Abstract
Recent studies suggest that T cell–based cellular immunity plays an important role in preventing and delaying progression of infectious and neoplastic diseases. Based on these findings, recent efforts in vaccine research are giving rise to a new generation of “T cell” vaccines. The development of T cell vaccines has been problematic. Current investigations are focusing on gene-based immunization strategies, including the development of non-viral “naked” plasmid DNA and recombinant viral vector–based genetic immunization approaches. Here, we briefly review recent progress in the development of recombinant viral vectors for genetic immunization and our own recent studies elucidating differences in mechanisms of genetic immunization. We propose that the mechanism of immune induction depends in part on unique features of specific viral vectors, and that a comparison of representative vectors mechanistically will enable a more informed understanding of the determining parameters of immune induction. Our initial studies have focused on the identification of antigen-presenting-cell subsets important for priming CD8+ T cell immunity, the effects of antigen persistence on immune responses, and the unique immunogenicity of skin as a target tissue for vaccine delivery. We review data suggesting that the unique properties of recombinant lentivectors make them appealing candidates as genetic immunization vehicles for eliciting T cell immune responses.
Keywords: Genetic immunization, Lentivector, Dendritic cell subsets, Skin DC network, T cell immunity
Introduction
Prophylactic vaccines have been heralded as one of the most important achievements of modern medicine and are effective in preventing a number of deadly infectious diseases (1,2). The effectiveness of traditional vaccines, typically based on delivery of attenuated or inactivated pathogens, has primarily been attributed to their ability to induce neutralizing antibodies. These antibodies can bind antigens on the exposed surfaces of microbes to block their entry into cells and facilitate the clearance of pathogens. However, for a number of microbial infections such as human immunodeficieny virus (HIV), tuberculosis (TB), and malaria, traditional vaccines designed to induce sterilizing humoral immunity have thus far failed to prevent infection (3). Recent studies suggest that T cell–based cellular immunity, including CD8+ cytotoxic T lymphocyte (CTL), play an important role in containing these intracellular infections (4,5) and delaying disease progression (6–8). In addition, T cell immunity has been shown to be critically important in the immunotherapy of chronic infections such as HBV, HCV, and HIV, and malignancies (8,9). Based on these findings, recent efforts in vaccine research are beginning to include strategies to induce cellular immunity, giving rise to a new generation of “T cell” vaccines.
The development of T cell vaccines has been problematic. Even though a variety of strategies for inducing T cell–mediated cellular immunity are being developed, as yet, there are no T cell vaccines approved for clinical applications. Currently, immunizations utilizing attenuated live microbes are the only traditional vaccines able to induce significant T cell immunity. However, the risks associated with live attenuated viruses preclude their use in immune-compromised patients, and production, storage, and distribution challenges present substantive obstacles to widespread use (10,11). To date, vaccines based on injection of inactivated virus or protein subunits alone have generally been shown to be inefficient stimulators of T cell immune responses. In light of the limitations of traditional vaccines, current investigations are focusing on gene-based immunization strategies, including non-viral “naked” plasmid DNA and recombinant viral vector–based genetic immunization approaches. Here, we briefly review recent progress in the development of recombinant viral vectors for genetic immunization and our own recent studies elucidating differences in mechanisms of genetic immunization. We review data suggesting that the unique properties of recombinant lentivectors make them appealing candidates as genetic immunization vehicles for eliciting T cell immune responses.
Recombinant Viral Vectors for Genetic Immunization
Genetic immunization with recombinant viral vectors is currently the most effective approach for eliciting T cell immunity (12). Expression of transgenic antigens by viral vectors has several theoretical advantages for the induction of T cell immunity. From a mechanistic perspective, a critical advantage of viral vectors appears to be their ability to enable substantial expression of transgenic proteins. Importantly, the expressed antigens have been shown to be presented directly by virally infected antigen-presenting cells, and/or cross-presented after secretion or release from apoptotic or necrotic infected somatic cells.
Viral vectors also share certain general disadvantages for genetic immunization. In addition to concerns related to infectivity, including their potential to cause infectious disease and insertional mutations, the immunogenicity of viral vectors can be severely limited by anti-vector immunity. This is problematic for vectors derived from viruses with widespread human exposure resulting in significant pre-existing immunity in the general population, and for boosting or subsequent immunizations regardless of prior population exposure. Furthermore, the immunodominance of vector-derived antigens compete with desired antigens, resulting in the diversion of most of the T cell responses to vector antigens rather than to desired antigens. It is also noteworthy that recent evidence suggests that an advantage of viral vectors, namely their ability to promote cross-priming by killing infected cells, can also be a disadvantage as the magnitude and duration of antigen exposure may be important variables influencing the strength of the primary immune response and the development of cellular memory responses (13–15).
A wide range of viral vectors, developed primarily as vehicles for gene therapy, have been utilized for genetic immunization (16). Representative vectors that are the focus of considerable ongoing efforts in vaccine design include pox virus–, alpha virus–, and adenovirus–based vectors. Of these, vectors based on recombinant pox virus were among the first vectors developed for genetic immunization and included replication competent vaccinia vector (17–19) and a number of highly attenuated replication-defective pox virus derivatives, such as modified vaccinia virus Ankara (MVA), NYVAC, and canarypox virus ALVAC (16). Recombinant pox virus vectors have been widely utilized in clinical trials (20). However, their efficacy for immunization has been shown to be limited by pre-existing anti-vaccinia immunity in the general population, and relatively weak and short-lived T cell immunity.
More recently, alpha viruses are being developed as vaccine vectors. Alpha viruses are positive-stranded RNA viruses that appear to cause no major illness in humans. In addition, in humans, pre-existing immunity against alpha viruses is rare. Three different alpha viruses are being developed as genetic immunization vectors, including Sindbis virus (SIN), Semliki forest virus (SFV), and Venezuelan equine encephalitis (VEE) (21). Alpha viral vectors replicate inside transduced cells to high copies, and thus infected cells express high levels of transgenic antigen. Importantly, engineered vectors are unable to form viral particles necessary to spread the infection without the provision of structural proteins, limiting risk potential. Another advantage of alpha viral recombinant vectors is that they are non-integrating, minimizing the potential of insertional mutations. Replication of alpha viral vector does lead to death of transduced cells, and the net effect of potentially enhanced cross-priming vs reduced direct priming and duration of antigen exposure has yet to be determined.
Adenovirus is another widely studied viral vector for genetic immunization and has significant potential due to its high transduction efficiency for both dividing and non-dividing cells, easy preparation of high titer viral particles, and weak pathogenicity in immunocompetent human adults (22–24). Comparative studies with vaccinia vector and naked DNA immunization suggest that adenoviral vector is the most effective vector for inducing CD8 T cell immunity specific for HIV or SIV gag and pol proteins in non-human primates (12,22,25,26). However, pre-existing immunity against adenovirus is widespread in human populations, and there is considerable concern that pre-existing immunity will neutralize the vectors before infection, thus minimizing their efficacy (27). To circumvent this problem, a number of new subtypes of adenoviruses, including adenoviruses derived from non-human primates, are being investigated for potential use in humans (28,29). Regardless of the development of these agents, the well-established immunogenicity of adenovirus in general is likely to limit its broad use as a vaccine, as both subsequent immunizations against different antigens, and boosting immunizations that may be necessary to enhance immunity, are likely to be problematic.
Development of Lentivectors for Genetic Immunization
We and others have begun to explore the potential use of lentivectors as genetic immunization vehicles for inducing Ag-specific T cell responses (30–33). Lentivectors have several additional advantages over other commonly used viral vectors. They are less immunogenic due to the absence of genes encoding viral proteins, and thus are less likely to stimulate immunodominant antivector responses that could compete with or interfere with antigen-specific immunity. Second, the prevalence of HIV infection is low in the general population, and generally directed against protein epitopes that are altered or missing from the lentivector surface, minimizing the potential effect of pre-existing antivector immunity. Finally, unlike oncoretroviral vectors, lentivector has the ability to transduce non-dividing cells, such as DCs, and thus has the potential to effectively express antigen-encoding genes in antigen-presenting cells. Clinical trials using lentivector transduced cells have recently been initiated. To date, no safety concerns have been raised [Humeau et al: Mol Ther, 7(5): S33 and virxsys.com web-site], somewhat alleviating concerns of insertional mutagenisis raised by studies utilizing integration-capable oncoretroviral vectors (34,35). With the initiation of other phase II clinical trials, the safety concerns associated with lentivectors will be thoroughly addressed. Furthermore, recent studies demonstrate that recombinant lentivectors can be produced as non-integrating vectors (36–38) while maintaining high-level gene expression in non-dividing cells (39). Taken together these advantages and safety modifications support the clinical development of lentivectors (40–42).
We have demonstrated that dendritic cells (DCs), the most potent professional antigen-presenting cells, can be effectively transduced in vitro by lentivector. More importantly, we and others have found that unlike most other viral vectors, lentivectors do not interfere with the antigen-presentation function of transduced DCs, nor do they affect their ability to respond to environmental stimuli. Immature DCs and their precursors can be transduced by lentivector in vitro without affecting their maturation process, or their capacity to respond to pro-inflammatory stimuli such as LPS or CpGs (32,43). Furthermore, our studies demonstrate that transgenic antigens synthesized by lentivector transfected DCs are efficiently presented through both the class II and class I restricted-processing pathways, resulting in efficient stimulation of both CD4+ and CD8+ T cell immunity (32). Lentivector transduced DCs stimulate antigen-specific T cell proliferation and cytokine production in vitro and induce potent and long-lasting CTL activity in vivo (32,44,45). In direct comparisons, we demonstrated that immunization with lentivector transduced DCs induces more potent and long-lasting immunity compared to peptide or protein-pulsed DCs, resulting in a more potent antitumor effect in a therapeutic murine melanoma model (32). In addition, others have shown that lentivector-transduced DCs are superior to adenovector-transduced DCs in eliciting CD8 T cell immunity in mice (31). In summary, these results suggest that lentivectors efficiently transduce DCs, and that the transduced DCs retain both their antigen-presentation function and their functional plasticity, stimulating potent antigen-specific T cell immunity in vivo.
The capacity of lentivectors to efficiently transduce DCs without altering their function makes them an attractive candidate for genetic immunization. It has been shown that injection of recombinant lentivector-stimulated potent HLA-Cw specific CD8 T cell immune responses in mice and melanin-A specific CD T cell immunity in HLA-A0201 transgenic mice (46). The efficacy of direct immunization with lentivector is as potent as ex vivo transduced DCs. Similar findings were also reported using human melanoma antigen NY-ESO in HLA-A2 transgenic mice (33). Using model OVA antigen, it has been shown that direct injection of recombinant lentivector is more efficient in stimulating T cell immunity compared to ex vivo transduced DCs. More importantly, the induction of both primary and memory CD8 T cell immunity by direct immunization with lentivector seems less dependent on CD4 T helper cells than ex vivo transduced DCs (44). Furthermore, directing the lentivector-encoded antigen into the MHC class II pathway enhances not only the CD4 but also the CD8 T cell immune responses (45). This corresponds to potent antitumor immunity in immunized mice that inhibits tumor growth and prolongs survival. We recently compared the efficacy and kinetics of CD8 T cell immune responses in recombinant lentivector and vaccinia vector immunization. We found that immunization with lentivector induced substantially stronger CD8 T cell immunity using either OVA model antigens or the HBV viral antigen. Importantly, we directly evaluated T-cell immunity and found that in lentivector immunization, CD8 T cell immunity is remarkably long-lasting and is associated with significantly greater memory CD8 T cell responses (100).
Taken together, progress thus far highlights potential immunologic and safety advantages of lentivectors, and supports the future development of lentivector-based genetic immunization. Although recombinant viral vectors have been extensively investigated as genetic immunization vehicles, our understanding of the mechanisms of immune induction in viral vector immunization remains superficial. Specific parameters affecting the efficacy of induced T cell immunity have not been identified. For example, it is unclear which antigen-presenting cells are responsible for T cell priming and whether this will affect the efficacy of induced T cell immunity. Similarly, the relative contributions of direct priming vs cross-priming to T cell induction and their effects on the nature of the immune response are unknown. Does the duration of antigen presentation affect the potency or durability of the immune response? Are there virus-specific intrinsic “adjuvant” effects on immune responses? Does the nature of the target tissue differentially effect mechanisms of immune induction and the efficacy of the vaccine? The answers to these questions and others will be critical to the future development of viral vector–based immunization and immunomodulation strategies. We have begun to approach these issues with the bias that the mechanism of viral vector–based genetic immunization is unlikely to be universal. We propose that the mechanism of immune induction will depend in part on unique features of specific viral vectors, and that by comparing representative vectors mechanistically, we will develop a more informed understanding of the determining parameters of immune induction. Our initial studies have focused on the identification of antigen-presenting-cell subsets important for priming CD8+ T cell immunity, the effects of antigen persistence on immune responses, and the unique immunogenicity of skin as a target tissue for vaccine delivery.
Role of DC Subsets in Priming Naïve CD8 T Cells in Lentivector Immunization
DC Subsets and the Classical Paradigm of Skin Immune Function
Defining the antigen-presenting cells responsible for T cell priming is critical for the rational development of vaccines. Until recently, the identity of antigen-presenting cells responsible for T cell priming following viral infections was largely unknown. Staerz and colleagues showed that CD8+ T cell activation by influenza virus was diminished if phagocytic cells were eliminated but could be restored by administration of peritoneal exduate cells or a macrophage-like cell line (47). Similarly, it has been shown that mice depleted of phagocytes by toxic liposomes fail to mount CD8+ T cell responses against vesicular stomatitis virus (48). Rock and his colleagues used bone marrow chimeric mice to dissect the role of antigen-presenting-cell subsets in vivo (49). Following infection with rVV-OVA virus, irradiated B6 mice reconstituted with TAP0/0 mouse bone marrow (Bm) cells failed to mount CD8+ T cell responses against VV antigen or transgenic OVA257–264, but responded to ER-targeted OVA257–264. By contrast, B6–B6 chimeric mice responded to all forms of OVA and vaccinia virus antigens. This experiment demonstrated that BM-derived cells are required to prime CD8+ T cells in a TAP-dependent classical cytosolic pathway. The conclusive data demonstrating CD11c+ DCs as antigen-presenting cells after microbial infections come from studies using mice in which DCs can be conditionally depleted. Jung et al. demonstrated that CD11c+ cells were responsible for in vivo priming of viral immune responses (50). They generated transgenic mice expressing diphtheria toxin receptor (DTR)–EGFP fusion protein under the control of CD11c promoter. This promoter is active in all conventional DCs. These transgenic mice are resistant to DT but CD11c+ cells can be depleted by administration of diphtheria toxin. By selectively depleting CD11c+ cells, they elegantly demonstrated a requirement for CD11c+ cells for priming CD8+ T cells responses against intracellular bacteria and parasites. Recent studies suggest that CD11c+ DCs are also the essential antigen-presenting cells for the activation of memory CD8 T cells (51,52). Thus, it has been widely accepted that CD11c+ DCs are the antigen-presenting cells not only for priming naïve T cell immune responses but also for stimulating memory T cell immunity.
Based on the surface cell markers, CD11c+ DCs are a heterogeneous group of cells with similar dendritic shape and the common function of antigen presentation. These DC subsets have distinct lineage, phenotype, and anatomical location, and possibly distinct functions. In mouse skin draining lymph nodes (DLN), at least six DC subsets have been identified (53–55). These subsets can be grouped into two major subdivisions based on their origins, i.e., tissue-derived DCs and blood-derived DCs. Based on phenotypic cell markers, the blood-derived DCs can be further divided into CD8+, CD4+, and CD8−CD4− DCs, which are believed to be derived from blood DC precursors and differentiate and reside in the lymphoid tissues such as LN and spleen, with the potential of acquiring and presenting blood-borne antigen. On the other hand, tissue-derived DC subsets are present in an immature state in the periphery and migrate to the DLN under both steady and inflammatory conditions. The skin DC subsets include epidermal Langerhans cells (LCs, CD11b+DEC205+CD8−/loLangerin+) and dermal DCs (CD11b+DEC205+CD8−Langerin−). In addition, plasmacytoid DCs with unique expression of the B220 marker and lower level of CD11c can also be found in skin DLNs and spleen. Even though the DC subsets have been comprehensively characterized phenotypically, the relative contributions of DC subsets to T cell priming or cross-priming immune responses after infection, and relevant to this discussion after immunization with viral vectors, remains to be determined.
Our general understanding of DC function is based in large part on the paradigm of skin immune function (56,57). Traditionally, skin DCs including dermal DCs and LCs, are thought to acquire antigen in the skin and then migrate to DLN and mature to prime naïve T cells. However, this classical paradigm has recently been questioned by a series of high impact studies. Collectively, these studies suggest that LN resident CD8+ DCs, rather than skin-derived DCs, are critical for the presentation of viral antigens to CD8+ T cells in the setting of viral infection. Specifically, these studies suggest that although skin DCs (LCs) are found in the DLN after epidermal HSV infection, they are not the APCs that prime naïve T cells. Rather, CD8+ LN resident DCs were shown to prime naïve T cells ex vivo and in vivo (58). Similar observations were made in subcutaneous (sc) infection by HSV, influenza (IAV), and vaccinia virus (VV) (59,60), after lung infection by HSV and IAV (53), and after parenteral infection with LCMV virus or Listeria monocytogenes bacteria (61). These results have led to a revised paradigm in which LCs acquire antigen in the periphery and then deliver antigen to LN resident DCs. These LN resident DCs that then cross-present antigen to naïve T cells, suggesting a “collaborative” antigen transfer and presentation model between tissue resident DCs and LN resident DCs (62–64). This “collaborative” cross-priming model offers the alluring possibility that antigen transfer between DC populations could efficiently distribute antigen to a large network of LN resident DCs for processing and presentation. However, it would require an efficient and as yet undiscovered mechanism for the transfer of small quantities of antigen to multiple DCs. Furthermore, a collaborative model would seemingly dissociate the “sentinel” function of skin DCs, which enables them to utilize their functional plasticity to skew immune responses based on environmental conditions, from their antigen-presentation function. This would require additional mechanisms to faithfully transfer and translate those inflammatory or tolerogenic signals from the periphery into appropriate T cell responses. Interestingly, the viruses focused on in those studies were either cytopathic (HSV, VV, IAV) or, like LCMV, had well-described mechanisms for immune evasion that directly or indirectly altered the antigen-presentation function of infected DCs (65–70). This suggested to us that it was possible that in situations where the antigen-presenting function of infected DCs is compromised, immune induction becomes dependent on cross-priming by other DCs or DC subsets that are not directly infected, including CD8+ DCs (71), providing an alternative default mechanism to that of the traditional paradigm. If this is the case, the classical paradigm of skin immune function would predict that following infection by non-cytopathic vectors, incuding lentivectors that have been shown not to interfere with the antigen-presenting function of transduced DCs (32), skin-derived DCs would be able to directly prime naïve CD8+ T-cells.
CD11c+ DCs Prime Naïve CD8 T Cells in Lentivector-Based Genetic Immunization
Both spleen CD11c+ DCs and B cells were found to be transduced after intravenous administration of recombinant lentivector (72). To identify the antigen-presenting cells that express lentivector-encoded genes after subcutaneous injection, we immunized mice with recombinant lentivector-expressing EGFP, EGFP-lvv, via footpad. Two days later, single-cell suspensions from the DLN were stained with antibodies against CD19 (B cells) and CD11c (DCs). Our initial in vivo experiments suggested that after cutaneous injection of lentivector, the DLN of immunized animals contained both DCs and B cells that expressed the delivered transgene. This is consistent with recent reports from Rowe et al. (45).
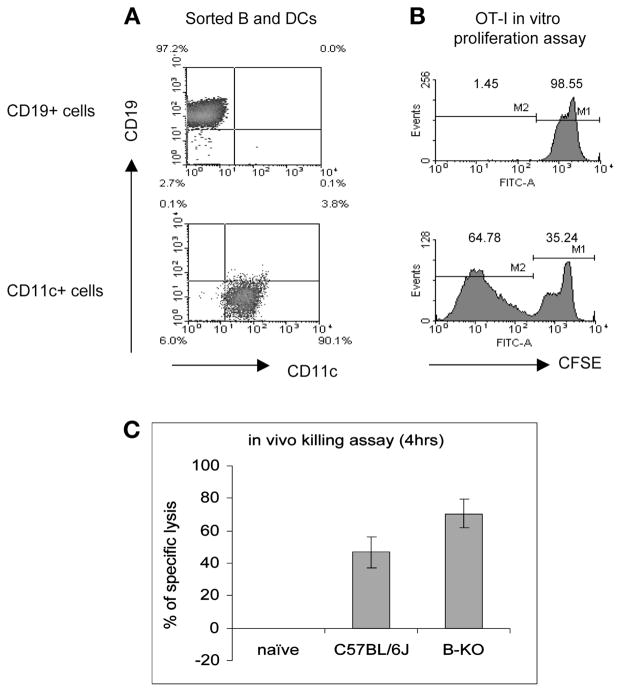
To determine which type of antigen-presenting cells primed naïve CD8 T cells, mice were immunized with OVA-expressing lentivector, 2 d later B cells and CD11c+ cells were isolated by cell sorting. Responder OVA-specific class I restricted OT-I cells were labeled with CFSE and co-cultured with isolated B cells or DCs. As exemplified in Fig. 1, we found that only CD11c+ DCs were able to prime naïve CD8 T cells ex vivo, as indicated by reduction of fluorescence intensity of proliferating OT-1 cells (Fig. 1B). Consistent with this result, we demonstrated that CTL induction by lentivector immunization was unaffected in B cell knockout mice, further supporting the interpretation that B cells are non-essential for CTL priming induced by lentivectors (Fig. 1C). Whether the transduced B cells play a role in generating or maintaining long-term memory, CD8 T cells remains to be elucidated.
Fig. 1.
CD11c+ DCs but not B cells are the APCs that prime naïve CD8 T cells ex vivo. Two days after cutaneous immunization with OVA-expressing lentivector, DCs and B cells were sorted (A) and utilized for stimulating OT-I cell proliferation ex vivo (B). Only the CD11c+ DCs were found to be able to stimulate naïve CD8 T cell proliferation although B cells were also found to express transgene. The induction of in vivo CTL activity was also unaffected in B cell knockout mice (C), further indicating that B cells did not play a measurable role in priming naïve CD8 T cell responses.
Skin-Derived DCs Play the Dominant Role of Priming Naïve CD8 T Cells
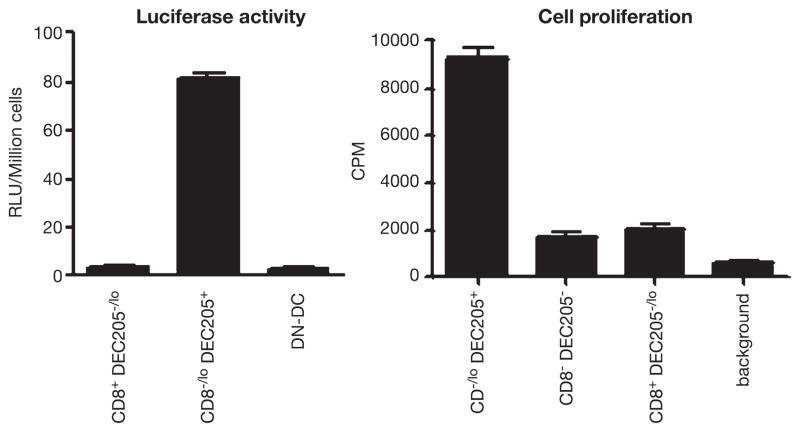
To specifically determine the DC subsets involved in antigen presentation, we immunized mice with recombinant lentivector-expressing luciferase, Luc-lvv, or OVA, OVA-lvv. Then, DC subsets were isolated by cell sorting after staining with a combination of cell surface markers. To corroborate the results, we utilized several combinations of cell markers to define and isolate DC subsets. We evaluated OVA gene expression in purified DC subsets by RT-PCR and separately confirmed expression of transgenic proteins by determining luciferase activity. We have found that the only DC subset that significantly expressed transgenes has a phenotype consistent with skin-derived DCs (CD11c+B220−CD8−CD11b+DEC205+). Furthermore, we found that this was the only DC subset that significantly primed naïve CD8 T cells ex vivo (Fig. 2) (He et al. in press). In contrast, but consistent with previous reports (59), we found that after recombinant vaccinia vector immunization, only the CD8+ LN resident DC subset is able to prime naïve CD8 T cells ex vivo even though transgene expression was also found in other subsets (100).
Fig. 2.
CD11c+CD8−DEC205+ skin DC subset is transduced in vivo by recombinant lentivector (left), which is able to prime naïve CD8 T cells ex vivo (right). Two days after footpad injection of lentivector, CD11c+ cells were enriched by microbeads and sorted based on CD11c–FITC, CD8–APC, and DEC205–PE. Luciferase activity was measured from isolated DC subsets (left). Isolated DC subsets were also co-cultured with naïve OT-I cells to measure their ability of inducing CD8 T cell proliferation (right).
In conclusion, in the recombinant lentivector immunization setting, our results support the traditional model of skin immune function, including the advantages of an “integrative” model by which skin DCs both present antigen to T cells and integrate antigen-presentation with an environmentally responsive program of signals that modulate and skew immune responses. Several recently reported studies are consistent with this “integrative” model and demonstrate that direct contact with invading microbes or other stimulatory signals are necessary to activate or program DCs to induce appropriate immune responses. For example, it has been shown that the direct interaction with TLR ligands or microbes is necessary to license DCs to fully activate the effector function of T-cells (73–75).
Cutaneous Immunization Elicits Strong CD8+ T Cell Activation
Skin DCs, which include LCs and dermal DCs, have long been recognized as playing an important role in initiating immune responses (57). Our own research using lentivector strongly indicates that skin-derived DCs play the dominant role of priming naïve CD8 T cell immune responses. These studies suggest that skin may be an important target for vaccine delivery. Recent demonstrations that cutaneous immunization induces stronger immunity against influenza virus infection than immunization by other routes, further supports this hypothesis and the importance of the skin DC network (76–78).
To directly asses the relative immunogenicity of skin immunization with lenti-vector, we evaluated and compared lentivector-based skin immunization (footpad) with immunization by the intramuscular (im), intravenous (iv), and intraperitoneal (ip) routes. Mice were immunized with same dose of recombinant lentivector expressing either OVA model antigen or HBV S antigen via different routes. The induction of CD8 T cell activation was measured by intracellular staining of IFN-γ ex vivo. As demonstrated in Fig. 3, cutaneous immunization elicits significantly higher levels of activated CD8+ T cells than all other immunization routes studied. This was true for both the model antigen OVA and for the HBV S antigen. These results further support the development of cutaneous genetic immunization and stress the importance of skin-derived DCs in priming CD8+ T cell immunity.
Fig. 3.
The effect of immunization routes on the magnitude of induced CD8 T cell responses. C57BL/6 mice (three mice in each group) were immunized with either 1 million TU of OVA-lvv or HBS-lvv through intramuscular (im), footpad (fp), intravenous (iv), or intraperitoneal (ip). Eight days later, mice were sacrificed and splenocytes were collected and restimulated in vitro for 3 h with either SIINFEKL or HBV S Ag S190–198 peptide. Cells were then intracellularly stained for IFN-γ.
Duration of Antigen Presentation
The effect of Ag-presentation duration on the potency and memory T cell responses has been investigated in recent years but remains controversial. Initial studies indicated that a brief exposure to Ag was enough to activate CD8 T cells (79–82), and more recent experiments are consistent with the notion that the initial dose rather than the duration of Ag presentation is critical for generating potent immunity (83,84). On the other hand, studies involving surgical removal of infection sites at different time points indirectly demonstrate that early termination of Ag exposure dramatically decreases the intensity of induced adaptive immunity, suggesting a prolonged period of Ag presentation may be required for eliciting appropriate T cell immunity (85). It has been suggested that brief antigen exposure may result in an abortive response resulting in cell death without accumulation of clonally expanded T cells (86). Consistent with this, other recent studies suggest that prolonged Ag exposure together with co-stimulation and signal 3 cytokines such as IL-12 are required to stimulate CD8 T cell clonal expansion and differentiation into effector cells (86–88). It has also been shown that prolonged interaction between T cells and DCs is critical in determining the fitness of activated T cells, with fitness defined as the ability to survive and respond to homeostasis cytokines IL-7 and IL-15 (89). The enhancement of DNA vaccine efficacy by co-administration of DNA encoding antiapoptotic protein also supports the idea that prolonged Ag presentation may be beneficial for inducing potent and persistent T cell immunity (90), as do other experiments demonstrating that CD4 T cell activation and CD4 T memory cell generation require prolonged and persistent Ag presentation (15,91). Furthermore, a very recent study suggests that prolonged antigen exposure is important for generating long-lived memory CD8 T cell responses overall (13).
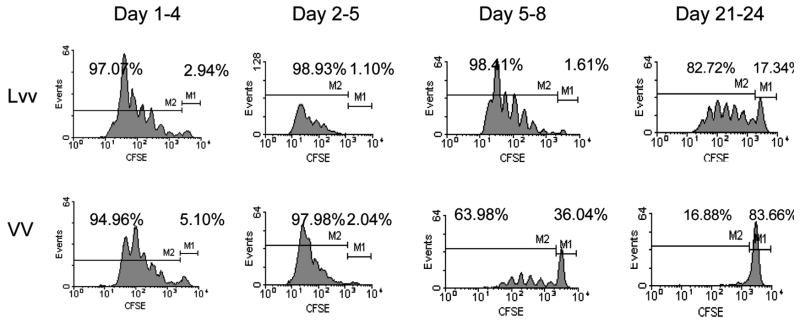
Overall, the data on the duration of in vivo antigen presentation after viral infections is limited. It has recently been shown that after HSV infection, antigen presentation can still be detected even after CTL activity has reached its maximum (85). Similarly, recent studies suggest that the duration of antigen presentation in vivo after replication-defective recombinant adenoviral vector immunization can last 40 d, even after antigen expression has terminated (92). This prolonged antigen presentation correlated with delayed contraction of CD8 T cell immune responses, suggesting that extended antigen presentation may be required for generating long-lasting T cell immunity. Analyzing correlations between the duration of antigen presentation and the potency and endurance of induced T cell immunity may help us design more effective genetic vaccines. We investigated the duration of antigen presentation in vivo subsequent to lentivector or vaccinia vector immunization. We sought to determine whether non-cytopathic vectors such as lentivector will have prolonged antigen presentation compared to cytopathic vectors, such as vaccinia vector. To address this directly, we immunized mice with recombinant lentivector or vaccinia vectors that express the OVA transgene. At different time points, we adoptively transferred CFSE-labeled OT-I cells into immunized mice. Three days after adoptive transfer, cells from DLN were collected and OT-I cell proliferation was analyzed by progressive reduction of CFSE intensity. As shown by a representative experiment in Fig. 4, antigen presentation in the DLN of mice immunized with lentivector could still be detected 3 wk after immunization. In contrast, antigen presentation after recombinant vaccinia vector immunization decreased rapidly and was only minimal evident after 5 d. In fact, the strength of antigen presentation 3 wk after lentivector immunization was stronger than that observed 5 d after vaccinia vector immunization. This in vivo antigen-presentation result correlated with more potent and prolonged CTL effector function in lentivector immunized mice, suggesting that the duration of antigen presentation in vivo may be a factor that affects the potency and persistence of CD8+ T cell immunity. Further investigation is required to determine if durable antigen presentation correlates with potent and persistent CD4+ and CD8+ T cell immunity and to determine other factors that may influence the immune response.
Fig. 4.
Duration of in vivo Ag presentation after immunization with lentivector or vaccinia vector C57BL/6 mice were injected with OVA-expressing lentivector (Lvv) or vaccinia vector (VV) on the footpad. At indicated time points, CFSE-labeled naïve OT-I cells were injected (iv) into the immunized mice to measure the antigen presentation manifested by the progressive reduction of CFSE intensity.
Summary: DC Networks in Cutaneous Genetic Immunization
In the lentivector-mediated genetic immunization, we have demonstrated that the skin-derived DC population expresses transgenes and primes naïve CD8 T cells. These data suggest that direct presentation by the transduced skin-derived DC subset may be a predominant mechanism of CD8 T cell induction. It is also likely that skin DCs cross-present antigens acquired from keratinocytes, fibroblasts, or other skin-resident cell populations. We suggest that the mechanism of CD8 T cell priming is dependent on the viral vector used, which may have cytopathic, inhibitory, or adjuvant effects on the relevant antigen-presenting cell populations. If a viral vector, such as vaccinia vector, has cytopathic effects on transduced APCs, then the antigen-presenting function of infected cells can be compromised, and LN resident bystander non-transduced DCs that acquire antigen from dying cells may be the predominant APC for cross-priming T cells. On the other hand, APCs transfected by non-cytopathic vectors such as lentivector should be able to present effectively either endogenously synthesized antigen to directly prime naïve T cells or cross-present antigen obtained from skin cells. It seems less likely that LN resident DCs could acquire antigens physiologically expressed in the skin, a process that would require considerable efficiency to enable both antigen transport and then cross-presentation. It is more likely that cross-presentation by LN resident DCs would be dependent on antigen-expressing DCs arriving from the periphery, or antigen transport through the lymphatics, as can occur following intradermal injection of antigen (74) or substantial death of skin cells. Interestingly, recent data suggest that proliferating T cells primed by LN resident DC subsets after acquiring antigen in the LN do not differentiate into effector cells (74), further substantiating the importance of skin-derived DCs, which have direct contact with invading pathogens in the process of priming functional T cell immunity.
The relative contributions of skin DC subsets to T cell priming resulting from lentivector immunization remain to be determined. The contributions of two skin DC subsets, LCs and dermal DCs, to the regulatory balance between induction of T cell immunity and the maintenance of peripheral tolerance is actively being investigated. Recent studies suggest that DC subsets other than LCs are responsible for priming naïve T cells after cutaneous infection with HSV (58) and leishmaniasis (93), or vaginal mucosal infection of HSV (94). In addition, using knockin mice expressing a toxin gene or toxin receptor gene under the control of the langerin promoter that allows specific deletion of LCs in vivo, it has been demonstrated that LCs are not essential for contact hypersensitivity (CHS), a CD8 T cell–mediated skin inflammatory reaction to topical application of haptens (95–97). The temporally distinct migration pattern of LCs and dermal DC into different anatomical locations of DLN (96) and the coincidence of early migration of dermal DCs with the potent T cell–priming capability of skin DC subsets in the DLN observed in our own study (He et al., in press) are consistent with the interpretation that dermal DCs may be a dominant antigen-presenting cell population after lentivector immunization. However, this does not rule out the possibility that multiple DC subsets may redundantly contribute to antigen presentation, enabling appropriate immune regulation in response to a wide range of environmental assaults. With the availability of current conditional LC-depletion transgenic mice and novel antigen-delivery approaches (98), it may be possible to further pinpoint the role of each skin DC subset in priming T cell immunity after viral vector–mediated genetic immunization.
Based on our observations, lentivector-transduced skin-derived DCs possess the ability to directly prime naïve T cells efficiently for extended periods of time due to factors including longevity of skin-derived DCs and persistent gene expression after lentivector transduction. This enables lentivector-mediated immunization to generate not only potent CD8 T cell immunity, but also long-lived memory responses. That is in contrast to the mechanism of cross presentation by LN-resident DCs after cytopathic vaccinia vector immunization, in which dependence on acquired antigen may allow only a limited period of time to prime naïve T cells (Fig. 4) (99,100). Clearly, presentation by migrating skin DCs would facilitate immune skewing based on the stimuli skin DC received in the peripheral, and thus generate environmentally responsive immunity. Additional insight into this priming mechanism and the role of the skin DC network in viral vector mediated genetic immunization will enable us to manipulate the process of T cell priming to develop more effective genetic vaccines.
Acknowledgments
The research conducted in our laboratory is supported by NIH grants to Y.H. and L.D.F. with the excellent technical support from Jiying Zhang and Cara Donahue in our group. We also acknowledge the provision of recombinant vaccinia vector from Dr. Jonathan Yewdell of NIAID, NIH.
Biography

Yukai He
References
- 1.Plotkin SA. Vaccines: past, present and future. Nat Med. 2005;11:S5–11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin SA. Six revolutions in vaccinology. Pediatr Infect Dis J. 2005;24:1–9. doi: 10.1097/01.inf.0000148933.08301.02. [DOI] [PubMed] [Google Scholar]
- 3.Burton DR, Desrosiers RC, Doms RW, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 4.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 5.Moore AC, Hill AV. Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol Rev. 2004;199:126–143. doi: 10.1111/j.0105-2896.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 6.Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- 7.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 8.McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–255. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 9.Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305:205–208. doi: 10.1126/science.1100600. [DOI] [PubMed] [Google Scholar]
- 10.Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 11.Learmont JC, Geczy AF, Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340:1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 12.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. Long-lived memory CD8(+) T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol. 2006;36:842–854. doi: 10.1002/eji.200535730. [DOI] [PubMed] [Google Scholar]
- 14.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 15.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voltan R, Robert-Guroff M. Live recombinant vectors for AIDS vaccine development. Curr Mol Med. 2003;3:273–284. doi: 10.2174/1566524033479816. [DOI] [PubMed] [Google Scholar]
- 17.Bennink JR, Yewdell JW, Smith GL, Moller C, Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984;311:578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- 18.Kieny MP, Lathe R, Drillien R, et al. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984;312:163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- 19.Moss B, Smith GL, Gerin JL, Purcell RH. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984;311:67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- 20.Adamina M, Daetwiler S, Rosenthal R, Zajac P. Clinical applications of recombinant virus-based cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1211–1224. doi: 10.1517/14712598.5.9.1211. [DOI] [PubMed] [Google Scholar]
- 21.Rayner JO, Dryga SA, Kamrud KI. Alphavirus vectors and vaccination. Rev Med Virol. 2002;12:279–296. doi: 10.1002/rmv.360. [DOI] [PubMed] [Google Scholar]
- 22.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 23.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer JL, Kobinger G, Wilson JM, Crystal RG. Adenovirus-based genetic vaccines for biodefense. Hum Gene Ther. 2005;16:157–168. doi: 10.1089/hum.2005.16.157. [DOI] [PubMed] [Google Scholar]
- 25.Casimiro DR, Chen L, Fu TM, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Zhai Y, Yang JC, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farina SF, Gao GP, Xiang ZQ, et al. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofmann C, Loser P, Cichon G, Arnold W, Both GW, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esslinger C, Chapatte L, Finke D, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003;111:1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esslinger C, Romero P, MacDonald HR. Efficient transduction of dendritic cells and induction of a T-cell response by third-generation lentivectors. Hum Gene Ther. 2002;13:1091–1100. doi: 10.1089/104303402753812494. [DOI] [PubMed] [Google Scholar]
- 32.He Y, Zhang J, Mi Z, Robbins P, Falo LD., Jr Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174:3808–3817. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- 33.Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V, Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- 34.Hacein-Bey-Abin S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 35.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 36.Lu R, Nakajima N, Hofmann W, et al. Simian virus 40-based replication of catalytically inactive human immunodeficiency virus type 1 integrase mutants in nonpermissive T cells and monocyte-derived macrophages. J Virol. 2004;78:658–668. doi: 10.1128/JVI.78.2.658-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saenz DT, Loewen N, Peretz M, et al. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J Virol. 2004;78:2906–2920. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas J, Jr, Gusella GL, Najfeld V, Klotman ME, Cara A. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum Gene Ther. 2004;15:361–372. doi: 10.1089/104303404322959515. [DOI] [PubMed] [Google Scholar]
- 39.Yanez-Munoz RJ, Balaggan KS, Macneil A, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- 40.Collins MK, Cerundolo V. Gene therapy meets vaccine development. Trends Biotechnol. 2004;22:623–626. doi: 10.1016/j.tibtech.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Lever AM, Strappe PM, Zhao J. Lentiviral vectors. J Biomed Sci. 2004;11:439–449. doi: 10.1007/BF02256092. [DOI] [PubMed] [Google Scholar]
- 42.Quinonez R, Sutton RE. Lentiviral vectors for gene delivery into cells. DNA Cell Biol. 2002;21:937–951. doi: 10.1089/104454902762053873. [DOI] [PubMed] [Google Scholar]
- 43.Breckpot K, Dullaers M, Bonehill A, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5:654–667. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- 44.Dullaers M, Meirvenne SV, Heirman C, et al. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13:630–640. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- 45.Rowe HM, Lopes L, Ikeda Y, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther. 2006;13:310–319. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Esslinger C, Chapatte L, Finke D, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003;111:1673–1681. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debrick JE, Campbell PA, Staerz UD. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991;147:2846–2851. [PubMed] [Google Scholar]
- 48.Ciavarra RP, Buhrer K, Van Rooijen N, Tedeschi B. T cell priming against vesicular stomatitis virus analyzed in situ: red pulp macrophages, but neither marginal metallophilic nor marginal zone macrophages, are required for priming CD4+ and CD8+ T cells. J Immunol. 1997;158:1749–1755. [PubMed] [Google Scholar]
- 49.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 50.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belz GT, Wilson NS, Smith CM, Mount AM, Carbone FR, Heath WR. Bone marrow-derived cells expand memory CD8(+) T cells in response to viral infections of the lung and skin. Eur J Immunol. 2006 doi: 10.1002/eji.200535432. [DOI] [PubMed] [Google Scholar]
- 52.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belz GT, Smith CM, Kleinert L, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 55.Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Sem Immunol. 2005;17:262–272. doi: 10.1016/j.smim.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 57.Larrengina AT, Falo LD. Changing paradigms in cutaneous immunology: adapting with dendritic cells. J Invest Dermatol. 2005;124:1–12. doi: 10.1111/j.1523-1747.2004.23554.x. [DOI] [PubMed] [Google Scholar]
- 58.Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 59.Belz GT, Smith CM, Eichner D, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 60.Smith CM, Belz GT, Wilson NS, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are preferentially involved in CTL priming after footpad infection with herpes simplex virus-1. J Immunol. 2003;170:4437–4440. doi: 10.4049/jimmunol.170.9.4437. [DOI] [PubMed] [Google Scholar]
- 61.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serbina NV, Pamer EG. Immunology. Giving credit where credit is due. Science. 2003;301:1856–1857. doi: 10.1126/science.1090613. [DOI] [PubMed] [Google Scholar]
- 63.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Heath WR, Belz GT, Behrens GM, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 65.Sevilla N, Kunz S, McGavern D, Oldstone MB. Infection of dendritic cells by lymphocytic choriomeningitis virus. Curr Top Microbiol Immunol. 2003;276:125–144. doi: 10.1007/978-3-662-06508-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 67.Engelmayer J, Larsson M, Subklewe M, et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163:6762–6768. [PubMed] [Google Scholar]
- 68.Larsson M, Beignon AS, Bhardwaj N. DC-virus interplay: a double edged sword. Sem Immunol. 2004;16:147–161. doi: 10.1016/j.smim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Larsson M, Fonteneau JF, Somersan S, et al. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur J Immunol. 2001;31:3432–3442. doi: 10.1002/1521-4141(200112)31:12<3432::aid-immu3432>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 70.Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8(+) T cells in vivo: the key to rational vaccine design. Annu Rev Immunol. 2005;23:651–682. doi: 10.1146/annurev.immunol.23.021704.115702. [DOI] [PubMed] [Google Scholar]
- 71.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.VandenDriessche T, Thorrez L, Naldini L, et al. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood. 2002;100:813–822. doi: 10.1182/blood.v100.3.813. [DOI] [PubMed] [Google Scholar]
- 73.Heath WR, Villadangos JA. No driving without a license. Nat Immunol. 2005;6:125–126. doi: 10.1038/ni0205-125. [DOI] [PubMed] [Google Scholar]
- 74.Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 75.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 76.Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 77.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 78.La Montagne JR, Fauci AS. Intradermal influenza vaccination—can less be more? N Engl J Med. 2004;351:2330–2332. doi: 10.1056/NEJMe048314. [DOI] [PubMed] [Google Scholar]
- 79.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 81.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 82.Wong P, Pamer EG. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol. 2001;166:5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 83.Hafalla JC, Sano G, Carvalho LH, Morrot A, Zavala F. Short-term antigen presentation and single clonal burst limit the magnitude of the CD8(+) T cell responses to malaria liver stages. Proc Natl Acad Sci USA. 2002;99:11819–11824. doi: 10.1073/pnas.182189999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–511. doi: 10.1016/s1074-7613(03)00081-5. [DOI] [PubMed] [Google Scholar]
- 85.Stock AT, Mueller SN, van Lint AL, Heath WR, Carbone FR. Cutting edge: prolonged antigen presentation after herpes simplex virus-1 skin infection. J Immunol. 2004;173:2241–2244. doi: 10.4049/jimmunol.173.4.2241. [DOI] [PubMed] [Google Scholar]
- 86.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 87.van Stipdonk MJ, Hardenberg G, Bijker MS, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 88.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 90.Kim TW, Hung CF, Ling M, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding anti-apoptotic proteins. J Clin Invest. 2003;112:109–117. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang TC, Millar J, Groves T, et al. The CD8+ T cell population elicited by recombinant adenovirus displays a novel partially exhausted phenotype associated with prolonged antigen presentation that nonetheless provides long-term immunity. J Immunol. 2006;176:200–210. doi: 10.4049/jimmunol.176.1.200. [DOI] [PubMed] [Google Scholar]
- 93.Ritter U, Meissner A, Scheidig C, Korner H. CD8 alpha-and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur J Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 94.Zhao X, Deak E, Soderberg K, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Bennett CL, van Rijn E, Jung S, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 99.Ramirez MC, Sigal LJ. Macrophages and dendritic cells use the cytosolic pathway to rapidly cross-present antigen from live, vaccinia-infected cells. J Immunol. 2002;169:6733–6742. doi: 10.4049/jimmunol.169.12.6733. [DOI] [PubMed] [Google Scholar]
- 100.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8+ T cell immunity in recombinant lentivector-medicated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]