SUMMARY
We report the unexpected finding that loss of Hh signaling through conditional deletion of Smoothened (Smo) in the adult hematopoietic compartment has no apparent effect on adult hematopoiesis, including peripheral blood count, number or cell-cycle status of stem or progenitor cells, hematopoietic colony-forming potential, long-term repopulating activity in competitive repopulation assays, or stress response to serial 5-fluorouracil treatment. Furthermore, pharmacologic inhibition of Hh signaling with a potent and selective small molecule antagonist has no substantive effect on hematopoiesis in the mouse. In addition, Hh signaling is not required for the development of MLL-AF9-mediated acute myeloid leukemia (AML). Taken together, these data demonstrate that Hh signaling is dispensable for normal hematopoietic development and hematopoietic stem cell function, indicating that targeting of Hh signaling in solid tumors is not likely to result in hematopoietic toxicity. Furthermore, the Hh pathway may not be a compelling target in certain hematopoietic malignancies.
INTRODUCTION
Multiple studies have explored the role of the Hh pathway in development, but relatively few studies have addressed the role of Hh in the hematopoietic system. Hh has been reported to play a role in hemangioblast formation (Dyer et al., 2001); B cell, T cell, and thymocyte development (El Andaloussi et al., 2006; Outram et al., 2000; Sacedon et al., 2005; Uhmann et al., 2007); erythrocyte proliferation and differentiation (Detmer et al., 2000); and the HSC and progenitor cell compartment (Bhardwaj et al., 2001; Gering and Patient, 2005; Trowbridge et al., 2006). Thus, the available literature indicates that there is an important contribution of Hh signal transduction in normal hematopoietic development. Of further interest, mutations in the Hh signaling pathway lead to severe developmental abnormalities and have been associated with several types of cancers. For example, germline mutations in PTCH1 result in Gorlin syndrome, which predisposes patients to medulloblastoma and basal cell carcinoma (Corcoran and Scott, 2001; Goodrich et al., 1997; Hahn et al., 1996; Johnson et al., 1996). More recent studies have shown that Hh signaling is important for survival of other tumors, including pancreatic adenocarcinoma and small cell lung carcinoma. Based in part on these observations, Hh antagonists are under development as potential therapeutic agents for such cancers (Pasca di Magliano and Hebrok, 2003; Rubin and de Sauvage, 2006).
To define the role of Hh signaling in normal hematopoietic development and leukemogenesis, we assessed the consequences of conditional deletion of the Smoothened (Smo) allele in an in vivo knockout mouse model. Smoothened is the central mediator of Hh signaling and is required for Hh pathway activation. Homozygous loss of function of Smo during development results in embryonic lethality at E9.5 (Zhang et al., 2001). To circumvent embryonic lethality, homozygous SmoC/C mice in which exon 1 is flanked by loxP sites (Long et al., 2001) were generated on an Mx1-Cre background, enabling inducible excision in the hematopoietic stem cell compartment using pIpC induction of interferon expression (Kuhn et al., 1995).
RESULTS
Treatment with pIpC Results in Complete and Persistent Excision of Smo
SmoC/C-Mx1Cre (hereinafter referred to as “SmoNull” mice) or SmoC/C mice (hereinafter referred to as “SmoWT”) were treated with pIpC as described in the Experimental Procedures, and complete excision was confirmed using a semiquantitative three-primer PCR analysis. We observed that hematopoiesis was supported in the absence of Smo for up to 18 months after pIpC treatment (see Figures S1A and S1B available online), indicating that there was no apparent deleterious effect of Smo deletion on long-term hematopoiesis or survival. Furthermore, the observation that the bone marrow remained fully excised at 6 and 18 months, respectively, indicates that there was no selective advantage for rare cells in which excision had not occurred.
Loss of Smo Has No Effect on Terminally Differentiated Hematopoietic Cells
In consonance with the above observations, there was no significant difference in peripheral blood cell counts obtained over time, or spleen, thymus, and liver weights between SmoNull and SmoWT animals at 6 months after pIpC induction (Figures S2A and S2B). Analysis of peripheral blood smears and histological tissue sections of bone marrow, spleen, and liver, as well as other major organ systems, also showed no apparent differences between the two groups (data not shown). Similar results were observed in cohorts of animals each containing at least five mice that were analyzed at 4 weeks, 12 weeks, and 6 and 18 months, respectively, after pIpC injection (data not shown). In addition, flow cytometry analysis showed similar numbers of T and B cells, erythroid and myeloid cells, and megakaryocytes between SmoNull and SmoWT animals based on lineage-specific cell-surface immunophenotyping (Figure S3).
Loss of Smo Does Not Alter the Number of Lin−Sac1+cKit+ Cells and Multipotent Progenitor Cells
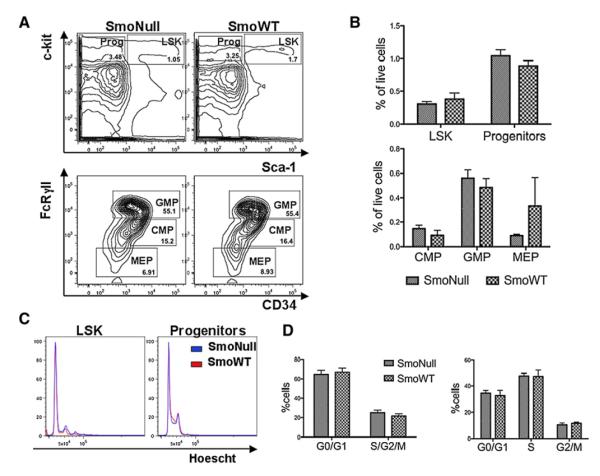
Multiparameter flow cytometry showed no significant differences in the number of Lin−Sca1+cKit+ (LSK) cells as a fraction of live or lineage-negative cells in SmoNull versus SmoWT mice (Figures 1A and 1B). Similarly, no differences were found in the absolute number or percent of live cells of myeloid progenitors (Figures 1A and 1B) or their subpopulations (common myeloid progenitors [CMPs], granulocyte-monocyte progenitors [GMPs], and megakaryocyte-erythroid progenitors [MEPs] (Figures 1A and 1B). There were also no apparent differences between Smo-deficient and wild-type cells in hematopoietic colony plating potential in methylcellulose (Figure S2C). In addition, flow cytometry-based cell-cycle analysis of LSK or myeloid progenitor cells showed no differences in the proportion of cells in G0/G1 and S/G2/M between SmoNull and SmoWT in absolute numbers or percent of live cells (Figures 1C and 1D).
Figure 1. Smo-Deficient Mice Show No Difference in Number of LSK or Progenitor Cells, or Their Cell-Cycle Activity.
(A)SmoNull or SmoWT mice (n = 2–3 per group) were treated with InvivoGen pIpC 200 μg and sacrificed 4 weeks after injection for complete endpoint analysis. Multiparameter flow cytometry analysis was used to identify myeloid progenitor (Prog) and LSK populations (top row) and the myeloid subsets CMP, GMP, and MEP (bottom row). There was no statistically significant difference between SmoNull or SmoWT groups in percentage of LSK cells (p = 0.5622) or myeloid progenitors (p = 0.2948) among live cells. Similarly, there were no differences in the percentage of CMP (p = 0.39), GMP (p = 0.5183), or MEP (p = 0.4823).
(B) Bar graph representation of data. Results were analyzed in an unpaired t test. Errors bars represent SEM.
(C) Fraction of LSK (left panel) or myeloid progenitors (right panel) in G0/G1 versus S/G2/M in overlapped cell-cycle histograms for SmoNull (blue) compared to SmoWT (red) mice.
(D) Cell-cycle analysis with Pyronin Y and Hoescht staining for LSK (left) and myeloid progenitors (right) shown as a percentage of live cells from the same animals as described in (A). An unpaired t test was used for analysis. Error bars represent SEM.
Loss of Smo Does Not Affect Bone Marrow Repopulation after Transplantation
We next assessed whether loss of Smo had any impact on repopulating the bone marrow after bone marrow transplantation. We transplanted 1 × 106 bone marrow cells from SmoNull and SmoWT animals into lethally irradiated recipients. After engraftment had occurred 3 weeks after transplantation, recipient mice were treated with pIpC 25 μg/g mouse weight to induce excision of Smo. Again, we confirmed that complete excision was achieved in the peripheral blood 4 weeks after the first dose of pIpC was given (data not shown). Complete blood counts were obtained 4 and 12 weeks after the pIpC was given and did not show any differences between mice that received bone marrow from SmoNull and SmoWT donors. Mice were sacrificed 12 weeks after pIpC injection for complete analysis. Again, no significant differences were observed in peripheral blood cell counts, spleen, thymus and liver weights, histopathology of all major organ systems, colony-forming unit (CFU) potential, and flow cytometric analysis of terminally differentiated hematopoietic cells (data not shown). We repeated the same experiment by treating SmoNull and SmoWT animals with pIpC first and then transplanting the harvested bone marrow into lethally irradiated recipient mice. We performed the same comprehensive analysis as outlined above and again observed comparable results, indicating that Smo is not required for repopulating the bone marrow after transplantation.
Hh Signaling Is Dispensable for Thymocyte Development
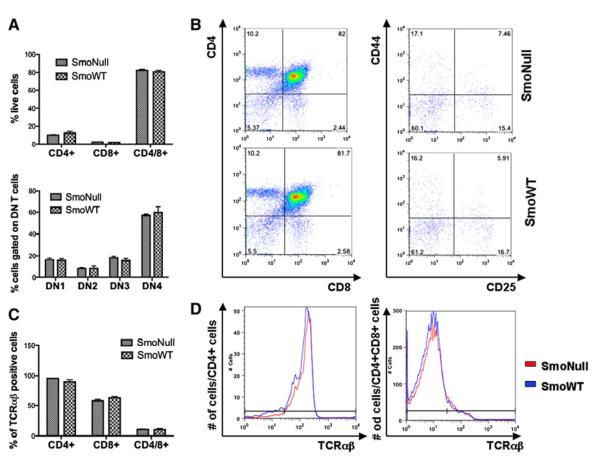
Given previous reports in the literature that loss of Smo plays a role in thymocyte development, we analyzed thymocyte development in mice after bone marrow transplantation. The differentiation pattern of CD4-CD8 double-negative (DN) thymocytes was assessed by flow cytometry and defined by immunophenotypic characteristics of CD25 and CD44 expression patterns leading to various stages of T cell maturation from DN1 (CD25−, CD44+), to DN2 (CD25+, CD44+), to DN3 (CD25+ CD44−), to DN4 (CD25−, CD44−) thymocytes. In addition, we analyzed the contributions of TCRαβ and TCRγδ of CD4-positive helper cells, CD8-positive cytotoxic T cells, or CD4- and CD8 double-positive T cells.
In contrast to the previously reported literature, we did not observe any differences in the absolute number or percent of live cells of CD4+ helper cells, CD8+ cytotoxic T cells, or CD4+CD8+ T cells (Figures 2A and 2B). Neither did we detect any abnormal maturation pattern of immature DN thymocytes by flow cytometry (Figures 2A and 2B). We also did not identify any aberrant T cell population by CD4/CD8 and TCRαβ characteristics (Figures 2C and 2D). The TCRαβ-positive T cells were negative for TCRγδ (data not shown). Comparable results were noted in a similar experiment in which pIpC was given prior to bone marrow transplantation.
Figure 2. Loss of Smo Does Not Affect T Cell and Thymocyte Development.
Bone marrow of SmoNull or SmoWT mice was harvested and transplanted into lethally irradiated B6.SJL recipients (n = 6 per group), which were treated with 25 μg/gram pIpC 3 weeks after engraftment. Thymi were harvested 12 weeks after pIpC treatment to analyze the T cell subpopulations and thymocyte development.
(A) Flow cytometry analysis was performed to identify CD4+ T helper cells, CD8 + cytotoxic T cells, and CD4+/CD8+ double-positive (DP) T cells, as well as thymocyte development (DN1-DN4) characterized by CD44 and CD25 characteristics. There was no difference between SmoNull or SmoWT mice in regards to T cell subpopulations (p = 0.9983) or thymocyte developmental stages (p = 1.0).
(B) Representative populations shown in flow scattergrams.
(C) Percentage of TCRαβ-rearranged cells gated on CD4+, CD8+, or CD4+/CD8+ T cells. There was no statistically significant difference between thymocytes obtained from SmoNull or SmoWT mice (p = 0.9917).
(D) Representative data for CD4+ and CD4+/CD8+ populations are shown in a histogram. An unpaired t test was used for statistical analysis. Error bars represent SEM.
Loss of Smo Does Not Affect Stem Cell Function in a Competitive Repopulation Assay
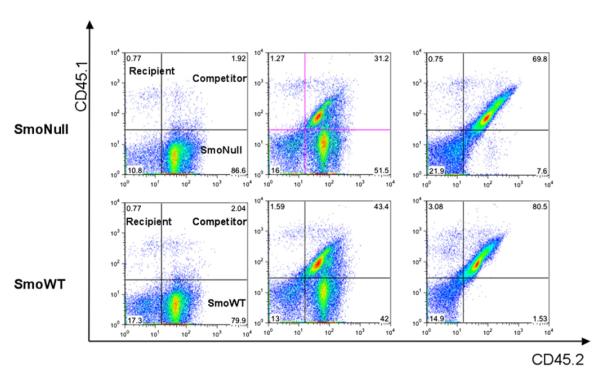
These data indicate that loss of Smo, the central cellular effector of Hh signaling, does not have any apparent effect on hematopoiesis in adult mice in an in vivo conditional knockout mouse model system. However, to formally test functional HSC activity in the context of Smo deficiency, we performed competitive repopulation assays. Whole bone marrow was harvested from SmoWT/CD45.2 or conditional deleted SmoNull/CD45.2 mice, mixed at varying ratios with competitor B6.SJLF1CD45.1/CD45.2 cells, and transplanted into lethally irradiated B6.SJLCD45.1 recipients. Baseline flow cytometric analysis of chimerism confirmed the expected ratios of SmoNull donor and SmoWT competitor cells 3 weeks after transplantation (data not shown). Mice were treated with pIpC 3 weeks after engraftment to eliminate any potential cytokine effect that pIpC might have on homing and engraftment. Complete excision of Smo was confirmed using semiquantitative three-primer PCR 4 weeks after treatment with pIpC (Figure S1C).
Flow cytometric analysis of the adult hematopoietic compartment at 8 weeks (data not shown), and at study endpoint 16 weeks after treatment with pIpC, showed no statistically significant difference between SmoNull and SmoWT donors (Figure 3). The observation that there is no disadvantage for Smo-deficient bone marrow cells in competitive repopulation assays in secondary recipient mice is in consonance with our previous experiment (Figure S1B) in primary animals showing no competitive disadvantage for Smo-excised cells 18 months after excision. In addition, there were no significant differences between the relative contribution of SmoNull versus SmoWT donors to lymphocyte, T cell, myeloid, erythroid cells, or megakaryocyte lineages (data not shown) and no differences in spleen or liver weight or histopathology of paraffin-embedded tissue sections of heart, lung, gastrointestinal tract, kidney, spleen, liver, or bone marrow (data not shown).
Figure 3. Loss of Smo Does Not Affect Competitive Repopulating Activity.
Flow cytometric analysis of whole bone marrow cells using CD45.1 and CD45.2 surface marker labeling to identify CD45.1-positive recipient (left upper quadrant), CD45.1/CD45.2 double-positive competitor (right upper quadrant), or CD45.2 donor (right lower quadrant) cell populations. SmoWT/CD45.2 or SmoNull/CD45.2 donor bone marrow was transplanted together with a competitor WT bone marrow from B6.SJL F1CD45.1/CD45.2 mice into B6.SJLCD45.1 recipients (n = 15 per group). The following donor:competitor ratios were used: 1:0, 3:1, 1:1, 1:3, and 0:1 (n = 3 per subgroup). Recipient mice were treated with pIpC 3 weeks after engraftment. Donor-to-competitor chimerism analysis of the bone marrow at 16 weeks is shown for SmoNull/CD45.2 (top) or SmoWT/CD45.2 mice at representative donor:competitor ratios of 1:0 (left), 1:1 (middle), and 0:1 (right). Analysis of the peripheral blood and spleen showed similar results.
Hh Signaling Is Dispensable under Conditions of Hematopoietic Stress
Overall, these data indicate that Hh signaling is dispensable for the reconstitution of long-term hematopoiesis in competitive repopulation assays using an experimental design in which Smo is excised after transplantation. To determine whether Hh signaling might be important for stress response in the hematopoietic system, we tested response to serial treatment with 5-fluorouracil (5-FU) (Cheng et al., 2000)(Figure 4). We observed no differences in complete blood counts or survival between SmoNull and SmoWT in response to 5-FU treatment.
Figure 4. Response to Stress with Serial 5-FU Experiment Does Not Lead to Any Changes in Peripheral Blood Counts or Overall Survival in SmoNull Mice.
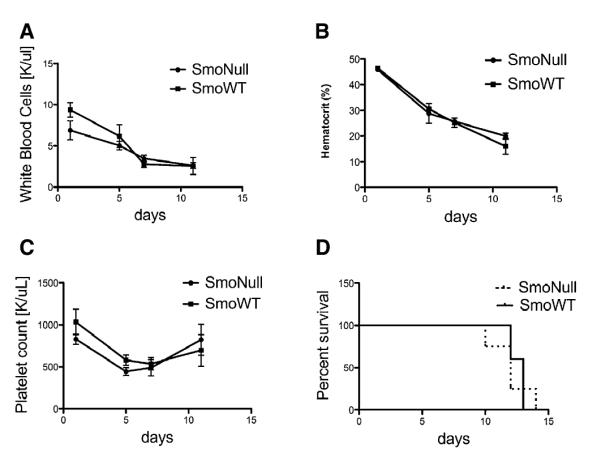
Peripheral blood counts of SmoNull or SmoWT mice that received sequential 5-FU treatment 3 weeks after pIpC treatment. No differences in (A) WBC (n = 5, p = 0.7130), (B) Hematocrit (Hct) (n = 5, p = 0.9524), or (C) platelet count (n = 5, p = 0.6867) was observed over time. An unpaired t test was used for statistical analysis. Error bars represent SEM. (D) Survival outcome after sequential 5-FU treatment. 5-FU was administered i.p. weekly at a dose of 150 mg/kg in a survival study. Results were analyzed using a log rank test and expressed as Kaplan-Meier survival curves (n = 5, p = 0.7569).
HhAntag and rShh Agonist Do Not Affect Hematopoietic Colony-Forming Potential In Vitro
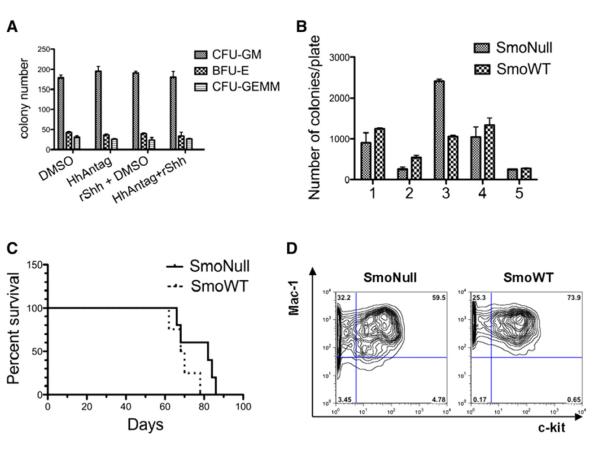
These genetic studies collectively indicate that Hh signaling plays a nominal role in normal adult hematopoietic development, maintenance, or stress response. These findings would suggest that systemic pharmacologic inhibition of the Hedgehog pathway, as a therapeutic approach for a spectrum of solid tumors, would be expected to have minimal hematopoietic toxicity. To formally test this hypothesis, we assessed the effect of pharmacologic inhibition of Hh on the hematopoietic system in vitro and in vivo. There are several low-affinity pharmacologic inhibitors of Hh signaling, such as cyclopamine, but it is difficult to ascertain the consequences of off-target effects at the high micromolar concentrations required for Hh inhibition (Taipale and Beachy, 2001). For this reason, we tested a high-affinity highly selective Hh antagonist (HhAntag) (Yauch et al., 2008), as well as a recombinant Sonic Hedgehog agonist (rShh), in hematopoietic colony-forming assays. We treated normal murine whole bone marrow cells with inhibitor HhAntag at a concentration ~7500-fold higher than the IC50 for cellular inhibition of Hh signaling alone, and rShh ligand at 100 ng/ml. We observed no effect on hematopoietic colony-forming (CFU-GM, BFU-E, and CFU-GEMM) potential for these agents, either alone or in combination (Figure 5A).
Figure 5. Loss of Smo Is Dispensable for MLL-AF9-Mediated Serial Replating Activity and Leukemogenesis; and Pharmacological Inhibition of Hh Signaling with HhAntag Has No Effect on Colony-Forming Potential.
(A) Modulation of the Hedgehog signaling does not impact colony formation. Normal murine bone marrow cells (1.3 × 105) per condition were plated in methylcellulose supplemented with SCF, IL-3, IL-6, and erythropoietin in the presence or absence of rSHH and/or the hedgehog antagonist HhAntag. There was no significant difference in CFU-GM, BFU-E, or CFU-GEMM when comparing vehicle versus HhAntag versus rShh agonist versus HhAntag + rShh (n = 3 per group, p = 0.5741, 0.6338, and 0.6935, respectively; one-way ANOVA test). Error bars represent SEM.
(B) SmoNull or SmoWT bone marrow cells were retrovirally transduced with MSCV-MLL-AF9/GFP and then serially replated on methylcellulose media for five rounds. Over the course of five rounds of replating, there was no significant difference in the absolute number of colonies observed (n = 7, p = 0.2424 using an unpaired t test). Error bars represent SD.
(C) SmoNull or SmoWT bone marrow cells retrovirally transduced with MLL-AF9/GFP were transplanted into lethally irradiated B6.SJL WT recipient mice. No significant difference in the disease latency or overall survival of SmoNull compared to SmoWT mice was noted (n = 5, p = 0.1448, log rank test). Results are shown in a Kaplan-Meier survival curve.
(D) Flow cytometric analysis demonstrating no difference in immunophenotype of Mac-1 and c-kit double-positive AML cells in SmoNull compared to SmoWT samples.
HhAntag or rShh Agonist Does Not Affect Blood Cell Counts In Vivo
To confirm these findings in vivo, we treated mice for 3 weeks twice daily by oral gavage with 100 mg/kg HhAntag or its vehicle, a dosing regimen that results in continuous in vivo suppression of the Hh pathway (Yauch et al., 2008). We confirmed a level of HhAntag of 4.1 ± 1.3 μM at 6 hr after oral dosing and a concomitant 2.3 ± 0.2-fold pharmacodynamic reduction in Gli1 expression in lung as a surrogate tissue. No significant differences in white blood cells, neutrophils, lymphocytes, or monocytes were noted in treated mice despite continued blockade of the Hh pathway (Table 1), although a slight reduction in hemoglobin was observed that reached statistical significance.
Table 1.
Hematologic Analysis of HhAntag-Treated Mice Following 21 Days of Continuous Treatment
| Treatment | Absolute Cell Count (1 × 103 Cells) | g/dL | % | |||||
|---|---|---|---|---|---|---|---|---|
| WBC | NEUT | LYMPH | MONO | RBC | HGB | HCT | ||
| MCT | Mean | 9.66 | 0.77 | 8.31 | 0.19 | 10.08 | 15.00 | 44.70 |
| Stdev | 0.84 | 0.13 | 0.96 | 0.23 | 0.39 | 0.53 | 2.00 | |
| HhAntag | Mean | 8.51 | 0.70 | 7.53 | 0.11 | 9.30 | 13.92 | 41.82 |
| Stdev | 0.71 | 0.24 | 0.74 | 0.08 | 0.42a | 0.55a | 1.58 | |
WBC, white blood cell; NEUT, neutrophil; LYMPH, lymphocyte; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit.
p < 0.05; unpaired t test.
Hh Signaling Is Dispensable for MLL-AF9-Mediated Leukemogenesis
These data convincingly demonstrate that the Hh pathway does not appear to play a central role in normal adult hematopoietic development, or at a minimum that there are highly redundant functional pathways that can compensate for either genetic or pharmacologic inhibition of Hh signaling in the hematopoietic system. However, these findings do not discount the possibility that Hh is important in leukemogenesis. Indeed, the limited literature available suggests that Hh may play an important role in hematologic malignancies. For example, Hh ligand promotes expansion, but not differentiation, of tumor stem cells in multiple myeloma (MM), whereas blockade of the Hh pathway inhibits clonal expansion of purified MM stem cells (Peacock et al., 2007). Hh derived from BM stroma acts as a survival factor for murine B cell lymphoma cells, and blockade of Hh signaling inhibits lymphoma growth in vivo (Dierks et al., 2007). Recent reports also indicate that mantle cell lymphoma cells show impaired proliferation after exposure to cyclopamine (Hegde et al., 2008) and that deregulation in Shh signaling appears to be associated with chronic myeloid leukemia (CML) progression (Sengupta et al., 2007). In addition, it has been reported that Hh signaling may play an important role in maintenance of leukemia stem cells in a murine model of CML-like disease (Dierks et al., 2008).
To characterize the role of the Hh pathway in leukemogenesis, we utilized in vitro serial replating assays and in vivo assays of leukemogenesis. Whole bone marrow derived from SmoNull or SmoWT animals was transduced with retrovirus harboring the leukemia-associated disease allele MLL-AF9, which confers serial replating potential in methylcellulose in the absence of stroma as an in vitro surrogate for leukemia stem cell activity (Krivtsov et al., 2006; Somervaille and Cleary, 2006). We observed no differences in replating potential for MLL-AF9-transduced cells when comparing bone marrow cells derived from Smo-deficient versus Smo wild-type animals (Figure 5B). We also performed in vivo analysis of leukemogenesis for the MLL-AF9 allele to assess the effects of loss of function of Smo. Bone marrow cells derived from SmoNull or SmoWT animals were transduced with MLL-AF9 retrovirus and transplanted into lethally irradiated wild-type recipient mice, leading to development of an acute myeloid leukemia (AML). Based on the Bethesda classification for nonlymphoid hematopoietic neoplasms in mice, the disease is best classified as monocytic leukemia (Kogan et al., 2002). Expression of dim Gr-1, and variable-to-bright Mac-1 (CD11b), is supportive of this diagnosis (data not shown). We observed no statistically significant differences in disease penetrance, latency, or phenotype between the two groups, with development of fully penetrant acute monocytic leukemia in recipients of MLL-AF9-transduced SmoNull versus SmoWT bone marrow (Figures 5C and 5D). To confirm that the MLL-AF9 leukemias could not be attributed to a high degree of selective advantage from transduction of rare unexcised cells, we demonstrated complete excision of Smo in MLL-AF9 leukemia cells (data not shown). These data indicate that Hh is dispensable for the development of AML mediated by MLL-AF9. However, it is important to note that other leukemogenic alleles should be tested in this system. Furthermore, these findings do not exclude the possibility that AMLs in humans might be sensitive to pharmacologic inhibition of Hh signaling. However, they do indicate that the pathway is not requisite for leukemogenic potential of an MLL fusion gene.
DISCUSSION
Taken together, these genetic and pharmacologic data support the unexpected conclusion that the Hedgehog pathway is not required for hematopoietic homeostasis in the adult mouse. These findings do not necessarily mitigate against findings from other investigators indicating that Hh signaling may play a role in proliferation of primitive human HSC through BMP regulation (Bhardwaj et al., 2001) or in modulation of cell cycle in regulation of HSC regeneration (Trowbridge et al., 2006). Our data would, however, indicate that Hh signaling is not required for these functions and that redundant mechanisms may be in play. The fact that Smo and Ptch1 are expressed in LSK and myeloid progenitors, but that their downstream transcription factor targets Gli1, Gli2, and Gli3 are not detectable (Gao et al., 2009 [this issue of Cell Stem Cell]), supports the hypothesis that Hh pathway components are expressed in the hematopoietic system but that the pathway is inactive in the adult murine hematopoietic system, given the lack of Gli expression.
This disparity between requirements during development compared with adult hematopoiesis is not without precedent. For example, the stem cell leukemia (SCL/tal-1) gene is essential for primitive hematopoiesis but is dispensable for HSC engraftment, self-renewal, and differentiation in the adult (Mikkola et al., 2003). On the other end of the spectrum, Etv6 (Tel) is not required for establishment of definitive hematopoiesis during development but is essential for maintenance of the HSC compartment in the adult (Hock et al., 2004). Smo is another example of genes that may play differential roles in developmental versus adult hematopoiesis.
Our data showing no effect on T cell development in the adult also vary from literature reports showing a defect in T cell development (El Andaloussi et al., 2006). The previous report assessed T cells derived from thymi of neonates 2 weeks after excision with pIpC, whereas we assessed T cells in adult mice 4–6 weeks after birth and then ~4 weeks after pIpC injection. These data might also suggest a differential requirement for Hh signaling during development compared with adult life. Finally, these findings vary from that observed in zebrafish where Hh signaling has been shown, using genetic strategies, to be requisite for adult blood stem cell formation (Gering and Patient, 2005). This difference may be a species-specific difference or may reflect a differential requirement for Hh signaling in the hematopioetic system during development compared with adult hematopoiesis.
Very recent data suggest that Hh signaling may play an important role in leukemia stem cell function in a BCR-ABL-induced model of murine hematopoietic disease, based in part on use of cyclopamine analogs (Dierks et al., 2008; Zhao et al., 2009). Although we observe no impact in MLL-AF9-mediated leukemogenesis in the absence of Smo, suggesting that the pathway does not play a critical role in initiation of leukemia, it remains plausible that Hh signaling could be effectively targeted in leukemias that arise de novo, or that there are allele-specific differences in requirement for Hedgehog signaling. For example, activated kinases like BCR-ABL that do not have the potential for activating self-renewal programs in stem cells may be more reliant on these pathways than alleles such as MLL fusion genes that can confer properties of leukemia stem cells to committed progenitors (Cozzio et al., 2003; Huntly et al., 2004; Krivtsov et al., 2006).
A recent publication from Zhao et al. indicates that Hh signaling plays an important role in hematopoietic stem cell renewal and in maintenance of cancer stem cells in CML (Zhao et al., 2009). The disparity in the stem cell phenotype between this report and the extensive in vitro and in vivo studies reported herein and independently by Gao et al. (2009) may be explained by methodological differences. These include the use of a nonconditional Vav-Cre allele that would be predicted to result in Smo loss during embryonic development, in contrast with conditional excision in the adult hematopoietic compartment induced by pIpC with the Mx1-Cre allele. In addition, the use of mixed background for competitive repopulation assays in the Zhao study compared with fully backcrossed C57/Bl6 in our study could influence competitive repopulation potential based on minor histocompatibility differences, among other possibilities.
Lastly, these findings do indicate that pharmacologic inhibition of the Hh pathway for the treatment of life-threatening diseases such as pancreatic cancer, colorectal cancer, and metastatic basal cell carcinoma should not be met with unmanageable hematopoietic toxicities.
EXPERIMENTAL PROCEDURES
CFU Assay
Bone marrow cells were plated in methylcellulose medium (MethoCult3434; Stem Cell Technologies) at a density of 20,000 cells/ml media according to the manufacturer’s instructions. Differential colony counts were scored 7–10 days after plating. For the serial replating assay, SmoNull or SmoWT bone marrow was retrovirally transduced with MLL-AF9 MSCV-GFP as previously described. Whole bone marrow cells were then plated at a concentration of 20,000 cells/mL. Colonies were counted after 7 days, removed from the plate by dilution with RPMI containing 10% FBS and 1% penicillin/streptomycin, washed in PBS with 2% FBS, and replated onto a new plate for up to five rounds.
For the pharmacological experiments, hematopoietic colony assays were carried out in complete methylcellulose medium (MethoCult GF, Stem Cell Technologies) according to the instructions of the manufacturer in the presence of vehicle, rSHH (final concentration 100 ng/ml), and/or the Hedgehog pathway antagonist HhAntag (final concentration 300 nM). Colonies were scored on day 8 after plating, and the experiment was carried out in triplicate.
Cell Staining and Flow Cytometry Analysis
The contribution of definitive hematopoietic cells of bone marrow, spleen, and peripheral blood was verified by staining with monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), allophycocyanin (APC), phycoerythrin (PE), or peridinin-chlorophyll-protein complex (perCP), all from BD Biosciences. The following antibodies were used: CD45, CD3, B220, CD4, CD8, Gr-1, Mac-1, c-kit, CD34, CD41, Ter119, CD45.1, and CD45.2. Surface marker expression was visualized with a FACSCalibur (Becton Dickinson) and was analyzed with FlowJo software. Stem and progenitor cell population analysis was done by lineage depletion of whole bone marrow cells using unconjugated murine lineage antibodies CD3, CD4, CD8, Gr-1, B220/CD45R, CD19, IL-7R, or Ter119, which were labeled using goat anti-rat PECy5. The following antibodies were used for the LSK compartment (containing long-term and short-term HSCs) and progenitor staining: CD34, FcRgII, c-kit, and Sca-1. LSK cells were defined by Sca-1+ and c-kit+ expression. Progenitor cells were defined by CD34+, FcRgII dim (CMP), CD34 dim, FcRgII dim (MEP), and CD34+, FcRgII+(GMP). Flow analysis for LSK or progenitor cells populations was done by using FACSAria (Becton Dickinson) and analyzed as above. For cell-cycle analysis, Pyronin Y and Hoescht staining were used as described elsewhere (Cheng et al., 2000).
Mouse Strains
Smoothened conditional knockout mice (Smotm2Amc), noted as SmoC/C (Long et al., 2001), were obtained from Jackson Laboratories. Mice were housed in a sterile barrier facility approved by the IUCAC at Children’s Hospital Boston. SmoC/C mice were backcrossed to C57/Bl6 for eight generations and then crossed with Mx1-Cre, also in a C57/Bl6 background (Kuhn et al., 1995).
Genotyping PCR
Routine PCR genotyping of the Smo alleles was performed on tail DNA by using a Promega PCR Master Mix kit. The total PCR volume was 13 μl, including 1 μlof 1:10 diluted tail DNA. The following primers were used to amplify the Smo floxed target gene: Smo-1 forward, 5′-GCAAGCTCGTGCTCTGGTC-3′; and Smo-2 reverse, 5′-CCGGTGGATGTGGAATGTG-3′, which generated a 250 bp band. For the Mx1-Cre genotyping, QIAGEN PCR Core kit was used. The primers used were Cre reverse, 5′-ACGACCGGCAAACGGACAGAAGCA-3′; and Mx1 forward, 5′-CCCAACCTCAGTACCAAGCCAAG-3′. The total PCR volume was 25 μl, including 1 μl of undiluted tail DNA.
Induction of Mx1-Cre Expression with pIpC
Animals were treated with polyinosine-polycytidylic acid (pIpC, Sigma) by intraperitoneal (i.p.) injection at ~10 weeks of age. Doses up to 25 μg/g mouse weight (or maximum dose 600 μg, whichever was less) were given every other day for a total of three doses. Mice were analyzed between 1 and 18 months after the first injection.
Detection of SmoC Excision
DNA was isolated from whole peripheral blood or from frozen single-cell suspensions of spleen, thymus, and bone marrow using the QIAamp DNA blood mini kit or tissue kit (QIAGEN). DNA was quantified and diluted to a standard concentration of 25 ng for PCR.
Semiquantitative Three-Primer PCR for Excision Analysis
A three-primer PCR was performed with the following primers: common reverse primer (CCATCACGTCGAACTCCTGGC) at a 1× concentration in the PCR reaction; forward primer for amplification of nonexcised (“floxed”) SmoC allele (CCGATTCGCAGCGCAT) at a 0.5× concentration; and forward primer for amplification of excised SmoC allele (GGCCTGCGCTGCTCAACA TGG) at a 0.5× concentration. Annealing temperature was 60°C.
Mouse Analysis and Organ Collection
Peripheral blood was collected from the retroorbital cavity using a heparinized glass capillary tube. Complete peripheral blood count analysis including a differential blood count was obtained by using Hemavet (Drew Scientific). For histological analysis, peripheral blood smears were stained with a standard Wright-Giemsa stain. Mice were euthanized, and all relevant organs were collected, fixed in 10% formalin, and subsequently paraffin embedded. Histological sections (4 μm) were stained by hematoxylin and eosin (H&E) in a histopathology core facility (Brigham and Women’s Hospital).
Bone Marrow Transplant Experiments
For the competitive bone marrow transplant experiment, SmoWT/CD45.2 or SmoNull/CD45.2 donor bone marrow was transplanted in various ratios together with a competitor WT bone marrow from B6.SJL F1CD45.1/CD45.2 mice into B6.SJLCD45.1 recipients. A total of 2 × 106 cells were transplanted with donor to competitor ratios of 1:0, 3:1, 1:1, 1:3, and 0:1, respectively. This approach enabled differentiation between donor, competitor, and recipient bone marrow populations using flow cytometry analysis with fluorochrome-labeled antibodies for CD45.1 or CD45.2. Helper bone marrow cells were generated by crossing C57/Bl6 and SJL mice and using offspring from the first generation that were CD45.1/CD45.2 double positive.
For retroviral bone marrow transplant experiments, SmoNull or SmoWT B6 F8 mice were injected i.p. with pIpC 25 μg/g every other day for three doses. Three weeks after the first pIpC injection, peripheral blood was collected. DNA was extracted to confirm Smo excision by using the three-primer PCR technique previously described. Mice were then treated with one dose of intraperitoneal 5-FU 150 mg/kg on day −8. Bone marrow cells were harvested on day −2 and “spin infected” twice with a MSCV retrovirus expressing MLL-AF9/GFP (kindly provided by Dr. S. Armstrong) (Krivtsov et al., 2006; Scholl et al., 2007). Retrovirally transduced SmoNull or SmoWT bone marrow was then transplanted into lethally irradiated B6.SJL WT recipient mice. A subset of bone marrow cells was used for serial replating on methylcellulose as described above. Peripheral blood was collected 4 weeks after transplantation to evaluate peripheral blood counts. Mice were euthanized at the clinical onset of leukemia determined by signs of distress, such as decreased activity and hepatosplenomegaly. Peripheral blood and all relevant mouse organs were collected as described above and used for comprehensive analysis, including CBC with differential, organ weight, histopathology, and flow cytometric analysis.
Serial 5-FU Experiment
5-FU was administered weekly at a dose of 150 mg/kg i.p. to SmoNull or SmoWT mice. Sequential blood counts prior to 5-FU therapy (day 1) and on days 5, 7, 11, and 14 were obtained, and survival was monitored.
Pharmacological Experiments with HhAntag
Female 6- to 8-week-old C57/BL6 mice were obtained from Charles River Laboratories (Wilmington, MA). All mice were housed and maintained according to the animal use guidelines of Genentech, Inc., conforming to State of California legal and ethical practices. Mice were treated with either control (MCT; 0.5% methyl cellulose, 0.2% tween-80 at 10 ml/kg) or 100 mg/kg HhAntag (10 mg/ml solution in MCT) twice daily for 21 days by oral gavage and blood collected 6 hr following the final dose by cardiac puncture for analysis. Analysis was performed on a Cell Dyn 3700 System from Abbott Laboratories, Inc. (Abbott Park, IL).
RT-PCR
Hh pathway genes were quantitatively assessed in frozen tissues by TaqMan using standard techniques. Transcript levels were normalized to the ribosomal protein L19 (RPL19) and results expressed as normalized expression values (= 2 – ΔCt).
Determination of HhAntag Concentration in Plasma
HhAntag was detected in plasma following oral administration 6 hr following the last daily dose. Whole blood was collected via cardiac puncture, placed in heparinized Eppendorf tubes on ice, and centrifuged at 10,000 × g for 4 min. The plasma supernatant was added to internal standard solution, mixed by vortexing, and centrifuged. Sample extract was assayed for HhAntag by LC/MS/MS analysis. Plasma levels were calculated using an authentic standard curve.
Supplementary Material
ACKNOWLEDGMENTS
We thank Emily Chan, Leslie Lee, and Maria Pitsiouni from Genentech and Allison Coburn, Elizabeth McDowell, and Sandra Moore from the Gilliland lab for technical support; and Thomas Mercher, Glen Raffel, Zuzanna Tothova, Claudia Scholl, and Stefan Frohling for valuable discussion. This work was supported in part by the Leukemia and Lymphoma Society and by National Institutes of Health (NIH) grants (D.G.G., E.H.S.). D.G.G is an Investigator of the Howard Hughes Medical Institute. Work in A.P.M.’s laboratory was supported in part by a grant from the NIH/National Institute of Neurological Disorders and Stroke (NINDS), R37NS033642. E.H.S. and I.H.Z. designed and performed research, analyzed data, and wrote the manuscript. F.J.d.S., N.G., and S.G. designed and performed research and analyzed data on the pharmacological experiments, participated in interpretation of data, and reviewed the manuscript. J.M. and A.P.M. generated the Smo mice, participated in design and interpretation of experiments, and reviewed the manuscript. S.A.A. participated in design and interpretation of experiments involving the MLL-AF9 fusion. D.G.G. designed research, analyzed data, and revised the paper. F.J.d.S., N.G., and S.G. are employees of Genentech, Inc. A.P.M. is a consultant for Merck.
Footnotes
SUPPLEMENTAL DATA Supplemental Data include three figures and can be found with this article online at http://www.cell.com/cell-stem-cell/supplemental/S1934-5909(09)00152-0.
REFERENCES
- Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat. Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Scott MP. A mouse model for medulloblastoma and basal cell nevus syndrome. J. Neurooncol. 2001;53:307–318. doi: 10.1023/a:1012260318979. [DOI] [PubMed] [Google Scholar]
- Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer K, Walker AN, Jenkins TM, Steele TA, Dannawi H. Erythroid differentiation in vitro is blocked by cyclopamine, an inhibitor of hedgehog signaling. Blood Cells Mol. Dis. 2000;26:360–372. doi: 10.1006/bcmd.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, Veelken H, Engelhardt M, Mertelsmann R, Kelleher JF, et al. Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat. Med. 2007;13:944–951. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- El Andaloussi A, Graves S, Meng F, Mandal M, Mashayekhi M, Aifantis I. Hedgehog signaling controls thymocyte progenitor homeostasis and differentiation in the thymus. Nat. Immunol. 2006;7:418–426. doi: 10.1038/ni1313. [DOI] [PubMed] [Google Scholar]
- Gao J, Graves S, Koch U, Liu S, Jankovic V, Buonamici S, El Andaloussi A, Nimer S, Kee BL, Taichman R, Radtke F, Aifantis I. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4(this issue):548–558. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev. Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- Hegde GV, Munger CM, Emanuel K, Joshi AD, Greiner TC, Weisen-burger DD, Vose JM, Joshi SS. Targeting of sonic hedgehog-GLI signaling: a potential strategy to improve therapy for mantle cell lymphoma. Mol. Cancer Ther. 2008;7:1450–1460. doi: 10.1158/1535-7163.MCT-07-2118. [DOI] [PubMed] [Google Scholar]
- Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, Orkin SH. Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–2341. doi: 10.1101/gad.1239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N, Rowan R, Amaral S, Curley D, Williams IR, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr., et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- Kogan SC, Ward JM, Anver MR, Berman JJ, Brayton C, Cardiff RD, Carter JS, de Coronado S, Downing JR, Fredrickson TN, et al. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood. 2002;100:238–245. doi: 10.1182/blood.v100.1.238. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature. 2003;421:547–551. doi: 10.1038/nature01345. [DOI] [PubMed] [Google Scholar]
- Outram SV, Varas A, Pepicelli CV, Crompton T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13:187–197. doi: 10.1016/s1074-7613(00)00019-4. [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl. Acad. Sci. USA. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- Sacedon R, Diez B, Nunez V, Hernandez-Lopez C, Gutierrez-Frias C, Cejalvo T, Outram SV, Crompton T, Zapata AG, Vicente A, et al. Sonic hedgehog is produced by follicular dendritic cells and protects germinal center B cells from apoptosis. J. Immunol. 2005;174:1456–1461. doi: 10.4049/jimmunol.174.3.1456. [DOI] [PubMed] [Google Scholar]
- Scholl C, Bansal D, Dohner K, Eiwen K, Huntly BJ, Lee BH, Rucker FG, Schlenk RF, Bullinger L, Dohner H, et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J. Clin. Invest. 2007;117:1037–1048. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Banerjee D, Chandra S, Banerji SK, Ghosh R, Roy R, Banerjee S. Deregulation and cross talk among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic myeloid leukemia progression. Leukemia. 2007;21:949–955. doi: 10.1038/sj.leu.2404657. [DOI] [PubMed] [Google Scholar]
- Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Trowbridge JJ, Scott MP, Bhatia M. Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc. Natl. Acad. Sci. USA. 2006;103:14134–14139. doi: 10.1073/pnas.0604568103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhmann A, Dittmann K, Nitzki F, Dressel R, Koleva M, Frommhold A, Zibat A, Binder C, Adham I, Nitsche M, et al. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.