Abstract
The isomerization of 11-cis retinal to all-trans retinal in photoreceptors is the first step in vision. For photoreceptors to function in constant light, the all-trans retinal must be converted back to 11-cis retinal via the enzymatic steps of the visual cycle. Within this cycle, all-trans retinal is reduced to all-trans retinol in photoreceptors and transported to the retinal pigment epithelium (RPE). In the RPE, all-trans retinol is converted to 11-cis retinol, and in the final enzymatic step, 11-cis retinol is oxidized to 11-cis retinal. The first and last steps of the classical visual cycle are reduction and oxidation reactions, respectively, that utilize retinol dehydrogenase (RDH) enzymes. The visual cycle RDHs have been extensively studied, but because multiple RDHs are capable of catalyzing each step, the exact RDHs responsible for each reaction remain unknown. Within rods, RDH8 is largely responsible for the reduction of all-trans retinal with possible assistance from RDH12. retSDR1 is thought to reduce all-trans retinal in cones. In the RPE, the oxidation of 11-cis retinol is carried out by RDH5 with possible help from RDH11 and RDH10. Here, we review the characteristics of each RDH in vitro and the findings from knockout models that suggest the roles for each in the visual cycle.
1. Introduction
The photoisomerization of 11-cis retinal to all-trans retinal in photoreceptors is the first step in vision. Of equal importance, however, are a series of enzymatic steps that replenish chromophore levels in photoreceptors by converting all-trans retinal back into 11-cis retinal. This process, known classically as the visual cycle, includes reduction and oxidation reactions carried out by retinol dehydrogenases (RDHs) in the photoreceptors and retinal pigment epithelium (RPE), respectively (Fig. 1). In the photoreceptor outer segment (OS), all-trans retinal is reduced to all-trans retinol by RDHs, and after all-trans retinol is transferred to the RPE and converted to 11-cis retinol, an oxidation reaction carried out by RDHs converts 11-cis retinol to 11-cis retinal. The multiple RDHs that function in the visual cycle are found in the retina and RPE (Fig. 2). In addition to the classical visual cycle, a cone-specific visual cycle (see below) is thought to exist in the retina to provide cones with a privileged source of 11-cis retinal, but the RDHs associated with the cone visual cycle have not been identified.
Fig. 1.
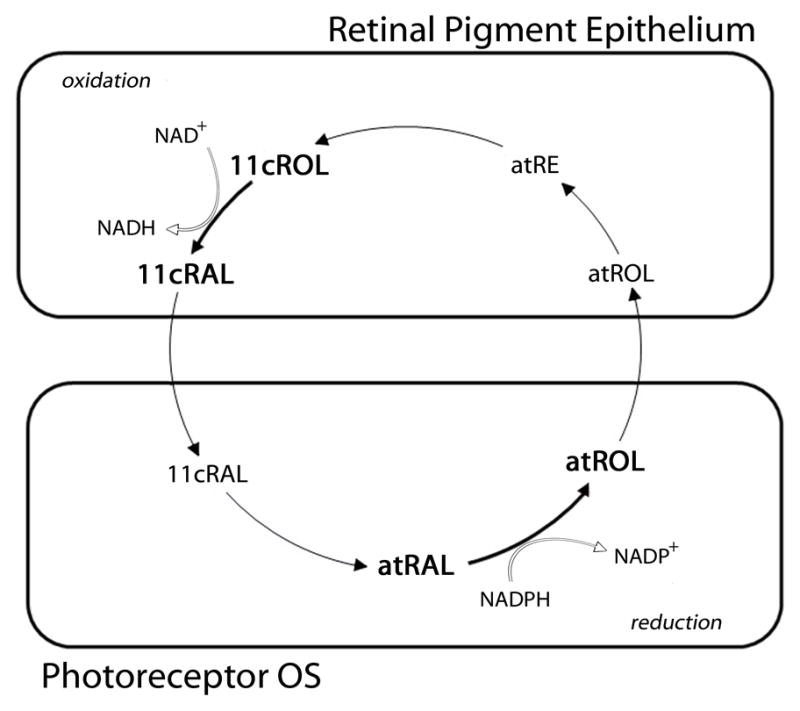
Oxidation and Reduction Reactions of the Visual Cycle. The classical visual cycle involves reduction and oxidation reactions carried out in the photoreceptors and retinal pigment epithelium (RPE), respectively. RDH enzymes are responsible for both reactions. In the photoreceptor OS, RDHs carry out the NADPH-dependent reduction of all-trans retinal (atRAL) to all-trans retinol (atROL). In the RPE, RDHs are responsible for the NAD+-dependent oxidation of 11-cis retinol (11cROL) to 11-cis retinal (11cRAL).
Fig. 2.

Distribution of Visual Cycle RDHs in the Eye. Multiple RDHs found in the eye contribute to retinoid metabolism. The proposed role of many RDHs is based on their location in the retinal pigment epithelium (RDH5, RDH11, RDH10), photoreceptor outer segments (RDH8, retSDR1), photoreceptor inner segments (RDH11, RDH12), and Müller cells (RDH10).
Visual cycle RDHs belong to the short-chain dehydrogenase/reductase (SDR) super-family of proteins. SDRs are divided into multiple families, but visual cycle RDHs fall into Clusters C2 and C3 of the classical SDRs (Bray et al., 2009). RDHs utilize NAD(H) or NADP(H) as cofactors in reactions that catalyze a hydride transfer between the nicotinamide group (S-face of the C4 position) and the retinoid substrate (Lukacik et al., 2006). The stereospecificity, substrate, and cofactor of known RDHs in the visual cycle are summarized in Table 1. Here, we review both the function of individual RDHs in vitro and the murine visual cycle in vivo in the absence of different RDHs.
Table 1.
RDHs Associated with the Visual Cyclea
| RDH | Location | Proposed Visual Cycle Substrate | Proposed Visual Cycle Cofactor | Additional Substrates | Stereospecificity | Disease | |
|---|---|---|---|---|---|---|---|
| Cofactor | Retinoid | ||||||
| RDH8 | ROS, COS | all-trans retinal | NADPH | — | Pro-S | Pro-R | — |
| RDH12 | RIS, CIS | all-trans retinal | NADPH | aldehydes | Pro-S | Pro-R | LCA |
| retSDR1 | COS | all-trans retinal | NADPH | — | Pro-S | Pro-R | — |
| RDH5 | RPE | 11-cis retinol | NAD+ | — | Pro-S | Pro-S | FA |
| RDH11 | RPE, IS | 11-cis retinol | NADP+ | aldehydes | Pro-S | Pro-R | — |
References are also noted in text but are as follows for each RDH: RDH8 (Palczewski et al., 1994; Jang et al., 2000; Rattner et al., 2000), RDH12 (Haeseleer et al., 2002; Janecke et al., 2004; Belyaeva et al., 2005), retSDR1 (Haeseleer et al., 1998), RDH5 (Simon et al., 1995; Romert et al., 1998; Yamamoto et al., 1999; Jang et al., 2001), RDH11 (Haeseleer et al., 2002; Kedishvili et al., 2002; Kasus-Jacobi et al., 2003). Abreviations: ROS (rod outer segment), COS (cone outer segment), RIS (rod inner segment), CIS (cone inner segment), RPE (retinal pigment epithelium), IS (inner segment, undefined), LCA (Leber’s Congenital Amaurosis), FA (fundus albipunctatus).
2. all-trans Retinal Reduction in Photoreceptors
The reduction of all-trans retinal to all-trans retinol occurs in the photoreceptors. Both rods and cones contain multiple RDHs capable of catalyzing this reaction. RDH8 and RDH12 are found in the OS and inner segment (IS), respectively, of rods and cones and contribute to all-trans retinal reduction.
2.1 RDH8 (prRDH)
RDH8 (prRDH) is localized to the photoreceptor OS, where it constitutes 0.2–0.5% of the total ROS protein (Rattner et al., 2000). Purified mouse RDH8 displays a substrate preference for all-trans retinal (Rattner et al., 2000) and a cofactor preference for NADPH (Palczewski et al., 1994), which support its role as an all-trans RDH.
Studies in Rdh8−/− mice have confirmed its role in all-trans retinal reduction but have also shown that it is not exclusively required for visual cycle function. In many ways, Rdh8−/− mice are unremarkable, as retina morphology and ERGs are normal through 8 months of age (Maeda et al., 2005). Although 11-cis retinal production is unimpeded in the absence of RDH8, all-trans retinal accumulation in rods slows the recovery of ERG responses. Thus, the phenotype of Rdh8−/− differs only mildly from wild-type (Wt) mice, but slower all-trans retinal reduction and a corresponding decrease in retinyl esters exist (Maeda et al., 2005). As such, RDH8 contributes to all-trans retinal reduction but is not essential for the reaction.
2.2 RDH12
RDH12 is localized to the IS of both rods and cones (Haeseleer et al., 2002). Recombinant human RDH12 utilizes both cis- and trans-retinoids as substrates (Haeseleer et al., 2002) and is also active towards medium chain aldehydes (Belyaeva et al., 2005). NADP(H) is the preferred cofactor (Haeseleer et al., 2002). While capable of all-trans retinal reduction in vitro, isolated ROS from Rdh12−/− and Wt mice have similar all-trans RDH activity, suggesting that the majority of all-trans retinal is not reduced by RDH12 (Maeda et al., 2006).
Although RDH12 mutations are associated with LCA (Janecke et al., 2004), the mild phenotype in Rdh12−/− mice suggests that the physiological role of RDH12 is not entirely homologous in murine and human retinae. Rdh12−/− mice have normal retina morphology through 7 months, although OS reductions appear after 10 months (Maeda et al., 2006; Kurth et al., 2007). As would be expected from an all-trans RDH, dark adaptation is slower, and all-trans retinal accumulates after extensive bleaching (Maeda et al., 2006). The effects, however, are less dramatic than those in seen in Rdh8−/− mice (Maeda et al., 2005), and it appears that RDH12 contributes to all-trans retinal reduction to a lesser extent than RDH8.
One theory regarding RDH12 function is that RDH12 protects the retina from excessive all-trans retinal accumulation during persistent illumination. In the presence of continuous illumination, photoreceptor loss occurs in the central retina of Rdh12−/− mice, and with intense illumination, photoreceptors in Rdh12−/− mice are more susceptible to apoptosis (Maeda et al., 2006). Regardless of its small contribution to all-trans retinal reduction under normal conditions, RDH12 appears to be important for photoreceptor health in the presence of excessive illumination.
2.3 RDH8 AND RDH12 in the rod visual cycle
Studies of Rdh8−/−Rdh12−/− mice provide additional information about the process of all-trans retinal reduction in the visual cycle and suggest a cooperative role for these two enzymes. Like single knockouts, six-week-old Rdh8−/−Rdh12−/− mice have normal retina morphology and ERGs, but shortened OS and diminished ERGs are seen by 3 months (Maeda et al., 2007). Additionally, all-trans RDH activity is 2% of Wt levels in Rdh8−/−Rdh12−/− retinae. RDH8 accounted for 70% of the total all-trans RDH activity in the retina after the first five minutes of the reaction (Maeda et al., 2007). Thus, both the RDH8 and RDH12 exhibit all-trans RDH activity, but RDH8 is the main contributor to total all-trans RDH activity in the retina.
3. Oxidation of 11-cis retinol in the RPE
A critical step of the visual cycle is the production of 11-cis retinol in the RPE, but to regenerate chromophore, 11-cis retinol must be oxidized to 11-cis retinal by RDH’s. RDH5 is largely responsible for this reaction, but 11-cis retinol oxidation is also facilitated by other RDH’s, among them RDH11.
3.1 RDH5
In the eye, RDH5 is most abundant in the RPE (Simon et al., 1995). RDH5 has no activity towards all-trans retinal (Simon et al., 1995), but it is capable of oxidizing or reducing all of the common cis-retinoid isomers (Romert et al., 1998). RDH5 is thought to oxidize cis-retinoids through an NAD+-dependent reaction in the RPE (Simon et al., 1995).
Studies in Rdh5−/− mice suggest that RDH5 contributes to 11-cis retinol oxidation but is not required for the reaction. Retina histology and ERGs are normal in Rdh5−/− mice, and ERG recovery following a bleach is similar to Wt (Driessen et al., 2000). Likewise, 11-cis retinol oxidation still occurs at a reduced rate in RPE membranes from Rdh5−/− mice. While function remains normal, retinoid profiles are unique in Rdh5−/− mice. Dark adapted retinoid profiles show increased levels of 11-cis retinol and cis-retinyl esters (Kim et al., 2005), and the recovery of ERG responses after intense bleaching is delayed (Driessen et al., 2000). Together, elevated levels of 11-cis retinol, retinyl esters, and delayed dark adaptation show that RDH5 does play a significant role in 11-cis retinol oxidation in the RPE. Similarly, human studies in a patient with an RDH5 R157W null mutation (Cideciyan et al., 2000) show that rod ERG amplitude and thresholds were unaffected, suggesting that the absence of RDH5 activity does not reduce the number or length of rod OS. Bleaches (0.5%) that expose early portions of rod dark adaptation were normal, but recovery of sensitivity from bleaches >2% were slowed, suggesting that RDH5 is needed for normal recovery from bleaches of >2%.
3.2 RDH11
RDH11 is found in the RPE, Müller cells (Haeseleer et al., 2002), and photoreceptor IS of mice (Kasus-Jacobi et al., 2003). Like other Cluster 2B RDHs, RDH11 can utilize both cis- and trans-retinoid substrates (Haeseleer et al., 2002). Mouse RDH11 (SCALD) can also utilize short-chain saturated aldehydes (such as nonanal) as substrates, but has no steroid activity (Kasus-Jacobi et al., 2003). Because nonanal is reduced faster than most retinoids, RDH11 is hypothesized to play a role in removing toxic aldehydes from the retina (Kasus-Jacobi et al., 2003). With regards to the visual cycle, both human (Belyaeva et al., 2003) and mouse (Kedishvili et al., 2002) RDH11 appear to be more efficient as retinal reductases than as retinal dehydrogenases, and NADP(H) is the preferred cofactor (Haeseleer et al., 2002). In the RPE, RDH11 is thought to facilitate 11-cis retinol oxidation.
Rdh11−/− mice have normal retinal morphology and ERGs under baseline conditions (Kasus-Jacobi et al., 2005), and retinoid profiles are equivalent to Wt (Kim et al., 2005). Dark adaptation studies, however, show that the kinetics of rod recovery is slowed in Rdh11−/− mice, but retinoid profiling shows that 11-cis retinal production is not impeded after bleaching (Kasus-Jacobi et al., 2005). Based on these findings, the etiology of delayed dark adaptation in Rdh11−/− mice is uncertain, but studies in Rdh5−/−Rdh11−/− mice suggest that diminished 11-cis RDH activity in the RPE does exist (see below).
3.3 Combined Effects of RDH5 and RDH11
While data about RDH11’s role as an 11-cis RDH are conflicting, studies in Rdh5−/−Rdh11−/− mice suggest a cooperative role between RDH5 and RDH11. Like Rdh5−/− and Rdh11−/− mice, Rdh5−/−Rdh11−/− mice have normal retina morphology, but only Rdh5−/−Rdh11−/− mice have diminished rod ERGs (Kim et al., 2005). Similarly, the recovery of rod responses measured with paired flash ERGs is normal in Rdh5−/− and Rdh11−/− mice but slow in Rdh5−/−Rdh11−/− mice (Kim et al., 2005). Retinoid profiling also suggests that both RDH5 and RDH11 contribute to 11-cis retinal production. After bleaching, a progressive attenuation of a-wave recovery among the three strains exists (Rdh11−/− < Rdh5−/− < Rdh5−/−Rdh11−/−) that correlates with 11-cis retinal concentrations measured after the same bleaching procedure (Kim et al., 2005). Thus, it appears that RDH11 plays a minor but complementary role to RDH5 in the flow of retinoids during dark adaptation.
4. Oxidation and reduction reactions in the cone visual cycle
A second visual cycle exists in the retina to provide cones with a privileged source of 11-cis retinal (Wang et al., 2009). This cone visual cycle begins with the reduction of all-trans retinal in the OS. all-trans Retinol is then transported to Müller cells, isomerized to 11-cis retinol (Mata et al., 2005), and transported to cones for oxidation to 11-cis retinal (Jones et al., 1989). As such, the cone visual cycle, like the classical visual cycle, involves one reduction and one oxidation reaction involving RDH enzymes.
4.1 retSDR1
Only one of the visual cycle RDHs is known to be cone-specific—retSDR1. Analogous to RDH8 in the OS of both rods and cones, retSDR1 is found in the OS of all murine cones (Haeseleer et al., 1998). As such, rod OS contain RDH8, while cone OS contain RDH8 and retSDR1. Like RDH8, the main function of retSDR1 appears to be all-trans retinal reduction. Recombinant retSDR1 reduces all-trans retinal to retinol but has no activity against 11-cis retinal or steroid compounds (Haeseleer et al., 1998). NADP(H) is the preferred cofactor and is not replaceable with NAD(H). Thus, retSDR1 appears to be analogous to RDH8 in the OS (Haeseleer et al., 1998), but this hypothesis is not supported by in vivo functional data.
4.2 RDH8 and RDH12 in Cone Function
RDH8 and RDH12 are located in the OS and IS of cones, respectively. In the OS, both RDH8 and retSDR1 utilize NADPH to reduce all-trans retinal to all-trans retinol. The presence of redundant all-trans retinol reductases in the cone OS might be needed for rapid all-trans retinal reduction under photopic conditions. Work is limited on RDH8’s role in cone function, but cone ERGs are normal in Rdh8−/− mice lacking the transducin alpha subunit necessary for rod photoreceptor signaling (Gnat1−/−Rdh8−/− mice). Similarly, studies of cone function are limited in mice lacking RDH12, but cone ERGs in Gnat1−/−Rdh12−/− mice are also normal (Maeda et al., 2007). However, it remains to be seen if the absence of RDH12 affects cone function under photopic conditions. Another possible role for RDH12 in cone function is the oxidation of 11-cis retinol in the cone visual cycle. RDH12 belongs to the sub-family of RDH enzymes with RDH11-14 with dual substrate specificity (Haeseleer et al., 2002). As such, RDH12 has the ability to reduce all-trans retinol or oxidize 11-cis retinol. Whether or not this role belongs to RDH12 or an unidentified oxidase has not been determined.
4.3 RDH5 and RDH11 in Cone Function
RDH5 and RDH11 are thought to be the main RDH’s responsible for 11-cis retinol oxidation in the RPE. While the cone visual cycle is believed to be independent of the RPE, the absence of cone function in the cone-like photoreceptors of Nrl−/−Rpe65−/− mice suggests that cones do rely on the RPE for some level of chromophore (Wenzel et al., 2007). As such, RDH5 and RDH11 have plausible roles in cone function. While photopic ERGs are normal in Rdh5−/−Rdh11−/− mice (Kim et al., 2005), cone function was found to be abnormal in a human patient containing a homozygous mutation of RDH5 (Cideciyan et al., 2000). Most interestingly, recovery of L/M cones following bleaching was delayed with a biphasic recovery that was 34x slower than normal. Thus, RDH5 does contribute to retinoid metabolism in human cones, and additional studies of cone function may reveal roles for RDH5 and RDH11 in murine cone function.
5. Additional RDHs in the Eye
Studies of knockout mice have helped identify specific roles of RDHs in the classical visual cycle, but other RDHs in the eye have unidentified functions. RDH10 is located in both the RPE (Wu et al., 2002) and Müller cells (Wu et al., 2004), and as such, has the potential to contribute to the classical visual cycle. Studies of recombinant RDH10 show that NAD+ is the preferred cofactor in vivo (Belyaeva et al., 2008a). While in vitro studies have shown that RDH10 can function as an oxidoreductase (Takahashi et al., 2009), the enzyme exhibits a preference for oxidation reactions and can utilize both cis- and trans-retinols similarly as substrates (Belyaeva et al., 2008a). Because RDH10 can substitute for RDH5 under experimental conditions (Farjo et al., 2009), it is possible that RDH10 accounts for the remaining 11-cis RDH activity in Rdh5−/−Rdh11−/− mice. Because of RDH10’s extra-ocular roles, Rdh10 deletions are lethal and knockouts cannot be studied.
RDH13 and RDH14 are expressed in the retina and have no known role in the classical visual cycle, but both are elevated in the retinae of Nrl−/− mice and may be important for cone function (Kanan et al., 2008). RDH13 is capable of utilizing retinoids as substrates, but its localization in the mitochondria seems to preclude its role as a visual cycle RDH (Belyaeva et al., 2008b). RDH14 has activity towards all-trans and 11-cis retinoids, and therefore, may participate in the oxidation of 11-cis retinol in the cone visual cycle.
6. Conclusion
RDHs are responsible for the oxidation and reduction reactions in the classical visual cycle. While knowing the preferred substrate, cofactor, and localization of RDHs suggests specific roles for each, studies of the visual cycle in knockout mice have increased our understanding of RDH function in vivo (Table 2) and revealed an amazing amount of redundancy in the oxidation and reduction reaction of the visual cycle. Thus, the absence of one RDH in knockout mice is often compensated for by others, and attempts to assign individual RDHs to specific reactions are difficult. Regardless, work in knockout mice has provided valuable information about RDH activity in the visual cycle and future studies will undoubtedly contribute further to our understanding of retinoid metabolism in the eye.
Table 2.
Summary of RDH Knockout Mouse Studiesa
| Model | Morphology | ERG | Dark Adaptation (ERG) | Retinoid Profile (dark) | Retinoid Profile (bleach) |
|---|---|---|---|---|---|
| Rdh8−/− | Normal | Normal | ↑↑ Delayed | Normal | ↑ all-trans retinal ↓ retinyl esters |
| Rdh12−/− | ↓ OS (10 months) | Normal | ↑ Delayed | Normal | ↑↑ all-trans retinal |
| Rdh8−/−Rdh12−/− | ↓ OS (3 months) | Decreased | ↑↑↑ Delayed | Normal | ↑ all-trans retinal |
| Rdh5−/− | Normal | Normal | ↑↑ Delayed | ↑ 11-cis retinol ↑ cis-retinyl esters |
↑ 11-cis retinol ↑ cis-retinyl esters |
| Rdh11−/− | Normal | Normal | ↑ Delayed | Normal | Normal |
| Rdh5−/−Rdh11−/− | Normal | Decreased | ↑↑↑ Delayed | ↑ cis-retinyl esters | ↑ cis-retinyl esters |
References are noted in text but are as follows for each knockout: Rdh8−/− (Maeda et al., 2005), Rdh12−/− (Maeda et al., 2006; Kurth et al., 2007; Maeda et al., 2007), Rdh8−/−Rdh12−/− (Maeda et al., 2007), Rdh5−/− (Driessen et al., 2000; Kim et al., 2005), Rdh11−/− (Kedishvili et al., 2002; Kasus-Jacobi et al., 2005; Kim et al., 2005), Rdh5−/−Rdh11−/− (Kim et al., 2005)
Acknowledgments
We thank Dr. Luanna Bartholomew for editorial assistance. Work was supported by grants from NIH EY04939 (to R.K.C.), EY14793 (MUSC vision core); Foundation Fighting Blindness, Inc. (Owings Mills, MD) (to R.K.C.); and an unrestricted award to the Department of Ophthalmology at MUSC from Research to Prevent Blindness (RPB; New York); R.K.C. is an RPB Senior Scientific Investigator. R.O.P. is the recipient of a RPB Medical Student Eye Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belyaeva OV, Johnson MP, Kedishvili NY. Kinetic analysis of human enzyme RDH10 defines the characteristics of a physiologically relevant retinol dehydrogenase. J Biol Chem. 2008a;283:20299–20308. doi: 10.1074/jbc.M800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva OV, Korkina OV, Stetsenko AV, Kedishvili NY. Human retinol dehydrogenase 13 (RDH13) is a mitochondrial short-chain dehydrogenase/reductase with a retinaldehyde reductase activity. FEBS J. 2008b;275:138–147. doi: 10.1111/j.1742-4658.2007.06184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44:7035–7047. doi: 10.1021/bi050226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyaeva OV, Stetsenko AV, Nelson P, Kedishvili NY. Properties of short-chain dehydrogenase/reductase RalR1: characterization of purified enzyme, its orientation in the microsomal membrane, and distribution in human tissues and cell lines. Biochemistry. 2003;42:14838–14845. doi: 10.1021/bi035288u. [DOI] [PubMed] [Google Scholar]
- Bray JE, Marsden BD, Oppermann U. The human short-chain dehydrogenase/reductase (SDR) superfamily: a bioinformatics summary. Chem Biol Interact. 2009;178:99–109. doi: 10.1016/j.cbi.2008.10.058. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Haeseleer F, Fariss RN, Aleman TS, Jang GF, Verlinde CL, Marmor MF, Jacobson SG, Palczewski K. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis Neurosci. 2000;17:667–678. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen CA, Winkens HJ, Hoffmann K, Kuhlmann LD, Janssen BP, Van Vugt AH, Van Hooser JP, Wieringa BE, Deutman AF, Palczewski K, Ruether K, Janssen JJ. Disruption of the 11-cis-retinol dehydrogenase gene leads to accumulation of cis-retinols and cis-retinyl esters. Mol Cell Biol. 2000;20:4275–4287. doi: 10.1128/mcb.20.12.4275-4287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo KM, Moiseyev G, Takahashi Y, Crouch RK, Ma JX. The 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle proteins. Invest Ophthalmol Vis Sci. 2009;50:5089–5097. doi: 10.1167/iovs.09-3797. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Huang J, Lebioda L, Saari JC, Palczewski K. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem. 1998;273:21790–21799. doi: 10.1074/jbc.273.34.21790. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Jang GF, Imanishi Y, Driessen CA, Matsumura M, Nelson PS, Palczewski K. Dual-substrate specificity short chain retinol dehydrogenases from the vertebrate retina. J Biol Chem. 2002;277:45537–45546. doi: 10.1074/jbc.M208882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, Wissinger B, Nurnberg P, Gal A. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- Jang GF, McBee JK, Alekseev AM, Haeseleer F, Palczewski K. Stereoisomeric specificity of the retinoid cycle in the vertebrate retina. J Biol Chem. 2000;275:28128–28138. doi: 10.1074/jbc.M004488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang GF, Van Hooser JP, Kuksa V, McBee JK, He YG, Janssen JJ, Driessen CA, Palczewski K. Characterization of a dehydrogenase activity responsible for oxidation of 11-cis-retinol in the retinal pigment epithelium of mice with a disrupted RDH5 gene. A model for the human hereditary disease fundus albipunctatus. J Biol Chem. 2001;276:32456–32465. doi: 10.1074/jbc.M104949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989;86:9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanan Y, Kasus-Jacobi A, Moiseyev G, Sawyer K, Ma JX, Al-Ubaidi MR. Retinoid processing in cone and Muller cell lines. Exp Eye Res. 2008;86:344–354. doi: 10.1016/j.exer.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasus-Jacobi A, Ou J, Bashmakov YK, Shelton JM, Richardson JA, Goldstein JL, Brown MS. Characterization of mouse short-chain aldehyde reductase (SCALD), an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2003;278:32380–32389. doi: 10.1074/jbc.M304969200. [DOI] [PubMed] [Google Scholar]
- Kasus-Jacobi A, Ou J, Birch DG, Locke KG, Shelton JM, Richardson JA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Edwards AO. Functional characterization of mouse RDH11 as a retinol dehydrogenase involved in dark adaptation in vivo. J Biol Chem. 2005;280:20413–20420. doi: 10.1074/jbc.M413789200. [DOI] [PubMed] [Google Scholar]
- Kedishvili NY, Chumakova OV, Chetyrkin SV, Belyaeva OV, Lapshina EA, Lin DW, Matsumura M, Nelson PS. Evidence that the human gene for prostate short-chain dehydrogenase/reductase (PSDR1) encodes a novel retinal reductase (RalR1) J Biol Chem. 2002;277:28909–28915. doi: 10.1074/jbc.M202588200. [DOI] [PubMed] [Google Scholar]
- Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson PS. Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280:8694–8704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth I, Thompson DA, Ruther K, Feathers KL, Chrispell JD, Schroth J, McHenry CL, Schweizer M, Skosyrski S, Gal A, Hubner CA. Targeted disruption of the murine retinal dehydrogenase gene Rdh12 does not limit visual cycle function. Mol Cell Biol. 2007;27:1370–1379. doi: 10.1128/MCB.01486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacik P, Kavanagh KL, Oppermann U. Structure and function of human 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2006;248:61–71. doi: 10.1016/j.mce.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA, Palczewski K. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci U S A. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44:11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- Romert A, Tuvendal P, Simon A, Dencker L, Eriksson U. The identification of a 9-cis retinol dehydrogenase in the mouse embryo reveals a pathway for synthesis of 9-cis retinoic acid. Proc Natl Acad Sci U S A. 1998;95:4404–4409. doi: 10.1073/pnas.95.8.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Hellman U, Wernstedt C, Eriksson U. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem. 1995;270:1107–1112. [PubMed] [Google Scholar]
- Takahashi Y, Moiseyev G, Farjo K, Ma JX. Characterization of key residues and membrane association domains in retinol dehydrogenase 10. Biochem J. 2009;419:113–122. doi: 10.1042/BJ20080812. 111 p following 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, von Lintig J, Oberhauser V, Tanimoto N, Grimm C, Seeliger MW. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest Ophthalmol Vis Sci. 2007;48:534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- Wu BX, Chen Y, Fan J, Rohrer B, Crouch RK, Ma JX. Cloning and characterization of a novel all-trans retinol short-chain dehydrogenase/reductase from the RPE. Invest Ophthalmol Vis Sci. 2002;43:3365–3372. [PubMed] [Google Scholar]
- Wu BX, Moiseyev G, Chen Y, Rohrer B, Crouch RK, Ma JX. Identification of RDH10, an All-trans Retinol Dehydrogenase, in Retinal Muller Cells. Invest Ophthalmol Vis Sci. 2004;45:3857–3862. doi: 10.1167/iovs.03-1302. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22:188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]


