Abstract
Background:
The use of electric current during application of etch-and-rinse adhesive systems has been recently introduced to decrease microleakage. This study investigated the effects of an electric field produced by an experimental device for the application of a two-step etch-and-rinse adhesive on moist dentin surface.
Methods:
Sixty freshly extracted human premolars were used for this study. In order to simulate real conditions, the pulpal pressure was set to 35 cm H2O for all the specimens. The teeth were divided into two groups: in group I, etch-and-rinse system (Single Bond) was applied with electric current while group II, etch-and-rinse system (Single Bond) was applied without electric current. Specimens were prepared for dye penetration test. The data were analyzed using Mann-Whitney U test.
Results:
The results showed that group I had less microleakage scores compared to group II (P = 0.047).
Conclusion:
Based on the result of this study, it could be concluded that using electric current for applying adhesive systems had a significant effect on reducing microleakage.
Keywords: Dental leakage, Dental pulp, Dentin-bonding agents, Electric current
Introduction
Despite recent developments in adhesive dentistry to reduce the number of working steps and to simplify the clinical procedure, bonding to dentin and complete sealing of the exposed dentinal surfaces remained problematic.1 The new simplified adhesives do not produce better results in in vitro tests2 or improve clinical efficacy.3 Different modifications to the application protocols of these simplified etchandrinse adhesives have been reported. They include application of multiple layers,4,5 enhanced solvent evaporation,6 and prolonged curing time.7
All dentin adhesives are currently applied mechanically to tooth structures using either disposable sponges or brushes. An adhesive application protocol, based on the use of an electric signal to enhance monomer infiltration in dentin has been introduced. This technique utilizes an electric field to enhance resin infiltration into the demineralized collagen matrices of acidetched dentin.8
Bonding to dentin and complete sealing of the exposed dentinal surfaces is troublesome because of highly hydrated and complex nature of the tissue. Dentin tubules constitute 20% to 39% of dentin, and the fluid within them represents 22% of dentin volume.9 The fact is easily overlooked in the design of most in vitro studies in which the effect of pulp pressure on the bonding interface is not considered. This study investigated the effects of an electric field produced by an experimental device for the application of a twostep etchandrinse adhesive on moist dentin surface. In order to simulate real conditions, the pulpal pressure was set for all specimens. The null hypothesis was defined as follows: “there is no difference in the microleakage between a conventional mechanical adhesive application technique and the use of an electric impulse assisted adhesive application technique under simulated pulpal pressure condition.”
Materials and Methods
Sixty fresh, cariesfree human premolars which were extracted for orthodontic purposes were selected. The teeth were cleaned thoroughly to remove both hard and soft deposits and were kept in distilled water at 4ºC for 24 hours. Class V cavities were prepared, with the gingival margin 1 mm below the CEJ, using a #4 round bur (Brasseler, Savannah, GA, USA) with a high speed handpiece and copious amounts of water. The preparations were standardized at 4 mm long, 3 mm wide and 2 mm depth and placed in the facial surfaces of each tooth. A 0.5 mm width bevel was placed on the enamel margins.
The apical half of each tooth was removed. Pulp tissue was removed by means of endodontic Kfiles (#20) taking care to avoid touching the pulp chamber walls. The pulp chambers were then irrigated with 2.5% sodium hypochlorite solution (NaOCl) for 30 s followed by immersion in distilled water for 30 min to neutralize the effects of NaOCl. All crown segments were luted with cyanoacrylate (Zapit, DVA, Anaheim, CA, USA) to a plexiglass plate through which an 18gauge stainless steel tube had been inserted. This tube permitted communication with the pulp chamber and was attached to an empty 20 ml plastic syringe barrel which had 10 cm height. The barrel was filled with distilled water and raised 25 cm from the tooth level in order to produce a pressure of 35 cm H2O at the dentine surface to be bonded (Figure 1).
Figure 1.
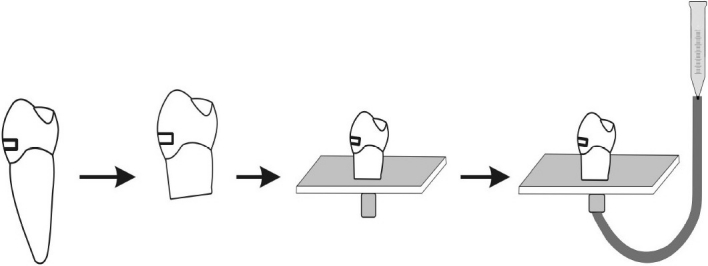
Schematic presentation showing how crown segments were created, attached to plexiglass and how fluid permeability was measured under 35 cm H2O pressure
Each prepared tooth was then etched with phosphoric acid (Ultraetch 35%, Ultradent, South Jordan, UT, USA) for 15 seconds, rinsed for 20 seconds, and then gently blown to remove excess water, being careful to maintain a moist surface. Then, the teeth were randomly divided into two groups. Thirty teeth were assigned to each group. In group I, the adhesive (Single Bond, 3M ESPE, St Paul, MN, USA) was applied using the experimental electric device (Multifrequency current source, Iran) which created an electric potential difference between the dentin substrate and the adhesive applicator tip. The electric applicator was used with a continuous brushing motion. The device induced an electric flow over 15 µA throughout the adhesive interface during the application procedure. In group II, the adhesive was applied in the same manner, but the electric generator switched off. A single blind study design was used, in which the operator performing the bonding procedure was not aware of the operating state of the electrical device (i.e., switchedon mode or switched-off mode). The adhesives were then light activated for 20 seconds at 600 mW/cm2 using a QuartzTungstenHalogen light (Coltolux 50, Coltene/Whaledent Inc, Cuyahoga Falls, OH, USA). Prior to this stage, the curing light was tested with a curing radiometer (Coltene/Whaledent Inc, Cuyahoga Falls OH, USA) and the output intensity was maintained at 600 mW/cm2 throughout the restorative procedures. One increment of microhybrid resin composite (Valux Plus A2, 3M ESPE, St Paul, MN, USA) was placed over the bonded dentin surface and polymerized for 40 seconds.
The restorations were wet finished using soflex disks (3M ESPE, St Paul, MN, USA). The teeth were stored in distilled water for 24 hours at 37ºC before thermocycling which comprised 1000 cycles (20 seconds in a 55ºC water bath, followed by 20 seconds in a 5ºC water bath, with a dwell time of 5 seconds). An acidresistant varnish (nail polish) was applied to all surfaces of the teeth except for 1 mm adjacent to the restoration margins. All specimens were then immersed in 0.2% basic fuschin for 24 hours at 37ºC and washed in running water.
The teeth were embedded in epoxy resin blocks then sectioned buccolingually through the center of the restoration with a diamond disk (KG Sorensen Ind Com Ltd, São Paulo, Brazil) at low speed. Dye penetration at the gingival margin was examined using a stereomicroscope at 40x and scored according to the following criteria: 0 = no dye penetration, 1 = dye penetration that extended up to 0 of the preparation depth, 2 = dye penetration greater than 1/3 and up to 2/3 of the preparation depth, 3 = dye penetration extending to the axial wall and 4 = dye penetration past the axial wall (Figure 2). Statistical analysis was performed using MannWhitney U test.
Figure 2.
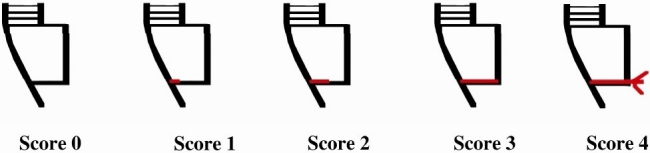
Diagram of microleakage evaluation criteria
Results
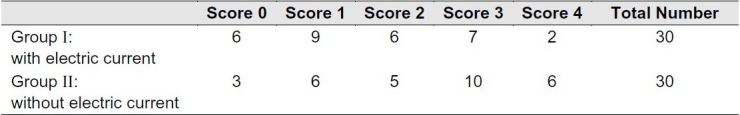
Table 1 shows microleakage scores for the two groups. When comparing the two groups, Mann-Whitney U test showed significantly less dye penetration in group 1 in which bonding agent was applied to dentin by the electric-current-assisted application technique(P = 0.047)
Table 1.
Frequency of microleakage scores
Discussion
The results of this study led to rejection of the null hypothesis (there is no difference in the microleakage between a conventional mechanical adhesive application technique and the use of an electric impulse assisted adhesive application technique under simulated pulpal pressure condition). According to results, using electric current for applying the etchandrinse adhesive (Single Bond) had a significant effect on reducing microleakage compared to the conventional application technique.
in vitro studies examining the microleakage are useful but most are not done under in vivo conditions; that is, the teeth are nonvital and are not subjected to pulpal pressure. In the present study, the entire evaluation was carried out under simulated pulpal pressure and the teeth were submitted to thermocycling to obtain a condition as similar as possible to the in vivo conditions. Interpretations of the better microleakage results that accompanied the use of an assisted electric field may be explained with different hypotheses. Since Single Bond contains HEMA, acrylic acid copolymer and itaconic acid which are polar components, it is speculated that this adhesive may interact with the electric field generated by the electric device used in this study. Polyalkenoic acid polymers contain ionizable carboxylic acid groups with following formula:
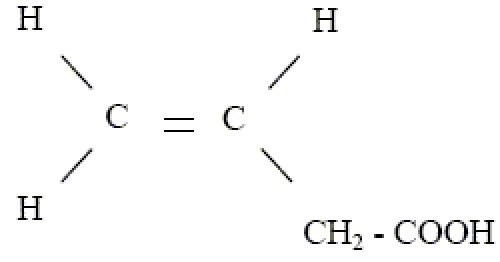
Thus, iontophoresis may enhance the movement of ions across dentin.10 This should be related to a faster rate of impregnation as ionic monomers are moving across the dentin with increasing ion mobilities that are caused by the imposed electrical gradient.11 Furthermore, because they are charged particles, these monomers are physically attracted by the electric field, thus increase the flow which is revealed by the reduced microleakage when applying the bonding agent with electric device. The difference in electric potential between the adhesive and etched dentin could enhance the penetration of adhesive monomers due to a biophysical modification of the organic matrix, or could enhance the wettability of the etched dentin surface, thereby improve the spreading of the adhesive.
Several devices that rely on the tooth's electrical properties are used in dentistry. Electronic root apex locators,12 pulp vitality tester,13 and early caries lesion detectors14 are some of such devices. Since dentin is not a pure capacitor or resistor, the flow of electricity depends on the relative humidity of the environment.15 Etching of the surface and subsequent exposure of the organic matrix in a wet environment increase the electric flow by reducing the resistance.16 Furthermore, in our study the use of simulated pulpal pressure condition increased the fluid flow toward the bonding interface. Thus, to ensure consistency of results, we subjected all teeth to the same electrical experimental conditions, regardless of the tooth dimensions. The experimental electric device was adjusted to electrical values that are compatible with in vivo use.13,15 It is worth mentioning that the intrinsic wetness of dentin and the perfusion of fluid from the pulp chamber should be considered when bonding to deep dentin. The density of waterfilled dentin tubules and hence intrinsic water content of dentin increases with dentin depth17 and these factors may increase the microleakage and affect the longevity of the restoration. Several investigations have also demonstrated the sensitivity of various bonding systems to pulpal pressure. Outward fluid flow from dentinal tubules in the mouth might contribute to a more rapid degradation of resindentin bonds in vivo.18,19 In conclusion, this study represents an attempt in reducing microleakage that was associated with the use of an electric impulse assisted application technique for the bonding of a etchandrinse adhesive to acid etched dentin. The use of simulated pulpal pressure was a further step towards simulation of in vivo condition.
Conclusion
The results of this in vitro study which was performed under simulated pulpal pressure showed that using electric current for applying the etchandrinse adhesive system (Single Bond) have a significant effect on reducing microleakage. Further in vivo studies should be carried out to confirm that an electric device is effective in improving the longevity of resindentin bonds through reducing the microleakage in vivo.
Acknowledgments
This report is part of a project financially supported and approved by the Research Deputy of Ahwaz Jundishapour University of Medical Sciences, Iran for which authors would like to thankfully acknowledge.
References
- 1.Nakabayashi N. 1st ed. Tokyo: Quintessence Publishing; 1998. Hybridization of Dental Hard Tissues; pp. 724–31. [Google Scholar]
- 2.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84(2):118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 3.Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69(11):726–31. [PubMed] [Google Scholar]
- 4.Hashimoto M, Sano H, Yoshida E, Hori M, Kaga M, Oguchi H, et al. Effects of multiple adhesive coatings on dentin bonding. Oper Dent. 2004;29(4):4 16–23. [PubMed] [Google Scholar]
- 5.Pashley EL, Agee KA, Pashley DH, Tay FR. Effects of one versus two applications of an unfilled, all-in-one adhesive on dentine bonding. J Dent. 2002;30(2-3):83–90. doi: 10.1016/s0300-5712(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto M, Tay FR, Ito S, Sano H, Kaga M, Pashley DH. Permeability of adhesive resin films. J Biomed Mater Res Part B: Appl Biomater. 2005;74(2):699–705. doi: 10.1002/jbm.b.30301. [DOI] [PubMed] [Google Scholar]
- 7.Cadenaro M, Antoniolli F, Sauro S, Tay FR, Di Le-narda R, Prati C, et al. Degree of conversion and permeability of dental adhesives. Eur J Oral Sci. 2005;113(6):525–30. doi: 10.1111/j.1600-0722.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 8.Pasquantonio G, Tay FR, Mazzoni A, Suppa P, Ruggeri A, Jr, Falconi M, et al. Electric device im-proves bonds of simplified etch-and-rinse adhesives. Dent Mater. 2007;23(4):513–8. doi: 10.1016/j.dental.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Mjor IA, Nordahl I. The density and branching of dentinal tubules in human teeth. Arch Oral Biol. 1996;41(5):401–12. doi: 10.1016/0003-9969(96)00008-8. [DOI] [PubMed] [Google Scholar]
- 10.Pashley DH, Livingston MJ, Outhwaite WC. Dentin permeability: changes produced by iontophoresis. J Dent Res. 1978;57(1):77–82. doi: 10.1177/00220345780570012701. [DOI] [PubMed] [Google Scholar]
- 11.Padula C, Colombo G, Nicoli S, Catellani PL, Massimo G, Santi P. Bioadhesive film for the transdermal delivery of lidocaine: in vitro and in vivo behavior. J Control Release. 2003;88(2):277–85. doi: 10.1016/s0168-3659(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 12.Sunada I. New Method for Measuring the Length of the Root Canal. Journal of Dental Research. 1962;41(2):375–87. [Google Scholar]
- 13.Daskalov I, Indjov B, Mudrov N. Electrical dental pulp testing.Defining parameters for proper instrumentation. IEEE Eng Med Biol Mag. 1997;16(1):46–50. doi: 10.1109/51.566152. [DOI] [PubMed] [Google Scholar]
- 14.Huysmans MC, Verdonschot EH, Rondel P. Electrical conductance and electrode area on sound smooth enamel in extracted teeth. Caries Res. 1995;29(2):88–93. doi: 10.1159/000262047. [DOI] [PubMed] [Google Scholar]
- 15.Krizaj D, Jan J, Valencic V. Modeling AC current conduction through a human tooth. Bioelectromagnetics. 2004;25(3):185–95. doi: 10.1002/bem.10189. [DOI] [PubMed] [Google Scholar]
- 16.Eldarrat A, High A, Kale GM. Age-related changes in cyclic voltammetry and potentiodynamic studies of normal human dentine. J Mater Sci Mater Med. 2003;14(11):979–84. doi: 10.1023/a:1026354816988. [DOI] [PubMed] [Google Scholar]
- 17.Pashley DH. Clinical correlations of dentin structure and function. J Prosthet Dent. 1991;66(6):777–81. doi: 10.1016/0022-3913(91)90414-r. [DOI] [PubMed] [Google Scholar]
- 18.Ciucchi B, Bouillaguet S, Holz J, Pashley D. Dentinal fluid dynamics in human teeth, in vivo. J Endod. 1995;21(4):191–4. doi: 10.1016/S0099-2399(06)80564-9. [DOI] [PubMed] [Google Scholar]
- 19.Toledano M, Osorio R, Ceballos L, Fuentes MV, Fernandes CA, Tay FR, et al. Microtensile bond strength of several adhesive systems to different dentin depths. Am J Dent. 2003;16(5):292–8. [PubMed] [Google Scholar]


