Summary
Encouraged by remarkable successes in preventing infectious diseases and by the well established potential of immune system for controlling tumor growth, active therapeutic immunization approaches hold great promise for treating malignant tumors. In recent years, engineered recombinant viral vectors have been carefully examined as genetic immunization vehicles and have been demonstrated to induce potent T cell mediated immune responses that can control tumor growth. Very recent efforts suggest that lentivectors possess important advantages over other candidate recombinant viral vectors for genetic immunization. Here we review the development of recombinant lentivectors and the characteristics of T cell immune responses elicited by lentivector immunization, including the mechanism of T cell priming with a focus on the role of skin dendritic cells (DC) and potential applications for tumor immunotherapy.
Keywords: lentivector (lentiviral vector), tumor immunotherapy, dendritic cell subsets, genetic immunization, gene delivery
1. Introduction
T cell mediated immunity is critical for the prevention and control of a broad range of infectious diseases and human malignancies 1, 2. Elimination of tumor is mainly dependent on the activation of tumor specific T cells, and especially CD8+ T cells, to recognize and kill tumor cells. Thus, active immunization against tumors is intended to elicit potent and durable T cell immune responses to eliminate existing tumors, and recognize and destroy evolving tumor cells as they arise. However, despite extensive efforts and many clinical trials, efficacy in tumor immunotherapy has been limited. The immunosuppressive condition induced by growing tumors and weakly immunogenic self-antigen (Ag) tumor targets likely contribute to the failure of tumor immunization 3–6, but also suggest that better Ag delivery systems are needed in order to elicit effective immune responses against weak Ag in immune compromised environments.
Genetic immunization, especially with recombinant viral vectors, is a promising approach for the elicitation of T-cell immunity, and genetic immunization strategies are now being developed in animal models and evaluated in clinical trials 1. A number of different recombinant viral vectors have been examined for the purpose of genetic immunization 7. Recombinant vaccinia viruses were among the first vectors developed for genetic immunization 8–10 and have been widely utilized in clinical trials 11. However, comparative studies suggested that vaccinia vectors is not as effective as other viral vectors in term of eliciting effector and memory T cell immunity in mice and in non-human primates 12–14. In addition, their efficacy for immunization has been shown to be limited by pre-existing anti-vaccinia immunity in the general population. In contrast, adenovirus based vectors, are generally considered to be the most potent gene delivery vehicles for inducing T cell immune responses 12, 15. However, the clinical application of adenoviral vectors for immunization is also limited by vector immunogenicity. In particular, the development of dominant immune responses against viral Ags is a major factor limiting the generation of transgenic Ag specific immune responses. This appears to be a problem both in the context of widespread pre-existing antivector immunity in targeted populations, and in regard to de novo generation of immunodominant immune responses against vector Ags following vector delivery. In this context, very recent studies suggest that lentivectors (lentiviral vectors) are promising vectors for eliciting Ag specific T-cell immunity 16–20. Immunization with lentivectors has been observed to induce remarkably potent and durable T-cell immunity. This is likely related to their capacity to transduce non-dividing cells, including DCs in vivo, and to enable persistent Ag presentation through high level expression of transgenes and low vector immunogenicity. Lentivector has recently been utilized in preclinical animal models for prevention of infectious diseases 21–23 and tumor immunotherapy. With improved bisosafety and better understanding of the basic mechanism of T cell priming induced by lentivector immunization, these vectors have great potential to effectively deliver Ag to induce potent T cell immunity against tumors. Here we provide a brief description of lentivector development and then review the recent progress in the development of lentivector based genetic immunization with emphasis on the immunobiology of lentivectors, the unique mechanistic features of lentivector-mediated immunization that may contribute to their observed potency, and the promising potential of lentivector mediated genetic immunization in tumor immunotherapy.
2. Lentivector development
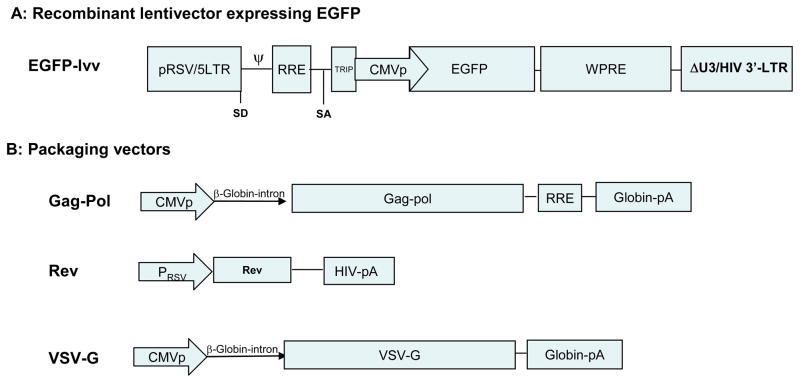
Recombinant lentivectors are replication-defective, hybrid viral particles made of RNA containing the cis-element of the lentiviral genome, lentivirus core protein and enzymes, and the envelope protein of a different virus, typically the vesicular stomatitis virus glycoprotein (VSV-G). Lentivector was first developed for the purpose of gene therapy because of the merits of infecting non-dividing cells and prolonging gene expression in infected cells through genome integration. The first generation of lentivector was described by Naldini et al in the middle of 1990s using three plasmid co-transfection methods 24. The HIV genome with deletion of the packaging signal and envelope protein was employed as a packaging construct under the control of the heterologous CMV promoter. A second plasmid encoded the envelope protein of VSV-G for generating pseudotyped hybrid viral particles. The third plasmid served as transfer plasmid carrying the desired gene and cis-acting elements such as packaging signals. RNA transcribed from the transfer plasmid is encapsulated by VSV-G envelope protein into the viral particle that is then released into medium from co-transfected cells to form recombinant lentivector. The utilization of VSV-G protein as envelope not only results in greater stability but also broadens the tropism of lentivector infections. The biosafety of recombinant lentivector was improved by deleting all the accessory proteins that are crucial for viral virulence (Vif, Vpr, Vpu, and Nef) of the lentiviral genome in the packaging plasmid, resulting in the “second generation” lentivector without reducing the efficiency of transduction 25. In both the first and second generation lentivectors, the regulatory protein Tat is essential for generating full length viral transcripts by binding the TAR (transactivation response element) stem-loop structure in the nascent RNA to recruit cyclin-kinase complex that stimulates transcription elongation by polymerase II to form full length lentivector genome.
The refinement of lentivector development led to the development of third generation of lentivectors. In the “third generation” of lentivector, the requirement of Tat is offset by using a constitutive chimeric promoter (RSV U3 enhancer joined to U5 region of HIV 5’ LTR) upstream of the vector transcript 26. The biosafety of third generation lentivectors was further improved by complete removal of U3 region of 3’ LTR, resulting in self-inactivating lentivectors 27. During reverse transcription, the deletion of U3 region of 3’ LTR is transferred to the 5’ LTR of the proviral DNA, which abolish the promoter activity of 5’ LTR and the production of full length vector RNA from the proviral DNA. Thus, after being integrated into the host cell genome, the recombinant lentivector is unable to produce the viral genome. In the third generation of lentivector, Rev is provided in-trans by a separate non-overlapping construct to further minimize the potential of generating replication-competent recombinants (RCR, also called replication-competent virus, RCV). Figure 1 depicts the current widely used third generation recombinant lentivector and its packaging plasmids. Recombinant lentivectors capable of expressing multiple genes have also been developed. These vectors utilize either different promoters 28, 29 or the internal ribosomal entry site (IRES) of encephalomyocarditis virus (EMCV) and/or foot-and-mouth disease (FMDV cleavage factor 2A 30 to drive gene expression. These improvements broaden the application potential of recombinant lentivector mediated gene delivery by enabling simultaneous expression of multiple genes in desired target cells.
Fig. 1.
Diagram of the recombinant lentivector. A represents the recombinant lentivector genome that will be packaged into viral particles. B are the packaging vectors expressing necessary components for assembling the recombinant lentivectors. RSV: Rous Sarcoma Virus; LTR: Long Terminal Repeat; RRV: Rev Responsive Element; TRIP: Triple-stranded DNA flap that contains a central polypurine tract sequence (cPPT) and the central termination sequence (CTS); CMVp, CMV promoter; WPRE:woodchuck hepatitis virus posttranscriptional regulatory element; VSV-G: Vesicular Stomatitis Virus Glycoprotein.
One of the major concerns of using integration competent viral vectors including conventional oncoretroviral vector and lentivector in gene delivery and genetic immunization is the insertional tumorigenesis. Indeed, infection of cancer-prone Cdkn2a deficient mice with retrovirus Moloney murine leukemia virus (MoMuLV) led to marked acceleration of myeloid and lymphoid tumors possibly by preferential integration into cancer-related loci or signaling pathway involved in cancer 31. Furthermore, high frequency of leukemia developed in the children of severe combined immuno deficiency (SCID) treated with ex vivo retroviral vector modified stem cells 32. In contrast, however, in the same cancer prone mouse model, injection of recombinant lentivector modified stem cells did not accelerate tumorigenesis 33. Consistent with this, patients with HIV infections are not associated with increased malignancy because of integration. In addition, there is no adverse effect in the mice transplanted with lentivector transduced hematopietic stem cells 34. Even though current data indicate that recombinant lentivector possess much safer profile compared to retroviral vector, the concern of insertional mutagenesis prompts development of the non-integrated recombinant lentivector. Saenz et al reported that in vitro if cell division was prevented, non-integrated lentivector can produce high level of transgene expression equivalent to integration competent lentivector 35. Similar results were also observed in vivo by demonstrating comparable level of transgene expression in non-dividing ocular and brain regardless of integration proficiency 36. Since DCs (the most potent Ag presenting cells) are non-dividing, it is likely that non-integrating lentivector will be equally effective in stimulating Ag specific immune responses. Indeed, Negri et al showed that single immunization with non-integrating lentivector stimulate potent and persistent T cell responses and antibody production at day 30 and day 90 comparable to that induced by integrated lentivector although they did not examine if effector function early time points was similar 37.
The commonly used approach for producing recombinant lentivector in laboratories utilizes transient co-transfection methods using calcium phosphate precipitation 38, 39. Although scale up of the co-transfection approach remains a challenge, this method is versatile in allowing production of lentivectors which can be pseudotyped with a variety of viral proteins with different cell type tropisms (see reviews by 40, 41. Recombinant lentivector pseudotyped with envelope protein from different viruses results in preferential transductions of restricted cell types, enabling partial targeting. Further specific cell targeting by lentivectors has been achieved by incorporating specific single chain antibodies into recombinant viral vectors which will recognize specific Ags whose expression is restricted to specific cell types 42, 43. These improvements in lentivector construction directly address biosafety concerns of HIV derived gene delivery vectors and are likely to enable clinical applications of lentivectors in near future.
The concentrations of recombinant lentivector are measured as a titer, either as transduction units (TU) or as p24 level in the preparations. The transduction units are determined by examining the infectious particles using easily infectable cell lines such as 293T cells. This method is time-consuming and only useful for lentivector expressing marker genes such as GFP or other genes whose proteins can be easily identified. Measurement of p24 levels in lentivector preparations is an alternative simple approach for approximating titers. However, the co-relation between transduction units and p24 level can be variable. A good vector preparation can be 10–100 transduction units per pg of p24. Other titration methods such as real time quantitative PCR based methods to measure the copy number of genomes have also been developed 44.
3. Lentivector mediated genetic immunization
For initiating (or priming) T cell responses, APCs have to acquire, process, and present antigenic epitopes in the form of MHC-peptide complexes to T cells that possesses the right TCR for recognizing the MHC-peptide complex. The most potent APCs are DCs. In fact, DCs are the probably the only APCs that prime naïve T cells in vivo 45. Recent studiee suggest that recombinant lentivector can effectively transduce DCs in vitro and in vivo and induce potent and persistent T cell immunity and long-lasting memory T cell responses underscored by prolonged in vivo Ag presentation. Mechanistic studies indicate that unlike wild type viral infections where cross presentation by LN resident DCs is important for eliciting antiviral T cell responses, migrating skin DCs can effectively present lentivector encoded Ag to naïve T cells following subcutaneous immunization of lentivector 46. This direct priming mechanism likely contributes to the elicitation of potent T cell immunity following lentivector immunization.
3.1 Transduction of APCs with recombinant Lentivector
Compared to conventional oncoretroviral vectors, a major advantage of lentivectors is their capability to infect non-dividing cells. Since DCs are the most effective APCs and non-dividing, a bona fides question is whether lentivectors can transduce DCs, resulting in effective processing and presentation of lentivector encoded Ags to immune cells. Following the first demonstration of transduction of CD34+ in vitro by lentivector and the transgene expression in the progeny DCs in 1998 47, several groups independently found that lentivectors are remarkably effective for transducing human peripheral monocyte derived DCs in vitro. Reported efficiencies of human DC transduction have varied considerably from 30–40% in some studies 48, 49, to as much as 70–100% in others 20, 50–52. Similar efficiencies were observed in the transduction of murine DCs, by lentivectors with transduction efficiencies reported from 40–50% to more than 90% 16, 19, 50, 53. The observed capacity of lentivectors to transduce non-dividing cells suggests that either mature or immature human DCs can be transduced by lentivector. This has generally been shown to be the case, but under some conditions higher transduction efficiencies can be obtained with immature DCs 48, 50. It is not clear why such wide variation in transduction efficiency has been observed, but it is likely that these differences relate to technical differences between investigators and systems such as the method of lentivector preparation, titer determination, and promoter used in the expression of transgenes, rather than to biologic variables. In addition, comparative studies found that lentivector was more effective at transducing both mouse BMDCs and human monocyte derived DCs than widely used adenoviral vectors 54. Importantly, we and others had further found that lentivector transduction of BMDCs had less effect on the maturation process of BMDCs in vitro as examined by the surface co-stimulatory molecules and cytokine production in responses to inflammatory stimuli 16, 19. Fonteneau et al also found that in vitro the maturation status of human CD11c+ DCs were not affected by exposure to HIV particles 55. However, pDCs were potently activated by HIV particles and produced type I IFN which induced the bystander maturation of myeloid DCs. Brown et al confirmed this finding in vivo showing that the lentivector could activate pDC to generate type I IFN which may limit transgene expression 56. Thus, it seems that lentivector can activate pDC which could then indirectly activate myeloid CD11c+ DCs.
Consistent with in vitro results, recent studies show that direct administration of lentivector via intravenous or subcutaneous injection results in transduction of Ag presenting cells (APCs) including DCs in the spleen 57–59, and in the draining lymph nodes 18, 46, 60. Besides transduction of DCs in vivo, recombinant lentivector also results in transduction of B cells in the spleen after intravenous injection 57, 59 or in the draining LNs after subcutaneous immunization. But, B cells isolated from mice immunized with recombinant lentivector did not induce naïve TCR transgenic T cells proliferation. More importantly, B cell knockout mice developed equally if not higher CD8 T cell immune responses following lentivector immunization, suggesting that B cells, even though being transduced in vivo, do not play a role in priming CD8 T cell immunity 61. However, based on available data, it is unclear whether transduced B cells contribute to the maintenance of T cell immunity and the development of memory T cell responses.
3.2 Role of skin DC subsets in CD8 T cell priming
Studies suggest that CD11c+ DCs are the only APCs that prime naïve T cells responses in vivo 45. However, CD11c+ DCs are a heterogenous group of cells that can have similar dendritic shape but variations in Ag presentation function. These DC subsets have distinct lineage, phenotype, and anatomical location, and possibly distinct functions. In mouse skin draining lymph nodes (DLN), at least 6 DC subsets have been identified which can be grouped into two major subdivisions based on their origins, i.e., tissue derived DCs and blood derived DCs. Both groups of DCs can be further identified by their surface markers 62–64. Even though the DC subsets have been comprehensively characterized phenotypically, the relative contributions of DC subsets to T-cell priming or cross-priming immune responses after infection, and relevant to this discussion of recombinant lentivectors, was unclear until recently.
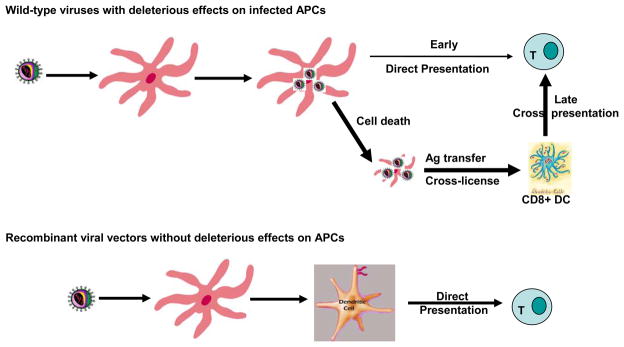
Our general understanding of DC function is based in large part on the paradigm of skin immune function 65, 66. Following skin viral infections or skin immunization with recombinant viral vectors, skin DCs (sDCs) including Langerhans cells (LCs) and dermal DCs (DDCs) are infected and migrate to DLNs. These migrating and maturing sDCs were suggested to be able to directly prime naïve T cells in the DLNs to initiate T cell immune responses. However, this so called skin DC or LC paradigm was recently challenged by a series of findings suggesting that skin migrating DCs were merely delivering the Ag captured in the periphery to LN resident DCs 67–69 in a number of skin viral infections including HSV, influenza virus (IAV), vaccinia virus (VV), lymphocytic choriomeningitis virus (LCMV) infections, and Listeria monocytogenes bacteria infection. This “collaborative” cross-presentation paradigm is appealing in the sense that Ag can be spread to a large DC subset network for processing and presentation, creating a higher chance of meeting cognate T cells and activating T cell responses. However, it apparently dissociates the sentinel function and Ag presenting function of the DC networks. More importantly, this cross presentation model also requires a subtle and undiscovered mechanism to faithfully transfer presumably minute amounts of Ag and also environmental cues from migrating DCs that have direct contact with invading microbes or other stimuli to the LN resident DCs that are going to present Ag. In order to outsmart the microbes which develop the mechanisms of evading the host immune system by either interfering with the Ag processing and presentation of infected DCs, or by inducing cytopathic effects in infected cells, the host could utilize this indirect mechanism to initiate defense against microbial invasion. In other words, the host could use this mechanism only when they have to, especially when viral infections interfere with the Ag processing and presenting function of APCs, such as in the cases of HSV, HAV, and vaccinia virus infections. Thus, we suggest that Ag transfer is not the primary pathway of Ag presentation. Further, we hypothesize that transduction of DCs with recombinant lentivector, which has minimal deleterious effect on infected DCs, will enable them to directly prime naïve T cells. Consistent with our hypothesis, we found, by utilizing a lentivector expressing the luciferase reporter gene, that the major DC subset expressing the reporter gene in the DLN was the migrating skin DCs. Furthermore, the same skin DC subsets migrating from the infected areas were able to prime naïve CD8 T cells ex vivo 46. We would propose that other genetic immunization strategies mediated by recombinant viral vectors that do not adversely affect DC Ag presentation function, including in theory some adenoviral and alpha viral vectors, could prime immune responses through similar Ag presentation mechanisms. Thus, in contrast to wild type virus infection in which the APC function of infected DCs is compromised, replication defective recombinant viral vectors engineered to have no or little deleterious effects on infected cells could enable directly infected DCs to prime naïve CD8 T cells (Fig. 2). This could occur in the presence or absence of cross-priming mechanisms. Further investigation of the immunologic significance of direct priming, cross-priming, the role of different DC subsets in priming and maintaining effector and memory T cell responses, and Ag transfer mechanisms including the properties (stability) of Ags is likely to enable the design of improved genetic immunization strategies.
Fig. 2.
Different DC subsets and mechanisms may be involved in priming CD8 T cell responses after immunization with engineered replication defective recombinant viral vectors and after wild type viral infections.
3.3 Characteristics of lentivector mediated genetic immunization
The transduction of DCs in vitro and in vivo by lentivector and the ensuing priming of naïve T cells by lentivector transduced DCs suggest that recombinant lentivector can be an effective vehicle for delivering Ag encoding genes into APCs for efficient expression, processing, and presentation to induce potent immune responses. These specific features of lentivector mediated gene transfer predict prolonged transgene expression and minimal interference with the functions of host cells. Prolonged Ag expression may directly contribute to the impressive potency and persistence of resulting T cell responses.
3.3.1 Prolonged Ag presentation in vivo after administration of lentivector transduced DCs or after direct immunization with lentivector
The effect of in vivo Ag presentation duration on the potency of effectors and the generation of memory T cell responses has been investigated in recent years. It has been suggested that extended interaction between APCs and T cells is required to elicit fit T cells that can respond to homeostasis cytokines for survival 70–72. In addition, prolonged Ag presentation might be required to have sufficient time for all possible cognate T cells to be screened by Ag presenting APCs and induce maximal CTL responses 73. Furthermore, it has recently been demonstrated that Ag presentation in the late stage of viral infection may be needed to generate memory CD4 and CD8 T cell responses 74–76. Conversely, other findings suggest that persistent Ag presentation may result in T cell exhaustion, such as that demonstrated in chronic viral infection where a large amount of Ag is present 77. Thus, the Ag presentation duration and its role on the ensuing immune response remain controversial. We have investigated the duration of Ag presentation in vivo and found that Ag presentation could be measured for up to two weeks after transfer of lentivector transfected DCs 16. This is consistent with reports that Ag expression can last at least 7 days in DCs after ex vivo transduction with lentivector 78. Our studies demonstrate that the duration of in vivo Ag presentation is even longer following direct skin immunization with lentivector. We observed significant OT-I proliferation in vivo three weeks after immunization with OVA expressing lentivector 46. Similar prolonged Ag presentation was also reported following adenoviral vector immunization 79. Importantly, this prolonged Ag exposure correlated with potent protective immunity and in general did not prevent recall responses 16, 46, 59, 79, 80, even though partially exhausted effector memory T cell phenotype was observed in some cases 79. It seems that the effect of prolonged Ag presentation on induced T cell responses after recombinant viral vector immunization is different from that observed in chronic viral infections where exhausted T cell responses were reported 77, 81.
These studies support further investigation and re-evaluation of the impact of Ag presentation duration on the effector and memory function of activated T cells. The persistent Ag presentation may contribute to the maintenance of sufficient levels of effectors to control either viral infections or tumor growth. Understanding these correlations will enable more informed manipulation of Ag presentation parameters in next generation genetic vaccines.
3.3.2 Persistent T cell immune responses following lentivector immunization
Prolonging Ag expression and presentation following lentivector-based immunization predicts the induction of potent and persistent T cell immunity. Indeed, immunization with lentivector transduced BMDCs induces stronger and longer lasting T cell immunity compared to that resulting from peptide or protein pulsed DCs 16. It was also found previously that the amplitude of CD8 T cell responses was greater in mice immunized with lentivector transduced BMDCs, than that induced by BMDCs transduced with adenoviral vector 54 or mRNA 78. In addition, they demonstrated that lentivector induced more potent CD8 T cell immunity than standard peptide/incomplete Freund adjuvant method 80. Using both an intracellular staining assay of IFN-γ and in vitro cytolytic activity assay, Dullaers et al demonstrated that direct immunization with lentivector and immunization with ex vivo lentivector modified BMDCs are equally potent in eliciting T cell immune responses 82. Further studies showed that direct injection of recombinant lentivector induce stronger CD8 T cell responses compared to other genetic immunization approaches, including naked DNA and vaccinia vector based immunization 46. In most cases, lentivector appears to be superior to adenovector in stimulating T cell immunity. Importantly, minimal vector specific immune responses are observed following lentivector immunization, making repeated immunization and/or boosting possible 46.
Although most studies have focused on CD8 T cell immunity, CD4 T cell responses can also be elicited by lentivector immunization, particularly when the encoded protein is either secreted or targeted to MHC II pathway by fusion with invariant chain (Ii) or transferrin receptor sequences 59. The relative dependence of CTL priming and memory responses on CD4+ T-cell help is unclear. Following direct lentivector immunization these responses have been shown to be partially dependent on CD4 T cell help 82. In contrast, stimulation of CTL activity and memory following immunization with ex vivo lentivector transduced DCs was totally dependent on CD4 T cell help 82. On the other hand, the in vitro stimulation of human blood Flu-specific CTL activity by lentivector transduced monocyte derived DCs was reportedly independent of CD4 T cell help 48.
Recently, several groups have investigated the kinetics of T cell immunity following lentivector immunization 46, 59, 82. After immunization, potent CD8 T cell immunity can be detected as early as five days after immunization and peaks between 8 to10 days. CTL activity remains at high levels for approximately one month and then gradually declines, but maintains a significant level. Depending on experimental systems, as many as 40% of target cells (10 million) can be lysed in vivo 1.5 months after immunization, suggesting potent and long-lasting T cell immunity 46. This is in contrast to vaccinia vector induced CD8 T cell immune responses that are relatively short-lived 46.
4. Generation of antitumor immunity with lentivector immunization in preclinical tumor models
The elicitation of a potent and persistent CD8 T cell mediated immune response, including effector function capable of lysing tumor cells, is important for both controlling tumor growth and eliminating established tumor cells. As illustrated above, lentivector appears to be an excellent immunization vehicle for inducing potent and persistent effector T cell immunity, perhaps due to the prolonged Ag presentation. It is not surprising that efforts to develop lentivectors for immunization have focused on antitumor immunity.
4.1. Induction of melanoma specific T cell immunity by ex vivo lentivector transduced DCs
The efficacy of stimulating antitumor immunity with lentivector immunization was first examined using ex vivo lentivector transduced DCs expressing the melanoma related Ags including Melan-A, NY-ESO, GP100, TRP2. Metharom et al reported the transduction of mouse BMDCs by lentivector with the transduction efficiency of 10–20% at an MOI of 2–4 83. Expression of mTRP2 gene in BMDCs was detected with immune staining. Importantly, they demonstrated that immunization with BMDCs transduced with lentivector expressing mTRP2 gene could protect mice from the challenge of B16 tumor cells 10 days later. In a therapeutic immunization model, significant results were obtained when the tumors were established by inoculating lower doses of B16 cells (1×104) and repeated immunizations were started early at 4 days after tumor inoculation. Results from this model suggested that tumor immunotherapy could be achieved when tumor burden was low and lentivector modified BMDCs were employed. Firat et al found that lentivector expressing combined multiple melanoma Ag epitopes derived from gp100, MelanA/Mart-1, tyrosinase, NA17-A, and MAGE-3 could transduce mouse BMDCs and human DCs effectively, especially when the lentivector was modified to include nuclear localization signal of central and polypurine tract sequence (cPPT). Importantly, those transduced DCs could effectively prime melanoma CTL responses in vitro 84. Similar findings were also reported by Palmowski et al who demonstrated that ex vivo lentivector transduced BMDCs could induce NY-ESO specific CD8 T cell immune responses after immunization of HLA-A2 transgenic mice, and these responses could be dramatically enhanced with a boosting immunization 58. Breckpot et al found that human DCs transduced with lentivector expressing invariant chain (Ii) fused melanoma Ag MAGE could prime both CD8 and CD4 T cell responses in vitro 19. In addition, mice immunized with murine BMDCs transduced with lentivector expressing Ii-OVA were protected from EL4-OVA tumor challenge and survived longer. Importantly, we and others have demonstrated that lentivector transduced BMDCs induce potent and long-lasting T cell immunity against tumor associated Ag that not only prevented tumor growth, but also prolonged survival of tumor bearing mice 16, 19. In one recent study, Chapatte et al also demonstrated that human and mouse DCs transduced ex vivo with recombinant lentivector expressing Melan A26-35 minigene could stimulate T cell activation in vitro and in vivo. However, human and mouse DCs could not effectively process full length Melan A self tumor Ag because of the presence of immunoproteasomes in DCs and thus failed to stimulate potent T cell responses in vitro and in vivo, which could possibly limit the applications of ex vivo transduced DCs in tumor immunotherapy 85.
4.2. Induction of tumor specific T cell immunity by direct lentivector immunization
As stated above, immunization with ex vivo lentivector transduced DCs could induce potent T cell immunity against tumors in most cases, but, the process of DC generation and transduction is laborious and expensive. Direct immunization with lentivector has considerable practical and theoretical advantages compared to DC adoptive transfer approaches. Firat et al first demonstrated that immunization of HLA-A2 transgenic mice with recombinant lentivector expressing polyepitopic melanoma Ag could stimulate potent CTL activity 84. Furthermore, subcutaneous injection of lentivector expressing a mini Melan-A gene into HLA-A2 transgenic mice has been shown to induce more potent CD8 T cell immune responses compared to peptide based immunization 80. Importantly, the CD8 T cell responses can be recalled by boosting immunization with the same lentivector 60 days later, indicating repeated immunization with lentivector is possible. Using both tetramer staining and in vivo CTL assays, Palmowski and colleagues demonstrated that direct immunization of HLA-A2 transgenic mice with lentivector encoding NY-ESO also stimulated CD8 T cell responses that could be dramatically enhanced by boosting immunization with vaccinia viral vector expressing the same NY-ESO Ag 58. We have demonstrated that direct immunization with lentivector could induce TRP2 specific CD8 T cell immunity in vivo when TRP2 epitope was fused with EGFP protein 46. Interestingly however, immunization with lentivector expressing native TRP2 protein did not elicit TRP2 specific T cell responses (He et al, unpublished results). Consistent with this finding, DNA vaccines expressing native murine TRP1 Ag were found to be ineffective at stimulating TRP1 specific CD8 T cell responses. On the other hand, plasmid DNA expressing mutated TRP1 could induce T cell responses 86, 87. In collaborating with Dr. Guevara-Patino, we investigated the induction of CD8 T cell responses against multiple TRP1 epitopes and found that after single lentivector immunization, potent and persistent CD8 T cell immunity against several TRP1 epitopes could be detected (He et al, unpublished results). More importantly, a single lentivector injection protected mice from B16 tumor challenge, and in 40% of tumor bearing mice tumors were eradicated after a single injection of lentivector expressing mutated mouse TRP1 Ag. These results suggest that with Ag modifications, lentivector-mediated gene delivery could become an effective genetic immunization strategy for stimulating potent T cell mediated antitumor immunity. However, one possible limitation is that recombinant lentivector or plasmid DNA expressing native full length self tumor Ag may be incompetent to stimulate potent T cell immunity under certain circumstances possibly due to the incapability of processing target Ag by DCs 85.
Even though it is debatable whether true memory T cell responses are established 1–2 months after immunization as the in vivo Ag presentation still exists, a rapid and more potent secondary CTL response has been observed following a second immunization 150 days after priming, suggesting an effective memory T cell responses developed five months after priming 82. The presence of tumor specific memory T cells has been implied in a number of studies based on the observation of rapid effector function following secondary immunization 58, 82. In a recent study, the memory CD8 T cell responses were further characterized 88. Chapatte et al compared the in vivo induction of Melan-A specific CD8 T cell responses following lentivector (expressing the Melan A26-35 minigene) immunization vs. peptide/adjuvant immunization approach in HLA-A2/H-2Kb mice. Using tetramer staining, they found that recombinant lentivector stimulated a stronger anti-Melan-A CD8 T cell response than peptide/adjuvant immunization. More importantly, majority of anti-Melan-A T cells generated following lentivector immunization expressed the memory marker CD127 at the peak of the primary response. In those mice, memory T cells were detectable 4 months after priming and could be activated by secondary peptide-based boosting immunization. This study suggests that lentivector was not only capable of inducing potent effector function, but also of stimulating long-lasting memory T cell responses that can be recalled to enhance antitumor immunity.
4.3. Delivery of tumor specific TCR gene into T cells for adoptive transfer into tumor bearing hosts
In addition to the active immunization approaches to stimulate antitumor immunity, lentivector based gene delivery can facilitate modification of T cells to target tumors. Adoptive transfer of T lymphocytes expressing high avidity tumor Ag specific TCRs enabled potent and reproducible clinical antitumor responses 89–93. Promising results like these have stimulated considerable interest in strategies based on modifying T cells with TCR genes specific for tumor Ags. Morgan et al first demonstrated that T cell modified with high avidity TCR genes delivered by retroviral vectors could sustain in the patients and resulted in significant therapeutic effect 94. However, the high risk pf insertional tumorigenesis of retroviral vector 32 and the low genotoxicity of lentivector integration 33 prompted broad interest of using lentivector for TCR transfer 95. Furthermore, early positive results from the therapy of AIDS patients treated by adoptive transfer of lentivector modified CD4 T cells 96, 97 strongly support the development of lentivectors for the modification of tumor specific T cells for adoptive transfer. Thus, it is not surprising that a number of laboratories have been actively investigating lentivector mediated TCR gene delivery into T cells for adoptive transfer (abstract of keystone meeting symposia, Banff, 2007). Lentivector could become an important vehicle for modifying immune cells to achieve robust antitumor immunity either through active immunization or adoptive transfer of lentivector modified T cells that can specifically target tumor cells.
5. Conclusion
Genetic immunization with recombinant lentivector is a novel gene delivery strategy for the purpose of stimulating Ag specific T cell immune responses. Studies to date suggest that recombinant lentivectors have several important advantages over other genetic immunization approaches. With regard to gene expression they are capable of: 1.) transducing non-dividing DCs, 2.) expressing desired Ags only, and 3.) inducing persistent Ag expression. These features allow prolonged Ag presentation which may be important in priming and maintaining potent effector function that could be crucial in controlling chronic infection and in eliminating all tumor cells the setting of therapeutic immunization. Mechanistically, lentivectors can transduce peripheral tissue resident DCs that can then migrate to draining LN where they contribute to potent T cell activation via direct presentation of endogenous Ag with stronger antigenic signals and for a prolonged periods of time. Effects of lentivector mediated immunization include maximal induction of potent and prolonged T cell immunity, and minimal stimulation of antivector immune response. Thus, lentivectors hold considerable promise for use in the development of effective genetic immunization strategies. Furthermore, lentivector has considerable potential as a vector for delivering tumor specific high avidity TCR genes into T cells for adoptive transfer tumor immunotherapy.
6. Five year view
The recent failure of HIV vaccine clinical trial of using recombinant adenoviral vector further dampen the hope of generating any usable T cell vaccines for HIV infection (News in Oct 5 issue of Science, 2007). However, it more reflects the value and relevance of T cell responses in preventing HIV viral infections rather than the fundamental flaws in developing T cell vaccines. There is a critical difference between conventional preventive immunization where the purpose is to elicit long-lasting memory humoral responses to prevent invasion of foreign microbes, and therapeutic immunization where the intention is to induce both potent effectors to kill already existed tumor cells and long-term memory responses to prevent evolving tumor cells 2. It is generally believed that in order to control tumor growth and eliminate existing tumor cells, tumor specific T cell immune responses especially the effector T cell responses play a critical role.
First, it is the authors’ opinion that our immediate future effort on developing genetic immunization for tumor immunotherapy should focus on understanding the parameters that govern the potency and persistency of effector and memory T cell responses. The authors anticipate that basic mechanistic studies will reveal the important parameters of T cell priming and allow manipulation of these factors to achieve optimal effector T cell responses and memory responses. These parameters include but not limit to the role of different DC subsets in priming T cell responses, the mechanism of T cell priming via direct vs cross presentation, and the effect of Ag expression level (Ag dose) and duration of Ag presentation following lentivector. The findings that certain subsets of DCs such as skin DCs are the potent APCs for stimulating CD8 T cell responses following lentivector immunization may promote development of targeted immunization strategy. Conversely, the discovery of CD19+B220+ pDCs are potent stimulators of regulatory T cells in the tumor bearing mice 98, 99 stress the importance and need to identify and avoid these regulatory DC subsets in designing genetic vaccines for tumor immunotherapy.
Second, the general immune suppressive environment in tumor bearing host and the weak self tumor Ag, two of the important issues in therapeutic immunization, are needed to be tackled in order to obtain clinical benefit of tumor genetic immunization. These problems may explain why the efficacy of therapeutic immunization using either protein or gene based approaches in treating malignant tumors has not been materialized despite the tremendous efforts of last decade. The immunologic milieu in the tumor-bearing host is very different from that of a naive host, and tolerogenic Ag-presentation pathways may be favored in hosts with tumors. Thus, it is imperative that investigations should be undertaken to understand the mechanism of immune suppression of tumor burden and if and how the immune tolerance can be broken following genetic immunization. Potentially, lentivectors may offer the advantage of by directly priming naïve T cells using activated transfected DCs. Previously we demonstrated that lentivector in vitro had little effect on DC activation 16. This minimal inflammatory effect of lentivector may be sufficient for activating DCs in vivo to induce potent T cell activation but not inversely hurt the ensuing memory T cell responses as that showed in CpG administration 100 However, it remains to be studied if additional stimuli for DC activation or maturation provided as part of lentivector immunization or delivered separately will be needed in reversing the immune suppressive condition of tumor bearing host.
Furthermore, conventional tumor therapies such as surgery, chemo, and radiation will be examined as approaches for removing the immune suppressive condition in tumor bearing mice and creating a window for genetic immunization to elicit potent T cell immunity that can prevent tumor recurrence.
In addition to all these potential research fronts of active genetic immunization, recombinant lentivector will be extensively investigated for the potential of delivering TCR genes for adoptive transfer tumor immunotherapy.
Regarding to the development of lentivector per se, we predict that research efforts and progresses will be made to further evaluate the biosafety in more clinical trials, to modify the lentivectors for achieving in vivo specific targeting to DCs, and to have more reliable methods for quantitative production.
Acknowledgments
Researches conducted in the authors’ lab are supported by NIH grants and by start-up fund from MCG Cancer Center.
Contributor Information
Yukai He, Email: yhe@mcg.edu, Immunology/Immunotherapy Program, MCG Cancer Center, Medical College of Georgia, CN-4150, 1120 15th St, Augusta, GA30912. Tel: 706-721-2728; Fax: 706-721-1670.
David Munn, Email: dmunn@mcg.edu, Immunology/Immunotherapy Program, MCG Cancer Center, Medical College of Georgia, CN-4150, 1120 15th St, Augusta, GA30912. Tel: 706-721-7141; Fax: 706-721-1670.
Louis D Falo, Jr, Email: lof2@pitt.edu, Department of Dermatology, University of Pittsburgh, School of Medicine, 190 Lothrop St, suite 145, Pittsburgh, PA15213. Tel: 412-648-3252; Fax: 412-648-8117.
References
- 1.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11(4 Suppl):S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 2•.Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305(5681):205–8. doi: 10.1126/science.1100600. This is a brief review with the focus on therapeutic immunization, which provides readers the introductory reading about therapeutic vaccines. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunological reviews. 2006;213:146–58. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 4.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunological reviews. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunological reviews. 2006;211:214–24. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. The Journal of clinical investigation. 2007;117(5):1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voltan R, Robert-Guroff M. Live recombinant vectors for AIDS vaccine development. Curr Mol Med. 2003;3(3):273–84. doi: 10.2174/1566524033479816. [DOI] [PubMed] [Google Scholar]
- 8.Bennink JR, Yewdell JW, Smith GL, Moller C, Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984;311(5986):578–9. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- 9.Kieny MP, Lathe R, Drillien R, et al. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984;312(5990):163–6. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- 10.Moss B, Smith GL, Gerin JL, Purcell RH. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984;311(5981):67–9. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- 11.Adamina M, Daetwiler S, Rosenthal R, Zajac P. Clinical applications of recombinant virus-based cancer immunotherapy. Expert Opin Biol Ther. 2005;5(9):1211–24. doi: 10.1517/14712598.5.9.1211. [DOI] [PubMed] [Google Scholar]
- 12.Casimiro DR, Chen L, Fu TM, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. Journal of virology. 2003;77(11):6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata K, Garcia-Sastre A, Tsuji M, et al. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173(1):96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, West K, Hayasaka D, Ennis FA, Terajima M. Recombinant adenovirus vector vaccine induces stronger cytotoxic T-cell responses than recombinant vaccinia virus vector, plasmid DNA, or a combination of these. Viral immunology. 2005;18(4):657–67. doi: 10.1089/vim.2005.18.657. [DOI] [PubMed] [Google Scholar]
- 15.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16(2):149–56. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 16.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174(6):3808–17. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 17.Collins MK, Cerundolo V. Gene therapy meets vaccine development. Trends Biotechnol. 2004;22(12):623–6. doi: 10.1016/j.tibtech.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18•.Esslinger C, Chapatte L, Finke D, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. Journal of Clinical Investigation. 2003;111(11):1673–81. doi: 10.1172/JCI17098. This is the first comprehensive study of using recombinant lentivector to stimulate T cell immunity against tumor and other antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breckpot K, Dullaers M, Bonehill A, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5(8):654–67. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- 20.Chinnasamy N, Chinnasamy D, Toso JF, et al. Efficient gene transfer to human peripheral blood monocyte-derived dendritic cells using human immunodeficiency virus type 1-based lentiviral vectors. Hum Gene Ther. 2000;11(13):1901–9. doi: 10.1089/10430340050129512. [DOI] [PubMed] [Google Scholar]
- 21.Zarei S, Abraham S, Arrighi JF, et al. Lentiviral transduction of dendritic cells confers protective antiviral immunity in vivo. Journal of virology. 2004;78(14):7843–5. doi: 10.1128/JVI.78.14.7843-7845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buffa V, Negri DR, Leone P, et al. A single administration of lentiviral vectors expressing either full-length human immunodeficiency virus 1 (HIV-1)(HXB2) Rev/Env or codon-optimized HIV-1(JR-FL) gp120 generates durable immune responses in mice. J Gen Virol. 2006;87(Pt 6):1625–34. doi: 10.1099/vir.0.81706-0. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, Charneau P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J Gene Med. 2006;8(3):265–74. doi: 10.1002/jgm.837. [DOI] [PubMed] [Google Scholar]
- 24.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science (New York, NY) 1996;272(5259):263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 25.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15(9):871–5. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 26.Dull T, Zufferey R, Kelly M, et al. A third-generation lentivirus vector with a conditional packaging system. Journal of virology. 1998;72(11):8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zufferey R, Dull T, Mandel RJ, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. Journal of virology. 1998;72(12):9873–80. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Zhan X, D’Costa J, et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7(6):827–38. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 29.Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol. 2005;23(1):108–16. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- 30.Chinnasamy D, Milsom MD, Shaffer J, et al. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virol J. 2006;3:14. doi: 10.1186/1743-422X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund AH, Turner G, Trubetskoy A, et al. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nature genetics. 2002;32(1):160–5. doi: 10.1038/ng956. [DOI] [PubMed] [Google Scholar]
- 32.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science (New York, NY) 2003;302(5644):415–9. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 33.Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–96. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 34.Biffi A, De Palma M, Quattrini A, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. The Journal of clinical investigation. 2004;113(8):1118–29. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saenz DT, Loewen N, Peretz M, et al. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. Journal of virology. 2004;78(6):2906–20. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanez-Munoz RJ, Balaggan KS, MacNeil A, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nature medicine. 2006;12(3):348–53. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 37.Negri DR, Michelini Z, Baroncelli S, et al. Successful Immunization with a Single Injection of Non-integrating Lentiviral Vector. Mol Ther. 2007;15(9):1716–23. doi: 10.1038/sj.mt.6300241. [DOI] [PubMed] [Google Scholar]
- 38.Follenzi A, Naldini L. HIV-based vectors. Preparation and use. Methods Mol Med. 2002;69:259–74. [PubMed] [Google Scholar]
- 39.Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–65. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- 40.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5(4):387–98. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breckpot K, Aerts JL, Thielemans K. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 2007 doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- 42.Morizono K, Xie Y, Ringpis GE, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nature medicine. 2005;11(3):346–52. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 43•.Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci U S A. 2006;103(31):11479–84. doi: 10.1073/pnas.0604993103. Reference 42 and 43 were the latest effort for developing targeting lentivector by incorporating antibody in the lentivector envelope. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delenda C, Gaillard C. Real-time quantitative PCR for the design of lentiviral vector analytical assays. Gene Ther. 2005;12 (Suppl 1):S36–50. doi: 10.1038/sj.gt.3302614. [DOI] [PubMed] [Google Scholar]
- 45.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24(5):643–56. doi: 10.1016/j.immuni.2006.03.014. In this paper, authors demonstrated that skin DC play a critical role in priming naïve CD8 T cells following genetic immunization with lentivector. It contrast to many other papers showing that skin DC merely ferry antigen to draining resident DCs following wild type virus infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Mukai T, Young D, Frankel S, Law P, Wong-Staal F. Transduction of CD34+ cells by a vesicular stomach virus protein G (VSV-G) pseudotyped HIV-1 vector. Stable gene expression in progeny cells, including dendritic cells. J Hum Virol. 1998;1(5):346–52. [PubMed] [Google Scholar]
- 48.Dyall J, Latouche JB, Schnell S, Sadelain M. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood. 2001;97(1):114–21. doi: 10.1182/blood.v97.1.114. [DOI] [PubMed] [Google Scholar]
- 49.Schroers R, Sinha I, Segall H, et al. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system. Mol Ther. 2000;1(2):171–9. doi: 10.1006/mthe.2000.0027. [DOI] [PubMed] [Google Scholar]
- 50.Firat H, Zennou V, Garcia-Pons F, et al. Use of a lentiviral flap vector for induction of CTL immunity against melanoma. Perspectives for immunotherapy. J Gene Med. 2002;4(1):38–45. doi: 10.1002/jgm.243. [DOI] [PubMed] [Google Scholar]
- 51.Lizee G, Gonzales MI, Topalian SL. Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Ther. 2004;15(4):393–404. doi: 10.1089/104303404322959542. [DOI] [PubMed] [Google Scholar]
- 52.Rouas R, Uch R, Cleuter Y, et al. Lentiviral-mediated gene delivery in human monocyte-derived dendritic cells: optimized design and procedures for highly efficient transduction compatible with clinical constraints. Cancer Gene Ther. 2002;9(9):715–24. doi: 10.1038/sj.cgt.7700500. [DOI] [PubMed] [Google Scholar]
- 53.Zarei S, Leuba F, Arrighi JF, Hauser C, Piguet V. Transduction of dendritic cells by antigen-encoding lentiviral vectors permits antigen processing and MHC class I-dependent presentation. J Allergy Clin Immunol. 2002;109(6):988–94. doi: 10.1067/mai.2002.124663. [DOI] [PubMed] [Google Scholar]
- 54.Esslinger C, Romero P, MacDonald HR. Efficient transduction of dendritic cells and induction of a T-cell response by third-generation lentivectors. Hum Gene Ther. 2002;13(9):1091–100. doi: 10.1089/104303402753812494. [DOI] [PubMed] [Google Scholar]
- 55.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. Journal of virology. 2004;78(10):5223–32. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown BD, Sitia G, Annoni A, et al. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109(7):2797–805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- 57.VandenDriessche T, Thorrez L, Naldini L, et al. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood. 2002;100(3):813–22. doi: 10.1182/blood.v100.3.813. [DOI] [PubMed] [Google Scholar]
- 58.Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V, Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172(3):1582–7. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- 59.Rowe HM, Lopes L, Ikeda Y, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther. 2006;13(2):310–9. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Majumder N, Lin H, Watkins S, Falo LD, Jr, You Z. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum Gene Ther. 2005;16(11):1255–66. doi: 10.1089/hum.2005.16.1255. [DOI] [PubMed] [Google Scholar]
- 61.He Y, Falo LD. Induction of T cell immunity by cutaneous genetic immunization with recombinant lentivector. Immunol Res. 2006;36(1–3):101–17. doi: 10.1385/IR:36:1:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belz GT, Smith CM, Kleinert L, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101(23):8670–5. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 64.Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin Immunol. 2005;17(4):262–72. doi: 10.1016/j.smim.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 65.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 66.Larrengina AT, Falo LD. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J Invest Dermatol. 2005;124(1):1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]
- 67.Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science (New York, NY) 2003;301(5641):1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 68•.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25(1):153–62. doi: 10.1016/j.immuni.2006.04.017. References 67 and 68 demonstrated elegantly that skin Langerhans cells simply transport antigen from skin to draining lymph nodes but could not prime naïve CD8 T cells directly. Instead, they transferred antigen to LN resident DCs for cross priming naïve CD8 T cells. [DOI] [PubMed] [Google Scholar]
- 69.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25(12):655–8. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171(10):5165–71. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 71.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nature immunology. 2003;4(4):355–60. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 72.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8(1):89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 73.Stock AT, Mueller SN, van Lint AL, Heath WR, Carbone FR. Cutting edge: prolonged antigen presentation after herpes simplex virus-1 skin infection. J Immunol. 2004;173(4):2241–4. doi: 10.4049/jimmunol.173.4.2241. [DOI] [PubMed] [Google Scholar]
- 74.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. The Journal of experimental medicine. 2005;202(5):697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Storni T, Ruedl C, Renner WA, Bachmann MF. Innate immunity together with duration of antigen persistence regulate effector T cell induction. J Immunol. 2003;171(2):795–801. doi: 10.4049/jimmunol.171.2.795. [DOI] [PubMed] [Google Scholar]
- 76.Spierings DC, Lemmens EE, Grewal K, Schoenberger SP, Green DR. Duration of CTL activation regulates IL-2 production required for autonomous clonal expansion. Eur J Immunol. 2006;36(7):1707–17. doi: 10.1002/eji.200635929. [DOI] [PubMed] [Google Scholar]
- 77.Gallimore A, Glithero A, Godkin A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. The Journal of experimental medicine. 1998;187(9):1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dullaers M, Breckpot K, Van Meirvenne S, et al. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: implications for cancer immunotherapy protocols. Mol Ther. 2004;10(4):768–79. doi: 10.1016/j.ymthe.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 79.Yang TC, Millar J, Groves T, et al. The CD8+ T cell population elicited by recombinant adenovirus displays a novel partially exhausted phenotype associated with prolonged antigen presentation that nonetheless provides long-term immunity. J Immunol. 2006;176(1):200–10. doi: 10.4049/jimmunol.176.1.200. [DOI] [PubMed] [Google Scholar]
- 80.Esslinger C, Chapatte L, Finke D, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. The Journal of clinical investigation. 2003;111(11):1673–81. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature reviews. 2005;5(3):215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 82.Dullaers M, Van Meirvenne S, Heirman C, et al. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13(7):630–40. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- 83.Metharom P, Ellem KA, Schmidt C, Wei MQ. Lentiviral vector-mediated tyrosinase-related protein 2 gene transfer to dendritic cells for the therapy of melanoma. Hum Gene Ther. 2001;12(18):2203–13. doi: 10.1089/10430340152710540. [DOI] [PubMed] [Google Scholar]
- 84.Firat H, Zennou V, Garcia-Pons F, et al. Use of a lentiviral flap vector for induction of CTL immunity against melanoma. Perspectives for immunotherapy. J Gene Med. 2002;4(1):38–45. doi: 10.1002/jgm.243. [DOI] [PubMed] [Google Scholar]
- 85.Chapatte L, Ayyoub M, Morel S, et al. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006;66(10):5461–8. doi: 10.1158/0008-5472.CAN-05-4310. [DOI] [PubMed] [Google Scholar]
- 86.Engelhorn ME, Guevara-Patino JA, Noffz G, et al. Autoimmunity and tumor immunity induced by immune responses to mutations in self. Nature medicine. 2006;12(2):198–206. doi: 10.1038/nm1363. [DOI] [PubMed] [Google Scholar]
- 87.Guevara-Patino JA, Engelhorn ME, Turk MJ, et al. Optimization of a self antigen for presentation of multiple epitopes in cancer immunity. The Journal of clinical investigation. 2006;116(5):1382–90. doi: 10.1172/JCI25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Chapatte L, Colombetti S, Cerottini JC, Levy F. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 2006;66(2):1155–60. doi: 10.1158/0008-5472.CAN-05-2597. This is a latest report on the memory CD8 T cells following lentivector immunization. The author demonstrated that lentivector immunization stimulated not only potent effector but also memory T cells against self tumor antigens. [DOI] [PubMed] [Google Scholar]
- 89.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science (New York, NY) 2002;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. The Journal of experimental medicine. 2003;198(4):569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nature reviews. 2006;6(5):383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature medicine. 2004;10(9):909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science (New York, NY) 2006;314(5796):126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Bobisse S, Zanovello P, Rosato A. T-cell receptor gene transfer by lentiviral vectors in adoptive cell therapy. Expert Opin Biol Ther. 2007;7(6):893–906. doi: 10.1517/14712598.7.6.893. This is a latest review of TCR gene transfer by lentivectors for adoptive cell therapy. [DOI] [PubMed] [Google Scholar]
- 96.Dropulic B, June CH. Gene-based immunotherapy for human immunodeficiency virus infection and acquired immunodeficiency syndrome. Hum Gene Ther. 2006;17(6):577–88. doi: 10.1089/hum.2006.17.577. [DOI] [PubMed] [Google Scholar]
- 97.Levine BL, Humeau LM, Boyer J, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103(46):17372–7. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. The Journal of clinical investigation. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang Q, Bluestone JA. Plasmacytoid DCs and T(reg) cells: casual acquaintance or monogamous relationship? Nature immunology. 2006;7(6):551–3. doi: 10.1038/ni0606-551. [DOI] [PubMed] [Google Scholar]
- 100.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature medicine. 2005;11(7):748–56. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]