Abstract
Experimental kinetics and computational modeling of human glutathione synthetase (hGS) support the significant role of the G-loop glycine triad (G369, G370, G371) for activity of this ATP-grasp enzyme. Enzyme kinetic experiments indicate that G369V and G370V mutant hGS have little activity (<0.7 and 0.3%, respectively, versus wild-type hGS). However, G371V retains ∼13% of the activity of wild-type hGS. With respect to G-loop:A-loop interaction in hGS, mutations at Gly369 and Gly370 decrease ligand binding and prevent active site closure and protection. This research indicates that Gly369 and Gly370 have essential roles in hGS, while Gly371 has a lesser involvement. Implications for glycine-rich ensembles in other phosphate-binding enzymes are discussed.
Keywords: glutathione synthetase, glutathione, negative cooperativity
Introduction
Glutathione (GSH) [1], an essential tripeptide antioxidant, is present in most bacterial, plant and mammalian cells. It protects against reactive oxygen species and participates in metabolism of drugs and carcinogens. GSH helps maintain protein thiols in their reduced states [2,3]. GSH is synthesized in two consecutive ATP-dependent steps, catalyzed by first γ-glutamylcysteine synthetase and then glutathione synthetase (GS; EC 6.3.2.3). Decreased levels of GSH are observed in Parkinson's disease, Alzheimer's disease [4], HIV, etc. [5]. Low levels of GSH occur in 5-Oxoprolinuria [6], a disease caused by inborn errors in GS. Improved understanding of GS is thus important to developing new therapies for GSH deficiencies.
Glutathione synthetase catalyzes the second and final step in GSH biosynthesis: ATP-dependent ligation of glycine with γ–glutamylcysteine. This process involves formation of a putative enzyme-bound acyl-phosphate that is attacked by glycine to form an enzyme-product complex. The latter dissociates with release of GSH, ADP and phosphate (Pi). Glutathione synthetase [7] belongs to the Glutathione synthetase ATP-binding domain-like (ATP-grasp) superfamily [8]. With two exceptions, structurally characterized ATP-grasp superfamily members are prokaryotic [9].
Analysis shows that the entire amino acid sequence of human GS (hGS) is only conserved in mammals, but identity remains between hGS and other eukaryotes. However, differences between human and prokaryotic GS sequences are much greater. Thus, prokaryotic GS sequences show little (<10%) sequence identity [10,11] when aligned against mammalian counterparts. However, there are specific regions (loops) of GS that are highly conserved in all species.
Structural data on hGS, co-crystallized with products [11], indicates three loops cover the active site: substrate-binding or S-loop (Phe266 - Ser276), glycine-rich or G-loop (Gln366 -Leu374) and alanine-rich or A-loop (Ile454 - Ala466). Our recent research [12] has shown that the amino acid sequence of the glycine-rich-loop (G-loop) is the most strictly conserved in eukaryotic GS.
Similar to eukaryotes, GS from E. coli has glycine-rich regions: small (164-GMGG-167) and large (226-IPQGGETRGNLAAGGR-241) loop. The structure indicates that the catalytically essential glycine-rich large loop of E. coli GS is very flexible and covers the substrate binding site [13]. Large loop residues are proposed to act as a “lid” to protect the acyl phosphate intermediate from hydrolysis during catalysis [13]. Mutagenesis studies suggested that two residues, Gly220 and Gly240, are important for substrate binding and catalytic activity [14,15]. Research [14,15] shows that the glycine-rich large loop of E. coli GS is important for enzyme activity. However, given significant differences in sequence identity and structure between human (dimer) and E. coli (tetramer) GS, it is essential to study the G-loop of hGS.
There are several examples of glycine-rich loops within enzyme catalysis that modify important conformational changes and ligand binding. For instance, X-ray crystallographic and kinetic studies of cytosolic aspartate aminotransferase containing pyridoxal 5′-phosphate (PLP) cofactor showed that mutation of a loop glycine residue (Gly38) significantly reduced enzyme activity and impaired loop structural flexibility, preventing small domain closure [16]. Triosephosphate isomerase (TIM) contains a glycine-rich flexible loop that closes the active site via interactions with the peripheral phosphate of the substrate [17]. The proposed role of the glycine residues in the loop of TIM is to bind and protect the enediol phosphate intermediate. Aminolevulinate synthase (ALAS), a PLP-containing enzyme in heme biosynthesis, has a conserved glycine-rich loop. Mutagenesis studies indicate that replacement of Gly142 or of Gly144 considerably decreases the binding affinities of PLP cofactor and also the catalytic efficiency of ALAS [18]. Dihydrofolate reductase (DHFR) [19], an important anticancer target, has a highly mobile loop with a conserved glycine residue. When Gly121 in the flexible loop is mutated to valine, binding of NADPH (nicotine adenine dinucleotide phosphate) and enzyme activity are significantly reduced [20]. Finally, there is a glycine-rich loop in the catalytic subunit of cAMP-dependent protein kinase (PKA) [21]. Several studies on PKA conclude that the glycine-rich loop positions the nucleotide triphosphate and also prevents water molecules from accessing the active site [22,23,24]. In summary, glycine residues are important in enzyme catalytic loops for controlling flexibility, ligand orientation and binding, and loop closure to protect reaction intermediates.
Based on the structure of hGS and the aforementioned literature precedents, we propose that glycine residues in the G-loop of human GS maintain loop flexibility essential for ligand binding and active site closure. We further hypothesize that glycine residues in the G-loop facilitate γ-phosphate transfer to substrate and protect the acyl-phosphate intermediate. We have constructed and characterized, by the use of experimental kinetics and modeling, mutant hGS enzymes that support the vital role of the glycine triad (G369, G370, G371) of the G-loop for hGS.
Materials and Methods
Computational Methods
Calculations were performed with MOE [25]. The AMBER94 force field [26] and PEOE atomic charges [27] were used. As in a previous theory-experiment study of hGS [12], the X-ray structure [11] for hGS (product form, PDB ID = 2HGS) [28] was used to initiate a molecular dynamics (MD) search for lowest energy conformations. Enzyme models were subjected to the MD protocol described previously [12,29]. Modeling was performed on product and reactant forms of hGS; only the latter are discussed here as the modeling results are similar and both corroborate experimental analyses.
Materials
Oligonucleotide primers for mutagenesis and sequencing were from Integrated DNA Technologies. Restriction enzymes were from New England Biolabs. Isopropyl-1-thio-β-D-galactopyranoside (IPTG) and lactate dehydrogenase (LDH) were from Amresco. The Quickchange™ site-directed mutagenesis kit was obtained from Stratagene. L-γ-Glutamyl-L-α-aminobutyrate (GAB) was synthesized as described [1,2]. All other reagents were obtained from Sigma.
Recombinant DNA methods
N-terminal His tag (His6) was added to wild-type hGS by subcloning it from plasmid pT7-7 into pET-15b vector (Novagen) at BamHI and NdeI sites (hGSpET-15b) [29]. Mutants of hGS were generated using the Quickchange™ kit (Stratagene). The internal primers used for synthesizing the mutants are shown in Table 1. The mutations were confirmed by sequencing hGS.
Table 1. Primers for site directed mutagenesis of the G-loop of glutathione synthetasea.
| Enzyme | DNA sequence |
|---|---|
| G369V | 5′–CCAGAGAGAGGTTGGAGGTAAC–3′ |
| 5′–GTTACCTCCAACCTCTCTCTGG–3′ | |
| G370V | 5′–CCAGAGAGAGGGTGTAGGTAACAACC–3′ |
| 5′–GGTTGTTACCTACACCCTCTCTCTGG–3′ | |
| G371V | 5′–CCAGAGAGAGGGTGGAGTTAACAACCTATATGGG–3′ |
| 5′–CCCATATAGGTTGTTAACTCCACCCTCTCTCTGG-3′ |
Underlined bases are changed nucleotide positions.
Enzyme purification
The growth and purification protocol adopted for the recombinant wild-type and mutant hGS was the same, as previously described [29]. All purification steps were performed at 4 °C [29]. Purified hGS proteins, wild-type and mutants, were apparently homogeneous by SDS-PAGE.
Enzyme assays and kinetic analysis
All kinetic assays were carried out in duplicate using at least two different recombinant hGS enzyme preparations. The enzyme activity was measured at 37 °C using a spectrophotometric assay that couples ADP production to NADH oxidation with continuous monitoring at 340 nm [29]. The Michaelis-Menten kinetic equation was used for glycine and ATP kinetic data analysis. For GAB kinetic analysis, the initial velocity (v), concentration (S) and Vmax are substituted into the Adair equation for negative cooperativity, and GAB Km and alpha (α), or interaction factor are calculated. The degree of negative cooperativity was assessed via a Hill plot (log[(V/Vmax - V)] versus log[S]) to obtain a Hill coefficient, h. Kinetic data was plotted and non-linear regression analysis was performed using SigmaPlot 8.0 (SPSS Science Inc.). A unit of enzyme catalyzes 1 μmol of product min-1 at 37 °C. Protein concentration was determined by the Lowry method [30] using bovine serum albumin as the standard.
Circular Dichroism
Circular dichroism was performed using a Jacso715. Enzymes in sodium phosphate buffer (10 mM; pH 7.5) were run for a total of three accumulations from 320 – 190 nm at 20 °C. Baseline was subtracted from each sample and subsequently underwent three Savitzky-Golay 25 point smoothings. The circular dichroism spectra of wild-type and mutant hGS were superimposable.
Results and Discussion
In Vitro Experiments
Our hypothesis that highly conserved glycine residues (Gly369, Gly370, Gly371) of the hGS G-loop are important for activity was evaluated by site-directed mutagenesis. Glycine triad residues were individually mutated to valine (to give G369V, G370V and G371V mutant GS), a bulky amino acid expected to hinder G-loop flexibility [20]. Kinetics indicate that purified G369V and G370V have little activity (<0.7 and 0.3%, respectively, versus WT-hGS) (Table 2). Interestingly, however, G371V retains ∼13% of the activity of the wild-type enzyme. Thus, individual mutations of glycine residues in the G-loop (Gly369, Gly370, Gly371) substantially reduce hGS activity.
Table 2. Activity and turnover number (kcat) of GS enzymesa.
| Enzyme | kcat (sec-1) | % Activity |
|---|---|---|
| Wild-type | 18.83 ± 0.77 | 100 |
| G369V | 0.133 ± 0.001 | 0.69 |
| G370V | 0.052 ± 0.026 | 0.27 |
| G371V | 2.36 ± 0.35 | 13.1 |
Results are an average of 3 different enzyme preparation, each assayed in duplicate. Control rates (lacking GAB) were subtracted to obtain kcat values. The lower limit of detection is 0.008 sec-1.
Highly purified wild-type hGS (WT-hGS) does not have activity unless all substrates (glycine, Mg-ATP, γ-glutamyl-α-aminobutyric acid (GAB), a non-thiol and non-oxidizable analog of γ-glutamylcysteine) are present. ATP hydrolysis activity is not observed in the absence of any of its substrates individually; notably WT-hGS does not hydrolyze ATP when glycine is absent, i.e., hGS not glycine-independent (Table 3). Similar to wild-type GS, the G371V mutant displays only 5% glycine-independent activity (Table 3). In contrast, both the slightly active G369V and G370V mutant hGS enzymes hydrolyze ATP in the presence of GAB, but absence of glycine (Table 3, GAB + ATP; Gly-Independent). Mutations G369V and G370V thus lead to a GAB-dependent ATPase activity (Table 3) (or “glycine-independent” GS activity). Since WT-hGS does not show ATPase activity, mutagenesis experiments indicate that highly conserved glycine residues of the G-loop have a substantial impact on enzyme function.
Table 3. Substrate dependent GS activity (kcat)a.
| Enzyme | GAB + Gly + ATP | GAB + ATP | % Gly Independent |
|---|---|---|---|
| Wild-type | 18.83 ± 0.77 | 0 | 0 |
| G369V | 0.133 ± 0.001 | 0.114 ± 0.017 | 85 |
| G370V | 0.052 ± 0.026 | 0.057 ± 0.024 | 100 |
| G371V | 2.36 ± 0.35 | 0.116 ± 0.065 | 5 |
Results are averages of replicate assays of two or three different enzyme preparations. For these studies the background rate subtracted to obtain Kcat values is that of ATP-Mg alone (Enzyme+ATP-Mg). There is no activity with glycine plus ATP.
The Michaelis constant (Km) of mutant hGS enzymes was determined for substrates (Table 4). The Km for ATP increased substantially over wild-type for both G369V and G371V mutant hGS enzymes (37- and 10-fold, respectively). The Km for glycine increased five-fold for G371V. The activity of the G370V mutant was too low for determination of Km. The little activity present for G370V was only dependent on GAB, not glycine. As previously observed [31,32], mammalian GS, a homodimer, displays negative cooperativity or non-linear double reciprocal plots for GAB. A measure of negative cooperativity is given by the Hill coefficient (h), which ranges from 0.5 to 1 for a homodimer. The G371V mutant is similarly negatively cooperative (h = 0.88) versus WT-hGS (h = 0.80). However, G369V no longer displays negative cooperativity for GAB binding. Mutations of conserved G-loop glycine triad residues thus substantially affect the Michaelis constant for the substrates of hGS.
Table 4. Km values for glutathione synthetase mutanta.
| Enzyme | ATP (mM) | Gly (mM) | GAB (mM)b |
|---|---|---|---|
| Wild-type | 0.012 ± 0.009 | 0.529 ± 0.009 | 1.26 ± 0.32 |
| Gly369Val | 0.44 ± 0.04 | N.D. | 0.89 ± 0.36 |
| Gly371Val | 0.12 ± 0.05 | 2.52 ± 0.30 | 0.100 ± 0.043 |
The results are the averages of replicate assays of two or three different preparations. N.D.: not determined.
GAB is estimated.
Based on the structure of hGS, mutations of glycine triad residues, which are near the enzyme surface, are not expected to greatly affect hGS secondary structure. Circular dichroism spectra of mutant and wild-type hGS are virtually identical (data not shown), supporting this contention, as does similarity of structures obtained from MD simulations (vide infra).
Modeling
We analyzed the geometry of reactant hGS to gain atomic-level insight into the effects of mutation on the G-loop glycine triad. Comparative structure analysis for WT-hGS and the three hGS mutants was performed.
Ligand Binding
Molecular dynamics of wild-type hGS (reactant) indicate that the G-loop has a flexible backbone that aids in substrate access to the active site. The backbone length (distance between nitrogen of Gly369 and carbonyl carbon of Gly371) is 7.92 Å – an extended conformation (Fig. 1.a). Structural analysis of the wild-type reactant shows all three glycine triad residues form hydrogen bonds with ATP γ-Pi (the phosphate lost to form acyl-phosphate intermediate). The amide of Gly369 and Gly370 each hydrogen bond (2.37 and 2.74 Å, respectively) with the same oxygen of γ-Pi, while the G371 amide hydrogen binds (2.94 Å) a different oxygen of the γ-Pi of ATP. Glycine triad residues in reactant hGS are thus oriented so that the backbone amides position the γ-Pi of ATP by forming hydrogen bonds during ligand binding.
Figure 1.
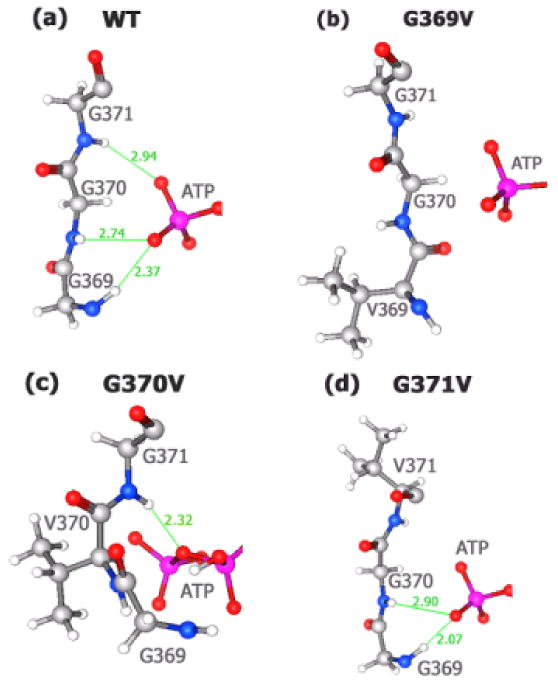
Hydrogen bonding between γ-Pi of ATP and G-loop residues (reactant). (a) wild-type; (b) G369V; (c) G370V; (d) G371V.
G369V Mutant hGS
Investigation of the modeled structure of hGS mutant G369V (reactant) reveals altered orientations of residues and ligands (Figure 1.b) versus WT-hGS. While the γ-Pi of ATP has a position similar to that of the wild-type structure, backbone amides of Val369, Gly370, and Gly371 are quite distinct in G369V. As a result of structural changes in G369V, the three backbone amide groups of the glycine triad now have an unfavorable orientation that prevents hydrogen bonding to the phosphates of ATP in G369V mutant.
G370V Mutant hGS
For reactant G370V, the orientations of ATP, the backbones of the remaining glycine residues (G369, G370), and mutated residue (G370V) are all altered. The distance between the N of Gly369 and the amide N of Asn372 in the reactant is shortened to 6.79 Å (wild-type distance = 7.24 Å). (Figure 1.c). Hence, the G-loop in G370V is in a less extended conformation than the G-loop for wild-type hGS. In G370V, γ-Pi has both a different orientation and altered hydrogen bonding arrangement vis-à-vis WT-hGS. Instead of each of the glycine residues forming a hydrogen bond with ATP as in wild-type hGS (Figure 1.a), there is only one hydrogen bond (2.32 Å, Figure 1.c) with ATP made by G371. This hydrogen bond is to the bridging oxygen between γ-Pi and β-Pi, rather than the γ-Pi of ATP. Interestingly, one of the γ-phosphate oxygens now forms a hydrogen bond (2.69 Å) with a glycine residue (Gly459) in the A-loop (vide infra).
G371V Mutant hGS
Analysis of G371V shows less drastic changes in loop orientation and ATP binding (vs. G369V and G370V) relative to WT-hGS. Even though the orientation of ATP is relatively unaffected (Figure 1.d), the valine side chain prevents Val371 from hydrogen bonding to ATP. The phosphate groups of ATP retain almost the same orientation in the active site of G371V as in the wild-type enzyme. The two remaining glycines, 369 and 370, form hydrogen bonds (2.07 Å and 2.90 Å, respectively) to the γ-Pi of ATP akin to those in wild-type hGS. The G371V mutant is thus the most structurally similar to WT-hGS of the three G-loop mutations studied in this research.
G-loop: A-loop Interaction
Our previous research [12,29] indicates that in addition to loop-ligand interactions, there are important loop-loop interactions in hGS. Interaction between the G-loop and A-loop of wild-type hGS occurs only in the reactant conformation (Figure 2.a). The carbonyl oxygen of Gly369 (G-loop) hydrogen bonds to the amide of both Val461 (2.99 Å) and Ala462 (2.44 Å) in the A-loop that lead to loop closure.
Figure 2.
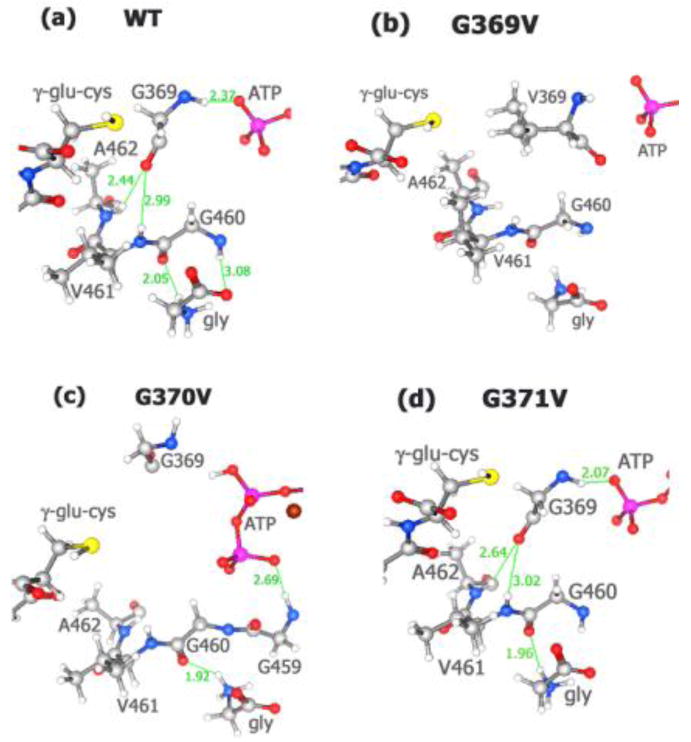
G-loop: A-loop interaction and glycine binding (reactant). The Gly370 and Gly371 residues were omitted for clarity. (a) wild-type; (b) G369V; (c) G370V; (d) G371V.
Based on substrate orientations and loop positions, we propose that G-loop:A-loop interaction [12] covers the active site, leading to loop closure, thus protecting from hydrolysis the acyl-phosphate intermediate (or the related transition state) in peptide bond formation. Hydrogen bonding patterns show that loop-loop interactions orient both the γ-Pi of ATP and the glycine substrate near (∼10 Å) of the γ-glutamylcysteine substrate. In the reactant form, Gly369, Gly370, and Gly371 bind the γ-Pi while Gly460 of the A-loop binds glycine by forming two hydrogen bonds. Thus, loop closure and glycine residues in the G-loop, particularly Gly369 and Gly370, substantially impact γ-Pi transfer from ATP to γ-glutamylcysteine while A-loop residues (Gly460, Val461, Ala462) together with Gly369 presumably facilitate glycine transfer to the acyl-phosphate. Nevertheless, further experiments are needed to confirm the role of A-loop residues.
G369V Mutant
Mutation of glycine 369 to valine alters the G-loop orientation of G369V (reactant) so that G and A loops no longer close (Figure 2.b). In the reactant G369V structure, the carbonyl oxygen of Val369 now points in the opposite direction to that found for WT-hGS, which prevents hydrogen bonding with both Val461 and Ala462. Hence, there is no longer A-loop:G-loop interaction in G369V, and thus no loop closure.
G370V Mutant
When Gly370 is mutated to valine (Figure 2.c), the distance between the Gly369 (G-loop) and A-loop residues increases significantly, from ∼3 to ∼8 Å! Hence, this mutation significantly disrupts loop-loop interaction in hGS.
G371V Mutant
Analysis of G371V shows that the geometry (Figure 2.d) for both ligand binding and for G-loop:A-loop interaction is similar to that of WT-hGS. In G371V, the oxygen atom of Gly369 still hydrogen bonds with the amide of Val461 (3.02 Å) and of Ala462 (2.64 Å) of similar length to those calculated for the wild-type enzyme. Similarly, Gly460 hydrogen bonds the glycine substrate, albeit less effectively because now there is only one hydrogen bond of 1.96 Å between the oxygen of G460 and the amide of glycine substrate in G371V instead of the two hydrogen bonds in wild-type hGS.
Conclusions
Analyses of conformational changes and hydrogen bonding variations elucidate experimentally observed decreased enzyme activity and altered binding affinity of glycine-triad mutant hGS relative to the wild-type enzyme. As a result, this research provides new and valuable insight into the function of Gly369-Gly371 residues of the hGS G-loop. Rotations around backbone C-C and C-N bonds in proteins are known to generate more flexibility for glycine than other amino acid residues [33]. Both G369V and G370V mutants have extremely low experimental activities, while G371V is less affected. However, the latter still shows a ∼10-fold decrease in kcat versus wild-type hGS. Single-point mutations (G369V, G370V, and G371V), analyzed via experiment and modeling, clarify the role of the three glycines in the G-loop of hGS. Simulations indicate that upon mutation of any of these residues, binding of γ-Pi of ATP is altered. Structural differences in mutant hGS (Figure 3) are manifested by local conformational changes that affect ligand orientation and presumably also impact transition state stabilization. In the reactant conformation, all three glycines bind the γ-phosphate of ATP. These favorable interactions detected in wild-type hGS, when combined with experimental results from site-directed mutagenesis, indicate that G369 and G370 are responsible for γ-Pi transfer to γ-glutamylcysteine, while G371 has only a minor contribution to the correct orientation of γ-Pi.
Figure 3.
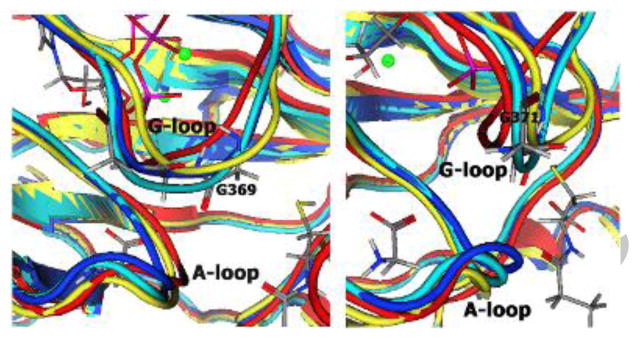
Distortion of G-loop backbone in reactant-complex for mutant enzymes. Front view (left) and side view (right) show G and A loops. Wild-type is rendered in blue, G369V in red, G370V in yellow, G371 in cyan.
Although a glycine-rich loop motif is found in many nucleotide binding enzymes, hGS displays a rare conserved Gly-Gly-Gly sequence. In contrast, the glycine-rich loop sequence in ALAS [18], DHFR [19], and the protein kinase family [34] is Gly-X-Gly-X-X-Gly, where X is a variable amino acid. Other phosphate binding loops [35] that interact with the γ-phosphate of ATP have the consensus sequence Gly-X-X-X-X-Gly, including the large loop of E. coli GS.
The present research indicates that the unusual glycine triad in the hGS G-loop greatly enhances flexibility of the backbone in close proximity to the γ-Pi of ATP. These factors facilitate a favorable orientation of ATP in the binding site, guide the transfer of γ-Pi to the substrate, and allow access of glycine substrate followed by its nucleophilic attack on the acyl-phosphate intermediate. Although the rate-limiting step is still under debate, glycine backbone atoms could easily form hydrogen bonds with the γ-phosphate of ATP in the transition state, lowering the activation energy for glutathione biosynthesis.
*Research Highlights
G-loop glycine-triad mutations greatly reduce the activity of human Glutathione Synthetase.
Mutations to glycine-triad residues decrease ligand binding and prevent active site closure.
G-loop glycines Gly369 and Gly370 have essential roles in hGS, while Gly371 has lesser involvement.
Acknowledgments
We thank V. Bhansali for technical assistance, D. Gray (UT-Dallas) for assistance in obtaining some of the circular dichroism data, K. Slavens for reading the manuscript, and the Chemical Computing Group for providing MOE.
Funding: M.E.A. acknowledges support by a Chemistry Department Welch Foundation Grant (TWU), a TWU New Faculty Research Enhancement Program award, the TWU A&S Faculty Development Fund, the NIH (grant 1R15GM086833-01), and the NSF (CHE-0820845). T.R.C. acknowledges a UNT Faculty Research Grant.
Abbreviations
- hGS
human glutathione synthetase
- GSH
glutathione
- GAB
γ-glutamyl-α-aminobutyrate
- IPTG
isopropyl-1-thio-β-D-galactopyranoside
- MCAC
metal chelate affinity chromatography
- Pi
phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson ME. Glutathione: An Overview of Biosynthesis and Modulation. Chem Biol Interact. 1998;111-112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 2.Meister A. Glutathione: Chemical, Biochemical, and Medical Aspects. John Wiley; New York, NY: 1983. [Google Scholar]
- 3.Meister A. Glutathione-Ascorbic Acid Antioxidant System in Animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 4.Bains JS, Shaw CA. Neurodegenerative Disorders in Humans: The Role of Glutathione in Oxidative Stress-mediated Neuronal Death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 5.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The Central Role of Glutathione in the Pathophysiology of Human Diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 6.Larsson A, Ristoff E, Anderson ME. The Metabolic and Molecular Bases of Inherited Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Vogelstein B, Childs B, Kinzler KW, editors. NY: McGraw-Hill; 2005. Online http://genetics.accessmedicine.com. [Google Scholar]
- 7.Meister A, Anderson ME. Glutathione. Ann Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 8.Galperin MY, Koonin EV. A Diverse Superfamily of Enzymes with ATP-Dependent Carboxylate-amine/thiol Ligase Activity. Protein Sci. 1997;6:2639–2643. doi: 10.1002/pro.5560061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John JPP, Chen WQ, Pollak A, Lubec G. Mass Spectrometric Studies on Mouse Hippocampal Synapsins Ia, IIa, and IIb and Identification of a Novel Phosphorylation Site at Serine-546. Proteome Res. 2007;6:2695–2710. doi: 10.1021/pr070157r. [DOI] [PubMed] [Google Scholar]
- 10.Huang CS, He W, Meister A, Anderson ME. Amino Acid Sequence of Rat Kidney Glutathione Synthetase. Proc Natl Acad Sci USA. 1995;92:1232–1236. doi: 10.1073/pnas.92.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polekhina G, Board PC, Gali R, Rossjohn J, Parker MW. Molecular Basis of Glutathione Synthetase Deficiency and a Rare Gene Permutation Event. EMBO J. 1999;18:3204–3213. doi: 10.1093/emboj/18.12.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinescu A, Anderson ME, Cundari TR. Catalytic Loop Motion in Human Glutathione Synthetase: A Molecular Modeling Approach. Biochem Biophys Res Comm. 2007;353:450–456. doi: 10.1016/j.bbrc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 13.Hara T, Kato H, Katsube Y, Oda J. A Pseudo-Michaelis Quaternary Complex in the Reverse Reaction of a Ligase: Structure of Escherichia coli B Glutathione Synthetase Complexed with ADP, Glutathione, and Sulfate at 2.0 Å Resolution. Biochemistry. 1996;35:11967–11974. doi: 10.1021/bi9605245. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Kato H, Nishioka T, Oda J. Mutational and Proteolytic Studies on a Flexible Loop in Glutathione Synthetase from Escherichia coli B: The Loop and Arginine 233 are Critical for the Catalytic Reaction. Biochemistry. 1992;31:2259–2265. doi: 10.1021/bi00123a007. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, Yamaguchi H, Kato H, Nishioka T, Katsube Y, Oda J. Flexibility Impaired by Mutations Revealed the Multifunctional Roles of the Loop in Glutathione Synthetase. Biochemistry. 1993;32:12398–12404. doi: 10.1021/bi00097a018. [DOI] [PubMed] [Google Scholar]
- 16.Pan QW, Tanase S, Fukumoto Y, Nagashima F, Rhee S, Rogers PH, Arnone A, Morino Y. Functional Roles of Valine 37 and Glycine 38 in the Mobile Loop of Porcine Cytosolic Aspartate Aminotransferase. J Biol Chem. 1993;268:24758–24765. [PubMed] [Google Scholar]
- 17.Pompliano DL, Peyman A, Knowles JR. Stabilization of a Reaction Intermediate as Catalytic Device: Definition of the Functional Role of the Flexible Loop in Triosephosphate Isomerase. Biochemistry. 1990;29:3186–3194. doi: 10.1021/bi00465a005. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Kay CJ, Barber MJ, Ferreira GC. Mutations at a Glycine Loop in Aminolevulinate Synthase Affect Pyridoxal Phosphate Cofactor Binding Catalysis. Biochemistry. 1996;35:14109–14117. doi: 10.1021/bi961296h. [DOI] [PubMed] [Google Scholar]
- 19.Miller GP, Benkovic SJ. Deletion of a Highly Motional Residue Affects Formation of the Michaelis Complex for Escherichia coli Dihydrofolate Reductase. Biochemistry. 1998;37:6327–6335. doi: 10.1021/bi972922t. [DOI] [PubMed] [Google Scholar]
- 20.Cameron CE, Benkovic SJ. Evidence for a Functional Role of the Dynamics of Glycine-121 of Escherichia coli Dihydrofolate Reductase Obtained from Kinetic Analysis of a Site-directed Mutant. Biochemistry. 1997;36:15792–15800. doi: 10.1021/bi9716231. [DOI] [PubMed] [Google Scholar]
- 21.Hanks SK, Hunter T. Protein Kinases 6. The Eukaryotic Protein Kinase Superfamily: Kinase (Catalytic) Domain Structure and Classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 22.Hirai TJ, Tsigelny I, Adams JA. Catalytic Assessment of the Glycine-rich Loop of the v-Fps Oncoprotein using Site-directed Mutagenesis. Biochemistry. 2000;39:13276–13284. doi: 10.1021/bi001216g. [DOI] [PubMed] [Google Scholar]
- 23.Grant BD, Hemmer W, Tsigelny I, Adams JA, Taylor SS. Kinetic Analyses of Mutations in the Glycine-rich Loop of cAMP-dependent Protein Kinase. Biochemistry. 1998;37:7708–7715. doi: 10.1021/bi972987w. [DOI] [PubMed] [Google Scholar]
- 24.Hemmer W, McGlone M, Tsigelny I, Taylor SS. Role of the Glycine Triad in the ATP-binding Site of cAMP-dependent Protein Kinase. J Biol Chem. 1997;272:16946–16954. doi: 10.1074/jbc.272.27.16946. [DOI] [PubMed] [Google Scholar]
- 25.Molecular Operating Environment (MOE), version 2003.02. Chemical Computing Group, Inc.; Montreal, Quebec, Canada: 2003. [Google Scholar]
- 26.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Jr, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- 27.Gasteiger J, Marsili M. Iterative Partial Equalization of Orbital Electronegativity—a Rapid Access to Atomic Charges. Tetrahedron. 1980;36:3219–3228. [Google Scholar]
- 28.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinescu A, Cundari TR, Bhansali VS, Luo JL, Anderson ME. Function of Conserved Residues of Human Glutathione Synthetase: Implications for the ATP-grasp Enzymes. J Biol Chem. 2004;279:22412–22421. doi: 10.1074/jbc.M401334200. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Njalsson R, Norgren S, Larsson A, Huang CS, Anderson ME, Luo JL. Cooperative Binding of Gamma-glutamyl Substrate to Human Glutathione Synthetase. Biochem Biophys Res Commun. 2001;289:80–84. doi: 10.1006/bbrc.2001.5961. [DOI] [PubMed] [Google Scholar]
- 32.Luo JL, Huang CS, Babaoglu K, Anderson ME. Novel Kinetics of Mammalian Glutathione Synthetase: Characterization of Gamma-glutamyl Substrate Cooperative Binding. Biochem Biophys Res Commun. 2000;275:577–581. doi: 10.1006/bbrc.2000.3337. [DOI] [PubMed] [Google Scholar]
- 33.Bright JN, Sansom MSP. The Flexing/Twirling Helix: Exploring the Flexibility about Molecular Hinges Formed by Proline and Glycine Motifs in Transmembrane Helices. J Phys Chem B. 2003;107:627–636. [Google Scholar]
- 34.Hirai TJ, Tsigelny I, Adams JA. Catalytic Assessment of the Glycine-rich Loop of the v-Fps Oncoprotein using Site-directed Mutagenesis. Biochemistry. 2000;39:13276–13284. doi: 10.1021/bi001216g. [DOI] [PubMed] [Google Scholar]
- 35.Saraste M, Sibbald PR, Wittinghofer A. The P-loop—A Common Motif in ATP-and GTP-binding Proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]


