factor (14, 34). IL-6 mRNA expression is normally low in normal intestinal epithelia cells, but increases in expression as tumor progression advances. IL-6 treatment in vitro induced cell proliferation in a concentration-dependent manner in colonic epithelial cells that were also heterozygous for Apc (Apc+/− cells), but not in colonic cells that were Apc+/+ (14). IL-6 facilitates tumor growth in prostate cancer cells as well (34). However, one study has shown that an anti-IL-6 antibody prevented muscle wasting in mice, but this type of treatment did not affect tumor size (40). This result would suggest that IL-6 may have the capability to directly cause muscle wasting, independent of tumor growth.
Data from our lab suggests that IL-6 has a potent effect on tumor growth and formation, and this is correlated with the severity of cachectic symptoms. We have demonstrated that the intestinal polyp burden correlates with plasma IL-6 levels (26). In addition, ApcMin/+ mice that lack IL-6 have fewer and smaller tumors than ApcMin/+ mice with IL-6 (7). Finally, when we over-express systemic IL-6 into ApcMin/+ / IL-6−/− mice, the tumor burden increases (See Figure 1). These data suggest that IL-6 has a potent effect on tumor multiplicity and growth, and treatments that inhibit either of these processes may have a positive effect on skeletal muscle mass. The question remains as to whether skeletal muscle wasting can be attenuated in the absence of an effective therapy at the level of the tumor.
Fat metabolism
Another potential indirect effect of IL-6 could be its effect as a lipolytic agent. IL-6 also affects lipid metabolism through oxidation and lipoprotein lipase activity (20). In general, adipose tissue mass precedes skeletal muscle mass loss and there is more adipose tissue loss than skeletal muscle mass loss (15). Circulating triglycerides also increase quite rapidly in ApcMin/+ mice, and these levels are correlated to the tumor burden (30).
Our data in the ApcMin/+ mouse shows that epididymal fat pad is about 50% less in young (3-month-old) ApcMin/+ mice compared to wild-type mice, but gastrocnemius muscle mass is unaltered at this early time point. This is the same time where we see increases in circulating IL-6 compared to wild-type mice (27). By 6 months of age, epididymal fat pad mass is ~75% less and gastrocnemius muscle mass is ~24% less than wild-type mice. It is quite possible that IL-6 induces the release of lipid from adipose tissue stores, and this state of hyperlipidemia is detrimental for skeletal muscle. It is currently unknown whether IL-6 affects hyperlipidemia in this mouse model. More work is necessary to delineate the effect of hyperlipidemia on skeletal muscle mass and the interaction between adipose tissue stores and skeletal muscle size.
EXERCISE, IL-6 AND CACHEXIA
The benefits of exercise are numerous. Health-related exercise guidelines released by the US government in 2008 call for 30 minutes of moderate intensity exercise most days of the week (1). The American Cancer Society has also published suggestions for physical activity levels in cancer patients undergoing treatment and during the recovery process (13). They suggest maintaining as much physical activity as possible to improve fatigue, anxiety, self-esteem, cardiovascular fitness, muscle strength, and body composition. However, they also caution older individuals and others that may have complications, such as anemia, compromised immune function, or musculoskeletal problems, that these symptoms may pose as contraindications to exercise. However, physical activity guidelines have not been established for those that also have cachexia. More research is needed to determine if exercise can be beneficial for cancer patients undergoing muscle wasting. The few studies that have been conducted in humans and rodents, as well as physical activity levels during cachexia and their relationship to circulating IL-6, are discussed below.
Physical activity levels during cachexia
Another indirect effect of IL-6 that could contribute to cachexia is its effect on activity. Cancer patients have been monitored for their physical activity levels. While overall physical activity did not differ between cancer and age-matched non-cancer patients, spontaneous physical activity was correlated to weight loss and blood hemoglobin concentrations, but circulating IL-6 was not measured (16). Others have shown that weight-losing cancer patients exhibit less physical activity than healthy controls (28).
When looking at rodent studies, our search of the literature did not uncover any studies that have measured physical activity levels during cancer cachexia. However, during high circulating IL-6 administration, IL-6 doses that were sufficient to cause muscle atrophy did not alter physical activity levels in rats (24). Likewise, when IL-6 was administered to rats via mini-osmotic pump directly to the muscle, the rats ran on average 4.5 km/d over the course of the study and ~5.5 km/d during the final 6 days of the study (10). While there was not a control group given access to wheels, the data suggests that physical activity levels are not affected by IL-6 alone.
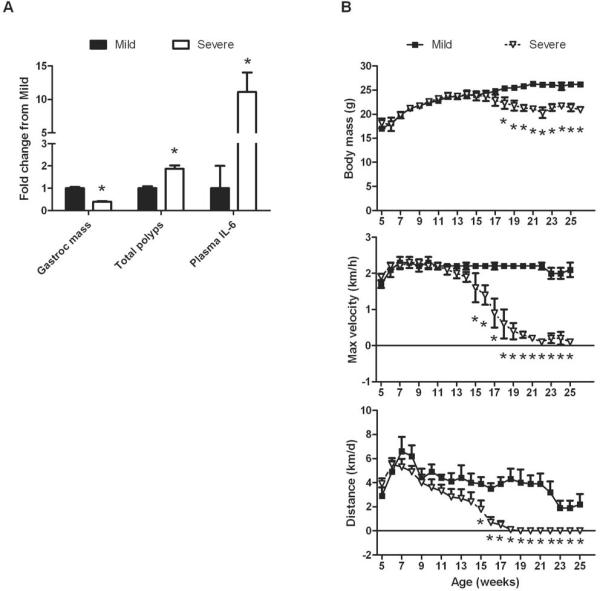
In work from our lab, we have shown that ApcMin/+ mice that become cachectic exhibit less voluntary wheel running activity, and this is associated with a greater polyp burden, higher circulating IL-6 levels, and gastrocnemius muscle atrophy (See Figure 2). Severely cachectic also start running less distance between 15 and 18 weeks of age (7). In more recent work, we have shown that maximum speed generated in voluntary activity wheels starts to decrease from wild-type mice by 15 weeks of age, while a change in body mass does not occur until 18 weeks of age (9). In another study, we have found that the number of mitochondria and many enzymes are reduced in severely cachectic mice (38). These data would suggest that changes in physical activity precede any changes in body mass.
Figure 2. The relationship between muscle wasting, circulating IL-6, and physical activity levels in cachectic ApcMin/+ mice.
ApcMin/+ mice were given access to voluntary activity wheels starting at 5 weeks of age until 26 weeks of age. At the study's end, mice were stratified by their degree of wasting, which was dependent upon body mass, gastrocnemius muscle mass, and epididymal fat pad mass. A. Gastrocnemius muscle mass, total polyps, and plasma IL-6 in mice with mild or severe cachexia. B. Body mass loss occurs after changes in physical activity. Physical activity was measured as maximum daily running velocity and daily distance run. *Signifies different from mice with mild cachexia. Figure 2 was created originally by the author and has not been previously published.
However, since others have not seen drastic reductions in physical activity levels with IL-6 administration into healthy rodents, it is possible that IL-6 indirectly affects activity levels. One variable could be anemia. In fact, we have shown that IL-6 is associated with the onset of anemia in ApcMin/+ mice (9). Additionally, the onset of anemia precedes the decrease in physical activity level. It is possible that physical activity levels are reduced because of the onset of anemia. In fact, others have shown that erythropoietin administration improves symptoms of cachexia, and decreases circulating IL-6 levels (25).
Exercise training effects on cachexia
While many articles have been written about the potential for exercise training to prevent or attenuate cancer cachexia, there is an extremely limited amount of published research in the area. Resistance training mimicked using electrical stimulation in mice bearing the colon-26 adenocarcinoma has been employed. This treatment increased muscle mass and protein content in the stimulated side compared to the unstimulated side, 62%, and 25%, respectively (3). Artificially increasing endurance capacity through erythropoietin in C-26-bearing mice can delay the loss of fat and muscle mass, in addition to reducing circulating IL-6, and improving survival (25).
We have used both treadmill and voluntary wheel running training paradigms for periods of 6 to 9 weeks to examine the effect of exercise on the tumor incidence and growth (See Figure 3). We have published that only treadmill training, not voluntary wheel running can reduce the tumor burden in male ApcMin/+ mice (27). However, circulating IL-6 levels were reduced with both treadmill training and voluntary wheel running. While gastrocnemius muscles were similar in mass between sedentary and treadmill-trained mice, mice with access to voluntary activity wheels actually had smaller muscle masses that the other two groups. These findings have important implications for cachexia. First, If IL-6 and the overall tumor burden are both important for muscle wasting, and treadmill exercise training can reduce both of these, we would speculate that moderate intensity treadmill exercise training may also be useful in preventing muscle wasting. However, we have not conducted any studies in older ApcMin/+ mice that are prone to cachexia. It is possible that low levels of exercise may be able to prevent cachexia in the early stages of cancer, but that exercise administered to a cachectic mouse may actually exacerbate the condition. Second, mice with access to voluntary activity wheels actually underwent atrophy. These mice also ran 4 times more than mice that were treadmill-trained. It is possible that moderate amounts of exercise are more beneficial than longer durations of exercise, despite positive effects on circulating IL-6 levels.
Figure 3. Exercise training reduces the polyp burden and circulating IL-6 in young ApcMin/+ mice.
Male ApcMin/+ mice were sedentary (Control), exercise trained (Treadmill), or given access to voluntary activity wheels (Activity Wheel) from 4 to 13 weeks of age. At the study's end, gastrocnemius muscle mass, total polyps, and circulating IL-6 levels were analyzed. Mice in activity wheels ran approximately 4 times more than mice on the treadmill. Daily spontaneous activity was not determined (ND) for Control mice. *Signifies different from Control. #Signifies different from Treadmill. Figure 3 was created originally by the author and has not been previously published.
It is not certain what role acute exercise-induced increases in IL-6 would have in the development and progression of cachexia. Muscle-produced IL-6 following a bout of exercise is thought to be a metabolic signal related to glucose metabolism and the replenishment of muscle glycogen stores. It is interesting to speculate that IL-6 could also have a regulatory role related to glucose metabolism in tumor cells, and this could occur at the expense of other tissues. These metabolic shifts related to IL-6 and tumor cells could explain why chronic circulating IL-6 levels do not cause atrophy in a non-tumor bearing mouse. It is also intriguing to speculate that these metabolic changes related to IL-6 may play a role in the susceptibility of type IIb fibers to cachectic stimuli (See Figure 4). Further work is needed to clearly define the relationship between exercise, IL-6, and the prevention of cachexia. Nevertheless, exercise of appropriate intensity and duration holds promise as a therapy for muscle and body mass retention with some types of cachexia.
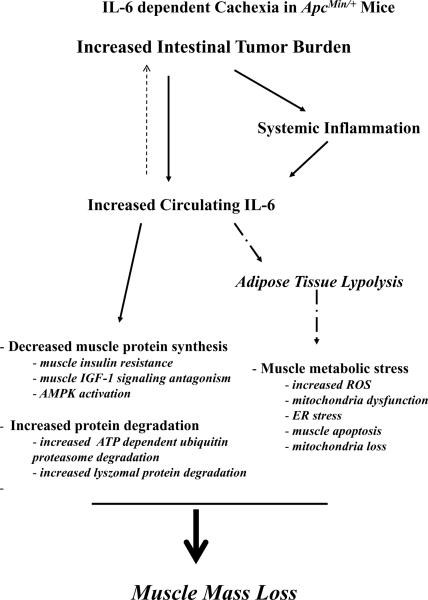
Figure 4. Theoretical model of IL-6 regulation of muscle wasting in the ApcMin/+mouse.
The increase in intestinal and colon tumor number and size increases systemic inflammation and circulating IL-6, which can act as a positive feedback signal to increase tumor growth and subsequent inflammation. IL-6 can directly influence muscle protein turnover through suppression of protein synthesis and activation of protein degradation. Indirect effects of IL-6 through the induction of adipose tissue lypolysis cause metabolic stress in the cachectic muscle. Figure 4 was created originally by the author and has not been previously published.
CONCLUSION
The biomedical research community is seeking treatments for cachexia which attenuate the loss of body weight without affecting the underlying disease. Treating wasting by improving the underlying disease state is a separate clinical issue. These criteria are also important when interpreting animal-based cachexia research. A true understanding of mechanisms related to the induction of muscle wasting is critically important for developing therapies that may improve outcomes related to patient health and quality of life. Essential to this goal is an understanding of the mechanism involved in different stages of cachexia. It has been acknowledged that there is little hope in reversing the severely cachectic condition. The critical time for intervention has been established as the period of pre-cachexia, or less than 5% body weight loss. Nutritional and physical activity interventions alone or in combination hold promise. Increased physical activity has the potential to lower chronic inflammation, increase muscle mitochondria content and function, and improve insulin sensitivity. Anabolic interventions such as testosterone and amino acid supplementation alone or in combination with exercise may provide an excellent paradigm for improving muscle protein maintenance and accretion. The future hope is that this research today will lead to specific science-based exercise and nutritional guidelines for maintaining body weight and muscle mass when living with and battling various diseases known to induce cachexia.
Acknowledgments
Disclosure The review was supported by NIH Grant 1 R01 CA121249-01A2 from the National Cancer Institute to James A. Carson. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.U.S.D.o.H.a.H. Services, editor. 2008 Physical Activity Guidelines for Americans. U.S. Department of Health and Human Services; Washington, D.C.: 2008. [Google Scholar]
- 2.Acharyya S, Ladner KJ, Nelsen LL, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–8. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Majid S, McCarthy DO. Resistance exercise training attenuates wasting of the extensor digitorum longus muscle in mice bearing the colon-26 adenocarcinoma. Biol Res Nurs. 2001;2:155–66. doi: 10.1177/109980040100200301. [DOI] [PubMed] [Google Scholar]
- 4.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290:C844–51. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 5.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104:1137–43. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 6.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. The interaction of a high-fat diet and regular moderate intensity exercise on intestinal polyp development in Apc Min/+ mice. Cancer Prev Res (Phila Pa) 2009;2:641–9. doi: 10.1158/1940-6207.CAPR-09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 8.Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Muscle wasting and interleukin-6-induced atrogin-I expression in the cachectic Apc (Min/+) mouse. Pflugers Arch. 2009;457:989–1001. doi: 10.1007/s00424-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baltgalvis KA, Berger FG, Pena MM, Davis JM, White JP, Carson JA. Activity level, apoptosis, and the development of cachexia in ApcMin/+ mice. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00442.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodell PW, Kodesh E, Haddad F, Zaldivar FP, Cooper DM, Adams GR. Skeletal muscle growth in young rats is inhibited by chronic exposure to IL-6 but preserved by concurrent voluntary endurance exercise. J Appl Physiol. 2009;106:443–53. doi: 10.1152/japplphysiol.90831.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth FW, Criswell DS. Molecular events underlying skeletal muscle atrophy and the development of effective countermeasures. Int J Sports Med. 1997;18(Suppl 4):S265–9. doi: 10.1055/s-2007-972723. [DOI] [PubMed] [Google Scholar]
- 12.De Benedetti F, Meazza C, Martini A. Role of interleukin-6 in growth failure: an animal model. Horm Res. 2002;58(Suppl 1):24–7. doi: 10.1159/000064757. [DOI] [PubMed] [Google Scholar]
- 13.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 14.Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507–15. doi: 10.1093/carcin/bgl018. [DOI] [PubMed] [Google Scholar]
- 15.Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care--correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 2005;103:2189–98. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- 16.Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13:6379–85. doi: 10.1158/1078-0432.CCR-07-1147. [DOI] [PubMed] [Google Scholar]
- 17.Franckhauser S, Elias I, Rotter Sopasakis V, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;51:1306–16. doi: 10.1007/s00125-008-0998-8. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto-Ouchi K, Tamura S, Mori K, Tanaka Y, Ishitsuka H. Establishment and characterization of cachexia-inducing and -non-inducing clones of murine colon 26 carcinoma. Int J Cancer. 1995;61:522–8. doi: 10.1002/ijc.2910610416. [DOI] [PubMed] [Google Scholar]
- 19.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–5. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg AS, Nordan RP, McIntosh J, Calvo JC, Scow RO, Jablons D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992;52:4113–6. [PubMed] [Google Scholar]
- 21.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–7. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase S, Murakami T, Saito Y, Nakagawa K. Steep elevation of blood interleukin-6 (IL-6) associated only with late stages of cachexia in cancer patients. Eur Cytokine Netw. 2004;15:312–6. [PubMed] [Google Scholar]
- 24.Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 25.Kanzaki M, Soda K, Gin PT, Kai T, Konishi F, Kawakami M. Erythropoietin attenuates cachectic events and decreases production of interleukin-6, a cachexia-inducing cytokine. Cytokine. 2005;32:234–9. doi: 10.1016/j.cyto.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J Appl Physiol. 2005;99:2379–87. doi: 10.1152/japplphysiol.00778.2005. [DOI] [PubMed] [Google Scholar]
- 27.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98:2219–25. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 28.Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90:996–1002. doi: 10.1038/sj.bjc.6601620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Niho N, Takahashi M, Kitamura T, et al. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–5. [PubMed] [Google Scholar]
- 31.Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513(Pt 3):889–94. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 33.Scott HR, McMillan DC, Crilly A, McArdle CS, Milroy R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br J Cancer. 1996;73:1560–2. doi: 10.1038/bjc.1996.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner H, Godoy-Tundidor S, Rogatsch H, et al. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am J Pathol. 2003;162:655–63. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–4. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- 37.van Hall G, Steensberg A, Fischer C, et al. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93:2851–8. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 38.White JP, Baltgalvis KA, Puppa M, Sato S, Baynes JW, Carson JA. Muscle phenotype and oxidative metabolic capacity during IL-6 dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00300.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh KY, Li YY, Hsieh LL, et al. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Jpn J Clin Oncol. 2010;40:580–7. doi: 10.1093/jjco/hyq010. [DOI] [PubMed] [Google Scholar]
- 40.Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111:592–5. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]