Abstract
Background:
When provisional restorations are worn for long term period, the adhesion of bacteria becomes a primary factor in the development of periodontal diseases. The aims of this study were to evaluate the surface roughness and bacterial adhesion of four different provisional fixed prosthodon-tic materials.
Methods:
Ten cylindrical specimens were prepared from bis-acrylic composites (PreVISION CB and Protemp 3 Garant), a light-polymerized composite (Revotek LC), and a polymethyl methacrylate-based (Dentalon) provisional fixed prosthodontic materials. Surface roughness was assessed by profilometry. The bacterial adhesion test was applied using Porphyromonas gingivalis (P. gingivalis) and spectro-fluorometric method. Statistical analysis was performed using ANOVA and Dunnett t-tests.
Results:
All tested materials were significantly rougher than glass (P < 0.05). Revotek LC had the greatest fluorescence intensity, PreVISION and Protemp 3 Garant had moderate values and all of them had significantly more bacterial adhesion compared to glass (P < 0.05). Dentalon had the lowest fluorescence intensity among the provisional fixed prosthodontic materials.
Conclusion:
The quantity of bacterial adhesion and surface roughness differed among the assessed provisional fixed prosthodontic materials. The light-polymerized provisional material Revotek LC had rougher surface and more bacterial adhesion compared with the others.
Keywords: Bacterial adhesion, Polymerization, Surface analysis
Introduction
Provisional crown and fixed partial prostheses rep-resent important elements in modern fixed prosthetic treatments.1–3 These prostheses are intended to enable prognoses and give the patient function, phonation, and good aesthetics while maintaining tissue compatibility until permanent restoration is achieved.4–6
For provisional restorations (PRs) to be successful, they must resist the adhesion of microorganisms, which facilitates surface colonization and plaque maturation and increases the risk of periodontal infections.7 While many studies have focused on microbiological adhesion on amalgams, glass ionomers, and composite resins,8,9 relatively few have investigated the bacterial adhesion on provisional fixed Prosthodontic materials (PFPMs).6,7
Many bacteria are able to adhere to hard sur-faces in the oral cavity,6,8 and the surface roughness of intraoral hard surfaces has a significant effect on primary and oral microorganism adhesion.10
In vitro studies have shown that a mean surface roughness greater than 0.2 μm in fixed restorations increases the degree of bacterial adhesion.10,11
Bacterial adhesion results in several physical and chemical consequences, such as leukotoxins, high levels of protease activity, and tissue invasion, which can contribute to the loss of gingival attachment that occurs as periodontitis progresses.12 Bacterial adhesion varies among species, but most previous studies have referred to Porphyromonas Gingivalis (P. gingivalis), an anaerobic species frequently associated with periodontal dis-ease.13–16 P. gingivalis is a gram-negative, black-pigmented, strictly anaerobic bacterium that has been implicated as a major etiological agent in the development and progression of periodontitis, particularly the chronic form.14,16
The present in vitro study evaluated the adhesive properties of P. gingivalis and assessed how these properties relate to surface roughness on four commonly used PFPMs using a spectrofluorometric method in combination with scanning electron microscopy (SEM).
Materials and Methods
Commercially available provisional materials were chosen such that each class of commonly used material was represented, as shown in Table 1. Glass, which is generally considered to be extremely smooth and is often used in bacterial adhesion studies, was selected as a control material. Ten cylindrical specimens (10 × 2.0 mm in height) were prepared with each of the four PFPMs (Dentalon, Revotek LC, PreVISION CB, Protemp 3 Garant) using a custom metal mold with calibrated circular holes. Specimens of Revotek LC were polymerized with a light-emitting diode (LED) light source (Blue Swan Digital, Dentanet, Turkey) held approximately 1 mm away at 400 mW/cm2 for 20 seconds. All specimens were wet-ground with 600-grit silicon carbide abrasive paper for 10 seconds on a 30-rpm grinding machine (Buehler Metaserv, Germany). After polishing, a mean surface rough-ness value (Ra) was measured at four randomly selected points on each of the specimen surfaces with a profilometer (Surf test 201, Mitutoyo, Ja-pan). A 7.5-mm field was scanned for every meas-urement with a study gap of 250 μm.
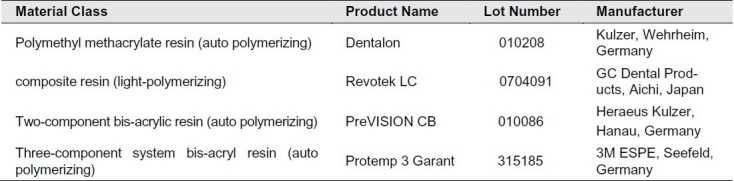
Table 1.
Material class and manufacturer information for the provisional fixed prosthodontic materials evaluated
All specimens were stored in distilled water for 10 days and then cleaned with ethanol (70%) and placed into 24-well plates (TPP, Switzerland) with one specimen per well. P. gingivalis (ATCC 33277; ATTC, USA) was cultured for 72 hours on non-selective anaerobe agar and colonies were resuspended in thioglycolate broth (Merck, Germany). The bacterial suspension was centrifuged at 18°C for 5 minutes at 2'000 rpm and the resulting bacterial pellet was washed twice with phosphate-buffered saline (PBS) solution. The final bacterial suspension was diluted in PBS to an optical density of 0.3 at 540 nm as determined with a spectropho-tometer (Bio-Tek-Synergy HT Microplate reader, Bio-Tek Instruments, USA). Alamar Blue/ Resazu-rin (0.007536 g/10 mL) (Sigma-Aldrich, USA) was used to determine the degree of bacterial adhesion. Before starting the experiment, 1 mL PBS was added to each well and the autofluorescence of the specimens was measured. The buffer was then re-moved and replaced with 1 mL bacterial solution and 15 μL resazurin. The plates were incubated at 37°C for 150 minutes under anaerobic conditions (85% N210% H2, and 5% O2). After incubation, the bacterial suspension and resazurin were extracted by suction, the wells were washed twice with distilled water, and 1 mL PBS was added to each well. Fluorescence intensities were recorded using a multidetection microplate reader, at excitation and emission wavelengths of 530 and 590 nm, respectively. Controls consisted of the fluorescence emission from pure PBS, PBS with resazurin, and pure bacterial suspension.
After incubation with P. gingivalis, one specimen of each material was rinsed with PBS and fixed with methanol to acquire SEM images. After solvent evaporation, specimens were coated with gold palladium and critical point dried, mounted on aluminium stubs, and examined via SEM (LEO 440, LeoElectron Microscopy, UK) operating at 20.00 kV.
To determine the significance of observed dif-ferences, the analysis of variance (ANOVA) was applied to the fluorescence and surface roughness data using statistical software (version 12.0.1 for Windows, SPSS Inc., USA). The mean values of testes materials and glass were compared by Dunnett t-test. P value less than 0.05 was considered significant.
Results
Surface roughness
The Radata for the tested PFPMs are presented in Table 2. The surface roughness ranged from 1.10 ± 0.49 μm (Protemp 3 Garant, the smoothest one) to 2.30 ± 0.43 μm (Revotek LC, the roughest one). All of the PFPMs were significantly rougher than the glass with the Ra < 0.01 μm (P < 0.05). Figures 1 and 2 show the SEM photographs of these two materials, respectively, which indicate the rougher surface of Revotek LC.
Table 2.
Statistical analyses of surface roughness of studied provisional materials
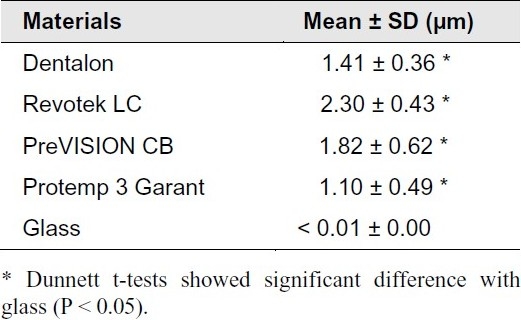
Figure 1.
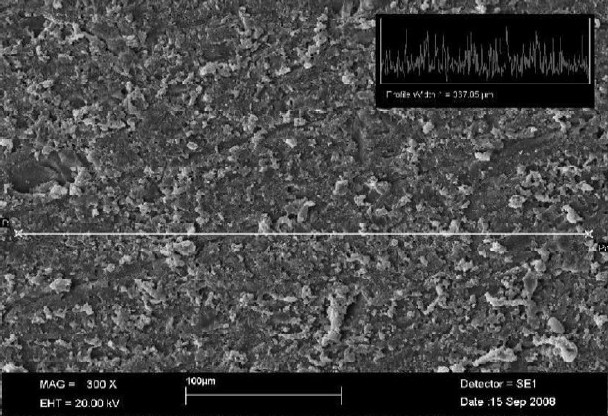
Scanning electron micrograph shows the surface roughness of Revotek LCs (profile width = 367.05 μm, 300X magnification)
Figure 2.
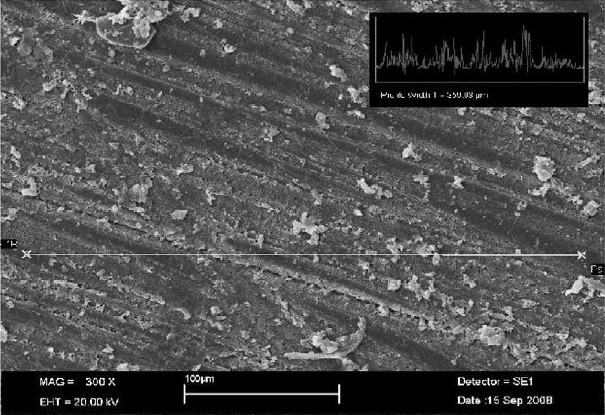
Scanning electron micrograph shows the surface roughness of Protemp 3 Garant (profile width = 359.03 μm, 300X magnification)
Bacterial adhesion
The mean fluorescence intensities of tested materials are shown in Table 3. The Dentalon specimens exhibited the least fluorescence, different from the controls by less than 10,000 counts, indicating low bacterial adhesion. No significant difference was detected between this material and glass. PreVISION CB and Protemp 3 Garant exhibited moderate adhesion and differed from the control by 10,000 to 30,000 counts (P < 0.05). Revotek LC gave the highest fluorescence of 30,000 counts above that of the control (P < 0.05). Significant differences were observed in the degree of fluorescence intensity among Revotek LC, PreVISION CB, and Protemp 3 Garant (P < 0.05). Among the PFPM samples evaluated herein, the light-polymerized composet resin and the outopolymerized poly methylmethacrylate (PMMA) specimens exhibited the highest and lowest fluorescence intensity, respectively.
Table 3.
Statistical analyses of fluorescence intensities of studied provisional materials
SEM images showed P. gingivalis as a coccobacillus-shaped cell with aggregates composed of chains and clusters. The colonization pattern of adhering bacteria was similar on all assessed materials, differing only by the number of adhering organisms. A bacterial monolayer was observed on all surfaces, indicating bacterial adhesion rather than accumulation. Only single bacterium and small aggregates were observed on Dentalon (Figure 3). In contrast, SEM images of aggregates on Revotek LC corroborated the results of the fluorescence analyses, which indicated a high degree of bacterial adhesion, with bacterial clusters formed by chain aggregation. This represents a more de-veloped stage of biofilm formation (Figure 4).
Figure 3.

Scanning electron micrograph shows P. gingivalis adhered to Dentalon
Figure 4.
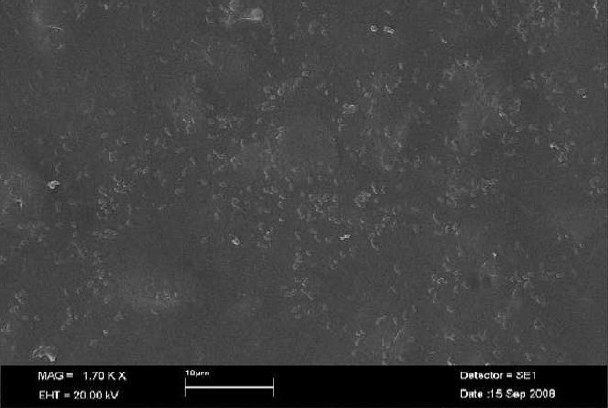
Scanning electron micrograph shows P. gingivalis adhered to Revotek LC
Discusssion
The placement of crown margins in intracrevicular spaces is an etiologic factor for gingival/periodontal inflammation.17 The specific plaque hypothesis suggests that certain oral bacteria are critical for initiation and progression of gingivitis/periodontitis.18 In vitro investigations have indicated that P. gingivalis, a gram negative bacterium and a factor in periodontitis, adheres to fixed prosthesis materials.17,18 In intracrevicular margin locations, the surface roughness and chemistry of PFPMs provide a potential niche for colonization by oral bacteria.19 As indicated by Borchers et al.20 when PR is used for longer periods, plaque prevention becomes increasingly important, necessitating a smooth surface.
In the present study, the relative degree of bac-terial adhesion was measured on two autopolymerized composites, a light-polymerized composite, and a PMMA-based PR material.
Fluorescence techniques offer quick and reproducible quantification with relatively few potential measurement errors. The resazurin fluorescence assay (Alamar Blue) can indicate the quantity of viable bacteria, since a direct correlation exists between the number of living microorganisms in the assay and the amount of resazurin that is reduced to the fluorescent resorufin. SEM analyses are especially well suited for microscopic charac-terization of bacterial morphology, material surfaces, and the interactions between them.21
The degree of bacterial adhesion varied significantly among materials. Relative to the other samples, greater fluorescence emission was detected with Revotek LC, Protemp 3 Garant, and PreVISION CB, indicating a relatively high susceptibility of P. gingivalis to adherence. The light-polymerized Revotek LC provisional composite material exhibited the highest surface roughness of all the specimens. This result agrees with Guler et al.22 who considered Revotek LC as being clinically unacceptable in this regard. Therefore, the other three PFPMs (Protemp 3 Garant, PreVISION CB, Dentalon) were expected to exhibit strong correlations between bacterial coverage and surface roughness. However, coverage by P. gingivalis on Protemp 3 Garant, which was the smoothest specimen (Ra= 1.10 μm), was greater than that on both Dentalon (Ra= 1.41 μm) and PreVISION CB (Ra= 1.82 μm). Thus, bacterial adhesion is influenced not only by the surface roughness but also by the composition of the resin matrix or the inherent chemistry of the materials.
The relationship between surface roughness and bacterial adhesion has been widely studied. Quirynen et al.23 demonstrated the existence of a roughness threshold (0.2 μm) below which no further impact on bacterial adhesion can be expected. The results of the fluorescence adhesion tests were verified in SEM images of the polymer surfaces. Bacterial colonization and more complex accumulation were found on Revotek LC, which exhibited high fluorescence intensity (Figure 4). Acrylic PMMA showed significantly lower bacterial adhesion and fluorescence than the bisacrylic composite resins (PreVISION CB and Protemp 3 Garant). Improving the chemical composition of these materials with the goal of reducing bacterial adhesion would allow PRs to be used for longer periods.
Although this study evaluated the surface roughness and the relative adhesion of only one bacterial species, it is a major periodontal pathogen and findings may assist in the future design of PRs boasting low bacterial adhesion. However, to account for the host of additional variables, such as pellicle proteins and the effect(s) of other bacterial species in the intracrevicular region, extensive in vivo assessments would need to be performed.
Conclusion
Surface roughness and the degree of bacterial adhesion differed significantly among the four PFPMs investigated. A light-polymerized composite provisional material (Revotek LC) exhibited the roughest surface and the greatest degree of adhesion, while the amount of bacteria observed on PMMA (Dentalon) was low relative to that on the bis-acrylic composite resins (PreVISION CB and Protemp 3 Garant).
Acknowledgments
We gratefully acknowledge the help of Anne Bryk for her expertise in obtaining P. gingivalis ATCC 33277. The authors would also like to thank the KTL National Public Health Institute (Helsinki, Finland).
References
- 1.Haselton DR, Diaz-Arnold AM, Dawson DV. Effect of storage solution on surface roughness of provisional crown and fixed partial denture materials. J Prosthodont. 2004;13(4):227–32. doi: 10.1111/j.1532-849X.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- 2.Tacir IH, Kama JD, Zortuk M, Eskimez S. Flexural properties of glass fibre reinforced acrylic resin polymers. Aust Dent J. 2006;51(1):52–6. doi: 10.1111/j.1834-7819.2006.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosentritt M, Behr M, Lang R, Handel G. Flexural properties of prosthetic provisional polymers. Eur J Prosthodont Restor Dent. 2004;12(2):75–9. [PubMed] [Google Scholar]
- 4.Sham AS, Chu FC, Chai J, Chow TW. Colour stability of provisional prosthodontic materials. J Prosthet Dent. 2004;91(5):447–52. doi: 10.1016/S0022391304001283. [DOI] [PubMed] [Google Scholar]
- 5.Rosenstiel SF, Land MF, Fujimoto J. 3rd. Philadelphia: Mosby; 2001. Contemporary fixed prosthodontics; pp. 318–416. [Google Scholar]
- 6.Hobo S, Whitsett LD, Jacobi R, Brackett SE, Shillingburg HT. 3rd. Chicago: Quintessence Pub Co; 1997. Fundamentals of fixed prosthodontics; pp. 225–56. [Google Scholar]
- 7.Sen D, Goller G, Issever H. The effect of two polishing pastes on the surface roughness of bisacryl composite and methacrylate-based resins. J Prosthet Dent. 2002;88(5):527–32. doi: 10.1067/mpr.2002.129335. [DOI] [PubMed] [Google Scholar]
- 8.Eick S, Glockmann E, Brandl B, Pfister W. Adherence of Streptococcus mutans to various restorative materials in a continuous flow system. J Oral Rehabil. 2004;31(3):278–85. doi: 10.1046/j.0305-182X.2003.01233.x. [DOI] [PubMed] [Google Scholar]
- 9.Svanberg M, Mjor IA, Orstavik D. Mutans streptococci in plaque from margins of amalgam, composite, and glass-ionomer restorations. J Dent Res. 1990;69(3):861–4. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 10.Maalhagh-Fard A, Wagner WC, Pink FE, Neme AM. Evaluation of surface finish and polish of eight provisional restorative materials using acrylic bur and abrasive disk with and without pumice. Oper Dent. 2003;28(6):734–9. [PubMed] [Google Scholar]
- 11.Heintze SD, Forjanic M, Rousson V. Surface roughness and gloss of dental materials as a function of force and polishing time in vitro. Dent Mater. 2006;22(2):146–65. doi: 10.1016/j.dental.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Tran SD, Rudney JD. Multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actino-mycetemcomitans and Porphyromonas gingivalis. J Clin Microbiol. 1996;34(11):2674–78. doi: 10.1128/jcm.34.11.2674-2678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62(4):1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontol. 2000;1999(20):168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 15.Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006;85(5):392–403. doi: 10.1177/154405910608500502. [DOI] [PubMed] [Google Scholar]
- 16.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man.A review of the literature. J Clin Periodontol. 1995;22(1):1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 17.Kancyper SG, Koka S. The influence of intracrevicular crown margins on gingival health: preliminary findings. J Prosthet Dent. 2001;85(5):461–5. doi: 10.1067/mpr.2001.115386. [DOI] [PubMed] [Google Scholar]
- 18.Bunetel L, Guerin J, Agnani G, Piel S, Pinsard H, Corbel JC, et al. In vitro study of the effect of titanium on porphyromonas gingivalis in the presence of metronidazole and spiramycin. Biomaterials. 2001;22(22):3067–72. doi: 10.1016/s0142-9612(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 19.Zortuk M, Kilic K, Uzun G, Ozturk A, Kesim B. The effect of different fiber concentrations on the surface roughness of provisional crown and fixed partial denture resin. Eur J Dent. 2008;2:185–90. [PMC free article] [PubMed] [Google Scholar]
- 20.Borchers L, Tavassol F, Tschernitschek H. Surface quality achieved by polishing and by varnishing of temporary crown and fixed partial denture resins. J Prosthet Dent. 1999;82(5):550–6. doi: 10.1016/s0022-3913(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 21.Buergers R, Rosentritt M, Handel G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J Prosthet Dent. 2007;98(6):461–9. doi: 10.1016/S0022-3913(07)60146-2. [DOI] [PubMed] [Google Scholar]
- 22.Guler AU, Kurt S, Kulunk T. Effects of various finishing procedures on the staining of provisional restorative materials. J Prosthet Dent. 2005;93(5):453–8. doi: 10.1016/j.prosdent.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: shortterm observations. Int J Oral Maxillofac Implants. 1996;11(2):169–78. [PubMed] [Google Scholar]


