Abstract
Rationale
In heart failure (HF), CaMKII expression and reactive oxygen species (ROS) are increased. Both ROS and CaMKII can increase late INa leading to intracellular Na accumulation and arrhythmias. It has been shown that ROS can activate CaMKII via oxidation.
Objective
We tested whether CaMKIIδ is required for ROS-dependent late INa regulation and if ROS-induced Ca released from the sarcoplasmic reticulum (SR) is involved.
Methods and Results
40 µmol/L H2O2 significantly increased CaMKII oxidation and autophosphorylation in permeabilized rabbit cardiomyocytes. Without free [Ca]i (5 mmol/L BAPTA/1 mmol/L Br2-BAPTA) or after SR depletion (caffeine 10 mmol/L, thapsigargin 5 µmol/L) the H2O2-dependent CaMKII oxidation and autophosphorylation was abolished. H2O2 significantly increased SR Ca spark frequency (confocal microscopy) but reduced SR Ca load. In wildtype (WT) mouse myocytes, H2O2 increased late INa (whole cell patch-clamp). This increase was abolished in CaMKIIδ−/− myocytes. H2O2-induced [Na]i and [Ca]i accumulation (SBFI and Indo-1 epifluorescence) was significantly slowed in CaMKIIδ−/− myocytes (vs. WT). CaMKIIδ−/− myocytes developed significantly less H2O2-induced arrhythmias, and were more resistant to hypercontracture. Opposite results (increased late INa, [Na]i and [Ca]i accumulation) were obtained by overexpression of CaMKIIδ in rabbit myocytes (adenoviral gene transfer) reversible with CaMKII inhibition (10 µmol/L KN93 or 0.1 µmol/L AIP).
Conclusion
Free [Ca]i and a functional SR are required for ROS activation of CaMKII. ROS-activated CaMKIIδ enhances late INa, which may lead to cellular Na and Ca overload. This may be of relevance in HF, where enhanced ROS production meets increased CaMKII expression.
Keywords: reactive oxygen species, Na+ current, Ca2+/calmodulin-dependent protein kinase II, Sodium channels, Sarcoplasmic Reticulum
Introduction
Reactive oxygen species (ROS) are generated during myocardial infarction and contribute to left ventricular remodelling and adverse outcomes.1, 2 In human failing myocardium increased oxidative stress is associated with reduced left ventricular function.3 These detrimental effects involve impairment of Na and Ca homeostasis4–8 resulting in contractile dysfunction, electrical instability and cell death.9 We and others have shown that H2O2, which generates ROS,7 enhances the late, slowly-inactivating current through cardiac Na channels (late INa), thereby leading to action potential (AP) prolongation and arrhythmias.6, 10 Na entering the cell via late INa leads to cytosolic Na accumulation,6 favouring Ca entry via sarcolemmal Na-Ca-exchange (NCX) and cellular Ca overload, contractile dysfunction, and hypercontrature.5, 11, 12
The mechanisms by which ROS increase late INa and initiate this detrimental cascade are not clear. We have shown that Ca/calmodulin-dependent protein kinase IIδ (CaMKIIδ) can phosphorylate cardiac Na channels leading to enhanced late INa.13 The binding of Ca/calmodulin (Ca/CaM) to its regulatory domain results in conformational changes that expose the catalytic domain,14 which can phosphorylate numerous targets including threonine 287 at its own regulatory domain. This autophosphorylation renders the protein to remain active even after Ca/CaM dissociation.14 It is known that expression and activity of CaMKII are increased in heart failure (HF), and we have shown that transgenic CaMKIIδ overexpression results in left ventricular dysfunction15 and arrhythmias,13 whereas CaMKIIδ inhibition/knockout was shown to prevent cardiac remodelling and heart failure.16–18
It has been recently shown that ROS oxidize methionine 281/282 at the regulatory domain of CaMKII, and oxidized CaMKII has properties equivalent to autophosphorylated CaMKII9 in mediating angiotensin-induced apoptosis9 and ROS-induced afterdepolarizations.19 Moreover, there is evidence that Ca/CaM-CaMKII interaction is a prerequisite for ROS-dependent oxidation of CaMKII,9 and ROS have been shown to directly induce sarcoplasmic reticulum (SR) Ca release via thiol oxidation of the ryanodine receptor 2 (RyR2).20
Thus, we hypothesized that 1) ROS-activated CaMKIIδ is responsible for the observed ROS effects on late INa, and 2) Ca released from SR Ca stores is involved in the ROS-dependent CaMKII activation process.
The results of the present study show that H2O2-induced augmentation of late INa requires CaMKIIδ, and the consequent cellular Na and Ca overload was significantly slowed in myocytes lacking CaMKIIδ. Moreover, we show that the H2O2-dependent CaMKII activation requires a functional SR. Finally, we establish CaMKIIδ as an important mediator of H2O2-dependent arrhythmogenesis and cellular death injury.
Methods
CaMKIIδ knockout mice and CaMKIIδ overexpression in rabbit myocytes
Ventricular myocytes were isolated from CaMKIIδ knockout mice (CaMKIIδ−/−)17 and wild-type (WT) littermates. For CaMKIIδ overexpression, isolated rabbit ventricular myocytes were transfected with CaMKIIδC adenovirus (β-galactosidase, βGal, as control).5, 13, 21
Patch-clamp experiments
Ruptured-patch whole-cell voltage-clamp or current clamp was used to measure INa or membrane potential, respectively. Myocytes were held at −120 mV and INa was elicited using a train of pulses to −20 mV. APs were continuously elicited by square current pulses of 1–2 nA amplitude and 1–5 ms duration at basic cycle length of 2 s.
Data analysis and statistics
All data are expressed as mean±S.E.M. For longitudinal data, two-way repeated measures analysis of variance (ANOVA) was run; else Student’s unpaired t test or one-way ANOVA with Student-Newman-Keuls multiple comparison was used. Double sided P-values of <0.05 were considered significant.
A detailed description of methods, including numerical simulations, can be found in the online supplement.
Results
H2O2-dependent CaMKII activation requires functional SR Ca stores
CaMKII autophosphorylation at serine 287 (p-CaMKII) and oxidation at methionine 281/282 (ox-CaMKII) were assessed using Western blotting in lysates of myocytes exposed to H2O2 (Fig. 1, Online Table I). Increasing cytosolic [Ca] to 1 µmol/L significantly increased p-CaMKII levels and inhibition of catalytic CaMKII activity using autocamtide 2-related inhibitory peptide (AIP) reversed this activation. In myocytes exposed to H2O2, p-CaMKII and ox-CaMKII levels increased in a concentration-dependent manner (40 vs. 200 µmol/L H2O2) and AIP significantly reduced H2O2-induced autophosphorylation but not oxidation of CaMKII. This suggests that CaMKII oxidation is accompanied by a significant autophosphorylation of CaMKII. Interestingly, when SR Ca stores were depleted using caffeine and thapsigargin (THA), both H2O2-dependent oxidation as well as autophosphorylation of CaMKII were prevented. This suggests that Ca derived from SR Ca stores is required for substantial H2O2-dependent CaMKII activation to occur. To further investigate the Ca-dependency of CaMKII oxidation, permeabilized myocytes were bathed in a mock intracellular solution, in which Ca was heavily buffered (5 mmol/L BAPTA/1 mmol/L Br2-BAPTA). Figure 2 and Online Table I show that no significant H2O2-dependent CaMKII oxidation or autophosphorylation were observed in the absence of intracellular free [Ca]. Additionally, heavily buffered low free [Ca] of 20 nmol/L prevented a substantial increase in p-CaMKII and ox-CaMKII levels after H2O2 exposure. This suggests that albeit the higher dose of H2O2 may oxidize a small amount of Ca/CaM-bound CaMKII at clamped free [Ca] of 20 nmol/L, large fluctuations of cytosolic free [Ca] are necessary for substantial oxidation to occur.
Figure 1.
ROS can activate CaMKII in permeabilized myocytes (free [Ca]i 20 nmol/L) Western blots of p-CaMKII (A) or ox-CaMKII (B) vs. CaMKII and GAPDH are shown. Mean densitometric values for p-CaMKII vs. CaMKII (C) or ox-CaMKII vs. CaMKII (D) are shown. One way ANOVA was used for statistical comparison. All densitometric values are summarized in Online Table I. CaMKII was stimulated with 1 µmol/L [Ca], 200 or 40 µmol/L H2O2. For some experiments, caffeine (10 mmol/L) and THA (5 µmol/L) were used to unload the SR or AIP (1 µmol/L) was present.
Figure 2.
ROS activation of CaMKII is Ca-dependent. Permeabilized myocytes bathed in heavy Ca buffers (5 mmol/L BAPTA/1 mmol/L Br2-BAPTA) were used for Western blots of p-CaMKII (A) or ox-CaMKII (B). All densitometric values are shown in Online Table I.
H2O2 effects on RyR2 function
We determined the effects of H2O2 on RyR2 function by analyzing the characteristics of SR Ca sparks. In permeabilized myocytes, the addition of 200 µmol/L H2O2 led to an immediate and massive SR Ca release throughout the whole cell, comparable to a caffeine transient, followed by an irreversible inhibition of RyR2 function (data not shown). Because intracellular H2O2 may reach only 1–15% of the applied exogenous concentration in case of an intact sarcolemma,22 we reduced the H2O2 concentration to 40 µmol/L and set the exposure time to 30 s for all experiments in permeabilized myocytes. Addition of H2O2 caused an immediate significant increase in CaSpF and FDHM, and a slight reduction in Ca spark amplitude, leading to a 3.5-fold increase in SR Ca leak (Fig. 3A–C). This ROS effect appeared to be transient. Indeed, 2.5 min after onset of H2O2 exposure CaSpF and amplitude had returned to baseline values, and SR Ca leak was significantly reduced despite a persistent prolongation of FDHM. Interestingly, at the time of maximal increased SR Ca leak, H2O2 exposure caused a significant reduction of SR Ca load that was not prevented by AIP (Fig. 3D, 30 s H2O2).
Figure 3.
Ca sparks were measured in permeabilized rabbit myocytes. A) Line scan images and typical corresponding Ca sparks. Mean data for CaSpF, Ca spark amplitude and FDHM (B) or calculated SR Ca leak (C) are shown. D) SR Ca content was estimated using caffeine (10 mmol/L).
SR Ca sparks were also measured in intact mouse myocytes during electrical field stimulation. Under baseline conditions, very few Ca sparks were detected (Fig. 4). Similar to permeabilized myocytes, 200 µmol/L H2O2 increased CaSpF but reduced Ca spark amplitude. The resulting SR Ca leak was markedly (about 15-fold) increased (Fig. 4 A–C). This was accompanied by reduced SR Ca load (Fig 4D). Notably, inhibition of CaMKII (KN93) did not influence the H2O2-dependent changes in CaSpF, SR Ca leak or SR Ca load, although Ca spark amplitude was slightly reduced (Fig. 4A–D). These results indicate that the H2O2-induced SR Ca leak does not require CaMKII.
Figure 4.
Ca sparks were measured in intact twitching mouse myocytes (BCL 2 s). A) Line scan images at baseline and 4 min after onset of H2O2 (200 µmol/L) without any pharmacological intervention (left) and in the presence of KN93 (10 µmol/L, right). Mean data for CaSpF, amplitude and FDHM (B) and calculated SR Ca leak (C) are shown. D) SR Ca load (caffeine 10 mmol/L) is shown.
CaMKII is required for H2O2-enhanced late INa
Our group and others have shown previously6, 10, 23, 24 that ROS enhance late INa, and CaMKII exerts a strikingly similar effect.6,13 To test whether H2O2-activated CaMKII is required for the effects of ROS on late INa, the current was measured in myocytes from WT and CaMKIIδ−/− mice (Fig. 5A). At baseline, no significant difference in late INa integrals was detected between the two groups (Fig. 5B, Table 1). When WT myocytes were exposed to H2O2, a significant time-dependent increase in the late INa integral, nearly doubling at 12 min after H2O2 treatment, was observed. This increase was absent in myocytes lacking CaMKIIδ (Fig 5 A+B, Table 1). Consistent with this, adenoviral CaMKIIδc overexpression in rabbit myocytes exhibit significantly more late INa at baseline and larger H2O2-dependent late INa increase (vs. βGal), both of which are prevented by AIP (Fig. 5 C+D, Table 1). In control myocytes, baseline late INa was not affected by AIP, which suggests that baseline CaMKII-induced late INa is negligible.
Figure 5.
Late INa after exposure to H2O2. Late INa was measured at BCL 2 s (protocol in inset) in myocytes from CaMKIIδ−/− mice (vs. WT littermates) and in isolated rabbit myocytes after CaMKIIδC overexpression (vs. βGal). Late INa was measured as the integral between 50 and 500 ms after onset of depolarisation. Original traces (A) and mean data (B) showed that H2O2 significantly increased late INa in WT myocytes. This increase was completely abolished in myocytes lacking CaMKIIδ. Similarly, H2O2 significantly increased late INa in rabbit myocytes (βGal, C&D). CaMKIIδC overexpression markedly enhanced H2O2-induced late INa, and AIP (100 nmol/L) significantly blunted this increase. *P<0.05 (two-way ANOVA) vs. WT or βGal, respectively; †P<0.05 (two-way ANOVA) vs. CaMKIIδC.
Table 1.
Late INa integral before (baseline) and during exposure to H2O2
| AmsF−1 [N] | Baseline | 12 min H2O2 |
|---|---|---|
| WT [15] | −203.5±21 | −380.1±101.1* |
| CaMKIIδ−/− [7] | −155.4±43.3 | −204.1±93.1† |
| βGal [17] | −99.3±7.4 | −331.8±77.2* |
| βGal+AIP [18] | −105.9±7.8 | −200.5±32.3† |
| βGal+TTX [4] | −66.4±22.6* | −54.5±32.1† |
| CaMKIIδC [18] | −249.1±23.3* | −719.7±192.2‡† |
| CaMKIIδC+AIP [14] | −158.7±12.4‡ | −277.0±37.4# |
| CaMKIIδC+TTX [8] | −101.9±29.4‡ | −121.4±63.5# |
Late INa integrals (50–500 ms; AmsF−1) at baseline and after 12 min of H2O2 exposure. For some experiments, AIP (100 nmol/L) or TTX (1 µmol/L) were used.
P<0.05 vs. WT (baseline) or βGal (baseline);
P<0.05 vs. WT (12 min) or βGal (12 min);
P<0.05 vs. CaMKIIδC (baseline);
P<0.05 vs. CaMKIIδC (12 min)
Low concentrations of the Na channel blocker tetrodotoxin (TTX; 1 µmol/L), which only slightly reduce peak myocyte INa, significantly reduced late INa at baseline (Table 1). Moreover, this TTX concentration abolished the H2O2-induced increase in late INa.
H2O2-induced Na and Ca overload is mediated via CaMKII
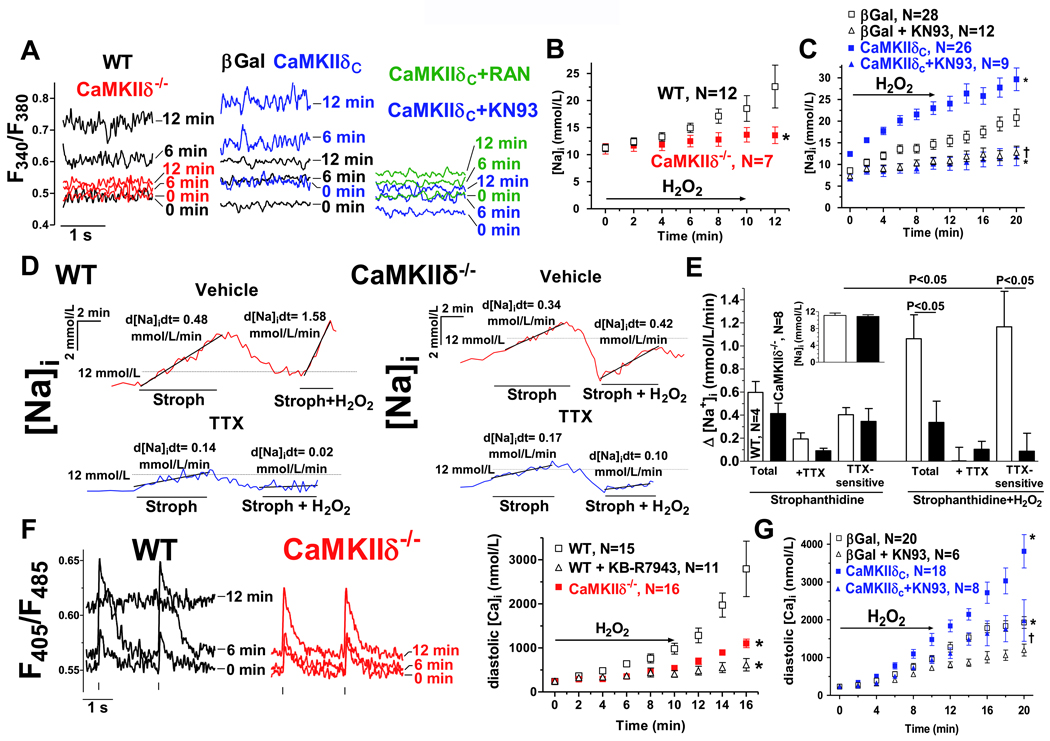
In WT myocytes (stimulated at BCL 2s), H2O2 increased [Na]i time-dependently, but this increase was much lower in CaMKIIδ−/− myocytes (Fig. 6A,B, Online Table II). Similarly, the H2O2-induced increase in diastolic [Ca]i was markedly reduced in CaMKIIδ−/− myocytes (vs. WT, Fig. 6F, Online Table II). In WT myocytes, the addition of reverse-mode NCX inhibitor KB-R7943 significantly blunted the H2O2-induced increase in diastolic [Ca]i (Fig. 6F, Online Table II). Likewise, CaMKIIδC overexpression enhanced H2O2-induced increases in [Na]i and diastolic [Ca]i significantly (vs. βGal), and KN93 slowed [Na]i and [Ca]i rise in both CaMKIIδC overexpressing and control myocytes (Fig. 6A,C&G). KN93 can also reduce ICa,L availability. The analogue KN92 (which does not inhibit CaMKII) shares this effect,25 but does not protect from H2O2-induced arrhythmogenesis.19 To control for possible ICa,L effects of KN93, experiments were also done with KN92, which did not alter the H2O2-induced increase of [Na]i and diastolic [Ca]i (Online Table II). Of note, at baseline there was no difference in [Na]i and diastolic [Ca]i between WT and CaMKIIδ−/− myocytes, or in control-transfected rabbit myocytes in the presence of KN93. This is consistent with the late INa data, arguing against an important role for CaMKII in the basal regulation of cellular Na and Ca homeostasis (Online Table II). Interestingly, at baseline, CaMKIIδC overexpression significantly increased [Na]i (reversible with KN93) but did not significantly alter diastolic [Ca]i (Online Table II).
Figure 6.
[Na]i and [Ca]i upon exposure to H2O2. A) Original traces of SBFI fluorescence in mouse (left panel) and rabbit ventricular myocytes (mid and right panel). Data is shown for baseline (0 min), 6 and 12 min after onset of H2O2 exposition (200 µmol/L). WT–black, CaMKIIδ−/−–red, CaMKIIδC overexpression–blue, βGal–black, KN93–blue, RAN–green. Mean data after conversion to [Na]i is shown for mouse (B) and rabbit myocytes (C). D) Original traces of Na influx (via [Na]i-converted SBFI fluorescence) in mouse ventricular myocytes (BCL 2s) exposed to 100 µmol/L strophanthidin (Stroph) (with and without 1 µmol/L TTX and/or 200 µmol/L H2O2). E) Mean data for the rate of rise of [Na]i (baseline [Na]i in inset). (one-way ANOVA). F) Original traces of Indo-1 fluorescence at baseline (0 min), 6 and 12 min after onset of exposure to H2O2 (myocytes from WT, left panel and CaMKIIδ−/−, mid panel). Vertical bars indicate the electrical stimulus. Right panel shows mean data after conversion to [Ca]i. G) Mean data for rabbit myocytes. CaMKIIδC overexpression significantly enhanced (vs. βGal) and CaMKII inhibition (10 µmol/L KN93) significantly slowed the increase in diastolic [Ca]i after H2O2. *P<0.05 (two-way ANOVA) vs. WT or βGal, respectively, †P<0.05 (two-way ANOVA) vs. CaMKIIδC
To further investigate the importance of late INa for H2O2-induced increase in [Na]i we conducted quantitative Na influx measurements in the presence of the NKA inhibitor strophanthidin (Fig. 6D+E). TTX (1 µmol/L) was used to specifically block Na influx via INa. At baseline, the TTX-sensitive Na influx was not different between WT and CaMKIIδ−/− myocytes, in accordance with our late INa data. The addition of 200 µmol/L H2O2 increased the TTX-sensitive Na influx nearly threefold in WT but not in CaMKIIδ−/− myocytes (Fig. 6E) again consistent with our late INa measurements (Table 1). These data is further supported by experiments using the late INa inhibitor ranolazine (RAN). RAN significantly reduced the H2O2-induced gain in [Na]i and diastolic [Ca]i after CaMKIIδC overexpression, but also in control (βGal, Fig. 6A, Online Table II). Interestingly, RAN also reduced [Na]i and diastolic [Ca]i before H2O2 exposure (Online Table II) consistent with a relevant TTX-sensitive Na influx at baseline (Fig. 6E).
To test the hypothesis that ROS-mediated SR Ca release is required for CaMKII activation leading to [Na]i and diastolic [Ca]i overload, SR Ca stores were depleted with THA. THA greatly slowed the H2O2-induced increase in [Na]i and diastolic [Ca]i for both CaMKIIδC overexpressing and control myocytes (Online Table II), completely eliminating the difference between CaMKIIδC and βGal (at 12 min, Online Table II). Interestingly, THA significantly reduced [Na]i in myocytes overexpressing CaMKIIδC not only after H2O2 exposure but also at baseline. THA may possibly interfere with a positive feedback loop involving CaMKII-dependent Ca spark augmentation26 leading to Ca-dependent CaMKII activation. On the other hand, THA did not alter [Na]i in control-transfected myocytes, in line with our data in permeabilized myocytes showing no significant reduction of CaMKII activity with caffeine/THA at baseline. This supports the concept that baseline CaMKII activity is low and reduction of SR Ca release does not further reduce its activity.
H2O2-induced arrhythmias and CaMKII
To test, whether CaMKII inhibition could prevent ROS-induced arrhythmias, mouse myocytes were exposed to H2O2 and membrane potential or sarcomer length were continuously monitored. H2O2 significantly prolonged action potential duration and depolarized the diastolic membrane potential in WT but not in CaMKIIδ−/− myocytes (Fig. 7A–C). At baseline, there was regular rhythmic activity for both WT and CaMKIIδ−/−. The addition of H2O2 induced EADs and DADs (Fig. 7D) as well as irregular contractile activity (Fig. 7E). These arrhythmias were significantly more frequent and more severe in WT vs. CaMKIIδ−/− (Fig. 7D,F). Cellular Ca overload results in the development of hypercontracture,5 which is a permanent reduction (<50%) of longitudinal cell length due to a persistent activation of myofilaments. Kaplan-Meier analysis (Fig. 7G) revealed that myocytes lacking CaMKIIδ were more resistant to ROS-induced hypercontracture.
Figure 7.
ROS-induced arrhythmias. A) Original traces of stimulated APs before and after H2O2 treatment. Mean data shows a significant H2O2-dependent increase in AP duration (APD 90, B), and a significant positive shift in the diastolic action potential in WT (C), but no change in APD and no positive shift diastolic action potential in CaMKIIδC−/− myocytes. Insets show baseline APD 90 and diastolic potential, respectively. D) Original traces of consecutive APs (left) in myocytes from WT or CaMKIIδC−/− mice. Mean data (right) shows that H2O2-induces EADs+DADs were significantly more frequent in WT vs. CaMKIIδC−/−. E) Original traces of sarcomere length in intact twitching myocytes from WT or CaMKIIδC−/− mice. F) Mean data. The propensity for H2O2-induced arrhythmias was significantly reduced in CaMKIIδC−/−. G) Kaplan-Meier analysis of hypercontracture development. *P<0.05 vs. WT. BCL 2 s, vertical bars indicate stimuli.
Discussion
We investigated the mechanisms by which the exposure of H2O2 enhances late INa in isolated ventricular myocytes. We found that 1) ROS-activated CaMKIIδ is required for late INa augmentation, which was abolished in the absence of CaMKIIδ expression, and 2) ROS-induced SR Ca release is a prerequisite for ROS-dependent CaMKII activation, based on the observation that the latter was inhibited after SR depletion with THA and caffeine.
These novel findings add to the growing body of evidence that CaMKII is activated under pathophysiological conditions being involved in signaling cascades that lead to dysregulation of cellular Na and Ca homeostasis, increased arrhythmogenesis, and ultimately cell death.
The role of the SR Ca stores in ROS-dependent CaMKII activation
In a fundamental study Erickson and colleagues have shown that ROS oxidize CaMKII at methionine 281/282, and this oxidation renders CaMKII autonomous of Ca/CaM levels similar to autophosphorylation at threonine 287.9 Direct oxidation of the kinase also produces a secondary increase in the fraction of autophosphorylated subunits.27, 28 We showed here that exogenous H2O2 applied to permeabilized myocytes increased CaMKII oxidation and autophosphorylation. We also showed that oxidation and autophosphorylation critically depend on free cytosolic Ca levels. Without free [Ca]i no significant H2O2-dependent CaMKII oxidation or autophosphorylation was observed (Fig. 2). This is in accordance with the concept that H2O2 requires initial interaction of Ca/CaM with CaMKII to expose the oxidation and autophosphorylations sites of CaMKII.9
Moreover, we showed that a free [Ca]i of 20 nmol/L in the presence of a strong and fast Ca buffer (BAPTA/Br2-BAPTA) is not sufficient to result in H2O2-dependent CaMKII activation. In contrast, if fast localized increases in free [Ca] (during diastolic SR Ca leak or Ca sparks) are allowed by a slower buffer (EGTA), the same free [Ca]i of 20 nmol/L facilitates a robust H2O2-dependent CaMKII activation (Fig. 1). This result is in accordance with Song et al.,27 who showed that high concentrations of BAPTA but not EGTA could prevent H2O2-induced CaMKII-dependent Ca current facilitation. Further evidence comes from Xie and colleagues showing that BAPTA loaded myocytes were resistant to H2O2-induced CaMKII-dependent formation of early afterdepolarizations (EADs).19 On the other hand, Palomeque at al. showed that neither extracellular application of 1 µmol/L BAPTA-AM in cultured myocytes nor zero Ca-EGTA used for an in vitro phosphorylation assay were sufficient to inhibit H2O2-induced CaMKII autophosphorylation.28 Methodological differences may account for this discrepancy: although it is likely that BAPTA was properly loaded into their cultured myocytes, its concentration may not be sufficient to avoid very fast localized increases of [Ca]i. Also, the isoosmotic homogenization buffer of their in vitro assay contained no detergent, thus leaving functional SR vesicles in the phosphorylation reaction. This suggests that SR Ca release may be involved in the rapid localized increases of free [Ca]i required for H2O2-dependent CaMKII activation. Here we show that unloading the SR with caffeine and THA abolished the H2O2-induced CaMKII oxidation and autophosphorylation. Moreover, in the presence of THA, the H2O2-dependent cytosolic Na and Ca overload was markedly reduced. This relates mechanistically to work showing that pre-incubation with caffeine and THA abolished the H2O2-activated CaMKII-dependent Ca current facilitation.27 Therefore, an SR-dependent mechanism appears to be required for ROS-dependent oxidation of CaMKII.
It has been shown that thiol-oxiziding agents like H2O2 activate RyR Ca release after oxidation of more than seven thiols per subunit.20, 29–32 In line with this, we show here that H2O2 immediately increased CaSpF and SR Ca leak. The SR Ca load was reduced, which may be a consequence of an increased SR Ca leak and/or inhibition of SERCA,33, 34 and may account for the observed reduction in Ca spark amplitude. Because activated CaMKII is known to phosphorylate RyR2 thereby increasing CaSpF,26 we cannot exclude that the observed H2O2-induced changes in CaSpF and SR Ca leak are secondary to CaMKII activation. However, acute CaMKII inhibition with KN93 did not affect the H2O2-induced increases in CaSpF and SR Ca leak indicating that direct H2O2-induced thiol-oxidation of RyR2 may be more important.
ROS enhance late INa via CaMKII activation
ROS have been shown to acutely enhance late INa leading to AP prolongation and EADs.6, 24, 35 To date, the mechanism of ROS action on Na channels is not fully understood. Changes in the lipid environment24 or oxidation of multiple methionine residues of the channel may be responsible.36 Here we show that the acute H2O2-dependent increase in late INa was absent in myocytes with genetic knock out of CaMKIIδ. Furthermore, subacute adenoviral CaMKIIδC overexpression enhanced whereas acute CaMKII inhibition with AIP slowed the H2O2-induced increase of late INa. Therefore, although redox modification of the Na channel or the lipid environment cannot be completely excluded, the dominating mechanism here involves acute CaMKIIδ activation.
We have shown that CaMKII can phosphorylate cardiac Na channels α isoform (NaV1.5)13. Aiba et al. showed that CaMKII phosphorylates the I–II linker but also to a lesser extent the C-terminus.37 Hund et al. found that CaMKII phosphorylates serine 571 in the I–II linker contributing to altered INa gating.38 Although phosphorylation does occur, ROS-activated CaMKII may also regulate late INa independent of its catalytic activity, as it was shown for oxidation-dependent facilitation of ICa,L.27 Because the cardiac Na channel is a macromolecular complex, CaMKII interaction with proteins of this complex could result in altered Na channel gating. For example, co-expression of NaV1.5 with cardiac β1 but not β2 subunit increased late INa,39 and it may be possible that oxidized CaMKII is involved in the interaction of α and β subunits.
Beside CaMKII, protein kinase A or C (PKA or PKC) are known to regulate Na channel gating. ROS have been shown to oxidize PKA regulatory subunit I, resulting in PKA translocation and activation.40 PKA increases peak INa current density via acceleration of channel trafficking to the plasma membrane,41 but does not change inactivation kinetics.42, 43 Mild oxidative stress has also been shown to activate PKC.44 The redox regulation of PKC is very specific for different isoforms and different cell types. Ward and Giles showed that the H2O2-dependent slowing of INa open state inactivation was blocked in the presence of the PKC inhibitor Bis-indolylmaleimide.10. In heterologeous expression systems (rat and human NaV1.5), however, PKC activation did not change INa inactivation.45, 46 The most consistent effect of PKC on cardiac Na channels is a reduction in peak INa current density47
Cellular Na and Ca accumulation
The present study shows that ROS-induced Na and Ca overload are blunted in myocytes lacking CaMKIIδ. We also show that SR Ca unloading with THA similarly reduces ROS-induced Na and Ca overload, suggesting that the CaMKII activation depends on SR function. These data suggest that CaMKIIδ is critically involved in ROS-induced [Na]i and [Ca]i overload. Quantitative Na influx measurements showed that TTX-sensitive Na entry is markedly increased by ROS exposure in WT but not in CaMKIIδ−/− myocytes and the late INa blocker RAN slowed the development of Na and Ca overload, thus suggesting that ROS-induced Na entry is mediated via Na channels. This is in line with data from us and others.6, 8, 48 We propose that the increase in [Na]i precedes the major rise in [Ca]i because reduction of extracellular [Na] or application of Na channel blockers slows the rise in [Ca],8 and inhibition of reverse mode NCX activity with KB-R7943 significantly blunts Ca overload in the present study. However, because RAN did not completely abolish the increase in [Na]i and [Ca]i, other mechanisms may also be involved. For example, ROS can significantly impair sodium-potassium ATPase (NKA) function,49 reduce SERCA2 activity and L-type Ca current (ICaL),50 and increase NCX activity.51, 52 In order to further understand these mechanisms we utilized numerical simulations of the cardiac action potential and Na and Ca handling.53 Incorporation of the measured ROS-induced late INa into the model led to a rise in [Na]i less than 1 mmol/L (Online Fig. IA: a vs. b), much less than measured experimentally. ROS may also (via CaMKII) increase diastolic TTX-sensitive Na influx, as reported in HF myocytes.54 Increasing background Na conductance can bring the model to match the measured [Na]i (Online Fig. IA, c)), but inclusion of ROS-dependent NKA inhibition49 limits the extent of background Na current that is required to explain the measured [Na]i increase (Online Fig. IA: c vs. d). If we also incorporate the above mentioned ROS-dependent effects on SERCA2a, RyR2, ICaL, and NCX function,50–52 the model reasonably mimics the ROS-induced alterations observed here in [Na]i and SR Ca content (Online Fig. I A, f, B and C). Three aspects merit comment. First, the elevated [Na]i and reduced Ca transient amplitude is predicted to cause substantial Ca influx via NCX during the action potential (i.e. reverse mode NCX; Online Fig. IB–C) and less Ca extrusion during diastole. This is also seen in human and rabbit HF myocytes which also exhibit elevated [Na]i and reduced Ca transients.54, 55 This agrees with our results that the NCX inhibitor KB-R7943 suppressed the ROS-induced increase in diastolic [Ca]i (Online Table II, Fig. 6F), that ROS-induced Ca overload depends on shifts of NCX activity,56 and that late INa is responsible for diastolic Ca accumulation in HF where ROS production is augmented.57 Second, turning off only the late and diastolic INa simulates reasonably the effects of ranolazine on [Na]i (Online Fig. IA, g). Third, the model does not predict the extent of diastolic [Ca]i elevation observed. Because intracellular Na and Ca are compartmentalized, part of the progressive measured rise in [Na]i and [Ca]i may be attributable to a consequent gradual rise in mitochondrial [Na] and [Ca].
Arrhythmogenesis
Here we show that the propensity for ROS-induced EADs/DADs and cellular arrhythmias were significantly reduced in myocytes lacking CaMKIIδC, although they were not completely abolished. ROS effects in myocytes are clearly multifactorial, and so are arrhythmogenic mechanisms. Therefore, activated CaMKII may be an important factor for ROS-induced arrhythmias but does not explain all arrhythmias that develop upon ROS or in HF. Possible mechanisms that contribute to the electrical instability are EADs and DADs. It was shown previously that enhanced late INa could prolong APD leading to EADs.6 Moreover, transient inward INCX (Iti), which leads to DADs may results from ROS-induced cellular Ca overload and increased RyR2 open probability. Here we show that both EADs and DADs develop less often after H2O2 exposure in myocytes lacking CaMKIIδC. While the reduction in the propensity for EADs in CaMKIIδ−/− myocytes may result from the lack in APD prolongation, less frequent DADs possibly indicate less cellular Na and Ca overload as shown here. The CaMKIIδ-dependent increase in late INa may be responsible for the observed H2O2-induced APD prolongation. Although ROS can decrease outward currents like Ito,58 CaMKII activation tends to increase Ito.21 In the absence of confounding CaMKII activity (CaMKIIδ−/− myocytes), we found that H2O2 did not increase APD, which argues against a significant role for inhibition of Ito in APD prolongation caused by H2O2. Interestingly, H2O2 rendered the diastolic membrane potential more positive increasing the likelihood that depolarizing currents would induce afterdepolarizations. This was not the case in CaMKIIδ−/− myocytes. Nevertheless, although we show here evidence that CaMKII may be involved in ROS-induced arrhythmias there was still H2O2-induced arrhythmogenic activity detectable in CaMKIIδ−/− myocytes suggesting that there are CaMKII-independent mechanisms as well. For instance ROS can directly oxidize RyR2 leading to increased CaSpF.31 While the present paper establishes a clear mechanistic working hypothesis for the role of CaMKIIδ in ROS-dependent regulation of late INa and its consequences, further studies are needed that aim at testing these mechanisms in HF.
In summary, we show that CaMKIIδ is required for the H2O2-induced augmentation of late INa and secondary changes of [Na]i and [Ca]i. The present results also suggest that ROS-induced SR Ca release may be a prerequisite for ROS-dependent CaMKII activation. In addition, CaMKIIδ appears to be involved in H2O2-induced arrhythmogenesis. These results are important, because expression and activity of CaMKII have been shown to be increased in HF,59–61 where ROS generation is enhanced.3 In addition, HF is associated with enhanced late INa62 leading to dysregulation of intracellular Na and Ca homeostasis, electrical instability and cell death.
Novelty and Significance.
What is known?
Heart failure (HF) is associated with increased reactive oxygen species (ROS) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) expression.
ROS can activate CaMKII by oxidation.
ROS and CaMKII can increase late INa and intracellular Na concentration.
What new information does this article contribute?
Ca2+ release from the sarcoplasmic reticulum (SR) is required for ROS-dependent CaMKII oxidation and autophosphorylation.
ROS-activated CaMKII enhances late INa leading to cellular Na+ and Ca2+ overload.
ROS-activated CaMKII is arrhythmogenic.
In HF, intracellular ROS are increased and CaMKII expression and activity is upregulated, contributing to electrical and functional remodeling. CaMKII was previously shown to be activated by ROS through Ca2+-dependent processes but the origin of Ca2+ remained unclear. In addition, ROS and CaMKII have both been shown to increase late INa leading to proarhythmogenic events but a mechanistic link was missing. In this study, we show that SR Ca release in required for ROS-dependent CaMKII oxidation and autophosphorylation, which leads to increased late INa, intracellular Na accumulation and Ca2+ overload. Moreover, ROS-activated CaMKII leads to action potential prolongation and cellular arrhythmias. These results provide novel insights into the Ca2+-dependence of the ROS activation process for CaMKII. Moreover, they highlight the importance of CaMKII for the ROS-induced disturbance of excitation-contraction coupling and arrhythmogenesis. Taken together, the present study suggests ROS-activated CaMKII as a promising new target for the treatment of heart failure.
Supplementary Material
Acknowledgment
We thank Dr. M.E. Anderson (Carver College of Medicine, University of Iowa, IA, USA) for kindly providing the ox-CaMKII antibody.9 We thank Ekaterina Skorova for assistance in the action potential experiments.
Source of Funding
Dr. Wagner was funded by the Research Program, Faculty of Medicine, Georg-August-University Göttingen. Dr. Bers is funded by NIH-P01-HL80101. Dr. Maier (MA 1982/4-1&2-2) and Dr. Backs are funded by the DFG (BA 2258/2-1). Dr. Maier was funded by the Fondation Leducq Transatlantic Network of Excellence on “Redox and Nitrosative Regulation of Cardiac Remodeling: Novel Therapeutic Approaches for Heart Failure”.
Non-standard Abbreviations and Acronyms
- CaMKII
Ca/calmodulin-dependent protein kinase II
- CaMKIIδ−/−
homozygous knockout of Ca/calmodulin-dependent protein kinase IIδ
- APD
action potential duration
- THA
thapsigargin
- RAN
ranolazine
- TTX
tetrodotoxin
- HF
heart failure
- ROS
reactive oxygen species
- SR
sarcoplasmic reticulum
- INa
Na current
- NAPDH
nicotinamide adenine dinucleotide phosphate oxidase
- NCX
sodium/calcium exchanger
- NKA
sodium/potassium ATPase
- SERCA
sarcoplasmic reticulum calcium ATPase
- RyR2
ryanodine receptor type 2
- CaSpF
calcium spark frequency
- AIP
autocamtide 2-related inhibitory peptide
- FDHM
full-duration-half-maximum
- [Na]i
intracellular sodium concentration
- [Ca]i
intracellular calcium concentration
- PKA
protein kinase A
- SBFI
sodium-binding benzofuran isophthalate
- AM
acetoxymethylester
- βGal
β-galactosidase
- FKBP
FK-binding protein
- BCL
basic cycle length
- EGTA
ethylene glycol tetraacetic acid
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Dr. Maier has a collaborative/research grant with Gilead Sciences, CA, USA. Dr. Belardinelli is an employee of Gilead Sciences, CA, USA.
References
- 1.Tsutsui H, Ide T, Hayashidani S, Suematsu N, Utsumi H, Nakamura R, Egashira K, Takeshita A. Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovasc Res. 2001;49:103–109. doi: 10.1016/s0008-6363(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 2.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: Role of oxidative stress. Circ Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 3.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin f-2 alpha in pericardial fluid of patients with heart failure - a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 4.Josephson R, Silverman H, Lakatta E, Stern M, Zweier J. Study of the mechanisms of hydrogen-peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem. 1991;266:2354–2361. [PubMed] [Google Scholar]
- 5.Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kögler H. Na+- Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. doi: 10.1016/j.cardiores.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Shryock J, Wagner S, Maier L, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- 7.Corretti M, Koretsune Y, Kusuoka H, Chacko V, Zweier J, Marban E. Glycolytic inhibition and calcium overload as consequences of exogenously generated free-radicals in rabbit hearts. J Clin Invest. 1991;88:1014–1025. doi: 10.1172/JCI115361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haigney MC, Lakatta EG, Stern MD, Silverman HS. Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation. 1994;90:391–399. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of camkii by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500:631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeitz O, Maass AE, Van Nguyen P, Hensmann G, Kogler H, Moller K, Hasenfuss G, Janssen PM. Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode na+- ca2+ exchange. Circ Res. 2002;90:988–995. doi: 10.1161/01.res.0000018625.25212.1e. [DOI] [PubMed] [Google Scholar]
- 12.Inserte J, Garcia-Dorado D, Ruiz-Meana M, Padilla F, Barrabes JA, Pina P, Agullo L, Piper HM, Soler-Soler J. Effect of inhibition of Na+/Ca2+ exchanger at the time of myocardial reperfusion on hypercontracture and cell death. Cardiovasc Res. 2002;55:739–748. doi: 10.1016/s0008-6363(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 13.Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase ii. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIδc overexpression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 17.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The δ isoform of CaM Kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-s-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 21.Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, Sowa T, Fabritz L, Kirchhof P, Bers DM, Maier LS. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009;2:285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: Issues and considerations. Curr Opin Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Wang GK, Wang SY. Modifications of human cardiac sodium channel gating by uva light. J Membr Biol. 2002;189:153–165. doi: 10.1007/s00232-002-1010-z. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, Amarnath V, Anderson ME, Boyden PA, Viswanathan PC, Roberts LJ, 2nd, Balser JR. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Blair L, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: Reversible inhibition of l-type calcium channels. Biochem Biophys Res Comm. 2006:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 26.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Brown J, Bers D, Maier L. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res. 2006;98:235–244. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 27.Song YH, Cho H, Ryu SY, Yoon JY, Park SH, Noh CI, Lee SH, Ho WK. L-type Ca2+ channel facilitation mediated by H2O2-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol. 2010;48:773–780. doi: 10.1016/j.yjmcc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 29.Boraso A, Williams AJ. Modification of the gating of the cardiac sarcoplasmic reticulum Ca2+-release channel by H2O2 and dithiothreitol. Am J Physiol. 1994;267:H1010–H1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- 30.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 31.Anzai K, Ogawa K, Kuniyasu A, Ozawa T, Yamamoto H, Nakayama H. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Comm. 1998;249:938–942. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- 32.Zissimopoulos S, Docrat N, Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. J Biol Chem. 2007;282:6976–6983. doi: 10.1074/jbc.M607590200. [DOI] [PubMed] [Google Scholar]
- 33.Rowe GT, Manson NH, Caplan M, Hess ML. Hydrogen peroxide and hydroxyl radical mediation of activated leukocyte depression of cardiac sarcoplasmic reticulum. Participation of the cyclooxygenase pathway. Circ Res. 1983;53:584–591. doi: 10.1161/01.res.53.5.584. [DOI] [PubMed] [Google Scholar]
- 34.Kourie J. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol Cell Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 35.Barrington P, Meier C, Weglicki W. Abnormal electrical-activity induced by free-radical generating systems in isolated cardiocytes. J Mol Cell Cardiol. 1988;20:1163–1178. doi: 10.1016/0022-2828(88)90596-2. [DOI] [PubMed] [Google Scholar]
- 36.Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, Heinemann SH. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch. 2008;456:1085–1095. doi: 10.1007/s00424-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aiba T, Hesketh G, Liu T, Carlisle R, Villa-Abrille M, O'Rourke B, Akar F, Tomaselli G. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010:454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hund T, Koval O, Li J, Wright P, Dian L, Snyder J, Gudmundsson H, Kline C, Davidson N, Cardona N, Rasband M, Anderson M, Mohler P. A βiv-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltsev VA, Kyle JW, Undrovinas A. Late Na+ current produced by human cardiac Na+ channel isoform Nav1.5 is modulated by its β1 subunit. J Physiol Sci. 2009;59:217–225. doi: 10.1007/s12576-009-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan J, Bardswell S, Burgoyne J, Fuller W, Schroder E, Wait R, Begum S, Kentish J, Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006:21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 41.Hallaq H, Yang Z, Viswanathan P, Fukuda K, Shen W, Wang D, Wells K, Zhou J, Yi J, Murray K. Quantitation of protein kinase A-mediated trafficking of cardiac sodium channels in living cells. Cardiovasc Res. 2006:250–261. doi: 10.1016/j.cardiores.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Murphy BJ, Rogers J, Perdichizzi AP, Colvin AA, Catterall WA. cAMP-dependent phosphorylation of two sites in the α subunit of the cardiac sodium channel. J Biol Chem. 1996;271:28837–28843. doi: 10.1074/jbc.271.46.28837. [DOI] [PubMed] [Google Scholar]
- 43.Frohnwieser B, Chen L, Schreibmayer W, Kallen R. Modulation of the human cardiac sodium channel α-subunit by cAMP-dependent protein kinase and the responsible sequence domain. J Physiol (Lond) 1997;498:309–318. doi: 10.1113/jphysiol.1997.sp021859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gopalakrishna R, Anderson W. Ca2+-independent and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Nat Acad Sci. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu Y, Rogers J, Tanada T, Scheuer T, Catterall W. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Nat Acad Sci. 1994;91:3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray KT, Hu N, Daw JR, Shin H-G, Watson MT, Mashburn AB, George AL., Jr Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res. 1997;80:370–376. doi: 10.1161/01.res.80.3.370. [DOI] [PubMed] [Google Scholar]
- 47.Qu Y, Rogers J, Tanada T, Catterall W, Scheuer T. Phosphorylation of S1505 in the cardiac Na+ channel inactivation gate is required for modulation by protein kinase C. J Gen Physiol. 1996;108:375–379. doi: 10.1085/jgp.108.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams IA, Xiao XH, Ju YK, Allen DG. The rise of [Na+]i during ischemia and reperfusion in the rat heart-underlying mechanisms. Pflugers Arch. 2007;454:903–912. doi: 10.1007/s00424-007-0241-3. [DOI] [PubMed] [Google Scholar]
- 49.Shattock MJ, Matsuura H. Measurement of Na+-K+ pump current in isolated rabbit ventricular myocytes using the whole-cell voltage-clamp technique. Inhibition of the pump by oxidant stress. Circ Res. 1993;72:91–101. doi: 10.1161/01.res.72.1.91. [DOI] [PubMed] [Google Scholar]
- 50.Goldhaber J, Liu E. Excitation-contraction coupling in single guinea-pig ventricular myocytes exposed to hydrogen-peroxide. Journal of Physiology-London. 1994:135–147. doi: 10.1113/jphysiol.1994.sp020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldhaber J. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol Heart Circ Physiol. 1996:H823–H833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 52.Soliman D, Hamming K, Matemisz L, Light P. Reactive oxygen species directly modify sodium-calcium exchanger activity in a splice variant-dependent manner. J Mol Cell Cardiol. 2009:595–602. doi: 10.1016/j.yjmcc.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Grandi E, Puglisi J, Wagner S, Maier L, Severi S, Bers D. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105:2543–2548. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 55.Weber C, Piacentino V, Houser S, Bers D. Dynamic regulation of sodium/calcium exchange function in human heart failure. Circulation. 2003:2224–2229. doi: 10.1161/01.CIR.0000095274.72486.94. [DOI] [PubMed] [Google Scholar]
- 56.Haigney MC, Miyata H, Lakatta EG, Stern MD, Silverman HS. Dependence of hypoxic cellular calcium loading on Na+-Ca2+ exchange. Circ Res. 1992;71:547–557. doi: 10.1161/01.res.71.3.547. [DOI] [PubMed] [Google Scholar]
- 57.Undrovinas N, Maltsev V, Belardinelli L, HN S, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Z, Abe J, Taunton J, Lu Y, Shishido T, McClain C, Yan C, Xu SP, Spangenberg TM, Xu H. Reactive oxygen species-induced activation of p90 ribosomal S6 kinase prolongs cardiac repolarization through inhibiting outward K+ channel activity. Circ Res. 2008;103:269–278. doi: 10.1161/CIRCRESAHA.107.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 60.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 61.Currie S, Loughrey CM, Craig MA, Smith GL. Calcium/calmodulin-dependent protein kinase IIδ associates with the ryanodine receptor complex and regulates channel function in rabbit heart. Biochem J. 2004;377:357–366. doi: 10.1042/BJ20031043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.