Abstract
This study explored the correspondence between implicit memory and the reactivation of encoding‐related brain regions. By using a classification method, we examined whether reactivation reflects only the similarities between study and test or voxels at the reactivated regions are diagnostic of facilitation in the implicit memory task. A simple detection task served as incidental encoding of object–location pairings. A subsequent visual search task served as the indirect (implicit) test of memory. Subjects did not know that their memory would be tested. Half of the subjects were unaware that some stimuli in the search task are the same as those that had appeared during the detection task. Another group of subjects was made aware of this relationship at the onset of the visual search task. Memory performance was superior for the study‐test aware, compared to study‐test unaware, subjects. Brain reactivation was calculated using a conjunction analysis implemented through overlaying the neural activity at encoding and testing. The conjunction analysis revealed that implicit memory in both groups of subjects was associated with reactivation of parietal and occipital brain regions. We were able to classify study‐test aware and study‐test unaware subjects based on the per‐voxel reactivation values representing the neural dynamics between encoding and test. The classification results indicate that neural dynamics between encoding and test accounts for the differences in implicit memory. Overall, our study demonstrates that implicit memory performance requires and depends upon reactivation of encoding‐related brain regions. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: implicit memory, incidental learning, reactivation, object locations, fMRI, SMLR classifier
INTRODUCTION
Memory performance may depend on subjects' ability to reconstruct the context of the encoding episode [e.g., Damasio, 1989; Kahn et al., 2004]. Recent neuroimaging studies supported this hypothesis by showing reactivation of encoding‐related brain regions during explicit retrieval [Habib, 2001; Johnson and Rugg, 2007; Kahn et al., 2004; Nyberg et al., 2000, 2001; Persson and Nyberg, 2000; Vaidya et al., 2002; Wheeler et al., 2000, 2006; Woodruff et al., 2005]. For example, words that were encoded with a sound context tended to reactivate the auditory cortex even though at test the words were presented without a sound context heard earlier [Nyberg et al., 2000]. Probe words that had been studied as pictures induced larger activity in the left fusiform gyrus, region critical for object processing [e.g., Köhler et al., 1998b], compared to probe words previously studied as words [Vaidya et al., 2002]. Probe words studied within a spatial context elicited greater neural activity at test in parietal and parahippocampal cortices compared to probe words studied within a nonspatial context [Kahn et al., 2004; Persson and Nyberg, 2000]. Because parietal and parahippocampal cortices are crucial for spatial processing [e.g., Rizzolatti and Matelli, 2003; Sommer et al., 2005], their activation during retrieval may indicate the reinstatement of spatial context originally presented with the words.
Explicit memory tests require subjects to consciously reconstruct previous experiences. Implicit memory tasks by definition test memory indirectly (e.g., by examining the effect of a previous exposure on performance in a task requiring subjects to identify or search for objects). If performance is facilitated (e.g., faster search), this is evidence of behavioral repetition priming [e.g., Maljkovic and Nakayama, 1994; Richardson‐Klavehn et al., 1994; Tulving and Schacter, 1990]. The finding that explicit memory increases neural activity in the task‐specific brain regions [Buckner et al., 1996; Cabeza and Nyberg, 1997], while implicit memory decreases it [Buckner et al., 1998; Grill‐Spector et al., 2006; Henson, 2003; Schacter and Buckner, 1998; Wig et al., 2005] has been taken as additional evidence for separate explicit and implicit memory systems [e.g., Schacter and Tulving, 1994].
While reactivation studies focus mostly on explicit memory tests, implicit memory tests may also involve reactivation processes. This issue, however, has not been directly addressed in previous research. The goal of our study was to investigate whether and how memory performance depends on the reactivation of encoding‐related brain regions during implicit memory testing. Arguably, the voxels in the reactivated brain regions may not only represent the similarities between study and test phases but also be diagnostic for memory performance. We used a classification method of data analysis to test whether subjects with a high level of performance on an implicit memory test may be distinguished from subjects with a low level of performance based on the patterns of reactivation.
Participants were scanned during both study and test phases. During the study phase, subjects incidentally learned objects and their spatial locations while doing an object detection task. During the test phase, subjects' implicit memory for object location was indirectly tested using a visual search task. Participants were asked to search for a target object on a 4 × 4 grid among 12 other objects. Objects were either old (appeared previously in the object detection task) or new. Old objects were either presented in the same locations as in the object detection task or in new locations.
Although implicit memory tests are extensively used in behavioral and neuroscience research, sometimes their validity is questioned. The major concern is the possibility that implicit performance may be “contaminated” by the use of explicit recall or explicit retrieval strategies [e.g., Beauregard et al., 1999, Bowers and Schacter, 1990]. One way to address this concern is to create experimental conditions that limit subjects' explicit memory for the stimuli. Our previous behavioral study [Manelis, 2009], which also used the object detection task in the study phase, showed that explicit memory after incidental learning is limited. In that study, subjects who encoded object locations intentionally were able to recall about 45% of locations in the cued recall task. In contrast, incidental learning subjects recalled only about 15% of object locations. Thus, to limit subjects' ability to explicitly recall object locations in this fMRI study, we assigned all subjects to the incidental learning condition.
Unlike explicit memory tests that allow a contrast of recalled and forgotten items, implicit memory tests often lack this opportunity. To contrast different levels of implicit performance, we assigned participants to the study‐test aware and study‐test unaware conditions. Previous studies have shown that the performance on associative, semantic, and spatial implicit memory tests is greater for study‐test aware participants compared to study‐test unaware participants [Bowers and Schacter, 1990; Mace, 2003b; Manelis, 2009; Richardson‐Klavehn et al., 1994]. We informed study‐test aware subjects that during the search task they are going to see the stimuli that appeared previously during the object detection task. Study‐test unaware subjects were not informed about the study‐test relationship. The instructions were given right before the onset of the visual search task.
The reactivation of encoding‐related brain regions during implicit memory test was investigated using a conjunction analysis implemented through inclusive masking [Johnson and Rugg, 2007]. Using this method we tested four possible patterns of reactivation: (1) brain regions that activated during encoding also activated during the testing, (2) brain regions deactivated during encoding also deactivated during implicit memory testing, (3) brain regions that deactivated during encoding activated during testing, and (4) brain regions that activated during encoding deactivated during testing. The first pattern of reactivation will closely resemble the reactivation patterns reported by the explicit memory studies; such similarity may indicate that implicit and explicit memory tests rely on the similar mechanisms of neural reactivation. The second pattern of reactivation would indicate that implicit memory tests reactivate encoding‐related brain regions, but in a different way than explicit memory tests. One study supported this hypothesis by reporting deactivation of left fusiform and bilateral inferior frontal gyri during both stimulus encoding and implicit memory testing [Schott et al., 2006]. Neither third nor fourth patterns of reactivation have been previously reported in explicit or implicit memory studies suggesting a low probability for observing these patterns in our study.
Processing of object and spatial information during the object detection and the search tasks may recruit fusiform and parietal brain regions [Köhler et al., 1998b; Sala et al., 2003]. If implicit memory reactivates encoding‐related brain regions, then fusiform and parietal cortices are the candidate areas for reactivation to occur. While implicit memory is usually thought to be hippocampus‐independent [Cave and Squire, 1992; Knowlton and Squire, 1994], recent studies revealed the important role of the hippocampus for implicit spatial memory [Chun and Phelps, 1999; Park et al., 2004]. Therefore, the hippocampus may be another candidate area.
The reactivation patterns may be predictive for performance on the implicit memory task. We expected that memory performance would be greater for study‐test aware, compared to unaware, subjects. Consequently, study‐test aware and study‐test unaware subjects might be classified based on the reactivation patterns. This question was addressed using a classification method that examines “the statistical relationship between patterns of brain activity and the occurrence of particular experimental conditions” [O'Toole et al., 2007, p 1736]. Unlike the traditional univariate analyses [e.g., general linear model (GLM)] treating each voxel as independent from other voxels, classifiers are able to account for the multivariate nature of neuroimaging data. By detecting the patterns of activity across multiple voxels, classifiers are able to extract more information from the fMRI dataset than traditional univariate methods [Cox and Savoy, 2003; Formisano et al., 2008; Hanson and Halchenko, 2008; O'Toole et al., 2007]. The classification approach may be especially useful when the standard univariate methods fail to detect differences between two experimental conditions [Diana et al., 2008]. In addition, classifiers use cross‐validation to test for generalizability of the results. Cross‐validation is performed by dividing the dataset into training and testing subsets. First, the classifier is trained on the training subset. Then, it is tested on the testing subset. The higher the prediction accuracy, the more the classifier's results accurately generalize to previously unseen trials. Accurate classification indicates that certain patterns of neural activity are diagnostic of a subject being in a particular condition.
METHOD
Participants
Sixteen undergraduates (9 female, M = 19.9 years, SD = 2.9) from Rutgers University participated for course credit. Participants were randomly assigned either to the study‐test aware (n = 8) or to the study‐test unaware (n = 8) conditions. All subjects were treated in accordance with Rutgers University and University of Medicine and Dentistry of New Jersey Institutional Review Board guidelines.
Design and Procedure
To disguise the memory‐related nature of our study, we invited subjects to participate in the “Speed of Detection” experiment. Participants were told that the aim of this experiment was to reveal how fast people detect objects on the computer screen. They were asked to respond as quickly as possible during all tasks of the experiment. Subjects did not know that their memory was tested until the debriefing after the experiment.
Study phase
The study phase included a dot detection task and an object detection task. Each trial lasted for 4,000 ms. The trial started with the presentation of an empty matrix for either 400 or 800 ms followed by object presentation. Participants had to press the key of the MRI‐compatible track‐ball as quickly as possible to indicate the presence of the object. Response time (RT) was limited to 1,000 ms. The empty matrix was presented again after the stimulus was detected (ITI) such that the total length of trial was 4,000 ms (Fig. 1a).
Figure 1.
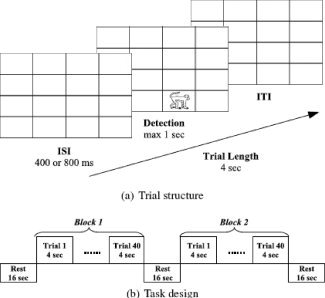
The design of the object detection task.
The scanning session began with two blocks of the dot detection task. Blocks consisted of 20 trials each and were preceded, separated, and followed by 16 s of rest. The dot detection task required subjects to respond as quickly as possible every time a small (0.5 × 0.5 in.2) white square (a dot) appeared on the screen. All dots were identical and were presented one at a time in a random location on a black background of a 4 × 4 invisible matrix. The frequency of stimulus presentation was the same for all spatial locations. Such stimulus uniformity was needed to persuade subjects that the only goal of our study was to examine their detection RT. The dot detection task was conducted only to establish a baseline measure of RT when subjects had no intention of memorizing object or spatial information. The neuroimaging data were not relevant to the goals of this study; therefore, we do not report them here.
After the dot detection task, subjects proceeded to the object detection task (see Fig. 1). During this task, all subjects incidentally learned spatial locations of real objects. Incidental learning was chosen to limit subjects' ability for explicit retrieval of object locations and to prevent subjects from verbalization of object and location information (i.e., “a monkey is in the lower right corner”). The object detection task consisted of two blocks of trials. Blocks consisted of 40 trials each and were preceded, separated, and followed by 16 s of rest (Fig. 1b). The object detection task required subjects to respond as quickly as possible every time when any object appeared on a screen. The objects were 16 black and white line drawings presented on a white background in one of 16 locations of the 4 × 4 matrix.
Repeated presentation of objects in constant locations leads to decrease in subjects' RT due to a priming effect [Maljkovic and Nakayama, 1996, 1994; Musen, 1996]. For this reason, objects that appeared in constant locations are referred to as primed. Eight other objects appeared in two to eight different locations that were not associated with the constant objects. These objects appeared once in each location and were introduced to disguise the real goal of the object detection task. For this reason, objects in variable locations are referred as foils. Fifty percent of trials were primed trials and 50% were foils. All trials were presented in random order. Each trial was analyzed as an event. While every object was presented several times during the object detection task, this article reports only the neuroimaging data for the last repetitions of primed stimuli because they directly preceded the implicit memory test. The full analysis of neuroimaging data in the object detection task will be reported elsewhere.
To make sure that subjects did not use more elaborate strategies in the object detection compared to the dot detection task, we compared subjects' RT on both tasks. If subjects did not exert any sufficient efforts for stimuli processing in the object detection task, we will detect no significant difference between dot detection RT and object detection RT.
Test phase
Subjects' implicit memory for object location was tested using a search task (see Fig. 2). The target was presented at the beginning of each trial and could be either an object that has already appeared during the object detection task or a new object. The search screen comprised 12 different objects, one of which was the target, that were placed along the outer border of the 4 × 4 matrix. Participants were asked to find and click on a target object as quickly as possible. To discourage subjects from selecting nontarget objects, the same search display was presented until participants found the correct object. This manipulation forced subjects' accuracy to be 100%. If, however, participants used more than one attempt to find the target, this trial was removed from the behavioral and neuroimaging data analysis.
Figure 2.
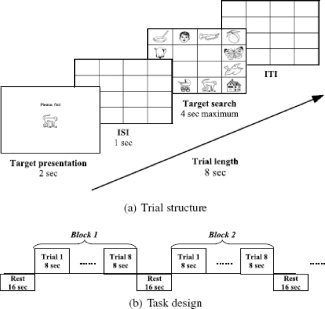
The design of the search task.
There were five types of trials in the search task. Primed trials were primed objects placed in the same locations as they appeared during the object detection task. Foil trials were foils placed in the same locations as they appeared during the object detection task. Unprimed trials were primed objects placed in the locations where foils appeared during the object detection task. Unprimed foil trials were foils placed in the locations where primed objects appeared during the object detection task. New trials were objects that never appeared during the object detection task. These objects could appear in any random location.
Eighteen blocks of the search task were preceded, separated, and followed by 16 s of rest (Fig. 2b). The first twelve blocks were composed of primed (37.5%) and foil trials (62.5%). During these blocks, each primed object appeared in its primed location six times. The last six blocks were composed of unprimed (25%), unprimed foil (50%), and new trials (25%). Trials were presented in a random order. Each trial was analyzed as an event.
Blocks consisted of eight trials each. Each trial in a block lasted for 8 s. The trial started with the presentation of a target object at the center of the computer screen for 2,000 ms followed by the presentation of an empty matrix for 1,000 ms. The search screen appeared after the empty matrix. The search screen was presented until subjects found a correct object but for no longer than 4,000 ms. After subjects responded, the empty matrix was presented again for the time needed to complete the 8,000‐ms time slot (ITI) (Fig. 2a).
To manipulate the implicit memory performance, we changed subjects' awareness of the relationship between the stimuli of the object detection task and that of the search task. After the object detection task but before the search task, subjects received either study‐test aware or study‐test unaware instructions. Study‐test aware subjects were informed that the stimuli that would be presented during the search task were the same as those presented during the object detection task. Study‐test unaware subjects were not informed about the relationship between the study and test stimuli. Participants in either test condition did not know that their memory would be tested during the search task. Moreover, all participants were reminded that they were to participate in the “Speed of Detection” study, and so they should respond as quickly as possible.
Implicit memory was indicated when search RT for the first repetition of primed trials was faster than for the first repetition of unprimed and new trials. Consistent with our previous findings [Manelis, 2009], we expected that primed trials would be significantly faster than unprimed trials and new trials in study‐test aware, but not in study‐test unaware, subjects. The first repetition of primed stimuli is the most important for the purposes of our study. First, these trials directly followed the stimulus encoding. Second, there was not learning effect from stimulus repetitions within the search task as it was for the stimuli repeated for the second, third, etc., times. In this article, we report only neuroimaging data for the primed stimuli presented for the first time in the search task. The analysis of neuroimaging data for the other types of trials will be reported elsewhere.
Image Acquisition
The fMRI experiment was conducted using a Siemens 3 T Allegra head‐only MR system. In all tasks, the stimulus presentation was synchronized with the image acquisition. In the beginning of the experiment, a high resolution structural image (TR = 2,000 ms, TE = 4.38 ms, slice thickness = 1 mm, FOV = 220, number of slices = 176, resolution = 0.8594 × 0.8594 × 1) was acquired using MPRAGE (a magnetization‐prepared rapid acquisition in gradient echo) sequence. Functional data (BOLD signal) were collected using a gradient echo, echo‐planar sequence (TR = 2,000 ms, TE = 30 ms, slice thickness = 4 mm, FOV = 220, number of slices = 32, resolution = 3.4375 × 3.4375 × 4.0). A total of 207 volumes were collected during the object detection task. A total of 800 volumes were collected during the search task for study‐test unaware subjects. A total of 830 volumes were collected during the search task for study‐test aware subjects. The search task was longer in the study‐test aware, compared to study‐test unaware, condition due to the longer set of instructions preceding the task. These differences could influence the performance of the classifier. Therefore, we removed volumes corresponding to the task instructions from both groups of subjects to have an equal number of volumes in the fMRI datasets for each condition.
fMRI Data Analysis
The images were processed and analyzed with FSL 4.0 (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl) software. On each raw BOLD dataset, nonlinear noise reduction [SUSAN (Smallest Univalue Segment Assimilating Nucleus)]; motion correction (MCFLIRT [Jenkinson et al., 2002]); non‐brain removal using BET [Smith, 2002]; spatial smoothing using a Gaussian kernel of FWHM 9 mm; multiplicative mean intensity normalization of the volume at each time point; and high‐pass temporal filtering (Gaussian‐weighted least‐squares straight line fitting, with sigma = 25.0 s) were applied. A hemodynamic response function was modeled using a Gamma function. The registration to high resolution structural (MPRAGE) and standard MNI (Montreal Neurological Institute) space images was carried out using FLIRT [Jenkinson and Smith, 2001; Jenkinson et al., 2002].
The FEAT (FMRI Expert Analysis Tool) was used for the first‐ and higher‐level analysis. The higher‐level analysis was carried out using ordinary least square mixed effects. Z‐statistics images were thresholded at P < 0.001 (uncorrected). Subjects' neural responses during the object detection and search tasks were analyzed using a GLM. Functional localization was determined using the Harvard‐Oxford cortical and subcortical structural atlases.
In the object detection task, we examined neural activity corresponding to the last repetition of primed stimuli. The first‐level analysis was used to compute the BOLD signal change on the last repetition of primed stimuli for every subject. The higher‐level analysis was used to compute the mean BOLD signal change associated with the last repetition of primed stimuli across all subjects.
In the search task, we analyzed subjects' neural activity during the first repetition of primed stimuli. A first‐level analysis computed the BOLD signal on these trials for every subject. A higher‐level analysis was used to compute the average neural activity for study‐test aware and study‐test unaware subjects. The higher‐level analysis also examined differences in neural activity between study‐test aware and study‐test unaware subjects on the first repetition of primed stimuli. In case the study‐test aware subjects have greater implicit memory than study‐test unaware subjects, the comparison of neural activity in these two groups will indicate the contrast between more and less successful implicit memory performance.
A conjunction analysis of encoding‐ and test‐related brain regions
We used a conjunction analysis to investigate whether and how brain areas involved during the last repetition of primed stimuli in the object detection task become re‐engaged during the first repetition of primed stimuli in the search task. The conjunction analysis was conducted using inclusive masking. First, the thresholded Z‐statistics images of encoding‐related and test‐related brain activity were binarized (Fig. 3a). Then, in each group of subjects, the encoding‐related and test‐related binarized images were added. The final image included only those brain regions whose neural activity passed threshold at encoding and at implicit memory testing (Fig. 3b).
Figure 3.
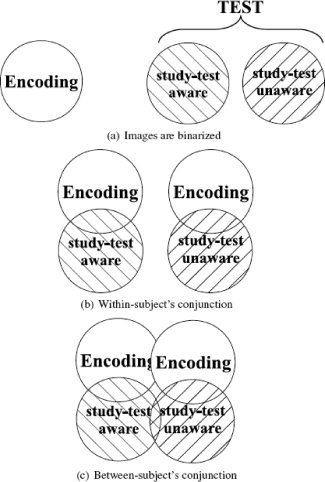
The conjunction analysis of encoding‐ and test‐related brain activity.
Four possibilities for the relationship between neural activity at encoding and testing were examined: encoding increase–test increase, encoding decrease–test decrease, encoding decrease–test increase, and encoding increase–test decrease. Each of these four resulting images were then subjected to a between‐subject conjunction analysis that revealed which brain areas were reactivated in both groups of subjects, and which brain areas were uniquely reactivated in one group of subjects (Fig. 3c).
A voxel‐based analysis of changes in neural activity between encoding and test
The first step in calculating reactivation values was to compute BOLD signal changes relative to the trial onset during the last primed trial of the object detection and the first primed trial of the search tasks. This analysis was implemented using the PyNIfTI module (http://niftilib.sourceforge.net/pynifti). PyNIfTI is a Python‐based software that provides an easy access to NIfTI images. For example, it allows computing the signal time course for conditions of interest for all voxels in a volume by averaging the time courses within specific experimental condition. Such computation may be implemented using the “pynifti_pst” (pst: peristimulus timecourse) script.
We used this script to calculate the percent BOLD signal change relative to the stimulus onset volume in trials of interest. The signal was extracted from the filtered functional data (nonlinear noise reduction, motion correction, spatial smoothing, intensity normalization, and high‐pass temporal filtering were applied) and averaged across three volumes in the object detection task (encoding) and four volumes in the search task (implicit memory test). The averaging across greater number of volumes in the search, compared to the object detection, task is explained by the longer trial latency in the former task. Reactivation values were calculated by subtracting the percent BOLD signal change during encoding from the percent BOLD signal change during implicit retrieval.
To examine whether reactivation values could predict implicit memory performance, we classified study‐test aware and study‐test unaware subjects using the sparse multinomial logistic regression (SMLR) [Krishnapuram et al., 2005]. The independent variables were reactivation values in voxels comprising the areas of conjunction. Our choice of the SMLR classifier was motivated by the ability of this classifier to optimize for performance by removing the least informative or redundant features (in our case, voxels) from the dataset.
The classification performance was optimized for the number of voxels in the activation pattern. The goal of optimization was to find the best classification performance with the largest amount of voxels (features) left in the dataset. The reason behind the searching for a model with the largest amount of features is to get a representation of all diagnostic voxels in the model (even if some of these voxels may be redundant for classification). Generalization of classification performance was tested by cross‐validating the model using a leave‐one‐out strategy. One subject from each group was “left out” for subsequent cross‐validation testing. The SMLR classifier was implemented through the PYMVPA (MultiVariate Pattern Analysis in Python) software (http://www.pymvpa.org). PYMVPA is a Python‐based framework for multivariate pattern analysis capable of running classification analysis of large datasets [Hanke et al., 2009a, b].
RESULTS
Behavioral Results
The analysis of behavioral data indicated no significant differences between RT in the dot detection (M = 292.35 ms, SD = 39.0) and object detection tasks (M = 297.13 ms, SD = 51.6) in the study phase, t(15) = 0.8, P = 0.43. These results suggest that subjects did not use more elaborate strategies to perform the object detection task compared to the dot detection task. In the test phase, subjects found 95% of target objects in the first attempt (SD = 0.03). The effect of individual object on search RT was examined using GLM with subjects' group as a between‐subject factor and object as a random factor. There was a significant main effect of object, F(25, 25) = 8.75, P < 0.001, but no significant main effect of group, F(1, 56.4) = 0.03, P = 0.86, and no group × object interaction, F(25, 2,064) = 1.3, P > 0.14. These findings suggest that although individual objects influenced subjects' performance, this effect was similar in both groups of subjects.
Subjects' search RT for all types of trials is presented in Table I. To focus on the main question of our study, we report here only statistics relevant to our question. Study‐test aware subject found primed stimuli significantly faster than study‐test unaware subjects on the first repetition trial (see Fig. 4), t(14) = 2.28, P = 0.039. Moreover, the priming effect was present in study‐test aware, but not in study‐test unaware, subjects. The search RT for the first repetition of primed stimuli was significantly faster than the search RT for the first repetition of unprimed stimuli, t(7) = −2.15, P = 0.035 (one‐tailed), and the first repetition of new stimuli, t(7) = −3.76, P = 0.007 (two‐tailed). It was also faster than search RT for foil stimuli; however, this effect did not reach statistical significance, t(7) = −1.5, P = 0.18. No priming effect was detected in study‐test unaware subjects. The search RT for the first repetition of primed stimuli was not different from the search RT for the first repetition of foils, t(7) = 0.12, P = 0.9, or from the first repetition of unprimed trials, t(7) = 1.1, P = 0.32, and was even slower than for the first repetition of new trials, t(7) = 2.4, P = 0.045.
Table I.
Search RT and standard errors of mean for all types of trial in the search task
| Trial type | Repetition | Study‐test unaware | Study‐test aware | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||
| Primed | 1 | 1,405.14 | 78.97 | 1,161.17 | 72.43 |
| Primed | 2 | 1,158.24 | 135.78 | 993.92 | 49.03 |
| Primed | 3 | 1,032.48 | 138.30 | 981.47 | 71.70 |
| Primed | 4 | 1,061.01 | 151.87 | 942.58 | 52.29 |
| Primed | 5 | 967.38 | 131.59 | 884.85 | 96.05 |
| Primed | 6 | 992.97 | 135.12 | 882.50 | 94.07 |
| Foil | 1 | 1,400.04 | 75.03 | 1,292.62 | 59.09 |
| Foil | 2 | 1,364.65 | 103.94 | 1,312.08 | 63.81 |
| Foil | 3 | 1,353.18 | 67.00 | 1,359.90 | 61.01 |
| Foil | 4 | 1,342.59 | 90.12 | 1,223.26 | 39.22 |
| Foil | 5 | 1,250.15 | 78.08 | 1,188.52 | 60.61 |
| Foil | 6 | 1,426.37 | 83.55 | 1,331.06 | 55.54 |
| Foil | 7 | 1,376.31 | 94.04 | 1,270.16 | 87.18 |
| Foil | 8 | 1,333.27 | 58.09 | 1,273.08 | 60.63 |
| Foil | 9 | 1,310.58 | 104.13 | 1,217.48 | 104.71 |
| Foil | 10 | 1,206.35 | 111.74 | 1,204.52 | 57.05 |
| Foil | 11 | 1,249.63 | 139.57 | 1,112.50 | 120.46 |
| Foil | 12 | 1,415.50 | 129.98 | 1,287.25 | 90.73 |
| Primed in new locations | 1 | 1,332.02 | 69.34 | 1,344.57 | 98.91 |
| Primed in new locations | 2 | 1,419.08 | 83.20 | 1,406.57 | 45.02 |
| Foils in new locations | 1 | 1,493.86 | 100.22 | 1,391.49 | 111.12 |
| Foils in new locations | 2 | 1,347.13 | 71.03 | 1,350.47 | 88.73 |
| New | 1 | 1,244.95 | 59.81 | 1,369.19 | 79.00 |
| New | 2 | 1,419.40 | 78.48 | 1,435.21 | 86.09 |
Figure 4.
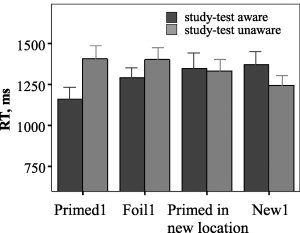
Search RT on the first repetitions of primed stimuli, foils, primed stimuli in new locations and new stimuli in study‐test aware and study‐test unaware groups of subjects.
Neuroimaging Results
Study phase
Relative to the baseline, the last repetition of primed objects in the object detection task increased neural activity in right middle frontal gyrus (MFG), right hippocampus, right thalamus, left superior frontal gyrus (SFG), left lateral occipital cortex (LOC) bordering with superior parietal lobule (SPL), bilateral inferior temporal gyrus (ITG), and bilateral occipital cortex that included occipital fusiform gyrus, lingual gyrus, and occipital pole. The neural activity decreased in right cuneus and precuneus, left lingual gyrus, and bilateral parahippocampal gyrus (Table II).
Table II.
Brain regions changed neural activity during the last repetition of primed stimuli in the object detection task
| Brain regions | Number of voxels | x | y | z | Z‐max | |
|---|---|---|---|---|---|---|
| Increase in BOLD signal | ||||||
| R | MFG | 78 | 38 | 1 | 51 | 4.80 |
| R | Precentral G. | 25 | 38 | −2 | 55 | 3.79 |
| R | Hippocampus | 22 | 28 | −10 | −26 | 3.41 |
| R | Thalamus | 19 | 14 | −24 | 7 | 3.50 |
| R | ITG, temporooc. | 33 | 50 | −40 | −16 | 3.78 |
| R | Temporal occipital fusiform C. | 15 | 46 | −48 | −20 | 3.47 |
| R | Occipital fusiform G. | 85 | 14 | −84 | −16 | 3.92 |
| L | Frontal Pole | 45 | −37 | 61 | 11 | 4.08 |
| L | SFG | 146 | −21 | 25 | 59 | 4.03 |
| L | ITG, anter. | 13 | −53 | −8 | −32 | 3.27 |
| L | SPL | 32 | −33 | −56 | 55 | 3.51 |
| L | LOC, super. | 144 | −35 | −64 | 61 | 3.75 |
| L | Lingual G. | 231 | −9 | −88 | −12 | 4.28 |
| L | Occipital Pole | 345 | −9 | −98 | −6 | 4.33 |
| Decrease in BOLD signal | ||||||
| R | Postcentral G. | 14 | 22 | −40 | 59 | 3.38 |
| R | Parahippocampal G., anter. | 11 | 14 | 3 | −22 | 4.19 |
| R | LOC, super. | 45 | 14 | −82 | 41 | 3.90 |
| R | Cuneal C. | 745 | 14 | −76 | 31 | 4.77 |
| R | Precuneus C. | 152 | 14 | −78 | 39 | 4.31 |
| R | Supracalcarine C. | 55 | 18 | −66 | 19 | 3.60 |
| L | Parahippocampal G., poster. | 14 | −15 | −40 | −10 | 4.02 |
| L | Intracalcarine C. | 48 | −9 | −78 | 19 | 3.84 |
| L | Lingual G. | 279 | −17 | −44 | −6 | 4.29 |
All coordinates are in MNI (Montreal Neurological Institute) template space.
G., gyrus; C., cortex; LOC, lateral occipital cortex; SPL, superior parietal lobule; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; R, right; L, left; anter., anterior; poster., posterior; super., superior; temporooc., temporooccipital.
Test phase
During the first repetition of primed stimuli, study‐test unaware subjects (Table III) activated right temporooccipital fusiform cortex; right inferior LOC; right insular cortex; small regions in right frontal cortex, left precentral gyrus, and left superior LOC; and large regions in left occipital cortex that included occipital fusiform and lingual gyri. The decreases were observed in right paracingulate gyrus; right central and parietal opercular cortices; right planum polare and temporale; right precentral gyrus; right posterior cingulate cortex and precuneus; small regions in left superior and middle temporal gyri; left insular cortex; left anterior cingulate gyrus; left postcentral gyrus; left hippocampus; and bilateral thalamus.
Table III.
Brain regions changed neural activity during the first repetition of primed stimuli in the search task: Study‐test unaware subjects
| Brain regions | Number of voxels | x | y | z | Z‐max | |
|---|---|---|---|---|---|---|
| Increase in BOLD signal | ||||||
| R | SFG | 26 | 4 | 13 | 55 | 3.73 |
| R | Insular C. | 81 | 32 | 21 | 5 | 4.20 |
| R | Frontal operculum C. | 11 | 34 | 23 | 7 | 3.49 |
| R | Temporal occipital fusiform C. | 249 | 42 | −44 | −26 | 4.67 |
| R | LOC, inferi. | 532 | 30 | −86 | −6 | 4.57 |
| L | Paracingulate G. | 195 | −3 | 11 | 49 | 4.66 |
| L | Juxtapositional L. (formerly SMC) | 71 | −5 | 9 | 51 | 3.99 |
| L | Putamen | 11 | −25 | 5 | 1 | 3.25 |
| L | MFG | 52 | −29 | −6 | 51 | 3.60 |
| L | Precentral G. | 767 | −39 | −12 | 65 | 4.19 |
| L | SPL | 57 | −29 | −58 | 55 | 4.11 |
| L | LOC, super. | 700 | −27 | −62 | 55 | 5.01 |
| L | ITG, temporooc. | 66 | −45 | −62 | −12 | 4.39 |
| L | Precuneus | 13 | −9 | −68 | 59 | 3.62 |
| L | Lingua G. | 865 | −9 | −78 | −10 | 5.06 |
| L | Occipital fusiform G. | 1,341 | −21 | −80 | −14 | 5.05 |
| L | Intracalcarine C. | 75 | −5 | −88 | −2 | 4.40 |
| L | Occipital pole | 1,309 | −5 | −92 | −4 | 4.84 |
| Decrease in BOLD signal | ||||||
| R | Frontal pole | 71 | 10 | 57 | 1 | 4.15 |
| R | Paracingulate G. | 232 | 12 | 55 | 3 | 4.4 |
| R | Frontal medial C. | 35 | 10 | 55 | −6 | 3.89 |
| R | Subcallosal C. | 36 | 6 | 15 | −12 | 3.52 |
| R | Juxtapositional L. (formerly SMC) | 40 | 10 | −14 | 49 | 4.00 |
| R | Planum temporale | 286 | 58 | −14 | 9 | 3.96 |
| R | Planum polare | 186 | 44 | −14 | −2 | 4.32 |
| R | Central opercular C. | 425 | 40 | −18 | 21 | 4.43 |
| R | Parietal operculum C. | 355 | 40 | −20 | 21 | 4.44 |
| R | Thalamus | 25 | 4 | −20 | 15 | 3.68 |
| R | Heschl's G. | 459 | 46 | −20 | 7 | 4.24 |
| R | Precentral G. | 313 | 10 | −24 | 49 | 4.66 |
| R | Supramarginal G., anter. | 217 | 64 | −28 | 43 | 4.29 |
| R | STG, poster. | 127 | 66 | −32 | 15 | 4.03 |
| R | SPL | 76 | 28 | −40 | 73 | 3.68 |
| R | Cingulate G., poster. | 466 | 8 | −52 | 23 | 4.61 |
| R | Precuneus | 1,126 | 10 | −54 | 21 | 4.85 |
| R | Cuneus | 90 | 4 | −72 | 31 | 4.19 |
| L | SFG | 23 | −19 | 35 | 45 | 3.53 |
| L | Temporal pole | 11 | −51 | 5 | −20 | 3.37 |
| L | STG, anter. | 70 | −51 | 1 | −20 | 3.78 |
| L | MTG, anter. | 61 | −53 | −4 | −22 | 4.06 |
| L | Cingulate G., anter. | 160 | −5 | −12 | 37 | 3.97 |
| L | Insular C. | 580 | −37 | −18 | 13 | 4.56 |
| L | Thalamus | 20 | −9 | −32 | 13 | 3.86 |
| L | Postcentral G. | 563 | −17 | −38 | 75 | 4.55 |
| L | Hippocampus | 42 | −29 | −38 | −6 | 3.57 |
| L | Angular G. | 33 | −43 | −52 | 23 | 3.45 |
All coordinates are in MNI (Montreal Neurological Institute) template space.
G., gyrus; C., cortex; LOC, lateral occipital cortex; SPL, superior parietal lobule; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; R, right; L, left; anter., anterior; poster., posterior; super., superior; temporooc., temporooccipital.
In the study‐test aware subjects (Table IV), the first repetition of primed stimuli activated right inferior LOC; left paracingulate gyrus; left MFG; left precentral gyrus; left superior LOC bordering SPL; and larger regions in left occipital cortex including occipital fusiform and lingual gyri. The decreases of neural activity were observed in right frontal medial and paracingulate gyri; right postcentral gyrus and right opercular cortex; right posterior cingulate gyrus; left SFG; left anterior cingulate and precentral gyri; superior regions in parietal cortex; bilateral anterior and posterior temporal cortices; bilateral hippocampus; amygdala; and putamen.
Table IV.
Brain regions changed neural activity during the first repetition of primed stimuli in the search task: Study‐test aware subjects
| Brain regions | Number of voxels | x | y | z | Z‐max | |
|---|---|---|---|---|---|---|
| Increase in BOLD signal | ||||||
| R | Insular C. | 28 | 38 | 23 | −4 | 4.00 |
| R | Frontal orbital C. | 27 | 38 | 23 | −6 | 3.96 |
| R | SFG | 29 | 4 | 13 | 55 | 3.75 |
| R | Temporal fusiform C., poster. | 42 | 34 | −38 | −24 | 4.09 |
| R | LOC, infer. | 595 | 32 | −84 | −16 | 4.66 |
| L | Paracingulate G. | 298 | −5 | 11 | 47 | 4.84 |
| L | Juxtapositional L. (formerly SMC) | 89 | −7 | 9 | 49 | 4.31 |
| L | Cingulate G., anter. | 28 | −7 | 9 | 43 | 3.85 |
| L | MFG | 176 | −31 | −4 | 51 | 4.26 |
| L | Thalamus | 19 | −17 | −8 | 13 | 3.48 |
| L | Precentral G. | 809 | −39 | −14 | 53 | 4.43 |
| L | Postcentral G. | 33 | −41 | −22 | 55 | 3.55 |
| L | SPL | 174 | −29 | −58 | 59 | 4.66 |
| L | ITG, temporooc. | 23 | −43 | −60 | −10 | 4.14 |
| L | Temporal occipital fusiform C. | 413 | −43 | −60 | −12 | 4.44 |
| L | LOC, super. | 1,398 | −27 | −62 | 57 | 5.16 |
| L | Precuneus | 16 | −9 | −68 | 59 | 3.56 |
| L | Occipital fusiform G. | 1,171 | −13 | −82 | −14 | 4.89 |
| L | Lingual G. | 614 | −9 | −82 | −14 | 5.13 |
| L | Intracalcarine C. | 107 | −7 | −90 | 1 | 4.80 |
| L | Occipital pole | 2,291 | −5 | −94 | −4 | 5.33 |
| Decrease in BOLD signal | ||||||
| R | Frontal pole | 651 | 10 | 57 | −2 | 4.92 |
| R | Frontal medial C. | 385 | 8 | 55 | −6 | 4.97 |
| R | Paracingulate G. | 1,055 | 10 | 53 | −4 | 4.91 |
| R | Subcallosal C. | 101 | 6 | 31 | −8 | 3.85 |
| R | Temporal pole | 36 | 48 | 13 | −36 | 3.32 |
| R | MTG, anter. | 138 | 56 | −4 | −16 | 4.78 |
| R | STG, anter. | 212 | 54 | −4 | −16 | 4.70 |
| R | Planum polare | 396 | 50 | −6 | −6 | 5.15 |
| R | MTG, poster. | 441 | 54 | −8 | −16 | 4.38 |
| R | Hippocampus | 43 | 28 | −10 | −2 | 3.66 |
| R | Insular C. | 801 | 44 | −10 | −2 | 4.96 |
| R | Putamen | 37 | 32 | −10 | −6 | 3.49 |
| R | Amygdala | 26 | 24 | −10 | −10 | 3.43 |
| R | Heschl's G. | 537 | 44 | −12 | 1 | 4.78 |
| R | Opercular C. | 737 | 42 | −18 | 21 | 4.81 |
| R | Parietal operculum C. | 783 | 44 | −20 | 23 | 4.69 |
| R | Cingulate G., poster. | 419 | 8 | −24 | 47 | 4.07 |
| R | Supramarginal G., anter. | 128 | 62 | −28 | 43 | 3.68 |
| R | Postcentral G. | 515 | 28 | −32 | 57 | 4.13 |
| L | SFG | 177 | −19 | 35 | 45 | 4.17 |
| L | Amygdala | 32 | −33 | −6 | −20 | 3.86 |
| L | Hippocampus | 82 | −33 | −10 | −20 | 3.90 |
| L | Cingulate G., anter. | 453 | −5 | −12 | 37 | 4.47 |
| L | Putamen | 55 | −33 | −18 | −2 | 4.38 |
| L | Precentral G. | 613 | −9 | −32 | 51 | 4.58 |
| L | Planum temporale | 477 | −57 | −32 | 17 | 4.29 |
| L | Precuneus | 319 | −7 | −36 | 49 | 3.96 |
| L | STG, poster. | 738 | −55 | −40 | 9 | 4.93 |
| L | Supramarginal G., poster. | 129 | −55 | −44 | 9 | 4.41 |
| L | MTG, temporooc. | 184 | −69 | −48 | −4 | 3.88 |
| L | Angular G. | 128 | −45 | −52 | 21 | 4.37 |
| L | LOC, super. | 125 | −47 | −62 | 29 | 4.01 |
All coordinates are in MNI (Montreal Neurological Institute) template space.
G., gyrus; C., cortex; LOC, lateral occipital cortex; SPL, superior parietal lobule; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; R, right; L, left; anter., anterior; poster., posterior; super., superior; temporooc., temporooccipital.
The comparison of subjects' performance on the search task revealed that study‐test aware, compared to study‐test unaware, subjects had higher activation of bilateral thalamus. Study‐test unaware, compared to study‐test aware, subjects had higher activation of right cerebellum, left paracingulate gyrus, and left middle temporal gyrus (Table V).
Table V.
The contrast between the study‐test aware and study‐test unaware conditions
| Brain regions | Number of voxels | x | y | z | Z‐max | |
|---|---|---|---|---|---|---|
| Study‐test aware > study‐test unaware | ||||||
| R | Thalamus | 52 | 6 | −20 | 13 | 3.89 |
| L | Thalamus | 20 | −1 | −18 | 13 | 3.73 |
| Study‐test unaware > study‐test aware | ||||||
| R | Cerebellum | 78 | 46 | −42 | −32 | 4.04 |
| L | Paracingulate G. | 19 | −3 | 47 | 25 | 3.48 |
| L | MTG, poster. | 8 | −59 | −16 | −14 | 3.20 |
| L | MTG, temporooc. | 17 | −69 | −48 | −4 | 3.56 |
All coordinates are in MNI (Montreal Neurological Institute) template space.
G., gyrus; C., cortex; LOC, lateral occipital cortex; MTG, middle temporal gyrus; R, right; L, left; poster., posterior; temporooc., temporooccipital.
A conjunction analysis of encoding‐ and test‐related neural activity
We found that independently of subjects' awareness, brain areas that showed an increase in neural activity during encoding never decreased in activity during the implicit memory test. Also the brain areas that showed a decrease in neural activity during encoding never showed an increase during the implicit memory test.
The conjunction analysis of the last repetition of primed stimuli in the object detection task and the first repetition of primed stimuli in the search task revealed that bilateral precuneus and cuneus showed reduced neural activity during both encoding and implicit memory testing in the study‐test unaware, but not aware, subjects (Fig. 5, right). In contrast, the region in the right MFG showed increased activity during both encoding and testing in study‐test aware, but not unaware, subjects (Fig. 5, left).
Figure 5.
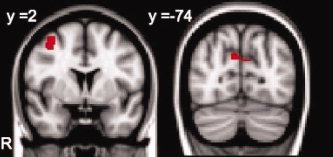
The region in the right middle frontal gyrus (left) activated during encoding and during testing in the study‐test aware group of subjects. The region in the bilateral cuneus and precuneus (right) deactivated during encoding and during testing in the study‐test unaware group of subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The between‐subject conjunction of encoding‐ and test‐related increases of neural activity revealed that the superior division of left LOC/SPL region [−30, −60, 56] and bilateral lingual/occipital fusiform gyri [±10, −84, −10] (see Fig. 7) activated during the last repetition of primed stimuli in the object detection task and during the first repetition of primed stimuli in the search task in both groups of subjects.
Figure 7.
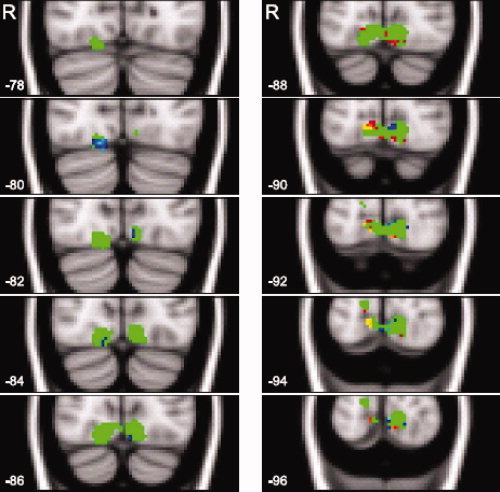
The voxel sensitivities for occipital fusiform and lingual gyri reactivated in both groups of subjects. Ten consecutive axial slices (from −78 to −96 mm) are shown. The numbers in the left‐lower corner of each image represent a slice coordinate along the y‐axis. The letter "R" in the upper left corner stands for the right hemisphere. The region of interest is depicted in green color. Positive sensitivities are shown in red color. Negative sensitivities are shown in blue color.
The changes in neural activity between the study and test phases are referred to as reactivation values. Reactivation values were calculated as the differences in the percent BOLD signal change relative to the trial onset on the first repetition of primed trials during the search task and the last repetition of primed trials during the object detection task. Figure 6 shows the relationship between activation at encoding and activation at testing in two selected voxels. One voxel [14, −90, −4] showed greater reactivation values in study‐test aware subjects. Another voxel [16, −80, −16] showed greater reactivation values in study‐test unaware subjects.
Figure 6.
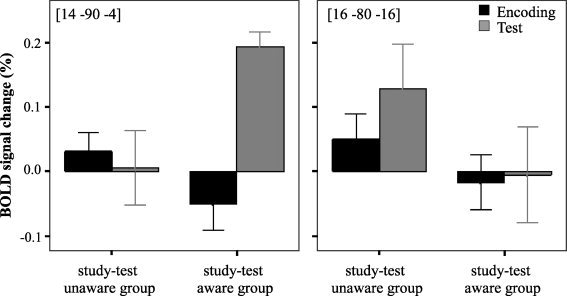
The differences in BOLD signal change relative to the trial onset between encoding and test in study‐test aware and study‐test unaware subjects.
To find out whether reactivation values may account for differences in implicit memory, we classified subjects using the SMLR method. The classifier's performance was optimized across nine lm parameters for the number of voxels left in the dataset. Tables AI, AII, AIII in Appendix reflect the process of optimization and report classification accuracies for all optimization parameters (lm). Herein we report only the best classification performance that was defined as the highest accuracy with the largest number of voxels left in the dataset. The areas of conjunction analysis were used as ROIs for this analysis. The chance performance was 50%.
The conjunction analysis showed that a large region in right MFG was reactivated in study‐test aware, but not in study‐test unaware, subjects. Mean reactivation value in these regions was 0.07% (SD = 0.15). The best classification performance was 69%, with 12 features out of 97 used for classification. Bilateral cuneus and precuneus regions decreased their neural activity during encoding and test in study‐test unaware, but not study‐test aware, subjects. Mean reactivation value in these regions was 0.016% (SD = 0.2). The best classification performance was 69%. SMLR selected 24 features out of 114 for classification. Voxels in the right precuneus discriminated larger reactivation values in the study‐test aware, compared to study‐test unaware, subjects. Voxels in the left precuneus discriminated larger reactivation values in the study‐test unaware, compared to study‐test aware, subjects.
The LOC/SPL and lingual/occipital fusiform regions reactivated in both groups of subjects (M = 0.019%, SD = 0.28). The best classification performance was 81.25%. SMLR used 98 features out of 657 to classify subjects. Voxels that were diagnostic for implicit memory performance were mostly located in lingual and occipital fusiform regions (see Fig. 7). As follows from Figure 7, the more anterior slices, which approximately correspond to the occipital fusiform region, had larger reactivation values in the study‐test unaware subjects. The more posterior slices that approximately correspond to lingual gyrus had larger reactivation values in the study‐test aware subjects. Neural reactivation in the parietal region was not very diagnostic for retrieval success. Only three voxels were sensitive to retrieval success by discriminating larger reactivation values in the study‐test aware, compared to study‐test unaware, subjects.1
Optimization of the classifier's performance implies that activation patterns may depend on optimization parameters. To address this issue we selected four voxels (two with positive sensitivities and two with negative sensitivities) that had shown the strongest diagnostic capability. We looked at the sensitivities of those voxels across several optimization parameters when classification performance was above 60%.
Figure 8 shows the change in voxel sensitivities under different optimization parameters. Arrows point to locations of selected voxels in the frequency distributions of sensitivities. Figure 8 clearly demonstrates that voxels with the strongest sensitivities remain in the model under all optimization parameters; for example, Voxel 1 was included in the activation pattern independently of optimization. Voxels with lower sensitivities may be removed from the model; for example, Voxel 4 was excluded from the model when the number of selected features became small.
Figure 8.
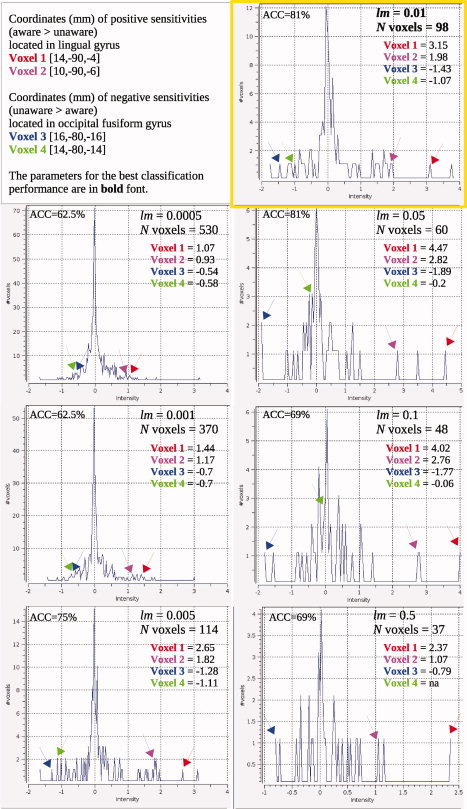
The distribution of voxel sensitivities observed under different optimization parameters. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Neuroimaging studies of explicit memory indicate that reconstruction of previous experiences during explicit retrieval reactivates encoding‐related brain regions [Habib, 2001; Johnson and Rugg, 2007; Kahn et al., 2004; Nyberg et al., 2000, 2001; Persson and Nyberg, 2000; Vaidya et al., 2002; Wheeler et al., 2000, 2006; Woodruff et al., 2005]. It was, however, previously unclear whether and how encoding‐related neural activity is reinstated during implicit memory tasks. Our fMRI study examined this question using traditional qualitative and new quantitative methods.
Subjects learned object locations incidentally during the object detection task. The incidental nature of learning was validated through the comparison of subjects' RTs in the dot detection and the object detection tasks. There were no significant differences in RT between these two tasks, suggesting that the processing the presence of unique objects took no more effort than processing the presence of identical dots. Subjects' implicit memory was tested indirectly using the search task. To manipulate implicit memory performance, we assigned subjects to either the study‐test aware or study‐test unaware conditions. Consistent with previous findings [Mace, 2003a, b; Manelis, 2009; Richardson‐Klavehn and Gardiner, 1995; Richardson‐Klavehn et al., 1994], study‐test aware, compared to unaware, subjects demonstrated greater implicit memory. The differences were indexed by faster search RTs in aware subjects. In addition, study‐test aware, but not unaware, subjects found previously seen objects presented in their prior locations (primed stimuli) significantly faster compared to previously seen objects presented in new locations, and new objects presented in random locations.
The main concern for implicit memory studies is the purity of implicit performance [e.g., Beauregard et al., 1999; Bowers and Schacter, 1990]. Arguably, study‐test awareness increases the likelihood that subjects use an explicit retrieval strategy during implicit memory test. The finding of the study‐test aware effect under deep, but not shallow, encoding provided support for this hypothesis [Mace, 2003b; Richardson‐Klavehn et al., 1994]. Other studies, however, demonstrated that study‐test awareness does not lead to the use of explicit retrieval strategies in the implicit memory paradigm [Fay et al., 2005]. We believe that our study also provides evidence for the latter point of view.
First, even though subjects were study‐test aware, they did not know that their memory would be tested. In addition, we analyzed only the first repetitions of the stimuli at test, in case subjects had figured out the real goal of the experiment midway through the search task. Second, unlike the studies of Mace [ 2003b] and Richardson‐Klavehn et al. [ 1994], we observed the effect of study‐test awareness under incidental encoding. Incidental encoding as used in our study should be considered as very shallow because subjects neither were instructed nor had time to use elaborate encoding strategies. Our previous study [Manelis, 2009] showed that subjects were able to recall only about 15% of incidentally encoded stimuli, compared to about 50% observed under deep encoding in Mace's and Richardson‐Klavehn et al.'s studies. These findings suggest that the likelihood of explicit “contamination” in our study is much lower than in the studies cited above.
Third, the previous studies showed that the use of explicit retrieval strategies during implicit memory test is a time‐consuming process [e.g., Richardson‐Klavehn and Gardiner, 1995; Richardson‐Klavehn et al., 1999]. Consequently, if study‐test aware subjects used explicit retrieval strategies, their search RT would increase compared to the search RT in study‐test unaware subjects. Contrary to these predictions, we observed faster search RT in study‐test aware, compared to study‐test unaware, subjects, implying that subjects did not use an explicit strategy for implicit memory task.
The analysis of neuroimaging data also provides evidence against usage of an explicit retrieval strategy by study‐test aware participants. Previous studies showed that explicit recall of locations cued by the objects elicited activation in dorsal extrastriate cortex, SPL, lingual and fusiform gyri, frontal eye fields, hippocampus, and parahippocampal cortex [de Rover et al., 2008; Piekema et al., 2006; Sommer et al., 2005]. If study‐test aware subjects tried to recollect spatial locations using target objects as a cue, they would have greater activity in these regions compared to study‐test unaware subjects. The comparison of neural activity in two groups of subjects did not reveal significant differences in any of these brain regions.
Searching for old objects in their corresponding old locations elicited greater activity in bilateral thalamus in study‐test aware, compared to study‐test unaware, subjects. The same trials elicited greater activity in left paracingulate, left MTG, and right cerebellum in study‐test unaware, compared to study‐test aware, subjects. While activity in cerebellum and left MTG may be related to item recognition [e.g., Lekeu et al., 2002], activation in paracingulate cortex is often associated with “mentalizing” [e.g., Gallagher and Frith, 2004]. In our study, the greater activity in these areas may suggest that study‐test unaware participants occasionally tried to figure out whether they previously encountered the search task stimuli in the object detection task. Study‐test aware subjects did not have to “mentalize” because they believed that all stimuli appeared previously.
Activation of the thalamus during implicit memory tests may be necessary for transformation of neural priming to its behavioral counterpart [Walla et al., 2003]. It was reported that thalamic lesions interfered with motor skill learning [Exner et al., 2001] and led to disconnection between processing streams of neural priming and behavioral response [Walla et al., 2003]. In our study, greater thalamic activity in study‐test aware, compared to study‐test unaware, subjects may indicate the facilitation of such transformation indexed by the decrease in RT in aware subjects.
Taken together, behavioral and neuroimaging data suggest that enhancement of implicit memory in study‐test aware subjects cannot be explained by the use of explicit retrieval strategies. We propose that behavioral facilitation could occur due to an unintentional reconstruction of the encoding episode. Arguably, the reconstruction may include not only properties that were presented during the memory test but also information that was absent from the test episode but was present in the encoding episode [Hintzman, 1986]. Thus, successful performance on the search task required the reinstatement of location information induced by presentation of target objects.
Brain reactivation may reflect a neural mechanism for reconstruction of previous experiences [e.g., Damasio, 1989]. If reactivation is a key factor for successful performance, then subjects with different levels of implicit memory may be classified based on the magnitude of reactivation. Implicit memory is often associated with reduction of neural responses in the task‐specific brain areas [e.g., Grill‐Spector et al., 2006; Henson, 2003]. Therefore, it was possible that the neural reduction during test may represent the reinstatement of neural decreases during encoding. Contrary to this prediction, we found that reactivation mechanisms of implicit memory resemble those of explicit memory. Specifically, bilateral occipital fusiform and lingual gyri along with left superior LOC and SPL were activated during encoding and during implicit memory testing in both groups of subjects. These brain regions are involved in processing of object and spatial information [e.g., Haxby et al., 1991; Köhler et al., 1998a; Sala et al., 2003]. Reactivation of these regions during an implicit memory task could indicate the reconstruction of incidentally learned associations between objects and locations.
While the brain regions that decreased their neural activity during encoding never increased it during the implicit memory test and vise versa, some brain regions were uniquely reactivated in study‐test aware or study‐test unaware subjects. In the study‐test aware subjects, this unique region was located in the MFG and adjacent precentral and superior frontal gyri, and approximately corresponded to the frontal eye fields (FEF). The FEF region plays an important role in planning and visual attention [Fincham et al., 2002; Kastner et al., 1999]. The re‐engagement of FEF during the search task in study‐test aware, but not study‐test unaware, subjects may suggest that study‐test aware instructions stimulated subjects' attention to the stimuli.
In the study‐test unaware subjects, the uniquely reactivated region was located in bilateral cuneus and precuneus. This region was deactivated during encoding and also during implicit retrieval. Recent studies suggested that activity in precuneus decreases when visual attention increases [Tomasi et al., 2006]. In our study, the decrease in precuneal activity may indicate the sustained visual attention required by encoding and implicit memory tasks for the stimuli detection and quick response. Another explanation for deactivation may be that precuneus is a part of a default‐mode network and activates during rest [Cavanna, 2007; Cavanna and Trimble, 2006]. This explanation, however, may be discounted because deactivation in this region was observed only in one group of subjects. Default‐mode networks are usually stable across different conditions [e.g., Damoiseaux et al., 2006] at least in healthy participants predicting the re‐deactivation of this region in both groups of subjects.
Although involvement of the hippocampus in processing object locations is well established [e.g., Burgess et al., 2002; Hartley et al., 2007; Postma et al., 2008], we did not find reactivation in hippocampus and adjacent regions. The right hippocampus increased its activity relative to the baseline during incidental encoding, while the left hippocampus reduced its activity relative to the baseline in both groups of subjects during implicit memory test. One explanation for these results may be related to the low memory load in the search task. This task did not require subjects to navigate, create spatial maps of object positions, or think about spatial relationships between objects. Previous studies have shown that spatial tasks with low memory load do not require involvement of the hippocampus. For example, patients with hippocampal damage performed normally if they had to memorize one or two locations. However, their performance declined when memory load was increased up to five locations [Shrager et al., 2007].
The quantitative analysis, implemented through the classification method, was able to classify study‐test aware and study‐test unaware subjects above the chance based on reactivation values in the regions that were commonly and uniquely reactivated. We found that even the occipital and parietal regions that were reactivated in both groups of subjects were not homogeneous in terms of their sensitivity to implicit memory performance. The fusiform part of this region included voxels that had greater reactivation values for lower implicit memory performance, while the lingual part of this region included voxels that had greater reactivation values for higher implicit memory performance.
We have to accept, however, that the method we used to calculate reactivation makes it difficult to interpret the absolute meanings of reactivation values. The current version of the pynifti_pst tool allows calculation of the per stimulus percent signal change relative to the stimulus onset. It is possible, however, that the neural activity at stimulus onset in encoding is different from that in the memory test. In the future, to ease the interpretation of absolute reactivation values, it would be beneficial to measure the percent BOLD signal change relative to neural activity during the rest periods surrounding the trials of interest.
To achieve the best classification performance, we optimized the classifier by changing the number of features left in the dataset. Optimization suggests that the observed activation pattern is not the only possible set of features. For example, some voxels could be removed from the model under different optimization parameters. We explored the stability of the activation pattern by looking at the changes in the voxel sensitivities across different optimization parameters. For this purpose, we chose four voxels from the activation pattern that provided the most accurate classification. Three of four chosen voxels remained in the model under all optimization parameters. The fourth voxel, which had the lowest sensitivity among chosen voxels, was removed from the model when the number of features left became small. These findings suggest that although optimizing the classifier changes activity patterns, the voxels with the strongest sensitivities are stable and remain in the model under all optimization parameters. Future work can address the issue of the pattern stability by developing an automated search method for stable voxels that remain in the model under the most optimization parameters.
Our fMRI study of neural reactivation differed from previous studies in several aspects. First, it demonstrated that reactivation is an important mechanism of implicit memory, which suggests similarity between explicit and implicit memory systems. Second, it evaluated the differences in reactivation between subjects with high and low implicit memory performance. Third, our study clearly showed the importance of the classification method for reactivation research.
In summary, our research suggests that reactivation of encoding‐related brain regions during implicit memory test enhances memory performance by inducing an unintentional reconstruction of the encoding episode. While occipital and parietal regions reactivated in study‐test aware and study‐test unaware subjects, the more refined analysis has revealed that some voxels within these regions are able to differentiate between two groups of subjects based on reactivation values. Reactivation patterns in occipital and parietal regions along with the uniquely reactivated FEF and test‐related activation in thalamus suggest a possible network for unintentional reconstruction of encoding episode during implicit memory tests. Further research needs to examine whether the reactivation pattern described here is unique for spatial processing or other stimulus properties, such as lexical or pictorial, also may be reconstructed without subject's intentions.
Acknowledgements
This work was supported in part by fellowship support to the first author in the form of an institutional training Grant 5T32‐MH019983‐12 to Carnegie‐Mellon University from the National Institute of Mental Health in support of Combined Computational and Behavioral Approaches to the Study of Cognitive Neuroscience. [Correction made here after initial online publication: Acknowledgments section inserted.] This research was supported by the James McDonnell Foundation. We thank Dr. Yaroslav Halchenko for helping with the PYMVPA software. We also thank Dr. Lynne Reder, Arielle Schmidt, and two anonymous reviewers for their constructive comments on the earlier versions of this manuscript.
Tables AI, AII, AIII below demonstrate the optimization of the SMLR classifier in the common and unique areas of reactivation in study‐test aware and study‐test unaware subjects.
Table AI.
Optimization of the classifier's performance
| lm parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0001 | 0.0005 | 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | 1.0 | |
| Number of voxels | 657 | 530 | 370 | 114 | 98 | 60 | 48 | 37 | 27 |
| ACC (%) | 56.25 | 62.5 | 62.5 | 75 | 81.25 | 81.25 | 68.75 | 68.75 | 50.0 |
Total number of voxels in the region is 657.
The best classification performance is reported in bold font.
ROI = LOC/SPL and lingual/occipital fusiform regions reactivated in both groups of subjects.
Table AII.
Optimization of the classifier's performance
| lm parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0001 | 0.0005 | 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | 1.0 | |
| Number of voxels | 97 | 97 | 97 | 74 | 55 | 31 | 28 | 12 | 6 |
| ACC (%) | 56.25 | 56.25 | 56.25 | 50 | 56.25 | 56.25 | 56.25 | 68.75 | 62.75 |
Total number of voxels in the region is 97.
The best classification performance is reported in bold font.
ROI = MFG reactivated in the study‐test aware group only.
Table AIII.
Optimization of the classifier's performance
| lm parameter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0001 | 0.0005 | 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | 1.0 | |
| Number of voxels | 114 | 114 | 114 | 82 | 64 | 29 | 24 | 15 | 6 |
| ACC (%) | 43.75 | 43.75 | 43.75 | 62.5 | 62.5 | 62.5 | 68.75 | 62.5 | 43.75 |
Total number of voxels in the region is 114.
The best classification performance is reported in bold font.
ROI = precuneus that decreased its activity in the study‐test unaware group during encoding and during implicit memory test.
This article was originally published online on 12 April 2010. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected 11 May 2010.
Footnotes
In the separate analysis, we used subjects' search RT as an additional explanatory variable. Because the addition of search RT to the model did not change the classifier's performance, we do not discuss these results further.
REFERENCES
- Beauregard M, Benhamou J, Laurent C, Chertkow H ( 1999): Word priming without awareness: A new approach to circumvent explicit memory contamination. Brain Cogn 39: 149–169. [DOI] [PubMed] [Google Scholar]
- Bowers JS, Schacter DL ( 1990): Implicit memory and test awareness. J Exp Psychol Learn Mem Cogn 16: 404–416. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE ( 1996): Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci 16: 6219–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM ( 1998): Functional‐anatomic correlates of object priming in humans revealed by rapid presentation event‐related fMRI. Neuron 20: 285–296. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J ( 2002): The human hippocampus and spatial and episodic memory. Neuron 35: 625–641. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L ( 1997): Imaging cognition: An empirical review of pet studies with normal subjects. J Cogn Neurosci 9: 1–26. [DOI] [PubMed] [Google Scholar]
- Cavanna AE ( 2007): The precuneus and consciousness. CNS Spectr 12: 545–552. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR ( 1992): Intact and long‐lasting repetition priming in amnesia. J Exp Psychol Learn Mem Cogn 18: 509–520. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA ( 1999): Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci 2: 844–847. [DOI] [PubMed] [Google Scholar]
- Cox DD, Savoy RL ( 2003): Functional magnetic resonance imaging (fMRI) "brain reading": Detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage 19: 261–270. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1989): Time‐locked multiregional retroactivation: A systems‐level proposal for the neural substrates of recall and recognition. Cognition 33: 25–62. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF ( 2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rover M, Petersson KM, van der Werf SP, Cools AR, Berger HJ, Fernández G ( 2008): Neural correlates of strategic memory retrieval: Differentiating between spatial‐associative and temporal‐associative strategies. Hum Brain Mapp 29: 1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C ( 2008): High‐resolution multi‐voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus 18: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner c, Weniger G, Irle E ( 2001): Implicit and explicit memory after focal thalamic lesions. Neurology 57: 2054–2063. [DOI] [PubMed] [Google Scholar]
- Fay S, Isingrini M, Pouthas V ( 2005): Does priming with awareness reflect explicit contamination? An approach with a response‐time measure in word‐stem completion. Conscious Cogn 14: 459–473. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, van Veen V, Stenger VA, Anderson JR ( 2002): Neural mechanisms of planning: A computational analysis using event‐related fMRI. Proc Natl Acad Sci USA 99: 3346–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Martino FD, Valente G ( 2008): Multivariate analysis of fMRI time series: Classification and regression of brain responses using machine learning. Magn Reson Imaging 26: 921–934. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD ( 2004): Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia 42: 1725–1736. [DOI] [PubMed] [Google Scholar]
- Grill‐Spector K, Henson R, Martin A ( 2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trends Cogn Sci 10: 14–23. [DOI] [PubMed] [Google Scholar]
- Habib R ( 2001): On the relation between conceptual priming, neural priming, and novelty assessment. Scand J Psychol 42: 187–195. [DOI] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollmann S ( 2009a): PyMVPA: A python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics 7: 37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Sederberg PB, Olivetti E, Frnd I, Rieger JW, Herrmann CS, Haxby JV, Hanson SJ, Pollmann S ( 2009b): PyMVPA: A unifying approach to the analysis of neuroscientific data. Front Neuroinformatics 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SJ, Halchenko YO ( 2008): Brain reading using full brain support vector machines for object recognition: There is no “face” identification area. Neural Comput 20: 486–503. [DOI] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha‐Khadem F, Burgess N ( 2007): The hippocampus is required for short‐term topographical memory in humans. Hippocampus 17: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI ( 1991): Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA 88: 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA ( 2003): Neuroimaging studies of priming. Prog Neurobiol 70: 53–81. [DOI] [PubMed] [Google Scholar]
- Hintzman DL ( 1986): Schema abstraction in a multiple‐trace memory model. Psychol Rev 93: 411–428. [Google Scholar]
- Jenkinson M, Smith S ( 2001): A global optimisation method for robust and accurate linear registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S ( 2002): Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD ( 2007): Recollection and the reinstatement of encoding‐related cortical activity. Cereb Cortex 17: 2507–2515. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD ( 2004): Functional‐neuroanatomic correlates of recollection: Implications for models of recognition memory. J Neurosci 24: 4172–4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, Weerd PD, Desimone R, Ungerleider LG ( 1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR ( 1994): The information acquired during artificial grammar learning. J Exp Psychol Learn Mem Cogn 20: 79–91. [DOI] [PubMed] [Google Scholar]
- Köhler S, McIntosh AR, Moscovitch M, Winocur G ( 1998a): Functional interactions between the medial temporal lobes and posterior neocortex related to episodic memory retrieval. Cereb Cortex 8: 451–461. [DOI] [PubMed] [Google Scholar]
- Köhler S, Moscovitch M, Winocur G, Houle S, McIntosh AR ( 1998b): Networks of domain‐specific and general regions involved in episodic memory for spatial location and object identity. Neuropsychologia 36: 129–142. [DOI] [PubMed] [Google Scholar]
- Krishnapuram B, Carin L, Figueiredo MAT, Hartemink AJ ( 2005): Sparse multinomial logistic regression: Fast algorithms and generalization bounds. IEEE Trans Pattern Anal Mach Intell l 27: 957–968. [DOI] [PubMed] [Google Scholar]
- Lekeu F, Marczewski P, der Linden MV, Collette F, Degueldre C, Del Fiore G, Luxen A, Franck G, Moonen G, Salmon E ( 2002): Effects of incidental and intentional feature binding on recognition: A behavioural and PET activation study. Neuropsychologia 40: 131–144. [DOI] [PubMed] [Google Scholar]
- Mace JH ( 2003a): Involuntary aware memory enhances priming on a conceptual implicit memory task. Am J Psychol 116: 281–290. [PubMed] [Google Scholar]
- Mace JH ( 2003b): Study‐test awareness can enhance priming on an implicit memory task: Evidence from a word completion task. Am J Psychol 116: 257–279. [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K ( 1994): Priming of pop‐out: I. Role of features. Mem Cognit 22: 657–672. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K ( 1996): Priming of pop‐out: II. The role of position. Percept Psychophys 58: 977–991. [DOI] [PubMed] [Google Scholar]
- Manelis A ( 2009): Implicit and explicit memory tests reactivate common memory traces: Support for a unitary memory system. Doctoral dissertation, Rutgers University, Newark, NJ. Ann Arbor: ProQuest/UMI (UMI Number: 3359481).
- Musen G ( 1996): Effects of task demands on implicit memory for object–location associations. Can J Exp Psychol 50: 104–113. [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E ( 2000): Reactivation of encoding‐related brain activity during memory retrieval. Proc Natl Acad Sci USA 97: 11120–11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Petersson KM, Nilsson LG, Sandblom J, Aberg C, Ingvar M ( 2001): Reactivation of motor brain areas during explicit memory for actions. Neuroimage 14: 521–528. [DOI] [PubMed] [Google Scholar]
- O'Toole AJ, Jiang F, Abdi H, Pnard N, Dunlop JP, Parent MA ( 2007): Theoretical, statistical, and practical perspectives on pattern‐based classification approaches to the analysis of functional neuroimaging data. J Cogn Neurosci 19: 1735–1752. [DOI] [PubMed] [Google Scholar]
- Park H, Quinlan J, Thornton E, Reder LM ( 2004): The effect of midazolam on visual search: Implications for understanding amnesia. Proc Natl Acad Sci USA 101: 17879–17883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L ( 2000): Conjunction analysis of cortical activations common to encoding and retrieval. Microsc Res Tech 51: 39–44. [DOI] [PubMed] [Google Scholar]
- Piekema C, Kessels RPC, Mars RB, Petersson KM, Fernández G ( 2006): The right hippocampus participates in short‐term memory maintenance of object–location associations. Neuroimage 33: 374–382. [DOI] [PubMed] [Google Scholar]
- Postma A, Kessels RPC, van Asselen M ( 2008): How the brain remembers and forgets where things are: The neurocognition of object–location memory. Neurosci Biobehav Rev 32: 1339–1345. [DOI] [PubMed] [Google Scholar]
- Richardson‐Klavehn A, Gardiner JM ( 1995): Retrieval volition and memorial awareness in stem completion: An empirical analysis. Psychol Res 57: 166–178. [DOI] [PubMed] [Google Scholar]
- Richardson‐Klavehn A, Lee MG, Joubran R, Bjork RA ( 1994): Intention and awareness in perceptual identification priming. Mem Cognit 22: 293–312. [DOI] [PubMed] [Google Scholar]
- Richardson‐Klavehn A, Clarke AJB, Gardiner JM ( 1999): Conjoint dissociations reveal involuntary "perceptual" priming from generating at study. Conscious Cogn 8: 271–284. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M ( 2003): Two different streams form the dorsal visual system: Anatomy and functions. Exp Brain Res 153: 146–157. [DOI] [PubMed] [Google Scholar]
- Sala JB, Rämä P, Courtney SM ( 2003): Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia 41: 341–356. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL ( 1998): On the relations among priming, conscious recollection, and intentional retrieval: Evidence from neuroimaging research. Neurobiol Learn Mem 70: 284–303. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Tulving E ( 1994): What are the memory systems of 1994? In: Schacter DL, Tulving E, editors. Memory Systems 1994. Cambridge, MA: The MIT Press; pp 1–38. [Google Scholar]
- Schott BH, Richardson‐Klavehn A, Henson RNA, Becker C, Heinze H‐J, Dzel E ( 2006): Neuroanatomical dissociation of encoding processes related to priming and explicit memory. J Neurosci 26: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Bayley PJ, Bontempi B, Hopkins RO, Squire LR ( 2007): Spatial memory and the human hippocampus. Proc Natl Acad Sci USA 104: 2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Rose M, Gläscher J, Wolbers T, Büchel C ( 2005): Dissociable contributions within the medial temporal lobe to encoding of object–location associations. Learn Mem 12: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L ( 2006): Common deactivation patterns during working memory and visual attention tasks: An intra‐subject fMRI study at 4 Tesla. Hum Brain Mapp 27: 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Schacter DL ( 1990): Priming and human memory systems. Science 247: 301–306. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JDE ( 2002): Evidence for cortical encoding specificity in episodic memory: Memory‐induced re‐activation of picture processing areas. Neuropsychologia 40: 2136–2143. [DOI] [PubMed] [Google Scholar]
- Walla P, Lehmer JP, Nasel C, Baumgartner C, Deecke L, Lang W ( 2003): Preserved memory traces within diencephalic amnesia. J. Neural Transm 110: 537–543. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL ( 2000): Memory's echo: Vivid remembering reactivates sensory‐specific cortex. Proc Natl Acad Sci USA 97: 11125–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Shulman GL, Buckner RL, Miezin FM, Velanova K, Petersen SE ( 2006): Evidence for separate perceptual reactivation and search processes during remembering. Cereb Cortex 16: 949–959. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM ( 2005): Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci 8: 1228–1233. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD ( 2005): Content‐specificity of the neural correlates of recollection. Neuropsychologia 43: 1022–1032. [DOI] [PubMed] [Google Scholar]


